Abstract
The brainstem locomotor system is believed to be organized serially from the mesencephalic locomotor region (MLR) to reticulospinal neurons, which in turn, project to locomotor neurons in the spinal cord. In contrast, we now identify in lampreys, brainstem muscarinoceptive neurons receiving parallel inputs from the MLR and projecting back to reticulospinal cells to amplify and extend durations of locomotor output. These cells respond to muscarine with extended periods of excitation, receive direct muscarinic excitation from the MLR, and project glutamatergic excitation to reticulospinal neurons. Targeted block of muscarine receptors over these neurons profoundly reduces MLR-induced excitation of reticulospinal neurons and markedly slows MLR-evoked locomotion. Their presence forces us to rethink the organization of supraspinal locomotor control, to include a sustained feedforward loop that boosts locomotor output.
The neural circuitry underlying vertebrate locomotion comprises spinal networks known as central pattern generators (CPGs)1,2 producing muscle synergies responsible for propulsion2. Supraspinal elements initiate and control these synergies2,3. The circuitry of the supraspinal control system for locomotion has been obtained mostly from anatomical and physiological studies in mammals. Despite a relatively broad knowledge of this organization, the detailed cellular connectivity and the neural mechanisms involved remain unknown in mammals. Forebrain structures are believed to be responsible for decision-making and selection of motor behaviors4. These structures activate centers dedicated to the control of locomotor output. One such center is the mesencephalic locomotor region (MLR), first described in cats5 and later in many vertebrates from fishes to mammals6-8.
The brainstem locomotor control system is believed to be organized serially3,9 whereby MLR neurons project to reticulospinal (RS) cells, which in turn send descending commands to spinal cord CPG neurons to generate locomotion. The MLR exhibits rheostat-like excitation onto RS neurons, thus controlling precisely the intensity of locomotor output10. It also controls the mode of locomotion5,8,10. For instance, as the intensity of MLR stimulation increases, locomotion in cats changes from walking, to running onto galloping4. In lampreys, the MLR sends glutamatergic and cholinergic monosynaptic excitatory inputs driving RS neurons11,12. The control is bilateral producing symmetrical locomotor behaviors13. This organization is believed to be common to all vertebrates and appears conserved in humans14.
Muscarinic agonists are powerful excitants of vertebrate brainstem locomotor command systems15,16, and we have recently demonstrated that they excite lamprey RS neurons for extended periods17. Similarly to the output from the MLR to RS neurons, excitatory activity in RS neurons driven by brainstem muscarine receptor activation is bilaterally symmetrical. However this excitation of RS neurons is indirect and neither its neuronal origins nor its physiological role in locomotor behaviors have been described. We now identify brainstem neurons that are directly activated by muscarine and by muscarinic excitation from the MLR, and which project back to RS cells to markedly amplify and extend MLR mediated locomotor behavior. Thus, a previously unknown feedforward pathway breaks the dogma of a serial activation of supraspinal locomotor elements. These neurons sustain very extended periods of excitation driven by muscarine receptor activity, which substantially boosts and sustains locomotor output mediated by a highly conserved locomotor region, the MLR.
Results
Muscarinoreceptive neurons project to the RS system
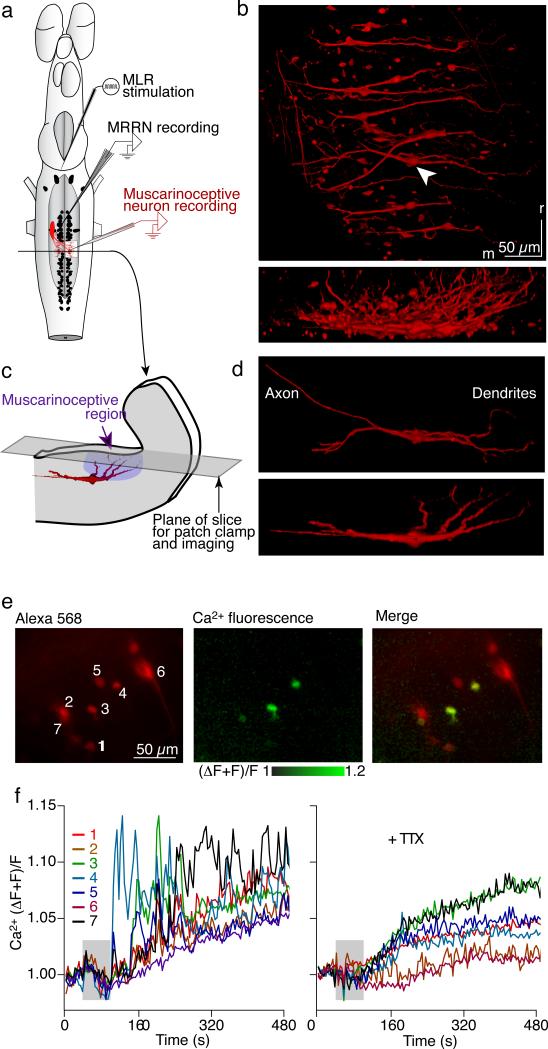
RS neurons show sustained excitation from a muscarine sensitive region (Fig 1a–c) at the basal/alar plate boundary caudal to the middle rhombencephalic reticular nucleus (MRRN) and rostral to the posterior rhombencephalic reticular nucleus (PRRN)17. We hypothesized that muscarinoceptive neurons project monosynaptically to the RS system. To visualize putative muscarinoceptive neurons in the muscarinoceptive region, we placed Alexa 568 dextran in one of their likely synaptic targets – the MRRN dendritic field (Fig 1a; n=14 preparations). The tissue was maintained alive for 24 hrs to allow retrograde dye transport, then fixed and imaged in whole mount. Confocal imaging of the brainstem ipsilateral and contralateral to the labeling site revealed spindle shaped neurons with dendrites extending laterally and to the dorsal surface of the 4th ventricle at the alar/basal plate boundary (Fig 1b,c,d). We imaged in detail the neurons projecting contralaterally because, while neurons were also readily identified ipsilaterally, the neuronal labeling was easier to utilize for physiological recordings away from the original labeling site. These neurons were mostly bipolar (67%), less often multipolar (33%) and either spindle shaped or pyramidal with their long axis oriented medial-laterally (mean soma length 18.0±0.8 μm, width 7.6±0.3 μm, n=40 cells, 14 preparations). Somata were located in a rostro-caudal line between the MRRN and PRRN and lateral to the RS cells in those nuclei. Their axons projected from the medial aspect of the cell body and crossed the midline slightly rostral to the soma (Fig 1d). In all cases, dendritic trees originated from the lateral aspect of the somata and projected dorso-laterally into the region that we have previously described as mucarinoceptive17 (Fig 1c). An example of a single neuron extracted from the nucleus by seeding and autosegmentation of the 3-dimensional image demonstrates the structure and location of these neurons (Fig 1c,d). The dendrites of these neurons showed a mean measureable projection of 128±6 μm from the soma laterally (n=40 neurons in 14 preparations). In each experiment, we observed labeled axons extending from the injection site to the fluorescent somata.
Figure 1. Muscarine-mediated activation of brainstem neurons projecting to the MRRN.
a) Schematic of the injection of retrograde tracers in MRRN and locations of stimulation and recording electrodes used in this study. Alexa 568 dextran crystals were inserted into the dendritic field of the MRRN. Neurons were imaged and recorded contralaterally.
b) Dorsal (top) and transverse (bottom) views of muscarinoceptive cell somata and dendrites labeled by the tracer.
c) Schematic demonstrating cross-sectional location of individually labelled cells in the rhombencephalon (red image from d), and the plane of tissue slicing used for imaging and patch clamp of these neurons.
d) Morphology of individually labelled neuron from (b, arrowhead). Images extracted by seeding and autosegmentation of the 3D data in (b) displayed in dorsal (top) and transverse (bottom) views.
e) Co-labelling of muscarinoceptive neuron somata with Alexa 568 (left) and Calcium Green dextran (middle). Co-labelling allowed soma localization and Ca2+ imaging (merged image, right).
f) Ca2+ transients in muscarinoceptive neuron somata in response to muscarine (25 μM, application marked by gray rectangle) superfusion in control (left) and in TTX (1 μM; right).
