Abstract
Human newborns are susceptible to microbial infection and mount poor vaccine responses, yet the mechanisms underlying their susceptibility are incompletely defined. We have previously reported that despite normal basal expression of Toll-like receptors (TLRs) and associated signaling intermediates, human neonatal cord blood monocytes demonstrate severe impairment in TNF-α production in response to triacylated (TLR 2/1) and diacylated (TLR 2/6) bacterial lipopeptides (BLPs) {Levy et al J Immunol 173: 4627}. We now demonstrate that in marked contrast, BLP-induced synthesis of IL-6, a cytokine with anti-inflammatory and Th2-polarizing properties, is actually greater in neonates than adults. Remarkably, newborn blood plasma confers substantially reduced BLP-induced monocyte synthesis of TNF–α, while preserving IL-6 synthesis, reflecting the presence in neonatal blood plasma of a soluble, low-molecular weight inhibitory factor (< 10 kDa) that we identify as adenosine, an endogenous purine metabolite with immunomodulatory properties. The neonatal adenosine system also inhibits TNF-α production in response to whole microbial particles known to express TLR2 agonist activity, including Listeria monocytogenes, E. coli (that express BLPs), and zymosan particles. Selective inhibition of neonatal TNF-α production is due to the distinct neonatal adenosine system, including relatively high adenosine concentrations in neonatal blood plasma and heightened sensitivity of neonatal mononuclear cells to adenosine A3 receptor-mediated accumulation of cAMP, a second messenger that inhibits TLR-mediated TNF–α synthesis but preserves IL-6 production. We conclude that the distinct adenosine system of newborns polarizes TLR-mediated cytokine production during the perinatal period and may thereby modulate their innate and adaptive immune responses.
Keywords: Human, Monocytes/Macrophages, Immunodeficiency, TNF-α, Inflammation
Introduction
Human newborns suffer a relatively high frequency and severity of invasive microbial infections compared to healthy adults (1). The challenge of addressing neonatal susceptibility is further compounded by the poor memory responses that neonates mount to most vaccines (2). This susceptibility necessitates a conservative diagnostic and therapeutic approach to newborns and is generally ascribed to “immaturity” of the newborn immune system. Recent studies suggest that neonatal immunity may be Th2-biased, presumably to avoid Th1-type inflammation-induced maternal rejection of fetal antigens that can result in spontaneous abortion or premature delivery and its attendant complications (3, 4). Thus both innate and adaptive immunity are distinct at birth relative to adulthood, but the molecular mechanisms underlying these differences are still being defined.
Toll-like receptors (TLRs) play crucial roles in the recognition of microbes by the innate immune system and trigger responses important to both acute inflammation and the instruction of adaptive immunity (5). Despite the growing appreciation of the importance of TLRs in recognition of microbes and their products, much remains to be learned about their expression and function at birth (6). We have recently shown that despite normal basal expression of TLRs, CD14, and TLR–associated signaling intermediates, neonatal blood monocytes demonstrate reduced TLR agonist-induced synthesis of TNF–α, a pro-inflammatory cytokine with Th1-polarizing activity (7). This deficiency in TLR-induced TNF–α was particularly pronounced for the bacterial lipopeptides (BLPs), including the triacylated BLP Pam3Cys-SSNA (TLR 2/1) and the diacylated BLP macrophage–activating lipopeptide (MALP; TLR 2/6), that required at least 2-3 logs greater concentrations to induce equivalent TNF–α synthesis in neonatal blood monocytes than in adult blood monocytes (7).
BLPs are expressed by a wide range of Gram-positive, Gram-negative and Mycoplasma species and contain a distinct, amino-terminal lipo–amino acid, N–acyl-S-diacylglycerylcysteine, that is the target of innate immune surveillance (8). During invasive infections, bacteria release BLPs into the bloodstream, thereby activating inflammatory responses that influence the outcome of infection (9). The immunostimulatory activities of BLPs have been effectively reproduced by synthetic bacterial lipopeptides corresponding to the N-termini of BLPs that activate host cells via TLR2 (10). Such synthetic BLPs not only model important inflammatory bacterial surface components, but are also candidate vaccine adjuvants (11, 12), further elevating the importance of characterizing neonatal innate immune responses to BLPs. Of note, in addition to its role in detecting pure BLPs, TLR2 also plays a sentinel role in detecting whole microbes, including important neonatal pathogens, such as E. coli (via detection of BLPs (9)), L. monocytogenes (13), as well as yeast such as Candida albicans (14).
Our previous study raised fundamental questions regarding the specificity and mechanism of altered TLR-induced cytokine production in human newborns (7). As that study was focused on the cytokine TNF–α, it was unclear whether the impairment in the inflammatory response to TLR agonists is a generalized phenomenon or cytokine-specific. Although the study indicated that differences in soluble factor(s) in neonatal and adult plasma account for decreased TLR-induced neonatal TNF–α production, it was also unclear whether the ability of neonatal plasma to limit TLR-induced TNF–α production reflected the absence of an activator or the presence of an inhibitor. Finally, the identity of such a soluble plasma modulatory factor was unknown.
We now report that in marked contrast to deficient TLR-induced TNF–α synthesis from neonatal blood monocytes, BLP- and whole microbe-induced production of IL-6, a cytokine with anti-inflammatory (15) and Th2-polarizing properties (16, 17), remains fully intact in newborns. Moreover, we demonstrate that adenosine, an endogenous purine metabolite with immunomodulatory properties (18, 19), significantly contributes to the impairment of the neonatal TNF–α response to BLPs and to whole microbial particles. Neonatal blood plasma contains relatively high adenosine concentrations and neonatal cells have heightened sensitivity to adenosine's actions. Adenosine, via engagement of A3 adenosine receptors, induces generation of cyclic adenosine monophosphate (cAMP), a second messenger that inhibits BLP- and microbe-induced TNF–α synthesis from neonatal monocytes while preserving BLP- and microbe-induced IL-6 production.
Materials and Methods
Blood
Peripheral blood was collected from healthy adult volunteers (mean age 26.2 years) and newborn cord blood (mean gestational age 38.3 weeks) collected immediately after cesarean section delivery of the placenta. Births at which antibiotics were administered during labor and/or delivery, and births to HIV-positive mothers were excluded. Human experimentation guidelines of the US Department of Health and Human Services, Children's Hospital, Boston, and the Brigham & Women's Hospital were observed, following protocols approved by local Institutional Review Boards. Blood was anticoagulated with 109 mM sodium citrate or, for preparation of serum, collected into sterile tubes without additives (Becton Dickinson, Franklin Lakes, NJ). Plasma was prepared by centrifugation of blood (930 g for 15 min) and serum by allowing blood to clot (30 min, room temperature) prior to centrifugation (930 g for 20 min). For experiments employing hemocytes (i.e., white and red blood cells), whole blood was centrifuged and the cellular fraction washed three times with sterile, pyrogen-free Hank's Balanced Salt Solution (HBSS) buffer without magnesium or calcium (Gibco BRL, Grand Island, NY) prior to cell resuspension in either autologous or heterologous citrated plasma, as previously described (7). Mononuclear cells (MCs) were isolated from newborn cord blood (CBMCs) and from adult peripheral blood (PBMCs), also as previously described (7). In brief, heparinized blood was layered onto Ficoll-Hypaque gradients (Sigma), and the MC layer collected and subjected to hypotonic lysis to remove red blood cells. MCs were subsequently cultured in suspension (106 cells/mL) in fresh autologous serum or citrated plasma. A similar pattern of TLR-mediated TNF-α and IL-6 production, of inhibition of BLP-induced TNF-α production, and of cellular cAMP content, was observed when culturing cells in either neonatal serum or citrated plasma.
