Abstract
Goal-directed behavior requires cognitive control to effect online adjustments in response to ongoing processing demands. How signaling for these adjustments occurs has been a question of much interest. A basic question regarding the architecture of the cognitive control system is whether such signaling for control is specific to task context or generalizes across contexts. In this study, the authors explored this issue using a stimulus–response compatibility paradigm. They examined trial-to-trial adjustments, specifically, the findings that incompatible trials elicit improved performance on subsequent incompatible trials and that responses are slower after errors. The critical question was, Do such control effects—typically observed within a single task context—occur across task contexts? The paradigm involved 2 orthogonal, stimulus–response sets: Stimuli in the horizontal direction mapped only to responses in the horizontal direction, and likewise for the vertical direction. Cues indicated that either compatible (same direction as stimulus) or incompatible (opposite to stimulus) responses were required. The results showed that trial-to-trial adjustments exist for both direction-repeat and direction-switch trials, demonstrating that signaling for control adjustments can extend beyond the task context within which they arise.
Keywords: cognitive control, attention, behavior, Simon task
Cognitive control, which involves the coordination of cognition and behavior in accordance with internal goals, mediates online adjustments in task performance that are responsive to ongoing processing demands. For instance, subjects are known to slow their responses after committing an error (Laming, 1968; Rabbitt, 1966) and focus their attention when interference increases (Gratton, Coles, & Donchin, 1992; Laming, 1968; Logan & Zbrodoff, 1979; Rabbitt, 1966; Tzelgov, Henik, & Berger, 1992). Although much of the theorizing about and empirical study of cognitive control has traditionally focused on the execution of control, there has been growing interest in characterizing the cognitive and neural mechanisms that signal for adjustments of control. The detection of error commission may be one such cognitive mechanism, and event-related potential (ERP) studies have demonstrated the existence of electrophysiologic signatures that may mark the brain’s detection of errors (Falkenstein, Hohnsbein, Hoorman, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). Complementing and extending this work, a more recently developed theoretical framework proposes that the monitoring of response conflict may also serve as a mechanism for adjustment of control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Yeung, Cohen, & Botvinick, 2004). Response conflict occurs when there is active competition between at least two mutually exclusive responses. This can result from interference due to distracting stimulus information or from attempting to overcome prepotent response tendencies (e.g., learned or due to recent priming) that favor the incorrect response.
A growing number of ERP (Nieuwenhuis, Yeung, Wildenberg, & Ridderinkhof, 2003; van Veen & Carter, 2002; Yeung et al., 2004) and fMRI (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Carter et al., 1998, 2000; Jones, Cho, Nystrom, Cohen, & Braver, 2002; Kerns et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001) studies have indicated that the dorsal anterior cingulate cortex (ACC) may be a critical brain structure for the detection of both errors and conflict. Such indexing of conflict by ACC activation has also been shown by Kerns et al. (2004) to be predictive of subsequent behavioral adjustments as well as activations of the dorsolateral prefrontal cortex, a frontal region known to mediate strategic aspects of control. More specifically, Kerns et al. showed that during an fMRI Stroop paradigm, the magnitude of ACC activation was correlated with the two primary behavioral measures of associated control adjustments that are the focus of the current study, namely, postconflict and posterror performance adjustments. Similarly, ERP studies have shown that activity thought to arise from the ACC during error trials is predictive of the degree of posterror slowing (Debener et al., 2005; Gehring et al., 1993). Together, the findings of these neuroimaging studies are consistent with the idea that the brain has mechanisms for monitoring performance and that this monitoring process can, in turn, lead to subsequent modulations in control.
Recruitment of Control: Context General Versus Specific
Various types of tasks have been employed in studies of conflict monitoring and control, including the Stroop task (MacDonald et al., 2000), the AX-CPT (Carter et al., 1998), the Eriksen flankers task (van Veen & Carter, 2002), simple forced-choice and go/no-go paradigms (Jones et al., 2002), and spatial incompatibility tasks (Fan, Flombaum, McCandliss, Thomas, & Posner, 2003). These tasks provide an indication of the scope of different contexts within which conflict can arise—ranging from basic stimulus–response relationships such as spatial incompatibility or “Simon effect” tasks (Simon & Berbaum, 1990), to contexts set up by recent stimulus history such as in the AX-CPT, and to task representation-level contexts, as in task-switching paradigms.
Although these tasks have shown both behavioral and neuroimaging evidence of processing conflict, it remains unclear whether there is a single, generic conflict detection and resolution system, or whether there exist distinct, parallel mechanisms that map independently to particular task contexts. A meta-analysis of fMRI studies of conflict tasks (Bush, Luu, & Posner, 2000) suggested that there is at least some anatomic variability in ACC activation by different conflict tasks. This anatomic variability in activations by the different tasks may suggest that the detection of conflict and associated recruitment of control occurs in a task-specific manner. Another possibility is that despite such anatomic variability, dorsal ACC activations are functionally equivalent in that they elicit control adjustments in any subsequent task context even if this context was different from that in which the conflict arose (e.g., in switching from one task to another).
Fan and colleagues (2003) attempted to address these issues in an fMRI study, arguing against a single unified network for processing conflict. Their study employed three conflict tasks—Stroop task, Eriksen flankers task, and Simon task—which elicited overlapping activations in the anterior cingulate and left prefrontal cortex, two regions commonly activated in conflict paradigms (Kerns et al., 2004; MacDonald et al., 2000). By itself, this result may lend support for a unified network that detects and resolves conflict across task contexts. However, failing to show consistent correlations in behavioral measures of conflict between task contexts, the authors concluded that despite sharing common anatomy in their patterns of activation, the contexts elicited unrelated forms of conflict. A lack of correlations in the conflict measures, however, may not be a definitive demonstration of functional independence between the forms of conflict. For instance, the lack of correlations may have been due to variability in subjects’ profiles of sensitivity to conflict across the different contexts.
More recently, Egner, Delano, and Hirsch (2007) examined the question of generalizability of control effects in an fMRI task paradigm task incorporating both Stroop and Simon effects. In their behavioral data, they found that stimulus conflict (induced by the Stroop effect) could elicit stimulus-based control, and that response conflict (induced by the Simon effect) could elicit response-based control, but that there were no control effects that generalized across conflict type (i.e., Stroop-based conflict did not elicit Simon-based control or vice versa). Consistent with the behavioral findings, their imaging data showed no overlap in the areas related to stimulus-based (parietal cortex) versus response-based (premotor cortex) control. Similarly, other behavioral studies have demonstrated that there were no cross-over control effects between Simon and flanker tasks (Stürmer, Seiss, & Leuthold, 2005; Wendt, Kluwe, & Peters, 2006). These findings, then, are consistent with there being distinct control mechanisms for different forms of conflict.
Also arguing against a unitary control system, other studies have shown that control mechanisms are sensitive to spatially local variations in the proportion of congruent trials in the Eriksen flanker (Corballis & Gratton, 2003) and Stroop (Crump, Gong, & Miliken, 2006) tasks. It may be asked, then, whether there are any conditions under which conflict and associated control mechanisms may operate generically across task contexts. Kunde and Wühr (2006), who found that prime–target correspondence affected subsequent spatial correspondence and vice versa, concluded that control can operate generically. Fernandez-Duque and Knight (2008) also found that control was task-specific but that cued voluntary modulations in control could generalize across task. To complement these previous findings of generic control modulations, we investigated in the current study whether such generic control effects may occur across task contexts that are not differentiated by the form of conflict or control mechanisms, but rather are defined by independence in their stimulus and response sets in the context of a stimulus–response spatial compatibility paradigm.
Study Overview
In the present study, we investigated whether conflict may signal for control across contexts by employing a stimulus–response compatibility paradigm to examine two well-established conflict-induced control effects. One involves posterror adjustments in behavior. Subjects are known to get slower after an error (Laming, 1968; Rabbitt, 1966; Smith & Brewer, 1995), an observation that has been termed the Rabbitt effect after Rabbitt, who first described the effect (Rabbitt, 1966). The Rabbitt effect is typically accompanied by decreases in error rate, thus suggesting that the posterror slowing is a temporary measure to accommodate suboptimal cognitive processing (that led to the error) to ensure accuracy on posterror trials. Some studies, such as Fiehler, Ullsperger, and Von Cramon (2005) and Rabbitt and Rodgers (1997), have shown increases in posterror error rates, although these findings can likely be explained by the task timing-related parameters particular to these studies that precluded adequate processing of the posterror trial stimulus. Fiehler et al. (2005) ensured a high error rate with adaptive response deadlines, resulting in very minimal posterror slowing, and Rabbitt and Rodgers (1997) used very short response to stimulus intervals (20 or 200 ms), resulting in “error correcting responses” in which a significant portion of the posterror responses were those that would have been correct for the just-committed error. As outlined above, it is thought that conflict as indexed by ACC activation drives such posterror adjustments, with both ERP (Cho et al., 2003; Debener et al., 2005; Gehring et al., 1993) and fMRI (Kerns et al., 2004) measures of ACC activity demonstrating such a relationship.
The other behavioral effect we examined in the current study involves increased control after high-conflict correct trials. For instance, improvements in behavioral performance are seen in incongruent trials following previous incongruent trials (iI) compared with incongruent trials following congruent (cI) trials (Gratton et al., 1992). iI trials are thought to show improved performance because the previous incongruent trial is associated with high conflict, which signals for increased control on the current incongruent trial. Such signaling is absent for cI trials with a previous congruent trial, resulting in relatively lower control on the current incongruent trial. Consistent with this behavioral finding, a number of fMRI studies have shown reliably greater activation in the ACC for cI trials (high conflict, low control) as compared with iI trials (low conflict, high control; Botvinick et al., 1999; Kerns et al., 2004), and that parametric manipulation in the number of previous congruent or incongruent trials elicits monotonic modulations in ACC activation in the expected directions (Durston et al., 2003). Conversely, congruent trials following congruent trials (cC) show better performance than those following incongruent trials (iC). For iC trials, the increased control due to previous conflict works against the facilitation that would otherwise be present for the current congruent trial because increased control results in a loss of compatible information from the irrelevant dimension; for cC trials, there is no increased control state to serve as such an impediment to facilitation. Such control effects that lead to relative improvements in performance for the iI and cC trials have collectively been termed the Gratton effect (Botvinick et al., 2001), after Gratton, who described the effect (Gratton et al., 1992). In this study, given that these sequential effects may not be solely due to control adjustments (see below), we use the more general term sequential compatibility effect.
