Abstract
Cyclophosphamide (CTX), a commonly used chemotherapeutic agent can enhance immune responses. The ability of CTX to promote the proliferation of effector T cells and abrogate the function of regulatory T cells (Tregs) has been described. In this study, we examined the effects of CTX treatment on dendritic cell (DC) subsets and the subsequent outcome on the effector and suppressive arms of adaptive immunity. In secondary lymphoid tissues, tissue-derived migratory DCs (migratory DCs), lymphoid tissue–resident DCs (resident DCs), and plasmacytoid DCs (pDCs) are well described. CTX has profound and selective cytotoxic effects on CD8+ resident DCs, but not skin-derived migratory DCs or pDCs in lymph nodes (LNs) and spleen, causing an imbalance among these DC subsets. CTX treatment increases the potency of DCs in antigen presentation and cytokine secretion, and partially inhibits the suppressor activity of Tregs. Adoptive transfer of CD8+ DCs can reconstitute this population in regional draining LNs and abrogate the immune-enhancing effects of CTX in vivo. These findings demonstrate that CTX may improve immune responses by preferentially depleting CD8+ lymphoid-resident DCs, which leads to diminished Treg suppression and enhanced effector T-cell function in vivo.
Introduction
Cyclophosphamide (CTX) is a chemotherapeutic agent, which is used for the treatment of leukemias, lymphomas, and solid tumors.1–2 In recent years, in part because of the challenges of stimulating effective immunity in the tumor-bearing host, the immunomodulatory effects of CTX have become an area of interest. Dose-dependent effects of CTX on the secretion of type I interferons (IFNs), homeostatic proliferation, and on depleting regulatory T cells (Tregs) have been proposed as mechanisms for the observed enhancement in immune responses.2–10 CD4+CD25+ Tregs are very sensitive to CTX, and exhibit increased levels of apoptosis and decreased ability to suppress T-cell proliferation in vitro after CTX treatment.11–13 A role for CTX in improving dendritic cell (DC) function has also been proposed.14 Recently, feedback control of regulatory T-cell homeostasis by DCs has been demonstrated in vivo, suggesting that therapeutic modulation of DCs could also lead to altered Treg function.15 Although immune responses are modified by the interplay among DCs, effector T cells, and Tregs,16 the effects of CTX on specific DC subsets has not been investigated.
DCs are specialized antigen-presenting cells that are critical in both initiating immunity to pathogens and promoting tolerance to self.17 DCs can be divided into 2 main categories: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). cDCs can be further divided into 2 main subsets: tissue-derived migratory DCs (migratory DCs) and lymphoid tissue–resident DCs (resident DCs). Migratory DCs access the local draining lymph nodes (LNs) from residing peripheral tissues, whereas resident DCs are replenished by blood-borne precursors from the bone marrow.18 Thus, resident DCs are found in lymph nodes (LNs) and spleen, but migratory DCs are found only in LNs. Resident DCs can be further subdivided into 3 types: CD8+ DCs, CD4+ DCs, and CD4− CD8− DCs.17 Because migratory DCs can transfer antigens to resident DC subsets and both can present antigens to naive CD8+ and CD4+ T cells, how the balance of DC subsets results in tolerance to self or reactivity to pathogens is not known.17,19
In this study, we examined the effects of CTX treatment on DC subsets in the LNs and spleen. We find that CTX causes the greatest decrease in the proportion of CD8+ resident DCs in these lymphoid tissues. DCs from CTX-treated mice are more potent antigen-presenting cells than DCs from naive mice and can decrease Treg suppressive function. When we correct the imbalance between DC subsets by adoptively transferring back CD8+ DCs, but not CD8− DCs, the immune-enhancing effects of CTX are significantly diminished. These results indicate that CTX enhances immune responses modulating the balance between DC subsets.