TLR Agonists
The synthetic triacylated BLP Pam3-Cys-SSNA corresponding to the N-terminus of a BLP from E. coli B/r (20) was from Bachem Bioscience (King of Prussia, PA) and the synthetic diacylated BLP macrophage–activating lipopeptide-2 (MALP) from Mycoplasma fermentans (21) (S-(2,3-bisAcyloxypropyl)-cysteine-GNNDESNISFKEK) was from Alexis Biochemicals (Lausen, Switzerland). Specificity of TLR agonists was previously confirmed using TLR-transfected Human Embryonic Kidney (HEK) 293 cells as well as a neutralizing mAb to TLR2 (7, 22). Microbial particles with known TLR2 agonist activity included E. coli, known to express BLPs that activate TLR2 (9), Listeria monocytogenes (13), and zymosan (23). E. coli K1/r, a bacteremic isolate, was a kind gift of Dr. Alan Cross (U. of Maryland) and was grown as previously described (24). L. monocytogenes serotype 4b (isolated from the cerebrospinal fluid of a child with meningitis; American Type Culture Collection strain 13932) was grown in Brain Heart Infusion broth (Becton Dickinson; Sparks, MD) overnight and then in sub-culture to an O.D. 650nm of 0.5 then re-suspended in sterile saline to 3 × 109/mL. Bacteria were heat-killed at 80°C for 60 min and an aliquot plated to confirm lack of viability. Zymosan (from Saccaromyces cerevisiae; InvivoGen) was prepared in 10% EtOH made up in sterile, endotoxin-free water.
Cell stimulation and cytokine measurement
BLPs were incubated in whole blood or in MC suspensions (106 cells/mL) in pyrogen-free polypropylene tubes (Posi-Click tubes, Denville Scientific; Metuchen, NJ) for 5 hours in room air at 37°C with end-over-end rotation, samples were diluted with five volumes of ice-cold RPMI medium (Gibco BRL) and centrifuged at 1,020x g at 4°C for 5 minutes. The supernatant was recovered and stored at –20°C until assay of TNF–α or IL-6 by ELISA (R&D Systems, Minneapolis, MN). In some experiments, cytokines were measured by flow cytometry on a MoFlo cytometer (DakoCytomation) using cytometric bead array according to the manufacturer's instructions (BD Biosciences).
cAMP measurement
cAMP was measured in lysates of MCs by competitive immunoassay using acetylation-enhanced detection as per the manufacturer's instructions (R & D Systems).
Plasma dialysis
For experiments employing dialyzed plasma, freshly prepared adult and neonatal plasma derived from citrated blood was dialyzed at 4°C in 10kDa dialysis cassettes (Pierce; Rockford, IL) against phosphate-buffered saline. After two buffer exchanges, the dialysate was recovered and anti-coagulated with sodium heparin (10 U/mL) for further testing.
Adenosine modulation experiments
For experiments employing selective adenosine congeners (25), cells were pre-incubated with adenosine antagonists for 30 min prior to addition of stimuli. Adenosine, or the A3-selective agonist N6-(3-iodobenzyl)ADO-5'N methyl uronamide (IB-MECA; (26)), the A3 receptor–antagonist MRS 1220 (27), the A1 receptor-selective antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; (28)), and the A1/A3 antagonist xanthine amine congener (XAC; (25)) were from Sigma (St. Louis, MO). The A2A–antagonist ZM-241385 (29) was from Tocris Cookson (Ellisville, MO). Dibutyryl cAMP (db-cAMP) was from Calbiochem (La Jolla, CA).
For experiments in which plasma was treated with adenosine deaminase (ADA), plasma was preincubated for 5 minutes at 37°C with (or, for purposes of control, without) 0.0125U/mL erythrocyte-derived Human ADA (Sigma). MCs were cultured in this pre-treated plasma (106 cells/mL) and stimulated with control buffer (MEM) or MALP-2 (1 μg/mL) with end-over-end rotation at 37°C for 5 h. After stopping the reaction with 4 volumes of ice-cold RMPI, samples were centrifuged at 1020 g at 4°C for 5 min and supernatants collected for subsequent TNF-α ELISA.
Measurement of plasma adenosine
Plasma adenosine concentrations were measured using a high-sensitivity modification of the HPLC technique previously described (30). 2.8 mL of adult peripheral and newborn cord blood were collected into syringes pre-coated with a stabilization buffer containing heparin (to inhibit blood clotting; final concentration 10 U/ml), dipyridamole (to prevent platelet aggregation; final concentration 10μM), and erythro-9-C2-Hydroxy-3-nonyl-adenine hydrochloride (EHNA; to inhibit adenosine deaminase; final concentration 10 μM). Blood was immediately centrifuged at 1500rpm for 30s at RT and plasma from like tubes collected and pooled. Plasma proteins were precipitated by addition of one volume of 10% trichloroacetic acid and incubation for 30 min at 4°C. After organic extraction with one volume of Freon-Trioctylamine, the top aqueous phase containing purine metabolites was collected by centrifuging at 1400rpm for 5m at 4°C and immediately frozen at –80°C. The aqueous phase was applied to a C-18 Sep-Pak cartridge (Waters, Milford, MA) and eluted off with methanol. After evaporation of the methanol, the samples were reconstituted in water, and the adenosine concentration was determined by reverse-phase high-performance liquid chromatography, as previously described (31). Samples were applied to a Bondapack C-18 column (Waters, Milford, MA) and eluted with a linear 0-40% gradient of 0.01M ammonium phosphate (pH 5.5) and methanol formed over 70 minutes with a flow rate of 1.5 ml/minute. Adenosine was identified by retention time and by the characteristic ultraviolet absorption spectrum, and the concentration calculated in comparison to standards, as previously described (31).
Statistical Analyses
Unless otherwise stated in the figure legend, comparisons were made with the non-parametric Mann-Whitney test using Prism 4 for MacIntosh v. 4.0a (GraphPad Software Inc.; San Diego, CA). Differences were considered significant for p values < 0.05.
Results
Low TNF–α but high IL-6 responses to BLPs in neonates
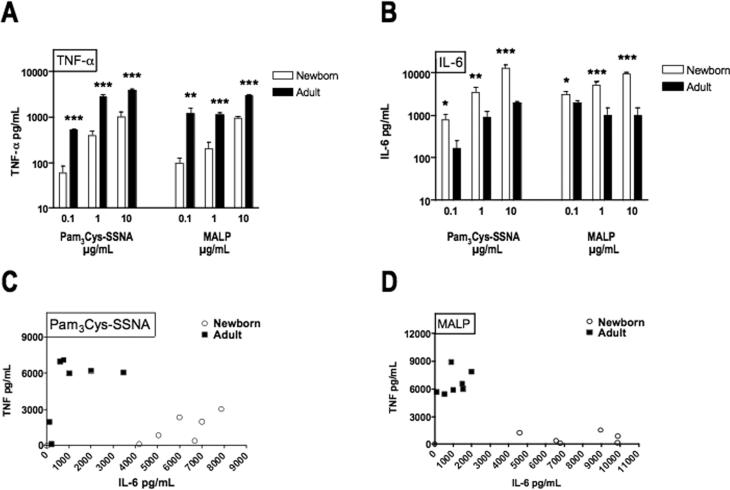
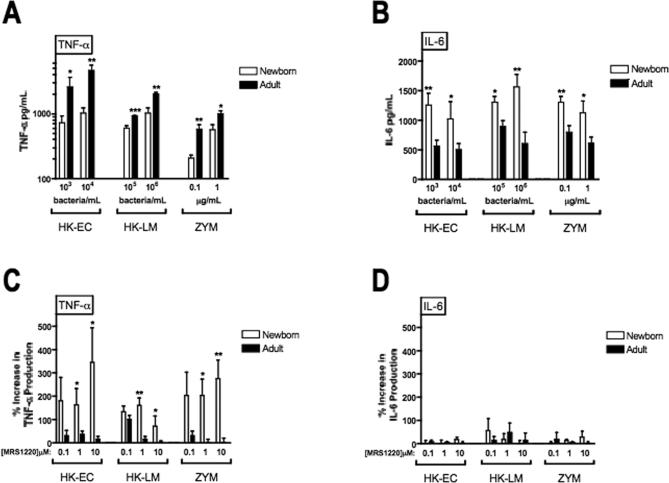
To characterize another facet of the neonatal innate immune response to BLPs, we compared BLP-induced production of TNF–α, whose synthesis is known to be impaired in newborns (7), with BLP-induced production of IL-6, a cytokine with distinct functional properties (15, 17) and whose synthesis is regulated differently from that of TNF–α. TNF–α production in response to Pam3Cys-SSNA and MALP was ~100-1000-fold greater (in relation to the agonist dose-response curve) in adult blood than in newborn cord blood (Fig. 1A), consistent with our previous observations (7). In marked contrast, these same BLPs induced greater concentrations of IL-6 in newborn blood as in adult blood (Fig. 1B). To further explore the discrepancy in the TNF–α to IL-6 ratio for neonates and adults, we plotted representative data points with respect to the concentration of TNF–α (y–axis) and IL-6 (x–axis; Fig. 1C & D), demonstrating that, in marked contrast to adults, neonatal samples consistently cluster at high concentrations of IL-6 but very low concentrations of TNF–α. Flow cytometry analysis of intracellular IL-6 production revealed that, similar to the results of our analysis for TNF-α production (7), BLP-induced IL-6 production in both neonatal and adult blood is predominantly confined to monocytes with no detectable production in granulocytes or lymphocytes (data not shown).