Alternative accounts suggest that these sequential effects are not due to adjustments in control but can be instead explained by priming (Mayr, Awh, & Laurey, 2003) or feature integration (Hommel, Proctor, & Vu, 2004; Wendt et al., 2006) effects. Mayr et al. (2003) showed that when stimulus repetitions were removed, such sequential effects disappeared, suggesting that these effects could be explained more simply by stimulus priming. Hommel et al. (2004) showed that such sequential effects could be explained by the extent to which the temporary integration of stimulus and response features on the previous trial was repeated on the current trial. However, studies that have controlled for such priming and feature-binding effects have still demonstrated such control adjustments (Botvinick et al., 2004; Egner & Hirsch, 2005; Ullsperger, Bylsma, & Botvinick, 2005; Verbruggen et al., 2005; Wühr, 2005). Furthermore, neuroimaging studies have shown findings consistent with modulations of control by previous conflict. As noted above, Kerns et al. (2004) examined incongruent trials preceded by incongruent trials, showing that the degree of previous conflict, as indexed by the ACC, was predictive of the degree of performance on the current trial. Administering transcranial magnetic stimulation of selected areas (premotor cortex, dorsolateral prefrontal cortex, posterior parietal cortex) during a Simon task, Stürmer and colleagues (2007) found that modulation of the Simon effect by previous incongruent trials was suppressed selectively by administering transcranial magnetic stimulation, 500–300 ms preceding the next stimulus, to the left dorsolateral prefrontal cortex, a structure implicated in preparatory control processes (MacDonald et al., 2000). Thus, although these studies do not definitively rule out alternative accounts, they are consistent with the existence of control-mediated sequential effects separate from those due to feature integration; the extent to which these two mechanisms each contribute to sequential compatibility effects depends on the specific task and task parameters (Egner, 2007).
In the current study, we aimed at avoiding priming and feature integration effects in the critical tests of across-context sequential compatibility effects, thus allowing an unconfounded investigation of the generalizability of control mechanisms. We employed the Rabbitt and sequential compatibility effects as behavioral probes to test whether the same modulations in control that have been observed typically within task contexts are also observed across contexts. We used variants of the Preparing to Overcoming Prepotency (POP) task (Cho, Konecky, & Carter, 2006; Snitz et al., 2005), a conflict paradigm that exploits conflict due to spatial stimulus–response incompatibilities, similar to the Simon effect. In the POP task, a cue is presented on each trial indicating whether to respond in the same or opposite side as the upcoming imperative stimulus that appears on the left or right side of the display screen. In the current study, all three experiments included a stimulus–response (S-R) mapping along the horizontal direction, as in the standard POP task, but also an orthogonal S-R mapping along the vertical direction. As such, there was no overlap between the stimulus or response sets of the horizontal and vertical components of the tasks. Task “context” was thus operationalized as independent S-R mappings, with the horizontal S-R mapping representing one context and the vertical S-R mapping the other context. We asked whether conflict elicited within one context could elicit control adjustments affecting the other, and vice versa. Specifically, we tested the following hypotheses:
Hypothesis 1: Previous trial conflict engendered in the context of one S-R mapping will elicit a sequential compatibility effect in the context of the other S-R mapping.
Hypothesis 2: Previous trial errors committed within the context of one S-R mapping will elicit a Rabbitt effect in the context of the other S-R mapping.
We conducted four experiments to test these hypotheses. Experiment 1 involved a cued POP task in which participants were cued on each trial to respond in the same or opposite direction of the imperative stimulus, regardless of whether the stimulus appeared along the horizontal versus vertical directions. Experiments 2 and 3 incrementally controlled for strategic factors that may have contributed to potential overlap between the two contexts in Experiment 1, thus possibly leading to spurious findings of across-context control effects. Because Experiment 1 used the same color cues to signal same or opposite response for both directions, Experiment 2 used separate cue sets for the horizontal versus vertical directions to reduce possible overlap in representations of the S-R contexts. Although Experiments 1 and 2 had separate response sets for the horizontal versus vertical directions, a single finger on a numeric keypad was used to respond for both directions. To further reduce any overlap in the response set representations, Experiment 3 used separate hands for each response set. In Experiments 1 through 3, although the cue and probe stimulus sets were uniquely mapped to their respective contexts, the overlap in their stimulus features may have blurred the distinctions between the task contexts. In Experiment 4, we eliminated such stimulus feature overlap to more definitively maintain task context separation.
General Method
Participants
Participants were undergraduate students at the University of Pittsburgh who participated for partial course credit. Nineteen subjects participated in Experiment 1 (10 men, 9 women; mean age = 21.2 years); 13 subjects participated in Experiment 2 (6 men, 7 women; mean age = 20.9 years); 44 subjects participated in Experiment 3 (20 men, 24 women; mean age = 19.9 years); 22 subjects participated in Experiment 4 (9 men, 13 women; mean age = 19.4 years). In Experiment 3, 21 of the subjects performed the task concurrent with EEG recording. All subjects had normal or corrected-to-normal vision and full-color vision (by self-report in Experiments 1 to 3 and explicitly tested in Experiment 4 with Ishihara color plates; Ishihara, 1980).
General Task Design
Subjects sat approximately 75 cm from a 15-in. CRT monitor All stimuli were generated using Eprime software (Psychology Software Tools, Pittsburgh, PA) on an IBM-compatible PC. On each trial, a cue (colored square), presented in the center of the screen, indicated how to respond to the upcoming probe stimulus The color of the cue indicated whether to respond in the same direction (congruent) or the opposite direction (incongruent) relative to the location of the upcoming probe stimulus (the letter X in the left, right, top, or bottom of screen). A probe appearing along the horizontal axis (left or right) would therefore elicit only left or right responses. A probe appearing along the vertical axis (top or bottom) would elicit only top or bottom responses. By assigning the S-R sets to separate axes (horizontal vs. vertical), we intended to introduce two independent task contexts to test hypotheses concerning across-context control adjustments.
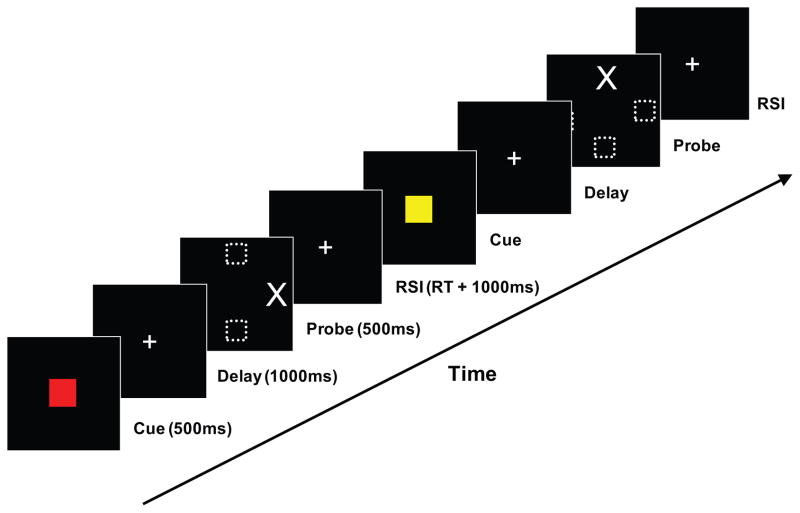
The timing and stimulus characteristics for each trial were as follows. The cue was presented for 500 ms and subtended a visual angle of 4.75 degrees. After a delay of 1,000 ms, during which only a small fixation cross was displayed in the center of the screen, the probe was presented for 500 ms. The probe subtended a visual angle of 1.9 degrees and was positioned 9.9 degrees left, right, up, or down, relative to the center of the screen (Figure 1). The reaction time (RT) was recorded from the onset of the probe, with the next trial starting 1,000 ms after the subject responded.
Figure 1.
Task format. In this two-trial progression, the subject first sees a red cue that, in this example, instructs him or her to make a response opposite to the location of the probe, which will be appearing along the horizontal axis. The probe appears on the right, so the subject is required to make a response with the left button. In the following trial, the subject sees a yellow cue, which, in this example, instructs the subject to make a response in the same direction as the probe, which will appear along the vertical axis. The probe appears in the top of the screen, requiring the subject to press the up button. RT = reaction time; RSI = response-to-stimulus interval.
The task was divided into eight blocks of 64 trials each. The proportion of horizontal and vertical trials was equal, as well as the proportion of congruent and incongruent trials. Trials were balanced across all possible previous and current trial type pair combinations. Context repeats, consisting of transitions from horizontal-to-horizontal and vertical-to-vertical trials, are denoted by H→H and V→V, respectively. Context transitions, consisting of transitions from horizontal-to-vertical and vertical-to-horizontal trials, are denoted by H→V and V→H, respectively.
Data Analysis
We used RT and accuracy as dependent measures. The overall effects of context (horizontal vs. vertical) and congruency (congruent vs. incongruent) on performance were analyzed in a 2 × 2 repeated measures analysis of variance (ANOVA).