To determine the population response of these neurons to muscarine, we included Ca2+-sensitive dye (Calcium Green dextran) with the Alexa 568 dextran as a cell marker. After 6–12 hours the dorsal surface of the basal plate and the alar plate were removed using a tissue slicer (Fig 1c). This improved image resolution by eliminating the refractive overlying epithelial cell layer. The tissue was placed in a superfused recording chamber. Labeled neurons in this region were identified using charge-coupled-device (CCD) imaging (Fig 1e, red). Muscarine (25 μM) was superfused over the preparation and these neurons responded with sustained increases in Ca2+ dye fluorescence (45 of 49 identified neurons, 10 preparations). While all responding neurons demonstrated a slow rise in Ca2+ lasting for minutes, many of the neurons also exhibited rapidly rising and falling Ca2+ events (Fig 1f, left panel). These latter neurons are seen clearly (Fig 1e, green) as the ratio of resting fluorescence to pre-muscarine control rose substantially in a subset of Alexa 568 labeled cell bodies. To determine whether these neurons responded directly to muscarine, action potential-evoked synaptic connectivity was blocked with tetrodotoxin (TTX; 1 μM; in 4 of these preparations plus 3 further preparations), and muscarine applied. All neurons showed a sustained Ca2+ rise, indicating that they respond directly to muscarine (Fig 1f, right panel). However, loss of the rapidly rising and falling Ca2+ activity in TTX, indicates that a component of the activity required action potentials and possibly recurrent synaptic input. In contrast, in RS neurons the response to muscarine was abolished by TTX17.
Temporal relationship between responses to muscarine
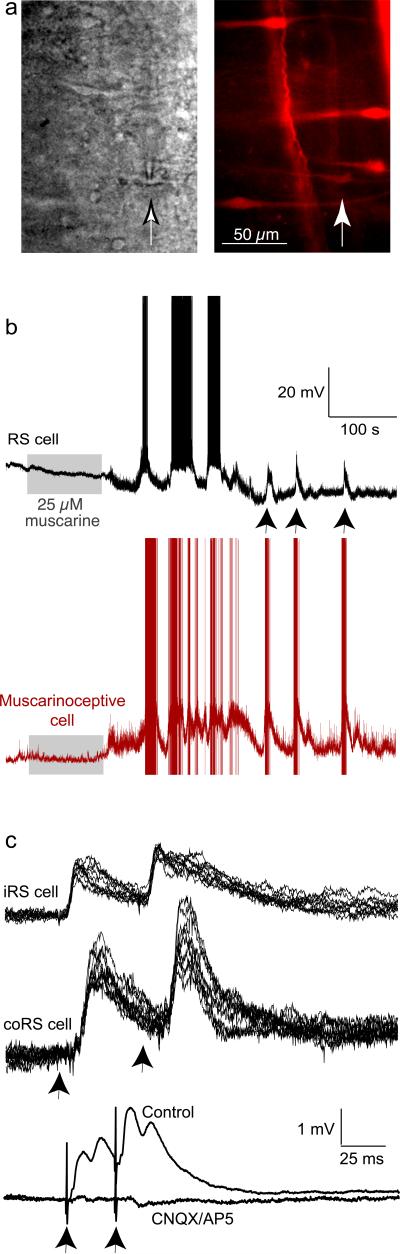
We assessed the temporal relationship between bath applied muscarine-evoked activity in these labeled neurons and the indirect activity evoked in RS neurons. Neurons in the muscarinoceptive region were again retrogradely labeled with dye (Alexa dextran 568) placed in the MRRN dendritic field. After 6–12 hrs the tissue was sliced as before (Fig 1c). We used fluorescence labeling to specifically target individual cells with projections to the MRRN dendritic field for whole cell patch recording within the intact brainstem. This is shown as two images (Fig 2a) of bright field (gray) and fluorescence (red) showing a whole cell patch electrode recording from the putative muscarinoceptive neuron at the tip of the electrode (arrow shows electrode and neuron in Fig 2a). The current clamp recording from this particular neuron is shown (Fig 2b, red trace). This recording arrangement ensured that we recorded only from the neurons in the brainstem muscarinoceptive region that project to the MRRN dendritic field and which we know respond directly to muscarine (Fig. 1f). Ipsilateral MRRN RS neurons were simultaneously recorded with microelectrodes (Fig 2b, black). Muscarine (25 μM; n=4 preparations, see Fig 1a for electrode locations) was applied and as previously demonstrated17 the RS neurons exhibited extended periods of excitation characterized by bursts of repetitive firing on depolarizing activity. The dye-labeled patch clamped and putative muscarinoceptive neurons invariably showed extended periods of depolarization and repetitive firing (Fig 2b, red) that correlated with RS activity. Correlated timing of later bursts in the muscarinoceptive neuron and MRRN neuron subthreshold depolarizations are arrowed.
Figure 2. Evoked activity within the muscarinoceptive region activates glutamatergic synapses on RS cells.
a) Imaging whole cell recording from a muscarinoceptive neuron retrogradely labelled with Alexa 568 dextran. Left – brightfield showing the tip of the patch electrode whole cell on a recorded neuron arrowed. Right – fluorescence of retrogradely labelled putative muscarinoceptive neurons used to target the patch electrode – recorded neuron arrowed.
b) Paired recording of RS cell and the whole cell patch clamped muscarinoceptive neuron from (a). Recording shows response to muscarine (25 μM, gray rectangle) superfusion in the RS neuron (black) and muscarinoceptive neuron (red).
c) Electrophysiological response of bilaterally recorded RS cells during electric stimulation of muscarinoceptive neurons on one side only.
d) RS cell responses in control and during superfusion of CNQX/AP5 (5 μM and 50 μM).
We have previously shown that block of muscarinic receptors, by antagonist (atropine) microinjection specifically to the region now identified as the dendritic field of these neurons that project to the MRRN, prevented bath-applied muscarine-mediated excitation of the RS system and locomotion17. This result suggests that neurons in this region project excitation to the RS system. Therefore, in intact brainstems, we recorded intracellularly from pairs of RS cells on both sides of the MRRN and extracellularly stimulated the muscarinoceptive region to assay synaptic connectivity. Paired pulse stimulation (1.5μA–10 μA, 1ms, 20–50 Hz) elicited short latency facilitating EPSPs in the RS neurons on either side of the brainstem midline. These synaptic responses were consistent with monosynaptic projections18. The responses were sustained with paired-pulse stimulation in which latencies were not significantly different for the 1st and 2nd responses (p<0.01; Fig 2c). The latencies of ipsilateral MRRN neuron EPSPs were 4.2±0.5 ms for the 1st response and 4.3±0.5 ms for the 2nd. In contralateral MRRN neurons, these latencies were 7.0±1.0 and 7.1±1.0 ms respectively (n=5 preparations, 6 ipsilateral and 6 contralateral RS neurons; paired pulse stimulation did not significantly alter the latencies). EPSPs were abolished by co-application of the AMPA and NMDA receptor antagonists (6-cyano-7-nitroquinoxaline-2,3-dione, CNQX, 5 μM and DL-2-amino-5-phosphonopentanoic acid, AP5, 50 μM); indicating that they are glutamatergic. This result is consistent with our previous finding that block of glutamatergic transmission abolished indirect muscarinic excitation of RS neurons17.