FIGURE 1. BLPs induce low TNF–α but high IL-6 production in newborn cord blood.
The triacylated BLP Pam3Cys-SSNA (TLR 1/2) or the diacylated BLP MALP (TLR2/6) were added to citrated whole blood at the indicated concentrations and incubated for 5 h at 37°C. Production of TNF–α (A) and IL-6 (B) was measured by ELISA or by flow cytometry using a cytometric bead array (BD Biosciences). Values represent the mean ± SEM, N = 13 - 19, *p < 0.05, **p < 0.01, ***p < 0.001. To demonstrate the marked discrepancy in neonatal and adult ratios of IL-6 to TNF–α, TNF–α is plotted as a function of IL-6 for neonatal (N = 3) and adult (N = 3) whole blood stimulated with Pam3-Cys-SSNA (C) or MALP (D) at 1 or 10 μg/mL.
Neonatal plasma inhibits BLP-induced TNF–α
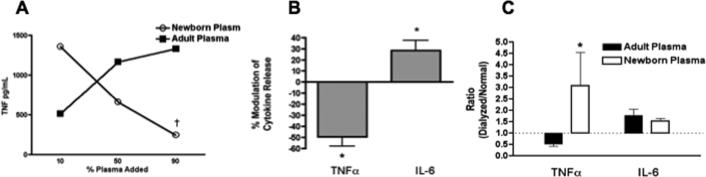
Our previous study suggested that differences in soluble factors between neonatal and adult blood plasma contributed to the differences in TLR-mediated TNF–α synthesis (7). Such an observation could indicate either that neonatal plasma lacks an activator or that it contains an inhibitor of BLP-induced TNF–α synthesis. The ability of neonates to produce robust amounts of IL-6 in response to BLPs suggested that the primary pathway for detection of BLPs by TLRs is intact in neonates. We therefore considered the possibility that neonatal plasma might contain a selective inhibitor of BLP-induced TNF–α (but not IL-6). To test this hypothesis, we added an increasing proportion of neonatal plasma to adult hemocytes (i.e., the cellular fraction of blood, prepared as described in Materials and Methods) cultured in 10% adult plasma as shown in the representative experiment depicted in Figure 2A. Whereas the addition of adult plasma resulted in increased BLP-induced TNF–α production, addition of neonatal plasma reduced BLP-induced TNF–α production in a dose-dependent manner. Composite analysis of several such experiments revealed a significant inhibition by neonatal plasma of BLP-induced TNF–α synthesis, but not BLP-induced IL-6 synthesis (Fig. 2B), indicating that newborn plasma contains an inhibitor of BLP-induced TNF–α production.
FIGURE 2. Neonatal plasma contains a low molecular-weight inhibitor of BLP-induced TNF–α production.
(A) Adult hemocytes (washed blood cells, prepared as described in Methods) derived from citrated blood were cultured in 10% autologous plasma and an increasing percentage (vol/vol) of adult or neonatal plasma prior to addition of Pam3Cys-SSNA (1μg/ml) and measurement of TNF–α production (representative experiment). The dagger symbol (†) at 90% neonatal plasma indicates the condition for the pooled analysis, shown in panel (B), that exerted a significant inhibition relative to the effect of adult plasma (N = 4). (C) Newborn or adult citrated plasma was dialyzed (10kDa cut-off) against 1X PBS and the dialysate added to hemocytes prior to addition of MALP (10 μg/mL). After 5 hours of incubation the extra-cellular medium was collected for TNF–α and IL-6 ELISAs. For comparison of the effects of dialyzed newborn vs. dialyzed adult plasma, a pooled analysis of cytokine responses of newborn and adult cells under each plasma condition is depicted, representing the mean ± SEM of the ratio of cytokine concentrations in the dialyzed to the normal plasma conditions. N = 4; * p < 0.05.
As several physiologic mediators capable of inhibiting monocyte TNF–α synthesis are of low-molecular weight (32-34), we evaluated the effect of dialyzing neonatal or adult plasma through a 10kDa pore-size membrane. Dialyzed or control undialyzed plasma was then added to washed neonatal or adult hemocytes prior to the addition of BLP and subsequent measurement of TNF–α production. Remarkably, dialysis of neonatal, but not adult, plasma substantially enhanced BLP-induced TNF–α production from adult and neonatal cells (Fig. 2C). In contrast, dialysis of newborn or adult plasma had little to no effect on BLP-induced IL-6 synthesis. These results indicate that neonatal plasma contains a low-molecular weight inhibitor of BLP-induced TNF–α.
Neonatal plasma increases cAMP levels in blood mononuclear cells
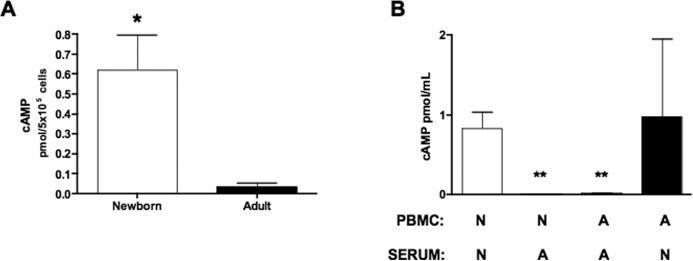
In characterizing the mechanism for low BLP-induced TNF–α production from newborns we considered published work indicating that agents that elevate intracellular cAMP concentrations inhibit stimulus-induced TNF–α synthesis but preserve (or even enhance) IL-6 synthesis (35, 36). To determine whether neonatal mononuclear cells may be exposed to cAMP-inducing factors in neonatal blood plasma, we measured intracellular cAMP concentrations in neonatal and adult PBMCs. Freshly isolated PBMCs were lysed and cAMP measured by competitive immunoassay (Fig. 3A). Remarkably, neonatal PBMCs contain nearly 20-fold higher concentrations of cAMP under basal conditions than do adult mononuclear cells (0.62 ± 0.18 vs. 0.035 ± 0.02 pmol/5 × 105 cells; N = 5, p = 0.01), raising the possibility that soluble factor(s) in neonatal blood plasma may enhance cellular cAMP synthesis. To explore this possibility, adult and neonatal PBMCs were cultured in the presence of autologous or heterologous serum prior to measurement of intracellular cAMP (Fig. 3B). When cultured in neonatal serum, neonatal PBMCs contained substantial amounts of intracellular cAMP whereas adult cells cultured in neonatal serum demonstrated variable cAMP accumulation. In marked contrast, PBMC, whether from neonates or adults, cultured in adult serum did not contain any detectable quantities of cAMP. These results indicate that soluble factor(s) in neonatal serum are able to induce cAMP accumulation, especially in neonatal cells. Given the known role of cAMP in inhibiting stimulus-induced TNF–α synthesis (37, 38), such results raise the possibility that induction of cAMP synthesis mediates low BLP-induced TNF–α synthesis.