Methods
Mice
Mice were maintained in a pathogen-free vivarium and all procedures were performed in accordance with institutional guidelines at Memorial Sloan-Kettering Cancer Center under an approved protocol. C57BL/6J mice (females, 6-8 weeks old), Balb/c mice (females, 6-8 weeks old), and OT-1 mice were obtained from The Jackson Laboratory. Thy1.1+ pmel-1 T-cell receptor (TCR) transgenic mice were bred in the Memorial Sloan-Kettering Cancer Center mouse facility and have been previously reported.20
Cyclophosphamide
Cyclophosphamide (Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) and administered in a single dose of 150 mg/kg as described previously21 at the specified time points.
Antibodies and flow cytometry
The following fluorochrome-labeled or biotin-conjugated anti–mouse monoclonal antibodies (mAbs) were from BD Biosciences: CD11c (HL3), I-Ab (AF6-120.1), B220 (RA3-6B2), Gr-1 (RB6-8C5), CD8 (53-6.7), CD4 (RM4-5), CD80 (16-10A1), CD86 (GL1), CD3 (145-2C11), CD44 (IM7), Thy1.1 (OX-7), interleukin-12p40/70 (IL-12p40/70; C15.6), and IFN-γ (XMG1.2). Foxp3 (FJK-16s) antibody was purchased from eBioscience. Splenocytes or LN cells were washed in fluorescence-activated cell sorting (FACS) buffer (PBS/2% bovine serum albumin/0.1% azide), and 106 cells/mL were incubated for 10 minutes at 4°C with CD16/CD32 Fc block (BD Biosciences). Subsequently, cells were incubated for 30 minutes at 4°C with primary antibody or antibodies (1 mg/mL) and washed twice with FACS buffer. Samples were acquired on an LSR I cytometer (Becton Dickinson) with CellQuest software (Becton Dickinson) and analyzed with FlowJo (TreeStar).
Isolation of dendritic cells
The isolation of DC subpopulations has been previously described.22–24 In brief, tissues were chopped and digested with collagenase (2 mg/mL; Roche) and DNase (1 mg/mL; Sigma-Aldrich) at 37°C for 60 minutes. Low-density cells were enriched by density centrifugation (1.083 g/cm3, Nycodenz). Non–DC lineage cells were coated with a biotinylated mAb cocktail (anti-CD2, anti-CD3, anti-CD49b, anti–murine immunoglobulin M, anti–Ter-119) and then removed using magnetic beads (Dynal). To obtain CD11c+ DCs from LNs, enriched DCs were then purified immunomagnetically by 2 to 3 rounds of positive selection with CD11c (N418) MicroBeads (Miltenyi Biotec) according to the manufacturer's instructions. Purity was analyzed by FACS and was between 90% and 94%. To obtain CD8+ or CD8− DC subsets from spleen, splenic DCs were enriched by magnetic bead (Dynal) negative selection and then sorted by magnetic-activated or flow cytometric cell sorting. For magnetic-activated sorting, enriched DCs were subjected to 2 to 3 rounds of selection using CD8 microbeads and CD11c microbeads. For flow cytometric sorting, cells were sorted based on the expression of CD11c and CD8. Sorting was performed on a MoFlo instrument (DakoCytomation). The purity of isolated CD8+ DCs was between 90% and 94% (magnetic-activated sorting) and between 96% and 98% (sorting).
T-cell preparations
Whole CD3+ T cells were purified with the Pan T Cell Isolation Kit (Miltenyi Biotec), a method of negative selection in a magnetic field, as instructed by the manufacturer. The purity of isolated T cells was greater than 95%. OT-1 CD8+ T cells, which recognize the OVA major histocompatibility complex (MHC) class I peptide (OVAp) SIINFEKL in the context of the MHC class I molecule H-2Kb, were harvested from spleen and LNs of OT-1 TCR transgenic mice by magnetic sorting using CD8 microbeads (Miltenyi Biotec). CD4+CD25+ Tregs were isolated from LNs by magnetic or flow cytometric cell sorting using a CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec) per the company protocol or by surface staining CD4+-enriched cells with anti-CD25–phycoerythrin (3C7) and anti-CD4–allophycocyanin (H129.19; PharMingen) and sorting on a MoFlo instrument (DakoCytomation). The purity of cells sorted by either method was more than 90%, and both methods of sorting were used in individual experiments for preparing Tregs for functional assays.