Using extracellular stimulation, we cannot rule out that axons of passage were not stimulated in addition to the muscarinoceptive somata. To overcome this limitation, paired cell recordings were made between neurons in the muscarinoceptive region and RS cells in the MRRN. In figures 1 and 2 we identified muscarinoceptive neurons by retrograde labeling from within the MRRN dendritic field. This approach destroys the target neurons. Instead, we applied tracer (Alexa 488 dextran) to the rostral aspect of the MRRN to avoid damage of most of the MRRN. After 6–12 hrs the tissue was sliced as before (Fig 1c) and imaged (Fig 3a, green) to reveal retrogradely labeled neurons in the region that we have previously identified as muscarinoceptive. This labeling was used to target the muscarinoceptive nucleus and individual cells with projections to the MRRN dendrites.
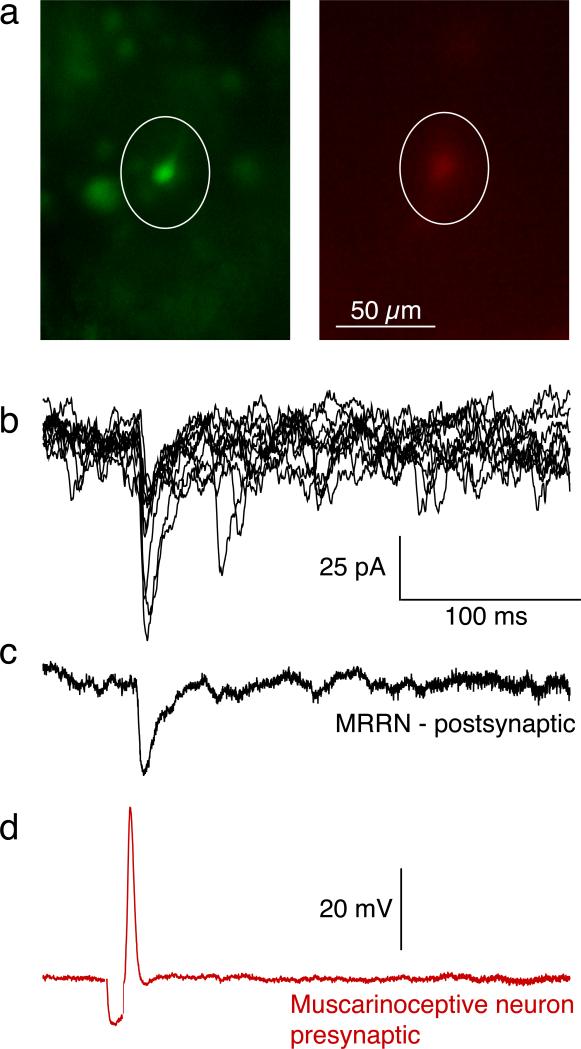
Figure 3. Paired cell recordings between muscarinoceptive region neurons and the MRRN reveal excitatory synaptic contacts.
a) Putative muscarinoceptive neurons were labeled with retrograde tracer (Alexa 488 dextran) placed at the rostral edge of the MRRN. After incubation (6–12 hours) and removal of the contralateral alar plate and dorsal-lateral tissue of the brainstem (Fig 1c), labelled neurons (green) were imaged. Paired cell recordings were made between these neurons with microelectrodes containing red dye (Alexa 594 hydrazide; red) and visually identified whole cell clamped RS neurons caudal to the dye application site. In the images shown the neuron labelled with red dye (circled) was seen to be co-labelled with retrograde tracer (green, circled).
b) Stimulation of presynaptic neurons through the recording microelectrode evoked EPSCs in the MRRN RS neuron. Individual EPSCs shown stimulated at 1 Hz.
c) The average of the EPSCs in (b).
d) Example of presynaptic action potential from red cell in (a) used to evoke the EPSCs in (b,c).
Paired cell recordings (n=5) were made between the muscarinoceptive nucleus neurons, and MRRN RS neurons (see Fig 1a for electrode placement). The muscarinoceptive neuron was recorded with a microelectrode containing red fluorescent dye (Alexa 594 hydrazide) to confirm this recording was from a cell body in the muscarinoceptive neuron. The MRRN RS neuron was whole cell patch clamped (voltage clamped at –70 mV). In two pairs, the presynaptic putative muscarinoceptive neuron was co-labeled with retrograde tracer that had been placed at the rostral edge of the MRRN (Fig 3a, green neuron circled) and the red dye in the electrode (Fig 3a, red neuron, circled). Stimulation of the presynaptic neuron evoked EPSCs in the postsynaptic RS neuron (Fig 3b; mean EPSC amplitude 54±13 pA, mean delay between presynaptic action potential peak and EPSC start 6.7±1.3 ms, this delay was not significantly different from that obtained with extracellular muscarinoceptive region stimulation, Fig 2c). These data indicate that neurons within the muscarinoceptive region make excitatory synapses with MRRN RS cells. These same neurons are clearly muscarinoceptive because they respond to muscarine both with a long-lasting Ca2+ transient in TTX (Fig 1f) and a sustained depolarization (Fig 2b).
MLR stimulation evokes long-lasting responses
The MLR projects cholinergic axons to the brainstem reticular formation in mammals19,20 and lamprey12,21. The MLR is, thus, one possible source of cholinergic inputs to brainstem muscarinoceptive neurons. Therefore, we determined whether projections from the MLR excite muscarinoceptive neurons. RS cells in the MRRN and retrogradely labeled putative muscarinoceptive neurons were simultaneously recorded as previously (Fig 2) and a micro-stimulating electrode placed in the MLR (electrode placement shown in Fig 1a). Single stimuli (3–5 μA, 1ms) to the MLR evoked EPSPs in MRRN RS cells as previously described11,12. The muscarinoceptive neurons also displayed EPSPs (Fig 4a; 4 pairs, 4 preparations) with a longer latency and slower time-to-peak (latency 26.0±10.7 ms, time-to-peak 21.0±0.7 ms) than EPSPs recorded in RS cells (latency 11.3±3.5 ms, time-to-peak 11.5±4.2 ms; latency and peak both significantly different, p<0.05).
Figure 4. Stimulation of the MLR activates the muscarinoceptive neurons.
a) Electrophysiological responses to a single MLR stimulus of an RS cell (top) and a muscarinoceptive neuron (bottom, recorded after retrograde labelling from the MRRN dendrites as for Figs 1 to 3).
b) Electrophysiological responses to train stimulation to the MLR of an RS cell (top) and a muscarinoceptive neuron (bottom, identified by retrograde labelling).
Stimulus trains to the MLR evoke sustained excitation of the RS system11,12. Therefore, we recorded similar pairs in a further 3 preparations in which we applied short stimulus trains (5Hz for 2s, 3–5 μA 1ms pulses) to the MLR using the same stimulation electrode type as above. This stimulation immediately activated EPSPs followed by long-lasting depolarization of MRRN neurons (Fig 4b, top). The whole cell patch clamp recorded muscarinoceptive cell (Fig 4b, bottom) exhibited a later but still long-lasting depolarization, and period of action potential firing that coincided with the later bursts of action potentials in the RS neuron. Thus, MLR stimulation activates excitatory responses in muscarinoceptive neurons, and similarly to RS neurons, this response following repetitive stimulation of the MLR is sustained.