FIGURE 3. Neonatal serum enhances cellular cAMP content.
A) Newborn CBMC or adult PBMCs (106 cells/mL) were briefly (~ 5min) cultured in 100% autologous serum then lysed in hydrochloric acid for cAMP measurement by competitive immunoassay (R&D Systems, Minneapolis, MN). Differences between newborns (N = 5) and adults (N = 5) were significant (P = 0.01). B) Newborn CBMCs (N) or adult PBMCs (A) were incubated in 100% autologous or heterologous serum for 5 minutes prior to measurement of intracellular cAMP. ** p < 0.01.
cAMP inhibits BLP-induced TNF–α but preserves BLP-induced IL-6
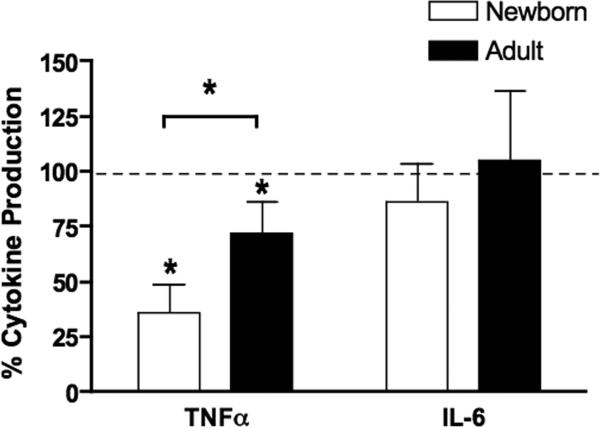
The greater intracellular concentration of cAMP in neonatal PBMCs (Fig. 3A) raises the possibility that elevated levels of this secondary messenger contribute to lower BLP-induced TNF–α production. Several studies have demonstrated that agents that induce intracellular accumulation of cAMP inhibit TNF–α production in response to “endotoxin” or LPS in leukocytes (36, 39) and other cells types (40). However, to our knowledge, such an inhibitory effect of cAMP has not yet been demonstrated with respect to BLP-induced TNF–α synthesis. To assess whether BLP-induced TNF–α production by neonatal mononuclear cells is inhibited by intracellular cAMP, we tested the effect of adding the cell-permeable cAMP analogue dibutyryl cAMP (db-cAMP) to neonatal CBMCs or adult PBMCs cultured in autologous serum. db-cAMP significantly inhibited MALP-induced TNF–α production from both neonatal and adult MCs without inhibiting MALP-induced IL-6 production (Fig. 4). Of note, db-cAMP exerted a significantly greater inhibitory effect on neonatal than adult TNF-α production. These results suggest that the inhibitory factor present in neonatal blood plasma (Fig. 2), may act via enhancing cellular cAMP levels.
FIGURE 4. Accumulation of cAMP in mononuclear cells inhibits BLP-induced TNF–α but not BLP-induced IL-6.
Neonatal CBMCs or adult PBMCs (106/mL) were cultured in 100% autologous serum prior to the addition of the cell-permeable analog dibutyryl-cAMP (10 μM). After a 5 h exposure to MALP (10 μg/ml), cytokine production was measured by ELISA. Cytokine activity was calculated by dividing the concentration of cytokine in the presence of db-cAMP by the concentration of cytokine in the presence of control buffer. Values represent the mean ± SEM; N = 4. db-cAMP significantly inhibited both adult and neonatal TNF-α production, with a greater inhibitory effect on neonatal cells. *p < 0.05.
Adenosine receptor A3 antagonists selectively enhance BLP-induced TNF–α (but not IL-6) production in neonatal blood
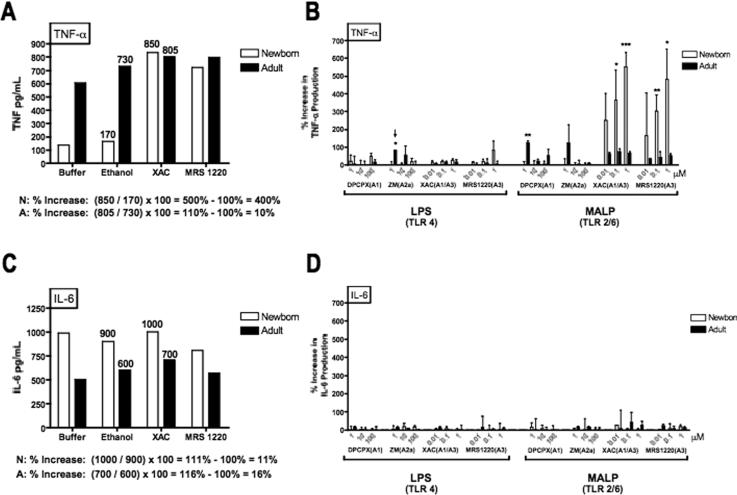
Several physiologic mediators of low molecular weight including catecholamines (32), prostaglandins (33) and purine metabolites (41), are known to limit stimulus-induced TNF–α production. These soluble physiologic mediators signal cells via cognate seven-transmembrane receptors that are G-protein-coupled and activate adenylate cyclase thereby enhancing concentrations of cAMP that inhibits stimulus-induced monocyte TNF–α synthesis. To determine whether one of these known inhibitory factors might contribute to the inhibitory effect of neonatal plasma on BLP-induced TNF–α synthesis, we tested a panel of selective antagonists, including antagonists of catecholamines, prostaglandins, and adenosine. Although inhibitors of epinephrine, norepinephrine and prostaglandins had little or no effect on BLP-induced TNF–α production in whole blood (not shown), the addition of xanthine amine congener (XAC), an adenosine receptor A1- and A3–antagonist, or MRS1220, an A3 receptor selective antagonist, to whole neonatal cord blood consistently and dramatically enhanced BLP-induced neonatal TNF–α production in a dose-dependent manner (Fig. 5), raising the possibility that adenosine, an endogenous purine metabolite with immunomodulatory properties (18), serves to severely limit BLP-induced neonatal TNF–α synthesis. To study the effects of adenosine antagonism on BLP-induced cytokine production in whole blood, the effect of a given antagonist on stimulus-induced cytokine production was analyzed relative to the amount of cytokine produced in the presence of control buffer (Fig. 5 A and C).
FIGURE 5. A3 adenosine receptor antagonists selectively enhance BLP-induced TNF–α production in neonatal cord blood.
Citrated neonatal cord or adult peripheral whole blood was pre-incubated with adenosine antagonists for 30 min at 37°C prior to addition of LPS (1 ng/mL) or MALP (1 μg/mL) and subsequent 5 h stimulation. A) MALP-induced TNF-α production (pg/mL) in a representative experiment demonstrates that addition of the A1/A3 selective antagonist XAC (in this example, 1 μM) or the A3-selective antagonist MRS1220 (1 μM) substantially increased MALP-induced TNF-α in newborn cord blood with a much more modest effect on adult peripheral blood. As an example, the percent increase in TNF-α production in the presence of XAC, defined as (the ratio of the concentration of TNF–α in the presence of the adenosine antagonist divided by the concentration of TNF–α in the presence of an equivalent amount of the ethanol solvent) – 100%, is calculated below the panel demonstrating a 400% increase in neonates but only a 10% increase in adults. B) Composite analysis of data (normalized as in panel A) reveals that XAC or MRS1220, but not DPCX (A1 antagonist), dramatically enhanced MALP-induced TNF–α from newborn but not adult blood (N = 2-6; * p < 0.05). The A2A antagonist ZM exerted a significant but modest enhancing effect on LPS-induced adult TNF-α production (arrow). C) Supernatants from samples pre-incubated with XAC or MRS1220 and stimulated with MALP were also analyzed for IL-6 by ELISA (R & D Systems). The y–axis range for % increase in cytokine production is kept the same to facilitate comparison between effects on TNF-α (panel B) and IL-6 (panel D). Values represent the mean ± SEM (N = 3-6); * p < 0.05; **p < 0.01; ***p < 0.001.