Cell proliferation assay in vitro
Various doses of purified DCs from LNs were cocultured with 1 × 105 allogeneic T cells from Balb/c mice or were pulsed with OVA peptide and cocultured with antigen-specific CD8+ T cells from OT-1 mice in triplicate wells of 96-well plates for 4 days. During the last 4 hours of incubation, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt and phenazine methosulfate (MTS/PMS) solution (CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega) was added to the cultures to measure proliferation of T cells. Absorbance at 490 nm was recorded using an enzyme-linked immunosorbent assay plate reader.
Intracellular cytokine staining
For intracellular IL-12 production of DCs, cells were fixed and permeabilized with the Cytofix/Cytoperm Kit (Pharmingen) after being stained with surface fluorochrome-conjugated antibodies. Cells were subsequently stained with phycoerythrin-conjugated anti–IL-12 p40/70 and anti–IL-10 antibodies. For intracellular IFN-γ production from T cells, lymphocytes were stimulated in vitro with phorbol myristate acetate and ionomycin for 6 hours and 10 μg/mL brefeldin A (Sigma-Aldrich) was added for the last 5 hours. Cells were stained for surface markers, fixed, and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences), and stained for intracellular IFN-γ.
CFSE-labeled T-cell proliferation assays
Purified CD3+ T cells were washed once in PBS and resuspended at 107 cell/mL. Carboxyfluorescein diacetate succinimidyl ester (CFSE, 5μM; Molecular Probes) was added (1 mL/107 cells), and incubated at 37°C for 10 minutes. RPMI-1640 medium containing 7.5% fetal calf serum was added, and cells were washed twice. T cells (5 × 104 cells/well) were incubated with various DC subsets (104 cells, or as otherwise stated) in U-bottomed 96-well plates in 200 μL of culture medium. Dead cells were excluded using 4,6 diamidino-2-phenylindole staining. Analysis was performed on an LSR II flow cytometer (Becton Dickinson). Proliferating T cells were identified by loss of CFSE fluorescence.
Treg suppression assay
Sorted Tregs (104) were cultured with 104 allogeneic DCs subsets (irradiated with 15 Gy) and 500 units/mL rhIL-2 in 96-well round-bottomed plates. After 1 week of expansion, DCs were depleted with anti-CD11c magnetic-activated cell sorting microbeads to provide more than 96% Tregs. Suppressive function of Tregs expanded with various DC subsets was tested using a modified Shevach suppression assay.25 Briefly, fresh CD25−CD3+ T cells (responders) were first labeled with 5μM CFSE. These responders were then cocultured with 104 lipopolysaccharide (LPS)–stimulated DCs. To these, we added graded numbers of DC-expanded Tregs. After 5 days of culture in 96-well round-bottom plates, CFSE dilution was assessed on live cells (dead cells gated out with 4,6 diamidino-2-phenylindole; Molecular Probes) and analyzed with FlowJo software (TreeStar).
Pmel-1 TCR transgenic adoptive transfer model
CD8+ pmel-1 T cells were isolated with CD8 microbeads (Miltenyi Biotec) from the spleens and LNs from Pmel-1 mice. Of the CD8 T cells in these mice, 60% to 80% express a transgenic TCR reactive against the immunodominant gp10025-33 Db epitope. CTX was administered by intraperitoneal injection at a dose of 150 mg/kg per mouse. Three days later, 1 × 105 naive thy1.1 pmel-1 T cells were adoptively transferred via tail vein injection. Mice were injected with either CD8+ or CD8− DCs into the ear on the same day. After 24 hours, mice were immunized with hgp100-pulsed activated mature DCs intradermally to the same ear. Draining retroauricular LNs were harvested 5 days after immunization and analyzed by flow cytometry. Mature DCs were obtained by culturing bone marrow in complete RPMI supplemented with recombinant granulocyte-macrophage colony-stimulating factor as described previously26 followed by treatment with LPS 1 μg/mL (Sigma-Aldrich).