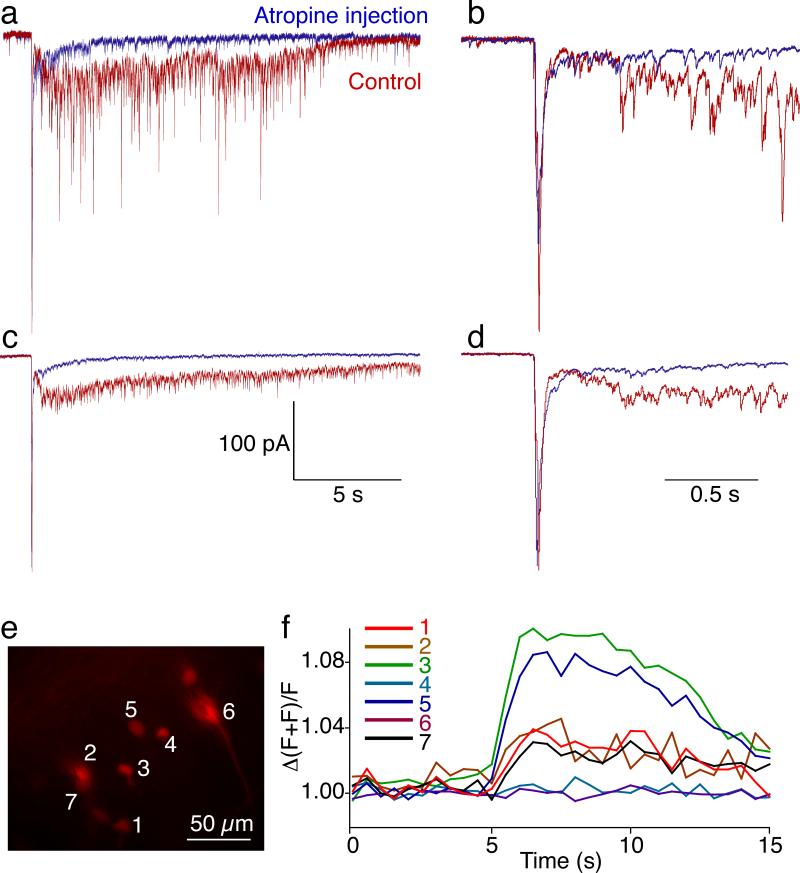
To determine whether a component of this MLR-evoked response required muscarine receptors, we whole cell voltage clamped 3 muscarinoceptive neurons targeted for recording using retrograde labeling as before. Single stimuli were applied to the MLR at higher intensities (10-15 μA for 2 ms). The stimulation elicited a compound response with early and late components (Fig 5a, red). Individual traces shown at a higher time resolution (Fig 5b) reveal that the late component comprises a sustained inward current with an overlay of asynchronous EPSCs. Bath application of the muscarinic antagonist atropine (10 μM) significantly reduced the late component of this response (Fig 5a,b, blue). Similarly, the mean evoked responses of 5 sequential stimuli in control and atropine demonstrated a marked inhibition of the later component (Fig 5c,d; area under current from mean of at least 5 stimuli, 3 preparations reduced to 20±2% of pre atropine control, p<0.01), but left the early component unaffected (Fig 5d; remained at 104±10% of control).
Figure 5. Muscarinoceptive neuron responses to MLR stimulation are inhibited by atropine.
a) Muscarinceptive neurons were targeted for whole cell recordings (labelled retrogradely, see Figs 1–4) and whole cell voltage clamped. Single, high intensity stimuli to the MLR evoked complex EPSCs. Response to a single stimulus is shown in control (red) and atropine (10 μM).
b) Expansion of recording in (a) to demonstrate composition of the late component and to demonstrate the lack of effect of atropine on the early component.
c) Mean response to 5 sequential stimuli in control and atropine.
d) Faster time base of data in (c)
e) Neurons labelled with dye (Alexa 568 dextran, shown and Ca2+ green dextran) by retrograde labelling from dye placed in the MRRN dendritic field.
f) Rise of Ca2+ green dextran fluorescence (Ca2+ concentration) in 7 muscarinoceptive cells when a train of stimulation (5 Hz, 2 s) was applied to the MLR.
To survey the population response of the dye labeled muscarinoceptive neurons to MLR stimulation, we measured neuronal responses by imaging Ca2+ transients. Muscarinoceptive neurons were dual-labeled with Alexa 568 dextran and Ca2+-sensitive dye (Calcium Green 488 dextran) by dye application in the MRRN dendritic field as before (Fig 5e). A stimulus train (5 Hz, 2s, 3–5 μA 1ms pulses; n=7 preparations) to the MLR led to a sustained rise in Ca2+ in most of the labeled neurons (7 neurons in one field shown, Fig 5f). The time-course of the Ca2+ response is consistent with the time-course of the electrophysiologically recorded MLR-mediated excitation. This response was abolished in all cases by TTX (not shown), indicating it was mediated by synaptic drive.
MLR Neurons project bilaterally to muscarinoceptive region
We have identified a group of rhombencephalic muscarinoceptive neurons. These neurons exhibit a long-lasting response following stimulation of the MLR. Furthermore, their stimulation or stimulation of the region in which they are located excites RS neurons. Therefore, we sought to determine whether neurons located in the MLR project directly to this muscarinoceptive region.
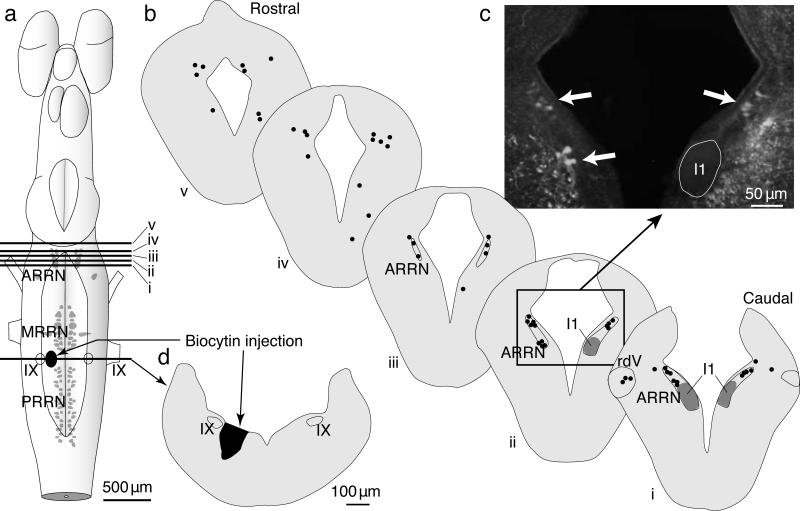
Biocytin was injected into the muscarinoceptive region of larvae (Fig 6a,d) and many neurons were labeled in the MLR. The distribution of those MLR neurons was the same in the three cases investigated, one of which is illustrated (Fig 6). In particular, many labeled neurons were observed in the caudal part of the MLR (Fig 6bi–iii) where we placed micro-stimulating electrodes during electrophysiological experiments. Other neurons were labeled in the more rostral part of the MLR (Fig 6biv,v). The majority of the labeled neurons were located medially, close to the ventricle. Most cells were pear-shaped with a diameter of less than 10μm and a few were slightly larger (15 to 20 μm; Fig 6c, photomicrograph). Thus, MLR neurons project to the muscarinoceptive region and their stimulation causes sustained depolarization of muscarinoceptive neurons recorded in this region. Muscarinoceptive neurons, in turn, project anatomically and electrophysiologically to the MRRN.
Figure 6. Neurons in the MLR project bilaterally to the muscarinoreceptive region.
Distribution of neurons located in the MLR region and projecting to the muscarinoceptive region are displayed.
a) The drawing represents a dorsal view of the whole brain of a larval stage lamprey with a 500 μm-scale bar.
b) Cross sections (100 μm-scale bar) were taken from the levels indicated on the whole brain (in a). Black dots represent the location of single cells.
c) The photomicrograph illustrates the retrogradely-labeled neuronal populations (arrows) found on schematized section (box drawn in b,ii).
d) Biocytin crystals were deposited just medial to the motoneurons of cranial nerve IX (IX), between the middle (MRRN) and the posterior (PRRN) rhombencephalic reticular nuclei, in the muscarinoceptive region. ARRN, anterior rhombencephalic reticular nucleus; rdV, descending root of the trigeminal nerve; I1, isthmic Müller reticulospinal cell 1 – a marker for the MLR location.