Remarkably, the pattern of modulation of MALP-induced TNF–α production by the panel of adenosine receptor antagonists was completely different for adults and newborns. XAC dramatically enhanced MALP-induced TNF–α production from newborn blood by ~200-500% while having no such effect in adult blood (Fig. 5B). Of note, DPCPX, a selective inhibitor of A1 adenosine receptors, did not enhance BLP-induced TNF–α production from neonatal blood, suggesting that the stimulatory effects of XAC on neonatal cells are mediated via blocking the adenosine A3-receptor. Consistent with this interpretation, as little as 100nM of the A3 receptor-selective antagonist MRS1220 greatly increased BLP-induced TNF-α production from neonatal but not adult blood (Fig. 5B). In accord with previous reports indicating the importance of the A2A receptor in moderating LPS-induced TNF-α from adult monocytes (42), the A2A-selective antagonist ZM-241385 modestly increased LPS-induced TNF-α from adult blood (Fig. 5B, see small arrow). However, ZM-241385 did not significantly enhance LPS- or BLP-induced TNF–α production from neonatal blood.
In marked contrast to the stimulatory effect of XAC and MRS1220 on BLP-induced TNF–α synthesis, neither XAC nor MRS1220 enhanced BLP-induced IL-6 production (Fig. 5C & D), demonstrating that the inhibitory effect of adenosine is selective to TNF–α. Overall, these data suggest that adenosine in newborn blood plasma serves to inhibit BLP-induced TNF–α while preserving BLP-induced IL-6.
Adenosine inhibits BLP-induced TNF-α production from mononuclear cells
Adenosine is known to inhibit LPS-induced TNF–α synthesis by human monocytes and macrophages (18, 43). To determine whether adenosine can modulate BLP-induced cytokine production from human MCs, we measured the effect of addition of exogenous adenosine on BLP-induced cytokine production from newborn CBMCs and adult PBMCs cultured in 5% autologous plasma. Addition of adenosine inhibited BLP-induced TNF–α production from both neonatal and adult MCs (Fig. 6A). However, whereas neonatal CBMCs were significantly inhibited at both 0.1 and 1 μM adenosine, inhibition of adult MCs was not significant at 1μM. Of note, adenosine did not affect BLP-induced IL-6 production from either adult or neonatal MCs at either concentration (Fig. 6B). Overall, these results demonstrate that adenosine can selectively inhibit BLP-induced TNF–α production from newborn and adult MCs and that upon exposure to adenosine at 0.1 to 1 μM range, neonatal CBMCs are consistently sensitive to adenosine's inhibition of BLP-induced TNF-α production.
FIGURE 6. Adenosine inhibits BLP-induced production of TNF–α but not of IL-6.
Neonatal CBMCs or adult PBMCs (106/mL) were cultured in 5% autologous plasma and pre-incubated with the indicated concentrations of adenosine for 30 min prior to stimulation with 1 μg/mL Pam3CysSSNA (N = 4 - 6). (A) Adenosine significantly inhibited TNF–α production by newborn CBMCs (at both 0.1 and 1 μM) and adult PBMCs (0.1 μM). (B) In contrast, production of IL-6 was unaffected. Values represent the mean ± SEM. Statistical comparison of each mean with the 100% value was made using a two-sided one-sample t test; **p < 0.01, ***p < 0.001.
The neonatal adenosine system limits whole microbe-induced neonatal TNF-α (but not IL-6) production
To confirm that the inhibitory effect of the neonatal adenosine system extends to whole microbes, we studied cytokine induction in whole blood by microbial particles known to express TLR2 stimulatory activity, including heat killed (HK)- L. monocytogenes (13), HK-E. coli (that express BLPs, (9)), and zymosan (23). Neonates exhibited markedly impaired microbe-induced TNF-α production (Fig. 7A) but produced greater concentrations of IL-6 than adults (Fig. 7B). Addition of A3 selective antagonist MRS1220 greatly increased microbe-induced TNF-α (Fig. 7C) without effect on microbe-induced IL-6 (Fig. 7D).
FIGURE 7. Neonatal pattern of low TNF-α but high IL-6 production extends to whole microbial particles and is reversed by an A3-adenosine receptor-selective antagonist.
Citrated neonatal cord whole blood (open bars) or adult peripheral whole (filled bars) was incubated for 5 h at 37°C with heat-killed E. coli (HK-EC), heat killed L. monocytogenes (HK-LM) or zymosan (ZYM) particles at the indicated concentrations prior to collection of the extracellular medium for determination of A) TNF-α and B) IL-6 by ELISA. N = 4-5. To determine the effect of adenosine antagonism on microbe-induced cytokine production, neonatal cord or adult peripheral blood were pre-incubated for 30 min with the A3 adenosine receptor-selective antagonist MRS1220 at the indicated concentrations prior to addition of HK-EC (103 bacteria/mL), HK-LM (105 bacteria/mL), or zymosan (0.1 μg/mL). After 5 h incubation to allow cytokine production, the extracellular medium was recovered for subsequent ELISA measurement of TNF-α (C) and IL-6 (D). N = 4-5. Statistical comparisons were made using the Student's t test (1 tailed; *p < 0.05, **p < 0.01, ***p < 0.001).
Neonatal blood plasma contains greater adenosine concentrations than that of adults
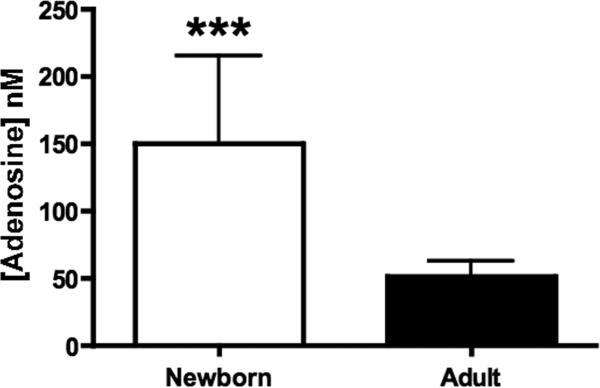
Our results indicate that adenosine receptor antagonism dramatically enhances BLP-induced TNF-α production in neonatal but not adult blood, raising the question of why this phenomenon is more evident in neonatal blood. One possibility is that neonatal plasma, derived from blood in the context of birth stress, might contain greater concentrations of adenosine than adult plasma. To assess this possibility, we measured plasma adenosine concentrations from extracted plasma by HPLC (Fig. 8). Mean plasma adenosine concentrations were higher in neonates than adults: 150.3 ± 64.5 vs. 52.0 ± 10.8 nM, respectively (p < 0.001). Higher basal plasma adenosine levels may partly explain the marked impairment in BLP-induced TNF-α production in neonatal blood.
FIGURE 8. Neonatal blood plasma contains a relatively high concentration of adenosine.
Neonatal cord (N = 8) and adult peripheral blood (N = 9) was collected in the presence of heparin, dipyridamole and EHNA to preserve adenosine levels. Plasma was subjected to organic extraction and the aqueous phase analyzed for adenosine content by HPLC as described in Methods. Mean plasma adenosine concentrations, represented ± SEM, were significantly higher in neonates than adults: 150.3 ± 64.5 and 52.0 ± 10.8 nM, respectively; ***p < 0.001.