The Rabbitt effect, describing posterror slowing, was examined for each of the four context transition types. The Rabbitt effect was evaluated on correct trials and calculated as posterror trial RTs minus postcorrect trial RTs. An accuracy version of the Rabbitt effect was also examined, calculated as posterror trial error rate minus postcorrect error rate; calculated as such, a negative number for this difference would be evidence of improvements in posterror accuracy compared with postcorrect trials. For both the RT and accuracy analyses, we performed a two-factor ANOVA with transition type (context repeat vs. switch) and performance type (posterror vs. postcorrect) as factors. For these ANOVAs, due to the relatively low error rates, we collapsed H→H and V→V trials into the context-repeat category, and H→V and V→H trials were collapsed into the context-switch category. Planned t test comparisons for each of the four individual transition types were performed with all available subjects who met the minimum data criterion.
The sequential compatibility effect has been proposed to describe the behavioral effects of higher control after a conflict trial (Botvinick et al., 2001). Thus, as explained above, iI trials show better performance than those preceded by cI trials; as well, congruent trials preceded by cC trials show better performance than those preceded by iC trials. These trial-to-trial effects manifest as a Previous Congruency × Current Congruency interaction in ANOVAs. As such, for each of the four context transition types (H→H, V→V, H→V, and V→H), 2 × 2 repeated measures ANOVAs with previous congruency and current congruency factors were conducted. Also, to verify directionality of any findings, we conducted planned comparisons (paired t tests) of iI versus cI trials and iC versus cC trials.
For all analyses, RT outliers, defined as RTs 3 slower than standard deviations above the mean or shorter than 200 ms, were also excluded. For analyses of accuracy, the error rate was arcsine transformed to approximate a normal distribution. For any analyses requiring indexing by accuracy information, a minimum of five errors for each condition-specific average (for each subject) was required. Despite emphasis of both speed and accuracy in the instructions to subjects, error rates were relatively low. Thus, Rabbitt effect analyses were conducted for Experiment 3 only because most subjects’ data were excluded by this criterion Experiments 1, 2, and 4. In examining the sequential compatibility effect for RTs, posterror trials were also excluded from analyses avoid inclusion of posterror slowing effects.
Experiment 1
Method
In the first experiment, two colors were used for the cue: green and red. A green square cued the subject to respond in the same direction as the X, regardless of whether the X appeared along the horizontal or vertical axis. A red square cued the subject to respond in the opposite direction in which the X appeared. The subjects made all responses using their right index finger on the numeric keypad of a standard PC keyboard. Subjects were instructed to keep their finger centrally placed on the 5 when not responding. Responses were made with the following mapping: 4 for left, 6 for right, 8 for up, and 2 for down responses. For instance, if preceded by a green cue, the required response for an X appearing on the right side would be a 6 key press; if preceded by a red cue, the required response would be a 4 key press for the same probe stimulus. Similarly, if preceded by a green cue, the required response for X appearing at the top of the screen would be an 8 key press; preceded by a red cue, the required response would be 2 key press for the same probe stimulus.
Results
Reaction Times
General
Overall mean reaction time was 658 ms (SD = 194). Context-switch trials (M = 702, SD = 217) were longer than repeat trials (M = 695, SD = 214), but this difference was not significant, t(18) = 1.04, ns. There were significant main effects of context, F(1, 18) = 8.98, p < .005, and congruency, F(1, 18) = 61.45, p < .001, with vertical and incongruent trials being slower; however, there was not a significant interaction between context and congruency, F(1, 18) = 1.03, ns. See Table 1 for summaries of performance broken down by context and congruency.
Table 1.
Performance Summary by Direction and Congruency for Experiment 1
| Direction | Reaction time (ms) | Error rate |
|---|---|---|
| Horizontal | ||
| Mean (SD) congruent | 664 (231) | .04 (.04) |
| Mean (SD) incongruent | 706 (212) | .04 (.05) |
| Vertical | ||
| Mean (SD) congruent | 686 (209) | .04 (.04) |
| Mean (SD) incongruent | 739 (206) | .04 (.04) |
Sequential compatibility effect
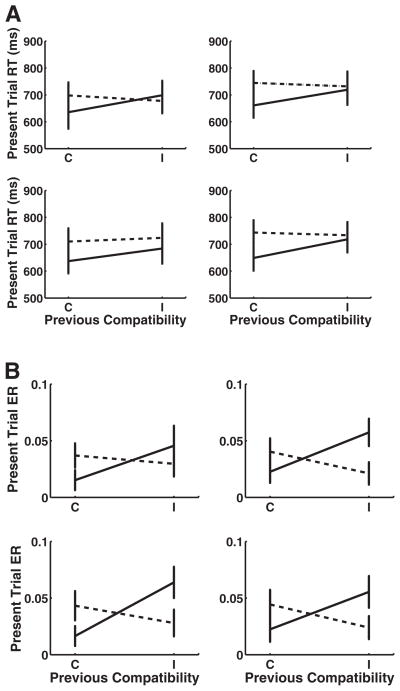
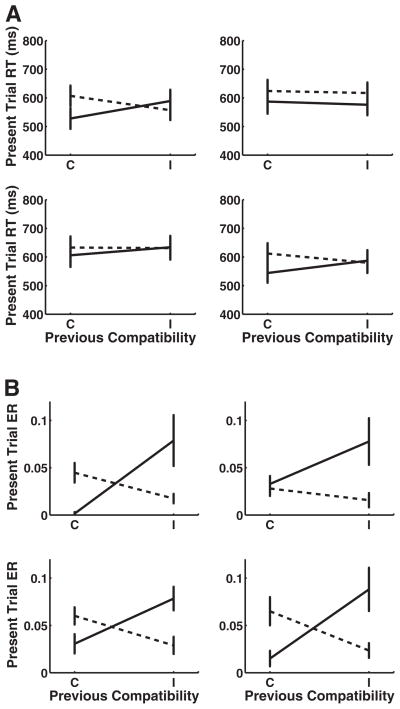
The overall sequential compatibility effect can be measured by the strength of the interaction between previous and current trial congruency. The Previous Congruency × Current Congruency interactions were significant for the context-repeat transitions, namely, the H→H and V→V transitions. Both context-switch transitions were also significant, namely, the H→V and V→H. There was no three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 18) = 1.93. Paired t tests showed that iI trials were faster than cI trials for the context repeats H→H but not for V→V; for context switches, no results were significant. The cC versus iC trial comparisons were significant for all of the context-repeat and context-switch transitions (see Table 2 for all sequential compatibility effect statistics and Figure 2 for sequential compatibility effect plots for each of the transition types).
Table 2.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Reaction Time Across Switch Types for Experiment 1
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 18) = 11.95, p < .005 | t(18) = 1.83, p < .05 | t(18) = 3.14, p < .005 |
| V→H | F(1, 18) = 4.72, p < .05 | t(18) = 0.75, ns | t(18) = 2.93, p < .005 |
| H→V | F(1, 18) = 8.22, p < .05 | t(18) = −1.05, ns | t(18) = 2.98, p < .005 |
| V→V | F(1, 18) = 10.39, p < .005 | t(18) = 0.62, ns | t(18) = 5.58, p < .001 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Figure 2.
Sequential compatibility effect in Experiment 1. Effect of previous trial congruence on present trial performance across context-switch types (error bars represent 1 standard error). Current incongruent trials are marked by the dotted lines, and current congruent trials are solid lines. (A) Results for reaction times (RT). (B) Results for error rates (ER). H = horizontal; V = vertical; C = congruent; I = incongruent.
Response Accuracy
General
The mean error rate was 3.9% (SD = 4.08). See Table 1 for summary of error rates for context and congruency. Horizontal and vertical trials showed no difference in error rate, t(18) = −0.32, ns, nor was there a difference in error rate across congruency, t(18) = 1.04, ns.
Sequential compatibility effect
The Previous Congruency × Current Congruency interactions were significant for the context-repeat transitions, namely, the H→H and V→V transitions. Both context-switch transitions, H→V and V→H, were also significant (see Table 3 for summary of statistics and Figure 2 for plots of each of the transition types). There was no three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 18) = 1.02. Paired t tests showed that iI trials were less error prone than cI trials for none of the transition types. The cC versus iC trial comparisons were significant for both the context-repeat transitions and for the context switches, the H→V transition (Table 3; Figure 2).
Table 3.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Accuracy Across Switch Types for Experiment 1
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 18) = 6.66, p < .05 | t(19) = 1.70, ns | t(19) = 2.12, p < .05 |
| V→H | F(1, 18) = 14.7, p < .005 | t(19) = 1.43, ns | t(19) = 4.43, p < .001 |
| H→V | F(1, 18) = 11.93, p < .005 | t(19) = 1.97, p < .10 | t(19) = 3.32, p < .005 |
| V→V | F(1, 18) = 9.72, p < .01 | t(19) = 1.71, p < .10 | t(19) = 3.17, p < .005 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Discussion
As hypothesized, the findings of this experiment provide evidence for control adjustments occurring not only within task context but also across task contexts, as evidenced by the significant Previous Congruency × Current Congruency interactions in the context-switch transitions. Planned comparisons showed these were primarily driven by iC versus cC performance differences, suggesting that facilitation effects figured more prominently in the sequential compatibility effect than the reduction of interference that manifests in the cI versus iI performance differences. Most notably, these across-context sequential compatibility effects were observed using completely orthogonal S-R mappings along the horizontal and vertical directions, respectively. The results suggest that conflict trials recruited control processes that affected performance during the next trial, regardless of whether it was the same or different context.
Despite the S-R mappings being orthogonal, however, the configuration of the cues may have led to a strategy confound that could have encouraged such context-general effects. Specifically, having one cue for indicating “respond same direction” and one cue for indicating “respond opposite direction” may have encouraged generic control processes that could apply equally to either of the two contexts. For example, after receiving a red cue, participants may have prepared a generic “respond opposite” strategy that could apply equally to either the horizontal or vertical contexts. The next experiment aimed at discouraging such generic preparation by employing cues that were unique to each context, as was the case for the stimulus and response sets in the first experiment. Specifically, two cues were used for the horizontal context to cue same versus opposite direction, and two different cues were used for the vertical context. As such, each cue would not only indicate whether to respond in the same versus opposite direction but would also definitively indicate which context was upcoming.