Atropine in muscarinoceptive region reduces RS output
We have identified a group of neurons within a region that projects glutamatergic excitation to RS neurons. These previously uncharacterized neurons respond to MLR stimulation and to bath-applied muscarine with sustained depolarizations and burst-like spiking. They also respond to muscarine in TTX, implying a direct muscarinic activation. RS neurons are excited by stimulation of these neurons and respond to muscarine and MLR stimulation with correlated sustained periods of excitation and burst firing. However, whereas RS neurons receive direct nicotinic and glutamatergic excitation from the MLR, they are only indirectly activated by muscarine17. Thus, we hypothesize that an augmenting input to the RS system is provided by muscarine receptor-mediated transmission through this newly identified group of brainstem neurons.
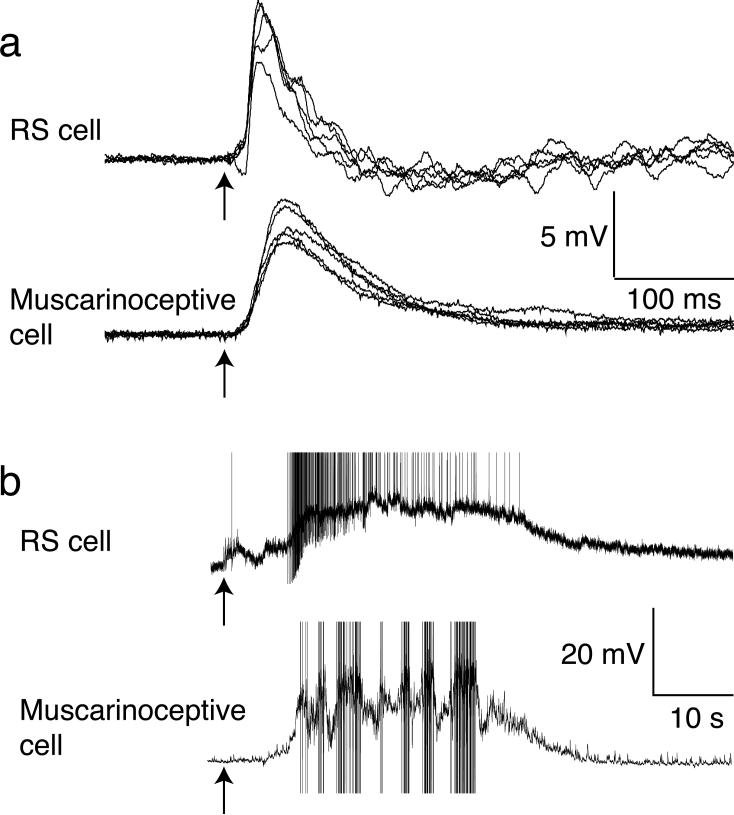
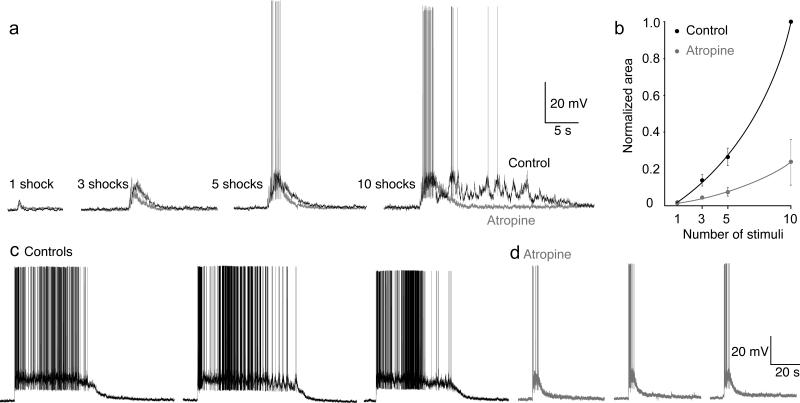
To test this hypothesis, MRRN neurons were recorded during microstimulation of the MLR (3 preparations). Two micropipettes both containing atropine (1mM) and fast green - to visually determine the injection site - were placed bilaterally in the muscarinoceptive regions. Single stimuli (1–10 μA, 1 ms) to the MLR evoked EPSPs in RS neurons. Stimulus trains (3–10 stimuli, 5 Hz, 3–5 μA, 1ms) to the MLR markedly extended the duration of the response recorded in the MRRN neurons that significantly outlasted the stimulus duration (Fig 7a). Atropine injected bilaterally through both pipettes, into the muscarinoceptive regions, substantially reduced the duration of these sustained stimulus train-evoked responses, such that depolarization of the RS neurons ceased rapidly after cessation of the stimulation (area under depolarization plotted against number of stimuli, Fig 7b). These responses were unaffected by atropine injected elsewhere in the rhombencephalon. To ensure that the sustained responses to stimulus trains were reliable, 10 shock stimulus trains (5Hz) were given at greater than 5 min intervals (Fig 7c,d, different preparation from Fig 7a). Sustained responses were reproducible. Atropine (Fig 7d) substantially and significantly reduced their duration (atropine reduced area under depolarization to 23±13% of control after 10 shocks, p<0.01). In contrast, neither the short-latency component of the response to stimulus trains nor responses to single stimuli were altered (area under the depolarization in atropine after 1 shock was 124±40% of control).
Figure 7. Muscarinoreceptive neurons provide a sustained excitatory input to RS neurons.
a) Electrophysiological responses of an RS cell to stimulation of the MLR with increasing stimulus numbers at 20 Hz in control (black) and during atropine microinjection (gray) bilaterally into the muscarinoceptive regions.
b) Graph of the RS cells electrophysiological response calculated as the area under the response, normalized to the control response following 10 stimuli to the MLR, as a function of the number of stimulus pulses in control and during atropine ejection.
c) Stimulus trains (10 shocks each) were applied sequentially to demonstrate that the extended response was reliably reproducible (black).
d) Atropine injection eliminated the extended response to MLR stimulation with the same stimuli as (c).
Errors are expressed as s.e.m.
Muscarinoceptive neurons boost MLR evoked locomotion
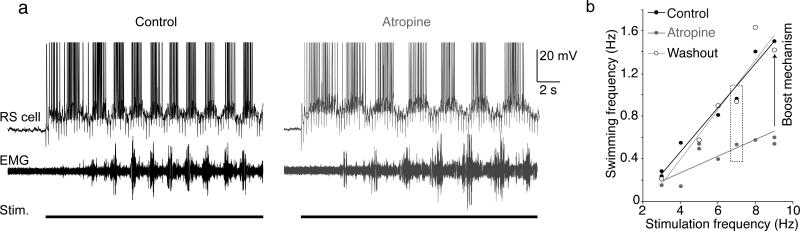
Activity of RS cells initiates and maintains locomotion3, but this activity following MLR stimulation is amplified substantially by muscarinoceptive neurons. Therefore, we evaluated the effect on locomotion of selectively blocking muscarine receptors in this muscarine-sensitive region (5 preparations). To determine effects on behaving animals, semi-intact preparations were utilized, in which the brain and rostral spinal cord were exposed and immobilized for recording, but body musculature and skin was retained. This arrangement allows simultaneous intracellular recording of supraspinal neurons responsible for the initiation and maintenance of locomotion, and recording of the resultant swimming movements and their underlying neural correlate22. An RS neuron in the MRRN was recorded with a microelectrode and an EMG electrode was inserted into the trunk musculature to monitor locomotor activity directly. Trains of stimuli were applied to the MLR using tungsten microelectrodes; the approach previously used to map synaptic connectivity within the isolated brainstem. These stimuli were maintained for extended periods over a range of frequencies in each preparation to evoke locomotion mimicking goal-directed activation of the MLR (3 to 9 Hz; threshold intensities to evoke locomotion at 3 Hz stimulation ranged from 2 to 6 μA and were subsequently used at all frequencies)10. Locomotion evoked by this stimulation was recorded as appropriate movements of the trunk and bursts of EMG activity from the musculature (Fig 8a, lower trace). The RS neurons depolarized and fired (Fig 8a, upper trace). Increasing the MLR stimulation frequency increased the locomotor burst frequency (Fig 8b, black).