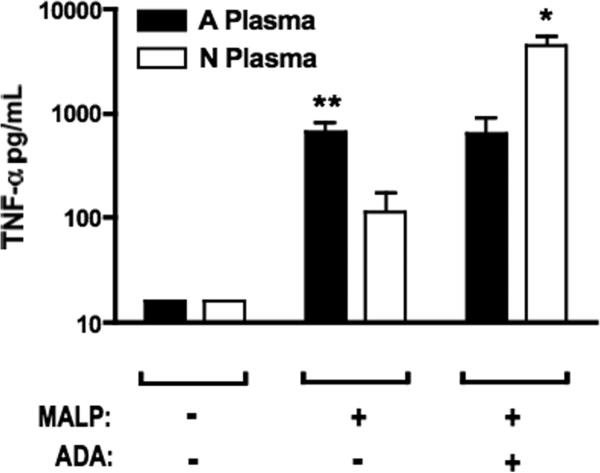
Adenosine deaminase treatment of neonatal plasma enhances BLP-induced TNF-α production
To confirm that high adenosine concentrations in neonatal blood plasma contribute to limiting BLP-induced TNF-α production, we tested the effect of pre-treatment of adult or neonatal plasma with adenosine deaminase (ADA) on the subsequent BLP-induced production of TNF-α (Fig. 9). Pre-incubation of neonatal, but not adult, plasma with ADA resulted in greatly enhanced MALP-induced TNF-α production, thereby confirming that soluble adenosine in neonatal blood plasma is capable of inhibiting BLP-induced TNF-α production.
FIGURE 9. Addition of adenosine deaminase to neonatal plasma enhances BLP-induced TNF-α production.
Adult PBMCs (106 cells/mL) were resuspended in neonatal cord- or adult peripheral blood-derived plasma that had been pre- incubated with buffer control or with adenosine deaminase (ADA) enzyme for 5 min at 37°C. MALP (1 μg/mL) was then added and incubated for 5 h to allow for cytokine production. Production of TNF-α was greater in the presence of adult than in neonatal plasma. Pre-incubation of neonatal plasma with ADA resulted in a substantial increase in MALP-induced TNF-α production. N = 3. *p < 0.05, ** p < 0.01.
Adenosine induces cAMP in neonatal mononuclear cells via the A3 adenosine receptor
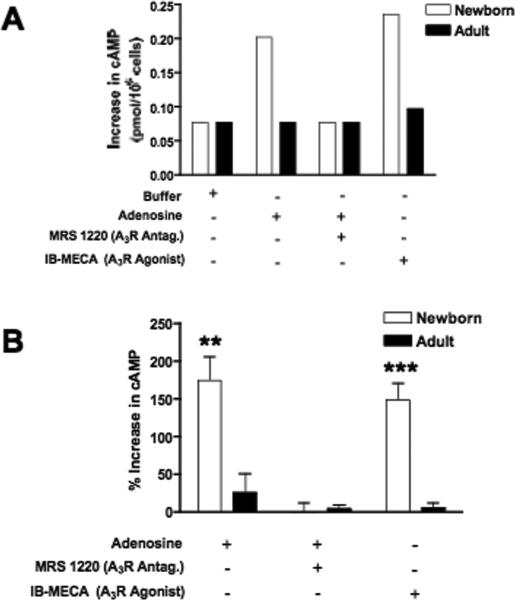
As adenosine exerts a profound inhibitory effect on neonatal TNF-α production (Figs. 5A, 6A, and 7C) and adenosine can act via cAMP induction (18), we posited that neonatal mononuclear cells may be more sensitive to adenosine-induced cAMP accumulation. To test this hypothesis, we determined whether addition of adenosine or adenosine receptor A3-selective modulators affected cAMP synthesis in neonatal CBMCs and adult PBMCs (Fig. 10). Intracellular cAMP concentrations in the presence of adenosine or adenosine congeners (see example in Fig. 10A) were normalized in relation to those in the presence of buffer for composite analysis (Fig. 10B). Incubation of MCs with adenosine triggered dramatic increases in cellular cAMP content for neonatal CBMCs, with a weaker effect for adult PBMCs. Co-incubation of cells with adenosine in the presence of the adenosine A3 receptor-selective antagonist MRS 1220 resulted in a complete inhibition of adenosine-induced cAMP accumulation, indicating that adenosine-induced cAMP accumulation proceeds via the A3 adenosine receptor. Moreover, the A3-selective agonist IB-MECA reproduced a similar pattern of cAMP synthesis by neonatal CBMCs as adenosine did. These results indicate that adenosine induces cAMP synthesis in neonatal CBMCs via the A3 adenosine receptor. Adenosine-induced cAMP accumulation provides a potential mechanism by which the neonatal adenosine system serves to limit BLP-induced TNF–α synthesis, while preserving BLP-induced IL-6 production.
FIGURE 10. Engagement of the adenosine A3 receptor enhances accumulation of cAMP in neonatal, but not adult, mononuclear cells.
Newborn CBMCs or adult PBMCs (106/mL) were cultured in 5% autologous plasma in the presence of adenosine (1 μM), adenosine with the A3 receptor inhibitor MRS 1220 (100 μM), or in the presence of the A3R-selective agonist IB-MECA (100μM). After a 35 min incubation at 37°C, cells were collected and lysed for measurement of cAMP by competitive immunoassay. (A) Representative experiment demonstrating actual cAMP concentrations (pmol/106 cells), (B) Composite analysis of normalized data expressed as a percent increase in cAMP concentration. Values represent the mean ± SEM (N = 4); ** p < 0.01; *** p < 0.001.
Discussion
Despite a good deal of work demonstrating impaired production of Th1-type pro-inflammatory cytokines from neonatal mononuclear cells (2), the molecular basis for the differences in cytokine production between newborns and adults is incompletely defined. Our current study has focused on defining the mechanism for severely impaired human neonatal TNF–α responses to TLR2 agonists, including pure BLPs (7) and whole microbial particles that can activate cells via TLR2 (9, 13, 23). We demonstrate that despite markedly impaired neonatal TNF-α production to such TLR agonists, neonatal production of IL-6 is actually greater than that of adults (Fig. 1B and Fig. 7B). Robust neonatal IL-6 production indicates that the intrinsic TLR pathway is intact at birth and suggests that the marked neonatal impairment in TLR-induced TNF–α production might reflect a distinct (Th2) polarization of the TLR-mediated response. Indeed, we demonstrate for the first time that the distinct nature of the neonatal adenosine system significantly contributes to the impairment in TLR-mediated TNF-α production. Both soluble and cellular aspects of the neonatal adenosine system are distinct from those of adults. Specifically, neonatal plasma contains about three-fold greater concentrations of adenosine than adult plasma (Fig. 8) and neonatal mononuclear cells are significantly more sensitive to adenosine-induced cAMP accumulation (Fig. 10) and are consistently and profoundly susceptible to adenosine-induced inhibition of TLR2-mediated TNF-α production (Figs. 5B, 6A, and 7C). Adenosine in neonatal plasma engages the A3 adenosine receptor on neonatal CBMCs resulting in an elevation of intracellular cAMP (Fig. 10), a second messenger that inhibits BLP-induced expression of TNF–α (Fig. 4) while preserving BLP-induced production of IL-6, a cytokine with anti-inflammatory (15) and Th2-polarizing (16) properties.