Concomitant immunity model
The murine melanoma cell line, B16F10 was provided by I. Fidler (M. D. Anderson Cancer Center). After a brief culture in vitro, B16 cells were inoculated by intradermal injection. For concomitant immunity experiments, the priming tumor was injected in the left ear (105) and the challenge tumor was given in the right shoulder (2.5 × 105) 6 days later. Tumor diameters were measured approximately every other day, and mice were killed when one of the tumors ulcerated or reached a maximum diameter of 1 cm, or when mice showed signs of discomfort.
Statistics
All values shown in graphs represent the mean (± SEM). Statistical differences between different groups were determined by 1-tailed Mann-Whitney U test for nonparametric variables. P value less than .05 was considered statistically significant. Correction for multiple comparisons was not performed because of the hypothesis-generating nature of these studies. For the concomitant immunity experiments, a generalized estimating equations model was fit to the data to evaluate whether there is an association among groups with respect to tumor growth over time (via Wald test). This model accounts for the longitudinal structure of the data and any inherent correlation within each mouse.27
Results
Effect of CTX on different DCs subsets in secondary lymphoid tissues
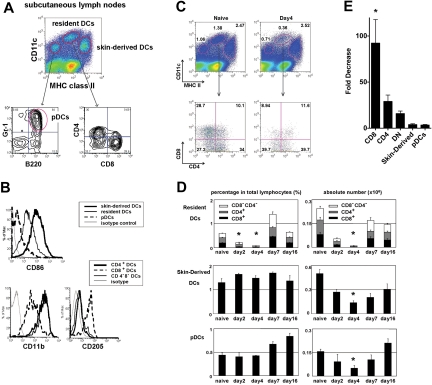
We first established the baseline level of each DC in lymphoid tissues in the steady state. In secondary lymphoid tissues, migratory DCs, resident DCs, and pDCs are identified by their relative expression of CD11c and MHC class II. In subcutaneous LNs, migratory DCs are skin derived, and contain Langerhans cells and dermal DCs.17 Skin-derived DCs have a CD11cint MHC class IIhigh phenotype, resident DCs have a CD11chighclass IIint phenotype, and pDCs have a CD11cintclass IIlow (B220+ Gr-1+) phenotype (Figure 1A). As described previously, skin-derived DCs expressed higher CD86 than resident DCs. Resident DCs are further divided into CD8+ DCs and CD8− DCs, and CD8+ DCs expressed higher levels of CD205 and lower CD11b than CD8− DCs (Figure 1B).
Figure 1.
Effect of CTX on the absolute and relative numbers of different DC subsets in lymph nodes. Mice were treated with CTX (150 mg/kg) by intraperitoneal injection and killed 2, 4, 7, and 16 days after drug administration. Subcutaneous LNs and spleen were harvested, digested, and assessed for the number and proportion of skin-derived migratory DCs (CD11cint, MHC IIhigh), resident DCs (CD11chigh, MHCint), or pDCs (CD11cint, MHClow, B220+, Gr1+) at the time points indicated and compared with untreated naive controls. (A) DC subsets in subcutaneous LNs and spleen of naive mice stained for CD4, CD8, and (B) CD86, CD11b, and CD205. Representative plots of naive LN DCs and LN DCs 4 days after CTX treatment (C). The proportions of skin-derived DCs, resident DCs, and pDCs in the LNs as measured by flow cytometry at various time points after the administration of CTX (D). Absolute cell number of different DC subsets was also counted using the total cell count of LN cells. Fold decrease in absolute number of LN DC subsets 4 days after CTX treatment (E). CD8 indicates CD8+ resident DCs; CD4, CD4+ resident DCs; DN, CD8−CD4− resident DCs; and pDC, plasmacytoid DCs. Data are representative of 4 independent experiments. *Statistically significant.