Experiment 2
Method
For horizontal trials, green and red cues indicated congruent and incongruent trials, respectively. For the vertical trials, blue and yellow cues indicated congruent and incongruent trials, respectively. The color of the cue therefore indicated the axis along which the probe would appear as well as whether to respond in the same or opposite direction as the probe. Responses were made using the same mapping as in Experiment 1.
Results
Reaction Times
General
The overall mean RT was 521 ms (SD = 85). Context-switch trials were slower than context repeats, but only at a trend level, t(12) = 1.35, p < .10. See Table 4 for descriptive statistics of context and congruency in Experiment 2. There were significant main effects of context, F(1, 12) = 17.4, p < .001, vertical > horizontal; and congruency, F(1, 12) = 11.2, p < .01, incongruent > congruent; however, there was not a significant interaction between context and congruency, F(1, 12) = 1.2, ns.
Table 4.
Performance Summary by Direction and Congruency for Experiment 2
| Direction | Reaction time (ms) | Error rate |
|---|---|---|
| Horizontal | ||
| Mean (SD) congruent | 541 (93) | .03 (.02) |
| Mean (SD) incongruent | 577 (100) | .02 (.03) |
| Vertical | ||
| Mean (SD) congruent | 587 (101) | .03 (.02) |
| Mean (SD) incongruent | 606 (108) | .03 (.02) |
Sequential compatibility effect
The overall sequential compatibility effect can be measured by the strength of the interaction between previous and current trial congruency. There was Previous Congruency × Current Congruency interaction for both context-repeat transitions H→H and V→V; for context switches, only V→H attained significance (see Table 5 for all sequential compatibility effect statistics and Figure 3 for sequential compatibility effect plots for each of the transition types). There was a three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 12) = 15.13, p < .005. Paired t tests of cI versus iI trials showed significance for both context repeats; for context switches, V→H attained significance. The same context transition types attained significance for iC versus cC comparisons (Table 5; Figure 3).
Table 5.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Reaction Time Across Switch Types for Experiment 2
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 12) = 21.01, p < .001 | t(13) = 2.48, p < .05 | t(13) = 3.21, p < .005 |
| V→H | F(1, 12) = 18.53, p < .005 | t(13) = 1.83, p < .05 | t(13) = 2.44, p < .05 |
| H→V | F(1, 12) = 0.479, ns | t(13) = −0.15, ns | t(13) = 1.63, p < .10 |
| V→V | F(1, 12) = 26.49, p < .001 | t(13) = 3.42, p < .005 | t(13) = 4.74, p < .001 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Figure 3.
Sequential compatibility effect in Experiment 2. Effect of previous trial congruency on present trial performance across context-switch types (error bars represent 1 standard error). Current incongruent trials are marked by the dotted lines, and current congruent trials are solid lines. (A) Results for reaction times (RT). (B) Results for error rates (ER). H = horizontal; V = vertical; C = congruent; I = incongruent.
Response Accuracy
General
The mean error rate was 2.7% (SD = 1.9). See Table 3 for descriptive statistics of context and congruency. Vertical trials were less accurate than horizontal trials, but only at a trend level, t(12) = 1.58, p < .10. Congruent and incongruent trials had comparable error rates, t(12) = .39, ns.
Sequential compatibility effect
Previous Congruency × Current Congruency interactions were significant for one of the context-repeat transitions, H→H, and one of the context switches, H→V, with a suggestive trend for V→H (see Table 6 for summary of statistics and Figure 3 for plots of each of the transition types). There was no three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 12) = 0.06. Comparisons of cI versus iI trials were not significant for any of the transition types. The cC versus iC trial comparisons were significant for one of the context-repeat transitions, H→H, and for one of the context switches, H→V (Table 6; Figure 3).
Table 6.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Accuracy Across Switch Types for Experiment 2
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 12) = 21.1, p < .001 | t(13) = 1.83, ns | t(13) = 4.1, p < .005 |
| V→H | F(1, 12) = 3.65, ns | t(13) = 0.93, ns | t(13) = 2.11, p < .10 |
| H→V | F(1, 12) = 9.14, p < .05 | t(13) = 1.57, ns | t(13) = 2.74, p < .05 |
| V→V | F(1, 12) = 1.49, ns | t(13) = 1.03, ns | t(13) = 0.36, ns |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Discussion
This experiment produced further evidence for control effects that carried over across contexts. In this case, only one of the two context-switch transitions (V→H) in the RT analysis had a significant Previous Congruency × Current Congruency interaction, suggesting that assigning unique cue sets to each of the contexts was effective in discouraging a strategy that generically modulated control for both contexts. Nevertheless, for the V→H transition, both the cI versus iI and iC versus cC planned comparisons were significant. Furthermore, the H→V transition also almost reached significance for the iC versus cC comparison. For the accuracy data, one of the context-switch transitions (H→V) also showed evidence of sequential compatibility effects, primarily driven by the iC versus cC comparison. Thus, even with a control for strategic effects that may result from cues being unambiguous about upcoming contexts, there was evidence for across-context control effects.
The results of Experiments 1 and 2 demonstrate the ability of conflict engendered in one context to elicit control effects in another, despite a complete separation of stimulus, response, and cue sets between the contexts. However, despite the response sets being orthogonal, all responses were produced by a single finger from one hand. Because mechanisms underlying the Simon effect are thought to include those at the response selection stage (Stürmer, Leuthold, Soetens, Schroter, & Sommer, 2002), the use of single digit as an effector for all responses may have encouraged across-context control effects despite separation of the specific response mappings across the two contexts.
Experiment 3
To address this concern, this experiment implemented a complete separation of the effectors for each of the respective responses, with two fingers from each hand mapping exclusively to one context or the other. This response set arrangement is similar to that of Kunde and Wühr (2006; Experiment 1) in which vertical and horizontal stimulus dimensions each mapped to separate response sets. However, in their study, each response hand had one finger mapped to one response set and another finger mapped to the other (e.g., left middle finger mapped to left responses and left index finger mapped to up responses). Although this arrangement may be an improvement over a single finger for all four directions, the nonuniqueness of response hand with respect to response set may still lead to representational overlap between the response sets, which would be less likely with exclusive mapping of each hand to one context or the other.
Method
To minimize any overlap in the response sets due to the use of a single finger for responding, Experiment 3 required participants to produce responses using separate hands for each of the two contexts. Responses were separated so that horizontal responses were always mapped to the right hand and vertical responses mapped to the left hand. Responses were made using a standard PC keyboard turned 90 degrees clockwise (cf. Meiran, Levine, & Henik, 2000). Left and right responses were made by pressing the 3 or 9 keys with the right index and middle fingers, respectively. Up and down responses were made with the left index and middle fingers using the left and right arrow keys, which, due to the orientation of the keyboard, were aligned up and down, respectively. In addition, the same four colors were used for cues as in Experiment 2, but the colors were counterbalanced across subjects to avoid any color-specific effects.
Results
Reaction Times
General
The overall mean RT was 460 ms (SD = 122). There was no significant difference in RTs for the subjects who performed the task concurrent with EEG recording versus behavioral alone, t(41) = 1.05, ns, and thus the behavioral results were pooled for the following analyses. Switch trials were longer than repeat trials, with a switch effect of 10 ms, t(42) = 2.22, p < .05. There were significant main effects of context, F(1, 42) = 30.5, p .001, and congruency, F(1, 42) = 98.5, p < .001, with vertical and incongruent trials being longer overall. Context and congruency also showed a significant interaction, F(1, 42) = 6.31, p < .05. See Table 7 for descriptive statistics of context and congruency.
Table 7.
Performance Summary by Direction and Congruency for Experiment 3
| Direction | Reaction time (ms) | Error rate |
|---|---|---|
| Horizontal | ||
| Mean (SD) congruent | 460 (128) | .04 (.03) |
| Mean (SD) incongruent | 513 (143) | .04 (.03) |
| Vertical | ||
| Mean (SD) congruent | 500 (144) | .04 (.04) |
| Mean (SD) incongruent | 535 (159) | .03 (.03) |
Sequential compatibility effect
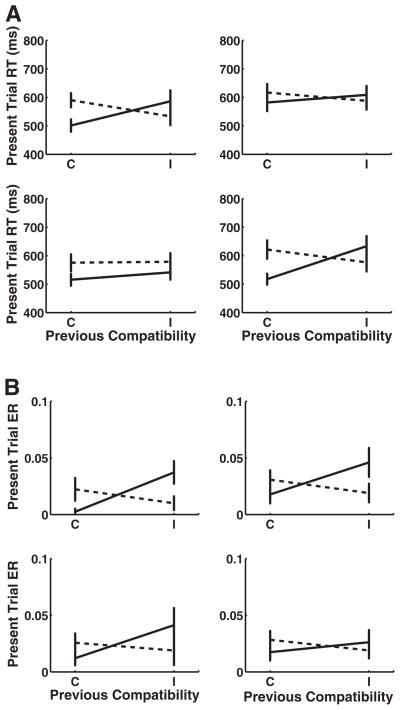
Across transition types there was a Previous Congruency × Current Congruency interaction both context-repeat transitions; for context switches, V→H attained significance (see Table 8 for all sequential compatibility effect statistics). There was a strong three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 42) = 33.86, p < .001. Comparisons of cI versus iI trials were significant for both context repeats; for context switches, neither transition attained significance. iC versus cC trial comparisons were significant for both context-repeat transitions and both context-switch transitions (see Figure 4).