Figure 8. “Fast swimming” is prevented by inactivation of the muscarinoceptive neurons.
a) In a semi-intact preparation (exposed brain with 10 most rostral spinal segments and the remainder of the animal intact), stimulation of the MLR induces RS cell discharges and swimming (EMG) that decreases in frequency from control (black) during atropine microinjection (gray)
b) Graph of swimming frequency as a function of the stimulation frequency in control (black, thick line), during atropine microinjection into the muscarinoceptive regions (gray) and after wash-out (open circles thin line). The muscarinoceptive region provides a substantial “boost mechanism” to locomotion activated by the MLR.
To determine the role that muscarinoceptive neurons play in this MLR evoked swimming, atropine was injected bilaterally into the brainstem muscarinoceptive regions as described above (Fig 7), and the stimulus pattern used to evoke locomotor behavior repeated. Atropine, microinjected around the muscarinoceptive cells, reduced locomotor output frequency (Fig 8a,b, gray). After cessation of atropine microinjection, and an extended period to allow wash out of the drug (120 minutes), locomotor activity recovered to control frequencies over the full range of stimulation frequencies (Fig 8b). Thus, MLR-stimulus evoked muscarinic activity in this muscarinoceptive region contributes markedly to the activation of locomotor behaviors.
Discussion
We have demonstrated a pathway parallel to generally accepted descriptions of vertebrate brainstem locomotor command circuitry. In addition to projecting directly to the RS system12,19,23-25 as part of the activation system for locomotion, MLR neurons also project to a group of rhombencephalic neurons located laterally in the basal plate. These neurons, identified in this study, are excited by a mixed input that originates in the MLR. Part of this signal comprises fast responses most likely mediated by ionotropic glutamate receptors, but perhaps also by cholinergic nicotinic transmission26. This early component is followed by a sustained depolarization lasting up to minutes, which is blocked by muscarine receptor antagonist applied to the brainstem in general, or specifically over the neurons. These newly identified neurons project back to RS cells eliciting glutamatergic responses to provide sustained excitatory drive during locomotion (Supplementary Fig 1). The discovery of this group of neurons explains earlier described excitatory effects of brainstem muscarine receptor activation on vertebrate locomotion15,17. This discovery also adds a previously unknown component to supraspinal locomotor command circuits with features complementary to known brainstem pathways.
We have anatomically identified bilaterally projecting neurons whose lateral dendritic fields are located precisely within a brainstem location in the rhombencephalon17, whose response to muscarine excites locomotor command RS neurons. Axons from these muscarinoceptive neurons project into the dendritic field of the RS neurons of the MRRN and paired cell recordings of these neurons and of MRRN RS neurons reveal a monosynaptic response in the RS neurons. The muscarinoceptive cells respond with a sustained Ca2+ signal and oscillating depolarization, both to muscarine, and to microstimulation of the MLR. This excitation in response to muscarine is direct; it is retained after block of action potential-evoked synaptic transmission with TTX in contrast to RS neurons17. During excitation mediated either by muscarine or electrical stimulation of the MLR, firing activity temporally corresponds to synaptic drive recorded simultaneously in RS neurons. Thus, a group of muscarinoceptive neurons, presumably a subset of brainstem muscarinoceptive neurons, caudal to the MRRN and rostral and lateral to the PRRN, act as a muscarinoceptive relay of sustained excitation from the MLR to the RS system. At low stimulus frequencies of the MLR, these neurons respond with a slower and delayed excitation compared to the response simultaneously recorded in RS neurons. However, during higher frequency MLR stimulation this late excitation corresponds with later and sustained excitation in RS neurons. This sustained component is muscarine receptor-mediated. To complete this parallel circuit, neurons of this muscarinoceptive region project monosynaptic glutamatergic excitation to RS neurons in the ipsilateral and contralateral MRRN.
Thus, transient activation of the MLR provides rapid excitation of the RS system by direct ionotropic glutamatergic and nicotinic receptor activation3,12, but additionally initiates sustained activation of muscarinoceptive neurons, which provide long-lasting excitation to RS cells. Similar persistent activity following muscarine receptor activation has been reported in thalamus27, and entorhinal cortex28. Muscarine directly induced sustained, recurring bursts of activity in a cell population in layer II of rat entorhinal cortex29. The depolarizing plateaus lasted 2–5s not unlike what we previously described in lamprey RS cells after muscarinic receptor activation17. Cell populations in entorhinal cortex were also made to oscillate synchronously upon muscarinic receptor activation30,31. Synchronization may also contribute to increased excitation of RS cells. We have previously shown that a unilateral muscarinic activation of the muscarinoceptive cells produced bilateral excitation in RS cells17. Therefore, the muscarinoceptive cells may provide a sustained and enhanced excitation to RS cells on both sides, maintaining bilateral coordination for locomotor output. Cellular properties linked directly to activation of muscarine receptors32 may thus play a crucial role in providing additional excitation to RS cells to boost the locomotor output.
Consequently, RS neurons receive two inputs originating from activity in the MLR. A direct and well-characterized component of serial activation of the locomotor system and an indirect, parallel pathway mediated by a very long-lasting muscarinic response in previously unremarked rhombencephalic neurons. This latter, newly identified parallel pathway markedly amplifies the output of the locomotor command system with a long-lasting response of the muscarinoceptive neurons, initiating sustained activity to provide a long-lasting boost to MLR driven motor output. This boost is clearly seen following brief intense stimuli applied to the MLR, in which early activation to the RS system remains but sustained activation of RS neurons is prevented by selective atropine application to the muscarinoceptive region.
Thus, transient activation of the MLR rapidly excites RS neurons by classical ionotropic monosynaptic drive, but can also sustain extended bouts of RS neuron excitation by a newly identified disynaptic pathway utilizing metabotropic muscarine receptors. Additionally, continuous activation of the MLR sustains locomotor activity over long periods3,10. Activation of command neurons implies an activation of locomotor behaviors. This hitherto unknown parallel pathway plays a substantial role in locomotor control. In the lamprey it is possible to resolve this role because we may access the functional, intact brainstems of semi-intact preparations in which continuous MLR stimulation leads to sustained locomotor activity. Atropine micro-application targeted to the muscarinoceptive region markedly reduced the frequency of the subsequent locomotor activity. This effect was non-linear with respect to stimulus frequency and subsequent locomotor frequencies. Thus, at high stimulus and high locomotor frequency, atropine profoundly reduced locomotor frequencies. At lower frequencies effects of atropine were much less marked.
A muscarinoceptive group of cells amplifying RS cell activity and locomotor output is likely to be common amongst vertebrates. In birds, locomotor behavior is induced by brainstem injections of carbachol, a nonspecific cholinergic agonist. This effect is blocked by the muscarine receptor antagonist, atropine15. Cholinergic inputs are also believed to activate brainstem neurones in mammals16,33. A group of muscarinoceptive neurons was recently described in mammals in the ventromedial medulla close to the pontine border34 at a location similar to that of the muscarinoceptive cells in lampreys. These cells receive cholinergic inputs from the pedunculopontine nucleus, known to be part of the mammalian MLR16. The role of these neurons was not described in relation to locomotion, but the similar properties of these neurons and their homologous location to those described in the present study strongly suggest that they could play a role in amplifying the RS descending signals to boost locomotor output. This would suggest that the muscarinic amplifying mechanism is conserved.
We conclude that brainstem supraspinal locomotor command systems are substantially more complex than previously thought. Anatomically simple serial pathways clearly excite brainstem command neurons. And yet, without a substantial amplification from muscarinoceptive neurons, the resulting locomotion may be short-lived and of much lower frequencies. These newly discovered rhombencephalic muscarinoceptive neurons provide a powerful augmentation of locomotor drive. The boost requires a sustained excitation driven by muscarine receptor activation.