We sought to investigate the effects of CTX on different DC subsets in spleen and subcutaneous LNs (Figure 1C and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Mice were assessed for the number and proportions of different DC subsets after a single injection of CTX (150 mg/kg). CTX treatment led to a transient decrease in the absolute number of all DC subsets in both the LNs and spleen (Figure 1D and supplemental Figure 1). DCs decreased until day 4 and then gradually approached baseline numbers by day 16. Interestingly, resident DCs, especially CD8+ DCs, were much more sensitive to CTX treatment than skin-derived DCs and pDCs (Figure 1E). The relative decrease after CTX administration was statistically significantly greater for CD8+DCs than skin-derived DCs and pDCs. This indicates that the administration of CTX causes a transient imbalance in DC subsets favoring skin-derived DCs over resident DCs, and that CD8+ DCs in particular are the most sensitive to CTX treatment.
DCs from CTX-treated mice are more potent than those from naive mice
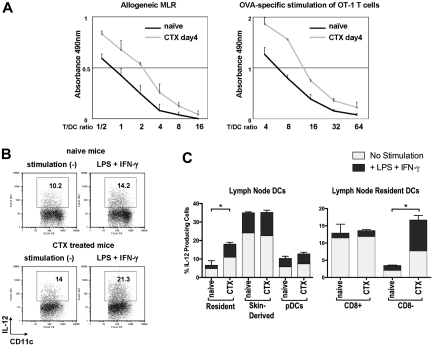
We next asked how this imbalance in DC subsets affects allogeneic or antigen-specific T-cell priming. Whole CD11c+ DCs from subcutaneous LNs of either naive mice or CTX-treated mice were purified to examine the function of DCs 4 days after CTX administration. Purified CD11c+ DCs contained 3 distinct DC subsets: skin-derived DCs, resident DCs, and pDCs (data not shown). Purified DCs from CTX-treated mice had more skin-derived DCs and far fewer CD8+ DCs than those from naive mice (data not shown). These DCs were cocultured with allogeneic T cells or pulsed with SIINFEKL and cocultured with syngeneic OT-1 CD8+ T cells. DCs from CTX-treated mice induced more potent allogeneic and antigen-specific proliferation of T cells than those from naive mice (Figure 2A). DCs from CTX-treated mice or naive mice were also examined by flow cytometry for cytokine production (IL-12, IL-10). DCs from CTX-treated mice secreted more IL-12 with or without stimulation than DCs from naive mice (Figure 2B). This difference was due to an increase in IL-12 secretion from CD8− DCs in CTX-treated mice compared with naive mice (Figure 2C). Interestingly, IL-12 secretion from skin-derived DCs, CD8+ DCs, and pDCs was unchanged by CTX treatment. IL-10 was secreted from very small number of DCs in both groups of mice (data not shown). Taken together, these results indicate that DCs from CTX-treated mice were functionally more potent and support the hypothesis that CD8+ resident DCs have a negative regulatory effect on immune responses.
Figure 2.
DCs from CTX-treated mice are more potent at antigen presentation. Whole CD11c+ DCs from CTX-treated or naive mice were sorted from LNs with anti-CD11c magnetic-activated cell sorting beads. (A) Graded doses of DCs were cocultured with allogeneic T cells from Balb/c mice or were pulsed with OVA peptide and cocultured with antigen-specific CD8+ T cells from OT-I mice. During the incubation, MTS/PMS solution was added to the culture to measure the proliferation of T cells. Absorbance at 490 nm was recorded using an enzyme-linked immunosorbent assay plate reader. Representative data from 3 independent experiments are shown. (B) DCs from CTX-treated or naive mice were sorted as described for panel A and cultured in vitro with granulocyte-macrophage colony-stimulating factor for 20 hours with or without stimulation (LPS + IFN-γ). Brefeldin A was present during the last 12 hours of the incubation. (C) DCs were stained with anti-CD11c, MHC class II, B220, Gr-1, and CD8 to identify different DC subsets as defined in Figure 1A and examined for production of IL-12 p40/70. Baseline levels and augmentation of IL-12 p40/70 with the addition of LPS and IFN-γ are depicted. Data are representative of 3 independent experiments. *Statistically significant.