Table 8.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Reaction Time Across Switch Types for Experiment 3
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 42) = 37.82, p < .001 | t(43) = 5.76, p < .001 | t(43) = 4.73, p < .001 |
| V→H | F(1, 42) = 6.08, p < .05 | t(43) = 0.69, ns | t(43) = 3.19, p < .005 |
| H→V | F(1, 42) = 13.25, p < .001 | t(43) = 0.7, ns | t(43) = 3.06, p < .005 |
| V→V | F(1, 42) = 38.07, p < .001 | t(43) = 4.85, p < .001 | t(43) = 6.56, p < .001 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Figure 4.
Sequential compatibility effect in Experiment 3. Effect of previous trial congruency on present trial performance across context-switch type (error bars represent 1 standard error). Current incongruent trials are marked by the dotted lines, and current congruent trials are solid lines. (A) Results for reaction times (RT). (B) Results for error rates (ER) H = horizontal; V = vertical; C = congruent; I = incongruent.
Rabbitt effect
An ANOVA with transition type (context repeat vs. switch) and RT type (posterror vs. postcorrect) showed main effect of RT type, F(1, 34) = 25.76, p < .001, no main effect of transition type, F(1, 34) = 0.02, ns, and no Transition × RT Type interaction, F(1, 34) = 0.42, ns. Planned tests of the posterror slowing effect for each of the four individual transition types were significant for both context-repeat (H→H and V→V) and both context-switch (V→H and H→V) transitions (see Table 9 and Figure 5a). The effect of congruency-level repeat versus alternation could not be added as an additional factor because an inadequate number of subjects had sufficient trials for each condition. separate test (in 25 subjects) of this factor showed that there were no differences, t(24) = 0.5, ns, between Rabbitt effects for congruency repeats (48 ± 16 ms) versus alternations (62 ± 22 ms)
Table 9.
Paired t Tests of Rabbitt Effect on Reaction Times for Experiment 3
| Switch type | Statistic |
|---|---|
| H→H | t(25) = 2.69, p < .01 |
| V→H | t(24) = 2.88, p < .01 |
| H→V | t(25) = 3.55, p < .005 |
| V→V | t(30) = 2.44, p < .05 |
Note. H = horizontal; V = vertical.
Figure 5.
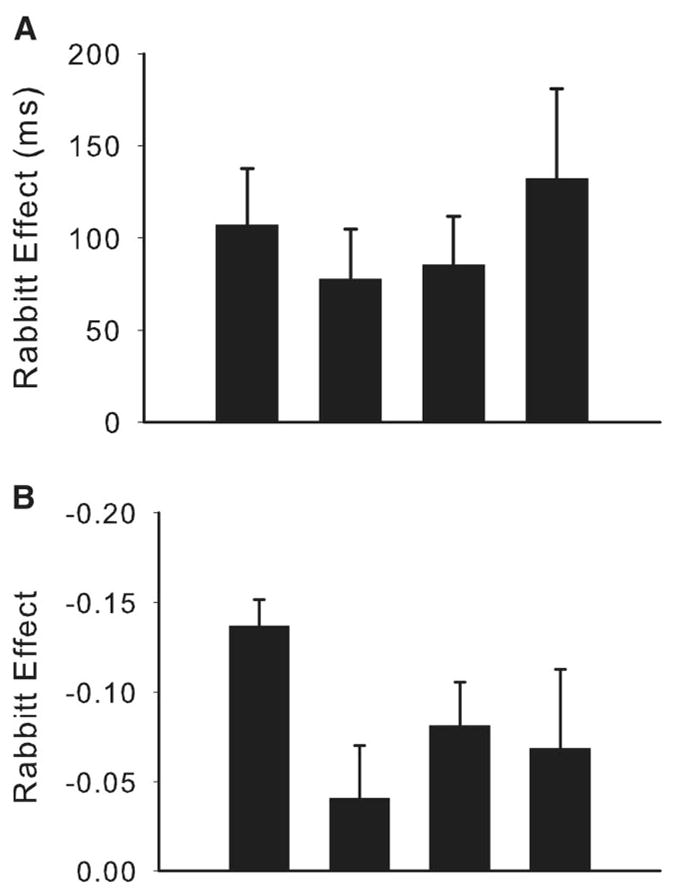
Rabbitt effect in Experiment 3. Differences between posterror and postcorrect trial performance, plotted for each context-switch type (error bars represent 1 standard error). (A) Results for reaction times (RT). (B) Results error rates (ER; arcsine transformed).
Response Accuracy
General
The mean error rate was 2.7% (SD = 1.5). See Table 7 for descriptive statistics of context and congruency. There was a main effect of context with vertical trials being less accurate than horizontal trials, F(1, 42) = 30.5, p < .001. There was no main effect of congruency.
Sequential compatibility effect
The Previous Congruency × Current Congruency interactions were significant for both context-repeat transitions, H→H and V→V, and both context switches, H→V and V→H (see Table 10 for summary of statistics and Figure 4 for plots of each of the transition types). There was no three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 42) = 0.39. Comparisons of cI versus iI trials were significant for all four transition types. The cC versus iC trial comparisons were also significant for both context repeats and both context switches (Table 10; Figure 4).
Table 10.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Accuracy Across Switch Types for Experiment 3
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 42) = 23.8, p < .001 | t(43) = 2.7, p < .005 | t(43) = 5.2, p < .001 |
| V→H | F(1, 42) = 23.65, p < .001 | t(43) = 3.26, p < .005 | t(43) = 4.2, p < .001 |
| H→V | F(1, 42) = 22.77, p < .001 | t(43) = 3.1, p < .005 | t(43) = 3.69, p < .001 |
| V→V | F(1, 42) = 21.23, p < .001 | t(43) = 3.12, p < .005 | t(43) = 3.83, p < .001 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Rabbitt effect
An ANOVA with transition type (context repeat vs. switch) and accuracy type (posterror vs. postcorrect) showed a trend-level main effect of accuracy type, F(1, 34) = 3.70, p = .06, no main effect of transition type, F(1, 34) = .26, ns, and no Transition × Accuracy Type interaction, F(1, 34) = 0.39, ns. Planned tests of the posterror accuracy improvements were significant only for one of the context-repeat (H→H) transitions and a trend toward significance in one of the context-switch (H→V) transitions (Table 11; Figure 5b). The effect of congruency-level repeat versus alternation was tested separately (25 subjects), showing no differences, t(24) = 0.8, ns, between Rabbitt effects for congruency repeats (0.057 ± 0.025 ms) versus alternations (0.035 ± 0.027 ms).
Table 11.
Paired t Tests of Rabbitt Effect on Error Rates for Experiment 3
| Switch type | Statistic |
|---|---|
| H→H | t(21) = −4.23, p < .001 |
| V→H | t(28) = −0.84, ns |
| H→V | t(27) = −2.00, p < .06 |
| V→V | t(26) = −0.99, ns |
Note. H = horizontal; V = vertical.
Discussion
The results of this experiment show that despite mapping each of the orthogonal response sets to the respective separated effector sets, there were robust across-context effects. There were significant sequential compatibility effects as evidenced by a previous by current congruency interaction for the H→V transition and by significant iC versus cC differences for both context-switch transitions. With the larger participant sample in this experiment, sufficient subjects’ data were retained to also conduct a Rabbitt effect analysis. These results showed that, in addition to the expected context-repeat posterror slowing, context-switch transitions also showed robust Rabbitt effects. In fact, the comparable magnitude of posterror slowing across all four transition types suggests that this form of performance adjustment may be a generic control mechanism that can be recruited and applied regardless of changes (or repetitions) in task context.
The results for posterror accuracy were less consistent, with only one of the transitions (H→H) attaining significance. This lack of uniformity in improvements in posterror versus postcorrect accuracy across all the transition types is consistent with the idea that posterror slowing is a temporary, strategic adjustment in control, implemented during a period of suboptimal cognitive processing (hence, the error), with the purpose of maintaining accuracy at levels sustained during periods of good performance. Posterror accuracy, then, could be expected to match, but need not necessarily improve on, postcorrect trial accuracy rates (especially in a task with relatively low error rates such as this one). Thus, even with the more rigorous control for overlap of representations of the respective response sets in this experiment, the results indicate robust evidence for both sequential compatibility and Rabbitt type control adjustments occurring across contexts.
Experiment 4
In Experiment 3, the respective response sets were further separated compared with the first two experiments by employing separate response hands for each of the response sets. However, questions may remain about how sharply defined the two task contexts were given the remaining overlap in features of the cue and probe stimuli. For instance, the cue stimuli were all visually presented colored squares shown in the identical spatial location; the probe stimulus was always an X regardless of the location of its appearance. So, despite the unique associations of the cue and probe stimuli to each respective task context, this overlap in stimulus properties may have blurred the distinction between the two contexts. To test whether the sequential adjustments observed in the first three experiments would be preserved despite elimination of such overlap in stimulus features, we further modified the cue and probe stimulus sets for the respective contexts, with the cues being auditory instead of visual and the probes being different symbols for each of the respective contexts.
Method
The cue stimuli in this experiment were auditory stimuli for one context and visual stimuli for the other context. The auditory stimuli were the words dog and cat, which indicated that the subject respond in the same or opposite direction as the location of the probe stimulus. The visual cues were colored (blue or yellow) squares as before. The meaning of the cues and which contexts the auditory versus visual cues were associated with were counterbalanced across subjects. Similarly, probe stimuli were differentiated between the contexts. For one direction, Xs were presented; for the other, Os were presented, with each symbol’s context counterbalanced across subjects. Responses were produced in the same manner as in Experiment 3.