Materials and Methods
In vitro isolated brainstem preparation
Experiments were performed on the isolated brainstem of larval lampreys (Petromyzon marinus). The animals were anesthetized with tricaine methanesulfonate (MS-222; 100 mg/l; Sigma Chemical, St. Louis, MO), decapitated, and dissected in a cold saline solution (Ringer) of the following composition (in mM): 100 NaCl, 2.1 KCl, 2.6 CaCl2, 1.8 MgCl2, 4 glucose, 26 HEPES, adjusted to a pH of 7.60. The skin, muscle, and viscera were removed. The dorsal surface of the cranium was opened, and the nervous tissue rostral to the isthmus and caudal to the 2th/3th spinal cord segment was removed. This arrangement is shown schematically in figure 2a. Also, a midsagittal, dorsal transection was made at the level of the isthmus. This opened the isolated brainstem providing clear access to the RS neurons. The cartilage containing the isolated brainstem was pinned down to the Sylgard bottom of a cooled, 5ml chamber. The recording chamber was continually superfused with cold oxygenated Ringer's (8-10°C). Procedures conformed to institutional guidelines and were approved by the University of Illinois at Chicago, Animal Care Committee and also to guidelines of the Canadian Council on Animal Care and were approved by the Animal Care and Use Committees at the Université de Montréal and the Université du Québec à Montréal. Special care was taken to limit any possible suffering to a minimum and to limit the number of animals used in the experiments.
Labeling of neurons with fluorescent dyes
Retrograde labeling of neurons with dextran-amine-conjugates of calcium-sensitive dyes has proven to be very effective to examine the activation of populations of neurons in the lamprey central nervous system 35. Changes in intracellular calcium concentration can be measured in response to single presynaptic action potentials to synaptic stimulation and to oscillations during rhythmic activity. Alexa 568 dextran, and Calcium Green dextran (10,000 MW, Molecular Probes, Eugene, Oregon) crystals were placed in the dendritic region of MRRN RS neurons lateral to their cell bodies. For paired cell recordings between muscarinoceptive neurons and neurons of the MRRN Alexa 488 dextran was placed immediately rostral to the MRRN dendritic field. The tissue was then maintained at 10C superfused with lamprey Ringer's solution for up to 24 hrs to permit in vitro retrograde transport of the dye in the dark. The preparation was then transferred and pinned down at the bottom of a small chamber. We perfused cold, oxygenated Ringer's solution throughout the subsequent electrophysiological experiments, which normally lasted 3–6 hrs.
Retrograde tracing of MLR neurons projecting to the muscarinoceptive region
Three larval lampreys ranging from 13.5 to 14 cm in total body length were used in this part of the study. Their brains were dissected as described above, except that the mesencephalon and the isthmic region were left intact. The neural tissue of the muscarinoceptive region was then disrupted with the tip of a glass micropipette and biocytin crystals were inserted into the lesion site. After 10 min, the whole preparation was rinsed thoroughly and kept overnight in a chamber as in the previous section. The next day, the preparation was immersed for 5 hours into a phosphate buffered saline (PBS: 0.1 M phosphate buffer + 0.9% NaCl, pH 7.4) solution containing 4% paraformaldehyde/0.4% picric acid, transferred into a 20% sucrose solution overnight, and then cut with a cryostat at 25 μm thickness. The sections were collected on ColorFrost Plus slides (Fisher Scientific) and left to dry overnight on a warming plate at 37°C. The next day, they were rinsed with PBS and incubated for 1 hr in a solution of Streptavidin conjugated to Alexa Fluor 488 (Molecular Probes) diluted 1:100 in PBS. The sections were then rinsed with PBS, followed by a brief rinse in distilled water, and left to dry for 15 min. The slides were then mounted with Vectashield medium containing DAPI (Vector Laboratories) and observed under an E600 epifluorescence microscope equipped with a DXM1200 digital camera (Nikon). Retrogradely labeled neurons were located on schematic drawings of the sections in the isthmic region. Only those with a clear DAPI-labeled nucleus were included in the analysis. No counts of cells were performed, as only their distribution was under investigation here.
Imaging
For confocal imaging of labeled neurons, the whole-mount brainstem was fixed (4% paraformaldehyde; 1 hr) and dehydrated in alcohols before clearing in methyl salicylate. The tissue was then imaged in whole mount under a custom built confocal microscope system. This system comprises a BioRad MRC 600 scan system, a 3 laser (488 nm, 568 nm and 633 nm fiber optic launch with intensities individually selected through an acoustic-optical tunable filter. Data from photomultiplier tubes was amplified through linear current amplifiers (Stanford Research Instruments, Sunnyvale, CA) and sampled directly via a 5 Mhz acquisition board (National Instruments, Schaumburg, IL). Scanning and acquisition software runs under Matlab (Mathworks, Natick, MA). Software is available for download from our website (http://alford.bios.uic.edu/Research/software.html).
For Ca2+ imaging of live cells the dorsal surface of the 4th ventricle was removed with a tissue slicer. For this the tissue was pinned within the ventral braincase to a sylgard block on the slicer and the blade advanced caudo-rostrally over the dorsal surface of the brainstem. The brain tissue was then removed from the braincase and pinned within a sylgard lined recording chamber. Labeled putative muscarinoceptive cells were imaged during bath application of 25μM muscarine using an Olympus microscope and recorded with an intensified CCD camera (Hamamatsu ORCA, Bridgewater, NJ). Image J36 imaging software was used to acquire and analyze the data. Calcium responses were expressed as relative changes in fluorescence (ΔF+F)/F after subtraction of background fluorescence immediately adjacent to the recorded neurons. All arithmetic manipulations performed on the image data were linear (background subtraction and alterations in gain) and were applied uniformly across the image under analysis.
Electrophysiology
Sharp electrodes intracellular recordings were made from retrogradely labelled muscarinoceptive neurons, and RS neurons in the MRRN and in the PRRN, using glass microelectrodes filled with 3M potassium acetate (80–120 MΩ). The signals were amplified by an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA; sampling rate: 2–10 kHz). Only RS neurons with a stable membrane potential, held for 15 min after impalement, lower than –65 mV were included in the study.
Putative muscarinoceptive neurons and MRRN RS neurons were also recorded under whole cell patch clamp. Patch pipette solution contained (mM): cesium methane sulphonate 102.5, NaCl 1, MgCl2 1, EGTA 5, HEPES 5, pH adjusted to 7.2 with CsOH and pipettes were pulled to a tip resistance of 5MΩ. These neurons were first labelled with fluorescent dye (Alexa 488 dextran) as described above by placing dye crystals in the dendritic field of the MRRN. After maintaining the tissue for 24 hours to allow retrograde labelling the tissue was prepared as for Ca2+ imaging experiments. Bright field views and fluorescence imaging of the labelled neurons were then combined to allow targeted whole cell patch recording of these retrogradely labelled neurons (Fig 2a). Positive pressure was applied to the pipettes to allow tissue penetration by the electrode37. This was removed to allow a seal against the fluorescently labelled neurons. Recordings were made with a patch clamp amplifier PC505B (Warner instruments, New Haven CT).
Semi-intact preparation
A semi-intact preparation was also used, in which the brain was exposed as well as the 10 most rostral spinal segments with the remainder of the animal left intact. The head region was pinned down on Sylgard as described above, while the body of the lamprey was left to swim freely in a Ringer's bath. Cold (8–10°C) Ringer's solution was perfused through the entire semi-intact chamber. At least 2 h were allowed between the end of the dissection and the beginning of recording sessions. Locomotion was monitored using electromyographic (EMG) recordings. Teflon coated stainless steel wires (diameter: 50 μm; California Fine Wire, Grover Beach, CA) were inserted into the side wall musculature between segments 20 and 25. Locomotor movements of the body/tail could be visualized to ascertain the quality of the swimming. The EMG signals were amplified (1000 times), filtered (bandwidth: 30 Hz to 1 kHz) and acquired with a sampling rate of 5 kHz.