The adenosine system has been shown to act as a physiologic brake on inflammation (18, 19) in part via its ability to inhibit neutrophil adhesion and recruitment (31). However, details of the impact of the adenosine system on the TLR system are just beginning to emerge. Adenosine inhibits LPS-induced TNF–α production (18) and a recent study of macrophages derived from adult mice has demonstrated that adenosine, acting via A2A receptors, can inhibit TNF–α production in response to several TLR agonists, including agonists of TLR2 (44). Studies of mice rendered genetically deficient in A2A receptors have revealed that adenosine can inhibit TNF–α production by murine macrophages via both adenosine A2A receptor-dependent (42) and independent mechanisms (45), thereby implicating additional adenosine receptors in the effects observed. Of note, adenosine (43, 46, 47) and its metabolites (48) can also act via the A3 receptor to inhibit LPS-induced expression of TNF–α in human and murine macrophages and LPS-induced expression of tissue factor in primary human monocytes. Indeed, our study focusing on human newborns implicates the neonatal A3 adenosine receptor as central to inhibition of BLP-induced TNF–α production by human neonatal CBMCs.
The selective effects of the neonatal adenosine system on TLR-mediated TNF-α production apparently arise from two mechanisms: a) greater basal plasma adenosine concentrations in neonates (Fig. 8) and b) greater sensitivity of neonatal (vs. adult) MCs to the effects of adenosine, as demonstrated by the selective enhancing effect of adenosine A3 receptor inhibitors on BLP- (Fig. 5) and microbe- (Fig. 7) induced neonatal TNF–α synthesis, the more consistent inhibition of TLR2-induced neonatal TNF-α production (Fig. 6), and to the greater adenosine A3 receptor-mediated accumulation of intracellular cAMP in neonatal CBMCs (Fig. 10). These results indicate that the adenosine system of human MCs has a distinct functional expression at birth. In addition to its anti-inflammatory potential, adenosine has also recently been found to play important roles in fetal and neonatal organ development (49), lending further conceptual support for the distinct function of the adenosine system during the perinatal period.
The more recently identified A3 receptor is broadly expressed yet regulates intracellular cAMP synthesis in a tissue-specific manner (50). Although the A3 receptor is coupled to inhibitory G proteins in some tissues (51), the A3 receptor has been found to be positively coupled to adenylate cyclase in primary human leukocytes (52). Kinetics of adenosine exposure may also play a role in some cell types in that brief exposure to adenosine can result in A3 receptor-mediated inhibition of cAMP synthesis (51) whereas prolonged exposure of cardiovascular cells to adenosine results in A3 receptor-mediated enhancement in coupling of stimulatory G proteins (Gs) to adenylate cyclase resulting in increased cAMP synthesis (53).
Although it has often been assumed that neonatal cytokine responses are generally impaired, recent evidence suggests that, with few exceptions (54), the pattern of stimulus-induced neonatal cytokine production is cytokine-specific, revealing a Th2-bias of the neonatal immune response (6). In accordance with such observations, we now demonstrate that, whereas BLP- and whole microbe-induced TNF–α is markedly impaired (7), production of IL-6, a cytokine with anti-inflammatory (15) and Th2-polarizing properties (17), is actually greater in neonates than adults. It has been recently demonstrated that neonatal MCs exposed to herpes simplex virus-1, that activates cells via TLR2, produce more IL-6 than do PBMCs of adults (55). Interestingly, responses of neonatal MCs to an array of microbial gastrointestinal flora are characterized by relatively high IL-6 production (56), further evidence that the pattern we have discovered for neonatal responses to purified BLPs and to whole microbial particles likely extends to a broad array of complex stimuli. In aggregate, these studies indicate that in response to multiple microbial stimuli, neonatal mononuclear cells produce lower amounts of TNF–α but higher amounts of IL-6 relative to adult cells.
What is the teleological rationale for the bias of neonatal mononuclear cells in cord blood to synthesize low amounts of TNF–α but high amounts of IL-6 in response to BLPs and to whole microbial particles? Given that IL-6 has some anti-inflammatory (15) and Th2-polarizing (16, 17) properties, such a pattern might protect the fetus from excessive inflammatory responses that drive allo-immune reactions and premature delivery (57). Given the inhibitory properties of IL-6 toward neutrophil migration (15), the relatively high IL-6 production in newborns is of interest with respect to the tendency of neonates with overwhelming sepsis to have reduced neutrophil recruitment to inflammatory sites (2). The distinct functional properties of TNF-α and IL-6 provide a teleological basis for the distinct transcriptional regulation of their genes (39, 58).
During gestation, adenosine is produced by the placenta (59) and, as a vasodilator, contributes to the maintenance of uterine placental vessels (60). Of note, adenosine is known to cross the placenta into the fetal circulation (61) and plasma levels of adenosine are known to increase with stress, including that associated with vaginal delivery (62). These observations imply an important physiologic role for adenosine and place this immunomodulator at anatomic sites where it may be poised to play important roles in modulating fetal and neonatal responses to TLR agonists. The duration of the neonatal adenosine system's inhibitory effects on TLR-mediated TNF-α production remains to be defined. It is also likely that additional maternal- and neonatal-derived factors contribute to inhibition of Th1-polarizing immunity (63, 64). Nevertheless, our study demonstrates a profound inhibitory effect of the human neonatal adenosine system on TLR-mediated TNF-α production during the perinatal period, when the newborn is first exposed to many new antigens (65) and, potentially, to maternal-derived pathogens, including enteric or urinary bacteria as well as viruses such as herpes simplex virus and cytomegalovirus (1).
If neonatal immunity is indeed biased against Th1-type responses in order to avoid potentially harmful inflammation, then the distinct functional properties of the neonatal adenosine system may serve as a key component of this protective polarization. However, such impairment in perinatal TNF-α production may render neonates more susceptible to microbial infection and impair neonatal responses to some vaccine–adjuvant combinations. Thus, our study suggests that modulation of the adenosine system, and in particular the A3 receptor, may present novel opportunities to enhance innate, and thereby acquired, immunity in the human newborn.
Acknowledgements
We thank Drs. Robert Munford, Jerrold Weiss, Anders Hakansson, and Richard Malley for helpful scientific discussions. Eugénie E. Suter and Camilo Chao provided expert technical assistance.
Footnotes
This work was supported by NIH KO8 AI50583-01 and RO1 AI067353-01A1 (OL) and by an unrestricted grant from Zephyr Sciences.