Results
Reaction Times
General
The overall mean RT was 600 ms (SD = 259) Switch trials were longer than repeat trials, with a switch effect of 40 ms, t(21) = 6.483, p < .001. There were significant main effects of context, F(1, 21) = 4.51, p < .05, and congruency, F(1, 21) = 16.12, p < .001, with vertical and incongruent trials being longer overall. Context and congruency did not show a significant interaction, F(1, 21) = 1.02, ns. See Table 12 for descriptive statistics of context and congruency.
Table 12.
Performance Summary by Direction and Congruency for Experiment 4
| Direction | Reaction time (ms) | Error rate |
|---|---|---|
| Horizontal | ||
| Mean (SD) congruent | 572 (255) | .05 (.07) |
| Mean (SD) incongruent | 605 (256) | .03 (.03) |
| Vertical | ||
| Mean (SD) congruent | 593 (267) | .05 (.05) |
| Mean (SD) incongruent | 617 (268) | .04 (.03) |
Sequential compatibility effect
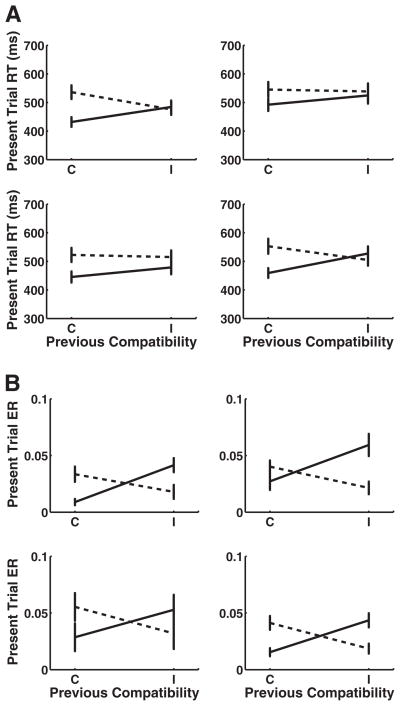
The Previous Congruency × Current Congruency interactions were significant for the context-repeat transitions, H→H and V→V, with the context-switch transition H→V approaching significance (see Table 13 for summary of statistics and Figure 6a for plots of each of the transition types). There was a strong three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 22) = 16.15, p < .001. Comparisons of cI versus iI trials were significant for both the context-repeat transitions but not the context switches. The cC versus iC trial comparisons were significant for both the context-repeat transitions and for one of the context switches, H→V (Table 13; Figure 6).
Table 13.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Reaction Time Across Switch Types for Experiment 4
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 21) = 19.79, p < .001 | t(21) = 3.87, p < .001 | t(21) = 4.36, p < .001 |
| V→H | F(1, 21) = 0.09, ns | t(21) = 0.67, ns | t(21) = −0.91, ns |
| H→V | F(1, 21) = 3.49, p < .10 | t(21) = 0.14, ns | t(21) = 2.30, p < .05 |
| V→V | F(1, 21) = 21.47, p < .001 | t(21) = 3.74, p < .001 | t(21) = 2.90, p < .005 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Figure 6.
Sequential compatibility effect in Experiment 4. Effect of previous trial congruency on present trial performance across context-switch type (error bars represent 1 standard error). Current incongruent trials are marked by the dotted lines, and current congruent trials are solid lines. (A) Results for reaction times (RT). (B) Results for error rates (ER). H = horizontal; V = vertical; C = congruent; I = incongruent.
Response Accuracy
General
The mean error rate was 4.1% (SD = 4.0). There were no significant main effects of context, F(1, 21) = 0.92, ns, or congruency, F(1, 21) = 2.02, ns, or Context × Congruency interactions, F(1, 21) = 2.48, ns. See Table 12 for descriptive statistics of context and congruency.
Sequential compatibility effect
The Previous Congruency × Current Congruency interactions were significant for both context-repeat transitions, H→H and V→V, as well as both context switches, H→V and V→H (see Table 14 for summary of statistics and Figure 6b for plots of each of the transition types). There was a three-way interaction between transition type (context repeat vs. switch) and previous and current congruency, F(1, 22) = 4.54, p < .05. Comparisons of cI versus iI trials were significant for both context-repeat and both context-switch transitions. Similarly, the cC versus iC trial comparisons were significant for both context-repeat transitions and for both context switches (Table 14; Figure 6).
Table 14.
Analysis of Variance and t Tests for Sequential Compatibility Effect on Accuracy Across Switch Types for Experiment 4
| Switch type | Previous × Current congruency | cI–iI | iC–cC |
|---|---|---|---|
| H→H | F(1, 21) = 15.21, p < .005 | t(21) = 2.18, p < .001 | t(21) = 4.37, p < .001 |
| V→H | F(1, 21) = 6.14, p < .05 | t(21) = 1.73, p < .05 | t(21) = 2.35, p < .05 |
| H→V | F(1, 21) = 17.34, p < .001 | t(21) = 2.76, p < .01 | t(21) = 4.44, p < .001 |
| V→V | F(1, 21) = 31.16, p < .001 | t(21) = 3.04, p < .005 | t(21) = 5.07, p < .001 |
Note. H = horizontal; V = vertical; cI = incongruent trials following congruent trials; iI = incongruent trials following incongruent trials; iC = congruent trials following incongruent trials; cC = congruent trials following congruent trials.
Discussion
Despite the unique mappings of cue, probe, and response sets to each respective context in the first three experiments, the remaining overlap in stimulus properties of the cue and probe stimuli may have encouraged a blurring of the two contexts into a common task representation. The larger switch costs observed in this experiment (40 ms) compared with those of Experiment 3 (10 ms) and the nonexistent costs of the first two experiments suggest that the manipulation to further separate the cue and probe sets, in addition to the already clear separation of the response sets, was effective in distinguishing the two contexts. The results of the current experiment demonstrate that, despite elimination of overlaps in the stimulus features, there continued to be robust across-context effects. In the RTs, there was suggestion of an across-context sequential compatibility effect as evidenced by a trend-level Previous Congruency × Current Congruency interaction and a significant iC versus cC difference for the H→V transition. In the accuracy data, evidence for across-context sequential compatibility effects was very robust and consistent, with significant Previous Congruency × Current Congruency interactions as well as significant cI versus iI and iC versus cC comparisons for both across-context transition types. Thus, even with the more rigorous control for overlap of representations of the respective cue and probe stimulus sets in this experiment, the results provide robust evidence for sequential compatibility effects occurring across contexts.
General Discussion
The current set of experiments examined whether signaling for recruitment of control generalizes across task contexts. We investigated this question specifically for four versions of a cued stimulus–response compatibility task, employing incrementally stringent controls for strategic effects that could have spuriously led to across-context findings due to possible blurring of the distinction between the contexts. Across all experiments, separate contexts were operationalized as orthogonal stimulus–response mappings. Instead of the single cue set for Experiment 1, Experiment 2 further separated the contexts by employing two separate cue sets that indicated the upcoming direction of stimulus as well as the required same or opposite response. Experiment 3 further separated the contexts by requiring a separate response hand for each of the response sets as opposed to the single right-hand digit that produced all of the responses for Experiments 1 and 2. Across all of these paradigm variants, evidence was found for across-context control adjustments. Experiments 1 through 4 all showed the sequential compatibility effect, and Experiment 3 which had sufficient errors for analysis, also showed the Rabbitt effect. These findings indicate that for task contexts defined by separated stimulus–response sets, conflict and errors that arise in one context can generalize as signals for control in other contexts.
Although there was evidence for across-context sequential compatibility effects, these effects were somewhat less robust compared with the context-repeat transitions, with Experiments 2, 3, and 4 showing significant interactions of the RT sequential compatibility effect with transition type (context repeat vs. switch) and with Experiment 4 also showing the same interaction for the accuracy measure. For context repeats, all of the Previous Congruency × Current Congruency interactions for RTs were significant, and all but one (the cI vs. iI comparison for V→V transitions in Experiment 1) of the planned comparisons were also significant across all of the experiments. In contrast, for context switches, there were significant Previous Congruency × Current Congruency interactions for RTs across all of the experiments but never for both the transition types (V→H and H→V). Similarly, the iC versus cC comparisons were significant for all of the V→H transitions for three of the four experiments, but the other planned comparisons attained significance less consistently.1
Compared with the RTs, the accuracy data showed much more consistent evidence of across-context sequential compatibility effects, but they showed a similar tendency to be less robust than the within-context effects. These results are consistent with the idea that there were common control mechanisms at work in both transition types, but that the task contexts may have been sufficiently differentiated to diminish the adjustment effects across the contexts. It may also be that the stronger effects observed during within-context versus across-context transitions were due to feature integration effects (Hommel et al., 2004) or priming (Mayr et al., 2003). On the integration account, the temporary integration of stimulus and response features from one trial leads to facilitation or interference depending on the extent to which such integration is replicated or violated on the next trial. Priming effects, which involve exact stimulus repetitions during cC and iI trials, may also confound interpretations of sequential compatibility effects. In our paradigms, such integration and priming effects would be possible during within-context transitions but not during across-context switches due to the separation of stimulus and response sets between the orthogonal directions. Although one cannot rule out the possibility that within-context effects are solely due to such integration or priming effects, it would seem unlikely that any control effects would operate only during across-context switches at the exclusion of within-context transitions. Using a similar spatial compatibility paradigm, Wühr (2005) demonstrated sequential compatibility effects (along a single directional axis) while controlling for integration and priming effects, consistent with a control account of these effects. However, while using stimuli that had vertical and horizontal spatial dimensions, the two required responses were mapped along the vertical dimension, precluding examination of the across-context effects investigated in the current study.
In contrast to the sequential compatibility effects, the Rabbitt effects for context switches were just as robust as those for context repeats. Together, these results indicate that, although the control adjustments were not always as robust as with repetitions of context, there was consistent evidence across all of the experiments for modulation of control across switches of context.