Drugs
All drugs were dissolved to their final concentration in the Ringer's solution. Muscarine chloride (muscarine, 25 μM), [Sigma-Aldrich], 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) [Tocris], DL-2-amino-5-phosphonopentanoic acid (AP5) [Tocris] and tetrodotoxin (TTX, 1 μM) [Calbiochem] were bath applied at the indicated concentrations per experiment. Atropine (1mM) [Sigma-Aldrich] was pressure-ejected through a glass micropipette with a Picospritzer (General Valve Corporation, Fairfield, NJ). For these microinjection experiments, the inactive dye Fast Green was added to the drug solution to monitor the extent of the application. The injection micropipette was positioned upon the surface of the tissue and injection parameters were set so a Fast Green stain of ~100μm diameter would be visible on the surface of the tissue right after injection. Control injections of Fast Green dissolved in Ringer's alone did not initiate any response in intracellularly recorded RS neurons.
Data acquisition and analysis
Electrophysiological data were acquired through a Digidata 1322A interface with Clampex 8.0 software (Axon Instruments, Molecular Devices, Sunnyvale, CA). Intracellular signals were analyzed with Clampfit 8.0 software. Data are expressed as means ± SEM unless otherwise noted. Statistical significance was determined using Students t tests.
Supplementary Material
Supplementary Information Titles
Please list each supplementary item and its title or caption, in the order shown below. Please include this form at the end of the Word document of your manuscript or submit it as a separate file.
Note that we do NOT copy edit or otherwise change supplementary information, and minor (nonfactual) errors in these documents cannot be corrected after publication. Please submit document(s) exactly as you want them to appear, with all text, images, legends and references in the desired order, and check carefully for errors.
Acknowledgements
We would like to thank D. Veilleux for assistance with experiments, C. Valiquette for his expertise in computer programming and to F. Bernard for help with the figures. We also thank E Hamid and M Alpert for comments and critical discussion of this manuscript. This work was funded by a grant from NINDS (R01 NS052699) to SA and Individual and Group grants from the Canadian Institutes of Health Research (CIHR), Grant Numbers: 15129 and 15176, the Natural Sciences and Engineering Research Council of Canada (NSERC), Grant Number: 217435-01, and the Groupe de recherche sur le système nerveux central (GRSNC) from the Fonds de la Recherche en Santé du Québec (FRSQ), Grant Number: 5249 to RD. LJ received a Jasper fellowship from the GRSNC.
References
- 1.Grillner S. Ion channels and locomotion. Science (New York, N.Y. 1997;278:1087–1088. doi: 10.1126/science.278.5340.1087. [DOI] [PubMed] [Google Scholar]
- 2.Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Dubuc R, et al. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26:273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- 5.Shik ML, Severin FV, Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain]. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- 6.Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 7.Bernau NA, Puzdrowski RL, Leonard RB. Identification of the midbrain locomotor region and its relation to descending locomotor pathways in the Atlantic stingray, Dasyatis sabina. Brain Res. 1991;557:83–94. doi: 10.1016/0006-8993(91)90119-g. [DOI] [PubMed] [Google Scholar]
- 8.Cabelguen JM, Bourcier-Lucas C, Dubuc R. Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J Neurosci. 2003;23:2434–2439. doi: 10.1523/JNEUROSCI.23-06-02434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- 10.Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Brocard F, Dubuc R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J Neurophysiol. 2003;90:1714–1727. doi: 10.1152/jn.00202.2003. [DOI] [PubMed] [Google Scholar]
- 12.Le Ray D, et al. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur J Neurosci. 2003;17:137–148. doi: 10.1046/j.1460-9568.2003.02417.x. [DOI] [PubMed] [Google Scholar]
- 13.Brocard F, et al. The transformation of a unilateral locomotor command into a symmetrical bilateral activation in the brainstem. J Neurosci. 2010;30:523–533. doi: 10.1523/JNEUROSCI.3433-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn K, et al. Supraspinal locomotor control in quadrupeds and humans. Prog Brain Res. 2008;171:353–362. doi: 10.1016/S0079-6123(08)00652-3. [DOI] [PubMed] [Google Scholar]
- 15.Sholomenko GN, Funk GD, Steeves JD. Avian locomotion activated by brainstem infusion of neurotransmitter agonists and antagonists. I. Acetylcholine excitatory amino acids and substance P. Exp Brain Res. 1991;85:659–673. doi: 10.1007/BF00231752. [DOI] [PubMed] [Google Scholar]
- 16.Homma Y, Skinner RD, Garcia-Rill E. Effects of pedunculopontine nucleus (PPN) stimulation on caudal pontine reticular formation (PnC) neurons in vitro. J Neurophysiol. 2002;87:3033–3047. doi: 10.1152/jn.2002.87.6.3033. [DOI] [PubMed] [Google Scholar]
- 17.Smetana RW, Alford S, Dubuc R. Muscarinic receptor activation elicits sustained, recurring depolarizations in reticulospinal neurons. J Neurophysiol. 2007;97:3181–3192. doi: 10.1152/jn.00954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J Neurophysiol. 1967;30:1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- 20.Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- 21.Pombal MA, Marin O, Gonzalez A. Distribution of choline acetyltransferaseimmunoreactive structures in the lamprey brain. J Comp Neurol. 2001;431:105–126. doi: 10.1002/1096-9861(20010226)431:1<105::aid-cne1058>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science (New York, N.Y. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Rill E, Skinner RD, Conrad C, Mosley D, Campbell C. Projections of the mesencephalic locomotor region in the rat. Brain research bulletin. 1986;17:33–40. doi: 10.1016/0361-9230(86)90158-9. [DOI] [PubMed] [Google Scholar]
- 24.Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 1984;307:263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]
- 25.Orlovsky GN. Connexions of the reticulo-spinal neuronswith the “locomotor regions” in the brainstem. Biofizika. 1970;1:171–177. [Google Scholar]
- 26.Jahn K, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. NeuroImage. 2008;39:786–792. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Curro Dossi R, Pare D, Steriade M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J Neurophysiol. 1991;65:393–406. doi: 10.1152/jn.1991.65.3.393. [DOI] [PubMed] [Google Scholar]
- 28.Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- 29.Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- 30.Dickson CT, Alonso A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J Neurosci. 1997;17:6729–6744. doi: 10.1523/JNEUROSCI.17-17-06729.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson CT, et al. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- 32.Tahvildari B, Alonso AA, Bourque CW. Ionic basis of ON and OFF persistent activity in layer III lateral entorhinal cortical principal neurons. J Neurophysiol. 2008;99:2006–2011. doi: 10.1152/jn.00911.2007. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987;411:1–12. doi: 10.1016/0006-8993(87)90675-5. [DOI] [PubMed] [Google Scholar]
- 34.Mamiya K, Bay K, Skinner RD, Garcia-Rill E. Induction of long-lasting depolarization in medioventral medulla neurons by cholinergic input from the pedunculopontine nucleus. J Appl Physiol. 2005;99:1127–1137. doi: 10.1152/japplphysiol.00253.2005. [DOI] [PubMed] [Google Scholar]
- 35.McClellan AD, McPherson D, O'Donovan MJ. Combined retrograde labeling and calcium imaging in spinal cord and brainstem neurons of the lamprey. Brain Res. 1994;663:61–68. doi: 10.1016/0006-8993(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 36.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 37.Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Titles
Please list each supplementary item and its title or caption, in the order shown below. Please include this form at the end of the Word document of your manuscript or submit it as a separate file.
Note that we do NOT copy edit or otherwise change supplementary information, and minor (nonfactual) errors in these documents cannot be corrected after publication. Please submit document(s) exactly as you want them to appear, with all text, images, legends and references in the desired order, and check carefully for errors.