References
- 1.Klein J, Remington J. Current Concepts of Infections of the Fetus and Newborn Infant. In: Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. 5th ed. W.B. Saunders Company; Philadelphia: 2001. pp. 1–23. [Google Scholar]
- 2.Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington J, Klein J, editors. Infectious Diseases of the Fetus and Newborn Infant. 5th ed. W.B. Saunders; Philadelphia: 2001. pp. 25–138. [Google Scholar]
- 3.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nature Reviews. Immunology. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DB, Tu W. The Physiologic Immunodeficiency of Immaturity. In: Stiehm ER, Ochs HD, Winkelstein JA, editors. Immunologic Disorders in Infants & Children. 5th ed. Elsevier; Philadelphia: 2004. pp. 687–760. [Google Scholar]
- 5.Akira S, Takeda K. Toll-like receptor signaling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 6.Levy O. Innate immunity of the human newborn: distinct cytokine responses to LPS and other Toll-like receptor agonists. J Endotoxin Res. 2005;11:113–116. doi: 10.1179/096805105X37376. [DOI] [PubMed] [Google Scholar]
- 7.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of Toll-like receptor-mediated innate immunity in human newborns: Neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod but preserves response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 8.Akira S. Mammalian Toll-like receptors. Curr Op Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 9.Hellman J, Roberts JD, Jr., Tehan MM, Allaire JE, Warren HS. Bacterial peptidoglycan-associated lipoprotein is released into the bloodstream in gram-negative sepsis and causes inflammation and death in mice. J. Biol. Chem. 2002;277:14274–14280. doi: 10.1074/jbc.M109696200. [DOI] [PubMed] [Google Scholar]
- 10.Sellati TJ, Bouis DA, Kitchens RL, Darveau RP, Pugin J, Ulevitch RJ, Gangloff SC, Goyert SM, Norgard MV, Radolf JD. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. Journal of Immunology. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 11.Bessler WG, Baier W, vd Esche U, Hoffmann P, Heinevetter L, Wiesmuller KH, Jung G. Bacterial lipopeptides constitute efficient novel immunogens and adjuvants in parenteral and oral immunization. Behring Institute Mitteilungen. 1997:390–399. [PubMed] [Google Scholar]
- 12.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 13.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 14.van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Molecular Immunology. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 17.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 18.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Linden J. Adenosine in tissue protection and tissue regeneration. Molecular Pharmacology. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 20.Biesert L, Scheuer W, Bessler WG. Interaction of mitogenic bacterial lipoprotein and a synthetic analogue with mouse lymphocytes. Isolation and characterization of binding proteins. European Journal of Biochemistry. 1987;162:651–657. doi: 10.1111/j.1432-1033.1987.tb10687.x. [DOI] [PubMed] [Google Scholar]
- 21.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. Journal of Experimental Medicine. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy O, Jean-Jacques R, Cywes C, Sisson R, Zarember KA, Godowski PJ, Christianson J, Guttormsen HK, Carroll MC, Nicholson-Weller A, Wessels MR. Critical role of the complement system in Group B streptococcus-induced tumor necrosis factor alpha release. Infect Immun. 2003;71:6344–6353. doi: 10.1128/IAI.71.11.6344-6353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy O, Sisson R, Kenyon J, Eichenwald E, Macone A, Goldmann D. Enhancement of neonatal innate defense: Effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein (rBPI21) on growth and TNF-inducing activity of Gram-negative bacteria tested in neonatal cord blood ex vivo. Infect Immun. 2000;68:5120–5125. doi: 10.1128/iai.68.9.5120-5125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 26.Gallo-Rodriguez C, Ji XD, Melman N, Siegman BD, Sanders LH, Orlina J, Fischer B, Pu Q, Olah ME, van Galen PJ, et al. Structure-activity relationships of N6-benzyladenosine-5'-uronamides as A3-selective adenosine agonists. J Med Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keddie JR, Poucher SM, Shaw GR, Brooks R, Collis MG. In vivo characterisation of ZM 241385, a selective adenosine A2A receptor antagonist. Eur J Pharmacol. 1996;301:107–113. doi: 10.1016/0014-2999(96)00020-9. [DOI] [PubMed] [Google Scholar]
- 30.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Critical Care Medicine. 2000;28:3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J. Clin. Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dooper MM, Wassink L, M'Rabet L, Graus YM. The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype. Immunology. 2002;107:152–159. doi: 10.1046/j.1365-2567.2002.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J. Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 35.Leal-Berumen I, Snider DP, Barajas-Lopez C, Marshall JS. Cholera toxin increases IL-6 synthesis and decreases TNF-alpha production by rat peritoneal mast cells. J. Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 36.Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J. Immunol. 1998;160:1811–1816. [PubMed] [Google Scholar]
- 37.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J. Biol. Chem. 1996;271(34):20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 38.Grandjean-Laquerriere A, Le Naour R, Gangloff SC, Guenounou M. Differential regulation of TNF-alpha, IL-6 and IL-10 in UVB-irradiated human keratinocytes via cyclic AMP/protein kinase A pathway. Cytokine. 2003;23:138–149. doi: 10.1016/s1043-4666(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 39.Leal-Berumen I, Snider DP, Barajas-Lopez C, Marshall JS. Cholera toxin increases IL-6 synthesis and decreases TNF-alpha production by rat peritoneal mast cells. J. Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 40.Wagner DR, Combes A, McTiernan C, Sanders VJ, Lemster B, Feldman AM. Adenosine inhibits lipopolysaccharide-induced cardiac expression of tumor necrosis factor-alpha. Circulation Research. 1998 Jan;:47–56. doi: 10.1161/01.res.82.1.47. 1999-1923. [DOI] [PubMed] [Google Scholar]
- 41.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 42.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J. Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 44.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. American Journal of Pathology. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB Journal. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 46.McWhinney CD, Dudley MW, Bowlin TL, Peet NP, Schook L, Bradshaw M, De M, Borcherding DR, Edwards CK., 3rd Activation of adenosine A3 receptors on macrophages inhibits tumor necrosis factor-alpha. European Journal of Pharmacology. 1996;310:209–216. doi: 10.1016/0014-2999(96)00272-5. [DOI] [PubMed] [Google Scholar]
- 47.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Adenosine inhibits tissue factor expression by LPS-stimulated human monocytes: involvement of the A3 adenosine receptor. Thrombosis & Haemostasis. 2002;88:123–130. [PubMed] [Google Scholar]
- 48.Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102:4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- 49.Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Molecular Genetics & Metabolism. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Z, Makaritsis K, Francis CE, Gavras H, Ravid K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochimica et Biophysica Acta. 2000;1500:280–290. doi: 10.1016/s0925-4439(99)00111-8. [DOI] [PubMed] [Google Scholar]
- 51.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- 52.Ezeamuzie CI, Philips E. Positive coupling of atypical adenosine A3 receptors on human eosinophils to adenylyl cyclase. Biochemical & Biophysical Research Communications. 2003;300:712–718. doi: 10.1016/s0006-291x(02)02910-8. [DOI] [PubMed] [Google Scholar]
- 53.Palmer TM, Harris CA, Coote J, Stiles GL. Induction of multiple effects on adenylyl cyclase regulation by chronic activation of the human A3 adenosine receptor. Molecular Pharmacology. 1997;52:632–640. doi: 10.1124/mol.52.4.632. [DOI] [PubMed] [Google Scholar]
- 54.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006 doi: 10.1182/blood-2005-12-4821. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurt-Jones E, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. The role of Toll-like receptors in herpes simplex infection in neonates. J Infect Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 56.Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infection & Immunity. 2002;70:6688–6696. doi: 10.1128/IAI.70.12.6688-6696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raghupathy R, Makhseed M, El-Shazly S, Azizieh F, Farhat R, Ashkanani L. Cytokine patterns in maternal blood after premature rupture of membranes. Obstetrics & Gynecology. 2001;98:122–126. doi: 10.1016/s0029-7844(01)01408-9. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, Saitoh T, Yamaoka S, Yamamoto N, Yamamoto S, Muta T, Takeda K, Akira S. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430:218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 59.Maguire MH, Szabo I, Slegel P, King CR. Determination of concentrations of adenosine and other purines in human term placenta by reversed-phase high-performance liquid chromatography with photodiode-array detection: evidence for pathways of purine metabolism in the placenta. J Chromatogr. 1992;575:243–253. doi: 10.1016/0378-4347(92)80152-g. [DOI] [PubMed] [Google Scholar]
- 60.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 1996;174:267–271. doi: 10.1016/s0002-9378(96)70406-4. [DOI] [PubMed] [Google Scholar]
- 61.Wheeler CP, Yudilevich DL. Transport and metabolism of adenosine in the perfused guinea-pig placenta. J Physiol. 1988;405:511–526. doi: 10.1113/jphysiol.1988.sp017345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irestedt L, Dahlin I, Hertzberg T, Sollevi A, Lagercrantz H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr Res. 1989;26:106–108. doi: 10.1203/00006450-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Semeniuk DJ, Boismenu R, Tam J, Weissenhofer W, Murgita RA. Evidence that immunosuppression is an intrinsic property of the alpha-fetoprotein molecule. Adv Exp Med Biol. 1995;383:255–269. doi: 10.1007/978-1-4615-1891-4_27. [DOI] [PubMed] [Google Scholar]
- 64.Olding LB, Papadogiannakis N, Barbieri B, Murgita RA. Suppressive cellular and molecular activities in maternofetal immune interactions; suppressor cell activity, prostaglandins, and alpha-fetoproteins. Curr Top Microbiol Immunol. 1997;222:159–187. doi: 10.1007/978-3-642-60614-4_8. [DOI] [PubMed] [Google Scholar]
- 65.Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3:125–132. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]