Relationship of Context-General Control to Task Switching
Task-switching paradigms have offered a unique set of tools to probe cognitive control mechanisms involved in the maintenance and updating of task sets. As the investigation of context-general control effects required a task-switching format in our experiments, it is natural to ask what relevance the mechanisms discussed in the task-switching literature may have to understanding the findings of the current study. However, due to the critical comparisons used to index across-context control effects, as well as the specific configuration of the stimulus and response sets in our experiments, the mechanisms typically invoked to explain performance costs inherent in task switching may not apply in explaining our results. The critical comparisons in probing for context-general control effects always involved a contrast between two quantities that were matched for the type of task switch. For example, in checking for a sequential compatibility type across-context control effect with a cI versus iI trial type comparisons during H→V switches, the two trial types were matched for type of task switch, in this case, H→V. This was true also for tests of across-context Rabbitt effects, in which posterror versus postcorrect reaction times would be compared, again matched for the type of task switch Controlling for task switch type in this manner precluded any simple explanations of across-context control effects by mechanisms that have been invoked to explain diminished performance during task switches.
Although controlling for generic task switch mechanisms should have avoided the influence of these factors on trial-to-trial adjustments, we nevertheless configured our stimulus and response sets to further minimize any possible interactions. Specifically, in defining each of the two task contexts for each experiment, we configured the two stimulus sets to be completely orthogonal to each other and likewise for the two associated response sets. In Experiment 4, we varied stimulus modality (for the cue) and identity (for the probe) for each context to further distinguish the task contexts. These task design manipulations aimed at avoiding the influences of a number of mechanisms thought to contribute to switch costs, defined as the increase in reaction time incurred when switching from one task to another in comparison with a repeat of the same task.
Switch costs have been attributed to various factors, including the time needed to reconfigure the task set (Logan & Gordon, 2001; Rogers & Monsell, 1995), task-set priming effects (Allport, Styles, & Hsieh, 1994), and episodic bindings of previous stimulus and response codes (Hommel, 1998). The effects of both task-set priming and episodic binding rely on at least some overlap in the features of stimuli that appear in the previous and current trials of a task switch. In our experiments, such overlap was purposefully avoided. It may be argued that if our attempts to avoid such overlap had failed, then the two contexts would be subsumed under a common representation, which would then open the findings to a task-set priming interpretation (Allport & Wylie, 2000), that is, repeats of congruency conditions (cC or iI) would confer performance advantages (by virtue of the repetition of task set and not because of control adjustments) over alternations (iC or cI, respectively). Although such an account could be consistent with our findings, the significant switch costs in Experiments 3 and 4 suggest that, at least in those experiments,2 the task parameters successfully separated the respective contexts. In addition, because a number of studies of unidirectional Simon tasks have provided evidence for sequential compatibility effects mediated by control mechanisms, it seems reasonable to assume that similar mechanisms may be operating during the across-context transitions in our tasks. For instance, ACC activity, as an index of conflict, predicted the degree of subsequent performance adjustments (Kerns, 2006) and the sequential compatibility effect was suppressed by transcranial magnetic stimulation of the left dorsolateral prefrontal cortex (Stürmer, Redlich, Irlbacher, & Brandt, 2007). Definitive demonstration, however, will require similar physiologic measures to show that the within-context findings of these previous studies can be replicated for across-context transitions in paradigms such as ours.
As for any potential task-set reconfiguration costs, these may have been present, but as noted above, would have been controlled for in the critical comparisons of interest (e.g., comparing cI vs. iI during a H→V transition, both the cI and iI trials are indexed by a task switch of the same type). Even if some more subtle interactions with task-switching dynamics were possible (e.g., previous congruent or incongruent trials during switches leading to stronger or weaker representations of the current task), it is unlikely that these influences were significant, because there were no significant switch costs in Experiments 1 and 2, and the significant switch cost in Experiments 3 and 4 were minimal (10 ms and 40 ms, respectively).3 Thus, the design of our experiments and data analytic approach likely minimized any direct or more subtle influences by mechanisms thought to contribute task-switch costs.
It should be noted that in a study of task switching, Goschke (2000) did examine the role of previous trial congruency on the switch cost, finding that previous incongruent trials increased the switch cost in comparison to previous congruent trials. This effect was primarily mediated by previous incongruent trials, leading to a large increase in the switch trial RTs as well as a modest decrease in the repeat trial RTs. This finding highlights the modulating effect of conflict in a previous task context on the establishment of a new task set. However, this result does not directly address the specific question of the current study, which asks whether the control mechanisms that support performance within a task context, as indexed by the sequential compatibility and Rabbitt effects, may also generalize to support performance across task contexts.
Generalizability of Current Findings
The current study defined task context at a very basic level, namely, by the unique associations between stimulus and response sets. Within each context, the same types of cognitive processes were engaged, specifically, those that would mediate mappings of spatial location to the appropriate responses or reversals of these mappings as prompted by the preceding cues. Using a flanker task that similarly alternated between vertical and horizontal stimulus arrangements, Mayr et al. (2003) reported finding no evidence of control-related sequential adjustments. However, in this paradigm, there was not a speed emphasis, which has been shown to be necessary for eliciting sequential compatibility effects (van Veen, 2006). In addition, there was no evidence of sequential control adjustments in a single-direction (horizontal) version of the flanker task in their study, so there would be no reason to expect control adjustment effects across direction switches. It can be asked, then, how well our findings would generalize to task context switches in which the cognitive processes are more differentiated across the component tasks. Findings from both our group (Cho et al., 2006) as well as others (Egner et al., 2007; Wendt et al., 2006) indicate that for task switches involving tasks that rely on the Stroop (Stroop, 1935) and Simon (Simon & Berbaum, 1990) or similar stimulus–response compatibility effects, there is no across-context sequential compatibility effect. In contrast, our Stroop–Simon task-switching paradigm demonstrated robust across-context Rabbitt effects. Together, these findings suggest that the Rabbitt effect may result from a more easily generalizable mechanism, such as the adjustment of generic response thresholds, and is therefore more easily applicable across task contexts. In contrast, the sequential compatibility effect may require direct involvement of more task-specific mechanisms, and thus, any across-context effects may depend on the similarity of the component tasks. Switching paradigms involving component tasks such as the Stroop and Simon tasks may involve forms of task-specific control mechanisms that are too differentiated to generalize across task contexts, whereas in the current study, there may have been sufficient similarity in the component task representations to elicit across-context sequential compatibility effects.
Consistent with this idea, Kunde and Wühr (2006) also found sequential compatibility effects across contexts defined by stimulus–response sets, eliciting conflict through the same mechanism for each stimulus–response set, namely, spatial incompatibilities between prime and target stimuli. However, as opposed to complete separation (using different response hands) implemented in Experiments 3 and 4 of the current study, their response sets were less separated as each response hand contributed a response finger to each of their two response sets. Notebaert and Verguts (2008) also examined this idea using a paradigm that incorporated spacial-numerical association of response codes (SNARC) (Dehaene, Bossini, & Giraux, 1993) and Simon effects as irrelevant stimulus features, finding that across-task Gratton effects occurred only when the relevant task feature was shared across SNARC and Simon trials.
It is of interest that Fernandez-Duque and Knight (2008) were able to elicit across-task sequential effects, even with component tasks that were quite different from each other (color Stroop vs. number Stroop task, as well as flanker vs. number Stroop task), but only when subjects were given warning cues regarding the congruency of trial N − 1 that would modulate performance on trial N. Although the cues used in the current study were meant to be instructional, it may be that they also served as warning cues as in the Fernandez-Duque and Knight study, thus augmenting across-context control effects. It may be of interest to study a task version in which the instructional cue is presented concurrently with the target stimulus (e.g., the color of the target itself indicating responding in the same or opposite direction).
An interesting possibility is that across-context sequential compatibility effects occur when there is a grouping of the component task contexts into a “meta-task” representation—one that encourages generic sequential compatibility-type control adjustments across task contexts so long as the component contexts are included in the meta-task grouping. In the current study, the component task contexts may naturally have led to such a meta-task representation because of their similarity with regard to the basic stimulus and response processing requirements. Very different task contexts (e.g., as defined by Stroop and Simon tasks) may not lead naturally to such a meta-task representation and thus prevent any associated across-context sequential compatibility effects. According to this view, however, such generic across-context control may be elicited even with two tasks that appear to be highly differentiated, if a meta-task representation is formed, for example, through extensive practice or appropriate reward contingencies.
Summary and Conclusions
In the current study, we asked a basic question regarding the architecture of the cognitive control system: Whether signaling for control is context-specific versus generalizing across contexts. With a set of experiments using orthogonal, stimulus–response sets, we found that control mechanisms, as indexed by the sequential compatibility and Rabbitt effects, behave in a context-general manner. Defining task context at such a basic level, while essentially keeping constant the required cognitive processes in each context, may have encouraged such context-general effects. While implementing a complete separation of the component stimulus and response sets afforded straightforward interpretation of our results, this commonality of cognitive processes in each context may be essential for such generalization of control effects to occur, although it may occur also with task contexts involving more differentiated cognitive processes when control mechanisms are appropriately activated by warning cues. In contrast, Rabbitt effects appear to generalize easily across contexts, perhaps owing to the generic nature of its possible mechanism (e.g., posterror adjustment of response thresholds). Thus, in answering the question of how generalizable control mechanisms are, the answer appears to depend on the degree of commonality of the cognitive operations in each context, the specific state that the control system is configured to be in, and which specific control mechanism is being examined.
Acknowledgments
This work was supported by National Institute of Mental Health Grants MH073955 awarded to Raymond Y. Cho and MH047073 awarded to Jonathan D. Cohen, and a NARSAD Young Investigator Award to Raymond Y. Cho. Portions of these data were presented at the Cognitive Neuroscience Society 2003 Annual Meeting, San Francisco, CA. Thanks to those who aided in data collection and processing for Experiment 4.
Footnotes
The sequential compatibility effects were strong enough in many cases that the congruency effect actually appeared to be not simply reduced but reversed by a previous incongruent trial (analyses not reported, but many of the pairwise tests of reversal were statistically significant). One might assume that cognitive control effects might reduce or eliminate congruency effects but not actually reverse them. However, a control-based account may still suffice in explaining such a result. For instance, assume that there are three main sources of influence on response production: (a) probe stimulus location (noted STIM); (b) cue information of the current trial (CUE); and (c) the control signal of the previous trial (CTRL), active only after a previous incongruent trial by biasing toward an incongruent response. If we further assume that the influence of CTRL is stronger than the other two sources, then the iC condition may actually elicit more conflict than iI. Consider, for example, a trial with a right-sided probe stimulus. For iC trials, CTRL biases the left response, whereas both CUE and STIM bias the right response. With the assumed relative strengths, the right and left responses are comparably active, thus leading to high conflict and long RTs. For iI trials, both CUE and CTRL bias the left response, whereas only the STIM biases the right response; the large asymmetry (left > right) in response biasing would lead to minimal conflict and short RTs. Clearly, this account rests on a number of assumptions, but it provides a possible explanation involving control adjustments that is open to testing against alternative accounts.
Having no switch costs in Experiments 1 and 2 does not necessarily mean that the two directions were not contextually distinct. Our aim was to set up two separate “task contexts,” not “two tasks” as is typically done in standard task-switching paradigms. In the latter case, switch costs usually arise when changing S-R mappings are applied to different features of exactly the same types of stimuli. In our study, we have attempted to create two separate “task contexts,” each defined by S-R sets that are independent from the other. Thus, with the stimulus arrays for the H versus V directions not overlapping, a lack of switch cost may not be surprising. Also note that for Experiments 3 and 4, the switch costs may simply be due to switches in “univalent” stimulus and response features (i.e., changes in stimulus identity or response hand) and, thus, not reflective of a further separation of task context. To eliminate such potential priming effects, future work employing redundant classes of stimuli and responses for each dimension (e.g., for stimuli, X and O stimuli for the horizontal direction, H and P for the vertical direction) may help to clarify this matter by permitting examination of direction switches without priming effects.
The question may arise why even a minimal switch cost would be incurred, given that both the stimuli and responses are univalent, that is, each stimulus and response unambiguously belongs to one of two orthogonal S-R sets. One could reasonably assume that this lack of overlap between S-R sets would prevent any cross-talk between them and thus eliminate any switch costs, in contrast with the substantial switch costs observed in other standard task-switching paradigms that employ bivalent stimuli and responses (e.g., Rogers & Monsell, 1995). One possible explanation for the modest switch cost observed in the current experiment may be that the task set is fully reactivated only on exposure to the imperative stimulus (the “stimulus-cued completion hypothesis” in Rogers & Monsell, 1995). A finding consistent with such a mechanism is the presence of a residual switch cost, even after foreknowledge of the required task over an extended preparatory period (e.g., 1,200 ms in Rogers & Monsell, 1995). In our paradigms, despite having a preparatory period of 1,500 ms, subjects may still have required the probe stimulus to fully reactivate the relevant task set during switch trials, thus resulting in a RT cost that would not be incurred during a repeat trial (the small magnitude of such a cost due to the lack of cross-talk between the S-R sets that would otherwise have increased the cost).
Contributor Information
Raymond Y. Cho, Department of Psychiatry, University of Pittsburgh
Joseph M. Orr, Department of Psychiatry, University of Pittsburgh
Jonathan D. Cohen, Department of Psychology, Princeton University
Cameron S. Carter, Department of Psychiatry, University of California, Davis
References
- Allport DA, Styles E, Hsieh S. Switching intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance XV: Conscious and nonconscious information processing. Cambridge, MA: MIT Press; 1994. pp. 421–452. [Google Scholar]
- Allport DA, Wylie G. Task switching, stimulus–response bindings, and negative priming. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 35–70. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver TS, Yeung N, Ullsperger M, Carter CS, Cohen JD. The conflict monitoring hypothesis: Computational and empirical investigations. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Press; 2004. pp. 91–102. [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999 November 11;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998 May 11;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Cohen JD, vanVeen V, Kerns J, Konecky RO, Clark K, et al. An ERP study and connectionist model of cognitive control deficits in schizophrenia. Paper presented at the 9th Annual Meeting of the Organization of Human Brain Mapping; New York, NY. 2003. Jun, [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impaired cognitive control and frontal cortical gamma-band synchrony in schizophrenia. Proceedings of the National Academy of the Sciences. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis PM, Gratton G. Independent control of processing strategies for different locations in the visual field. Biological Psychology. 2003;64:191–209. doi: 10.1016/s0301-0511(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Crump MJ, Gong Z, Milliken B. The context-specific proportion congruent Stroop effect: Location as a contextual cue. Psychonomic Bulletin & Review. 2006;13:316–321. doi: 10.3758/bf03193850. [DOI] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Bossini S, Giraux P. The mental representation of parity and number magnitude. Journal of Experimental Psychology: General. 1993;122:371–396. [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Egner T. Congruency sequence effects and cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:380–390. doi: 10.3758/cabn.7.4.380. [DOI] [PubMed] [Google Scholar]
- Egner T, Delano M, Hirsch J. Separate conflict-specific cognitive control mechanisms in the human brain. NeuroImage. 2007;35:940–948. doi: 10.1016/j.neuroimage.2006.11.061. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoorman J, Blanke L. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Knight M. Cognitive control: Dynamic, sustained, and voluntary influences. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:340–355. doi: 10.1037/0096-1523.34.2.340. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, Von Cramon D. Electrophysiological correlates of error correction. Psychophysiology. 2005;42:72–82. doi: 10.1111/j.1469-8986.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Goschke T. Intentional reconfiguration and involuntary persistence in task-set switching. In: Monsell S, Driver J, editors. Control of cognitive processes: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 331–335. [Google Scholar]
- Gratton G, Coles MGH, Donchin E. Optimizing the use of information: Strategic control of activation and responses. Journal of, Experimental Psychology: General. 1992;4:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Hommel B. Event files: Evidence for automatic integration of stimulus–response episodes. Visual Cognition. 1998;5:183–216. [Google Scholar]
- Hommel B, Proctor RW, Vu KPL. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Ishihara S. Ishihara’s tests for colour-deficiency. Tokyo: Kanehara; 1980. [Google Scholar]
- Jones AD, Cho RY, Nystrom LE, Cohen JD, Braver TS. A computational model of anterior cingulate function in speeded response tasks: Effects of frequency, sequence, and conflict. Cognitive, Affective, & Behavioral Neuroscience. 2002;2:300–317. doi: 10.3758/cabn.2.4.300. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Anterior cingulate and prefrontal cortex activity in an fMRI study of trial-to-trial adjustments on the Simon task. NeuroImage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, III, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004 February 13;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kunde W, Wühr P. Sequential modulations of correspondence effects across spatial dimensions and tasks. Memory and Cognition. 2006;34:356–367. doi: 10.3758/bf03193413. [DOI] [PubMed] [Google Scholar]
- Laming DRJ. Information theory of choice reaction times. London: Academic Press; 1968. [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychological Review. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7:166–174. [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000 June 9;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Sequential compatibility effects in the absence of executive control. Nature Neuroscience. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Meiran N, Levine J, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Wildenberg WVD, Ridderinkhof R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial-type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Verguts T. Cognitive control acts locally. Cognition. 2008;106:1071–1080. doi: 10.1016/j.cognition.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Errors and error correction in choice reaction time tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Rodgers B. What does a man do after he makes an error? An analysis of response programming. Quarterly Journal of Experimental Psychology. 1977;29:727–743. [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Simon JR, Berbaum K. Effect of conflicting cues on information processing: The “Stroop effect” vs. the “Simon effect. Acta Psychologica. 1990;73:159–170. doi: 10.1016/0001-6918(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Smith GA, Brewer N. Slowness and age: Speed–accuracy mechanisms. Psychology and Aging. 1995;10:238–247. doi: 10.1037//0882-7974.10.2.238. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: Functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1935;18:643–662. [Google Scholar]
- Stürmer B, Leuthold H. Control over response priming in visuomotor processing: A lateralized event-related potential study. Experimental Brain Research. 2003;153:35–44. doi: 10.1007/s00221-003-1579-1. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schroter H, Sommer W. Control over location-based response activation in the Simon task: Behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Redlich M, Irlbacher K, Brandt S. Executive control over response priming and conflict: A transcranial magnetic stimulation study. Experimental Brain Research. 2007;183:329–339. doi: 10.1007/s00221-007-1053-6. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Seiss E, Leuthold H. Executive control in the Simon task: A dual-task examination of response priming and its suppression. European Journal of Cognitive Psychology. 2005;17:590–618. [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling Stroop effects by manipulating expectations for color words. Memory and Cognition. 1992;20:727–735. doi: 10.3758/bf03202722. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM. The sequential compatibility effect: It’s not just priming. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- van Veen V. Unpublished doctoral dissertation. University of Pittsburgh; Pittsburgh, PA: 2006. A neuroimaging approach to the relationship between attention and speed–accuracy tradeoff. [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Liefooghe B, Notebaert W, Vandierendonck A. Effects of stimulus–stimulus compatibility and stimulus–response compatibility on response inhibition. Acta Psychologica. 2005;120:307–326. doi: 10.1016/j.actpsy.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Wendt M, Kluwe RH, Peters A. Sequential modulations of interference evoked by processing task-irrelevant stimulus features. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:644–667. doi: 10.1037/0096-1523.32.3.644. [DOI] [PubMed] [Google Scholar]
- Wühr P. Evidence for gating of direct response activation in the Simon task. Psychonomic Bulletin and Review. 2005;12:282–288. doi: 10.3758/bf03196373. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]