CD8+ DCs inhibit the allostimulatory capacity of DCs from CTX-treated mice in vitro
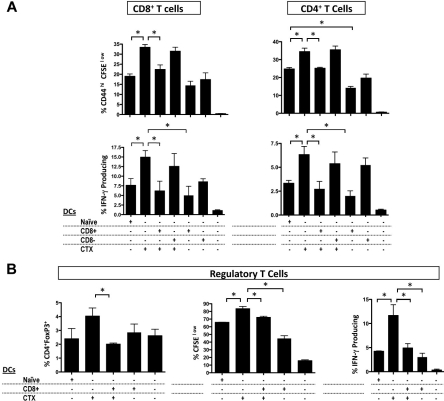
Because prior studies suggested that CD8+ DCs could have immunoinhibitory or tolerogenic functions,28–30 we asked how CD8+ or CD8− resident DCs affect the immune-enhancing effects of CTX using an in vitro allogeneic mixed lymphocyte reaction (MLR) assay. Whole CD11c+ DCs were purified from LNs of CTX-treated or naive mice. CD8+ or CD8− DCs were purified from the spleens of naive mice. As expected, DCs from CTX-treated mice induced more activation, proliferation, and IFN-γ secretion both in CD4+ and CD8+ T cells than DCs from naive mice (Figure 3A). Adding CD8+ DCs inhibited the effects of CTX on cell proliferation and IFN-γ secretion. Adding CD8− DCs did not change the effects of CTX in this setting. Thus, CD8+ DCs have immunoinhibitory properties in vitro, suggesting that their depletion in vivo could enhance immune responses after CTX treatment. Unexpectedly, examination of Tregs after allogeneic MLRs revealed that DCs from CTX-treated mice induced more potent proliferation and IFN-γ production by Tregs, and that these effects were inhibited by CD8+ DCs (Figure 3B). Tregs from CTX-treated mice have been shown to have decreased suppressor activity, but the interactions between DCs and Tregs have not been investigated.11,13 Recent studies have demonstrated that Treg homeostasis is directly affected by DC number, and Tregs in turn regulate DC numbers.15,31 Thus, our results suggest 2 possibilities: (1) DCs from CTX-treated mice may enhance immune responses by inhibiting Treg suppressive activity, or (2) DCs from CTX-treated mice may expand Tregs, which perhaps help restore homeostasis after CTX treatment.
Figure 3.
CD8+ DCs inhibit the allostimulatory capacity of DCs from CTX-treated mice in vitro. CD11c+ DCs from CTX-treated or naive mice were magnetically sorted from LNs. CD8+ and CD8− DCs of naive mice were also magnetically or FACS sorted from the spleen. DCs (naive DC indicates DCs from naive mice; CTX DCs, DCs from CTX-treated mice; CD8+, CD8+ DCs; and CD8−, CD8− DCs) were cocultured with the combinations indicated with CFSE-labeled CD3+ T cells from allogeneic Balb/c mice for 4 days. (A) CD4+ and CD8+ T-cell proliferation was examined by measurement of CFSE dilution and the T-cell activation marker, CD44. To assess IFN-γ production, T cells were stimulated with phorbol myristate acetate and ionomycin for 6 hours; brefeldin A was present during the last 5 hours, and then stained for intracellular IFN-γ. Results are the mean ± SEM of triplicate wells. Data are representative of 3 experiments. (B) The proportion, proliferation, and IFN-γ secretion of CD4+ Foxp3+Tregs were also examined. Results are the mean ± SEM of triplicate wells. Data are representative of 3 experiments. *Statistically significant.
Tregs expanded with DCs from CTX-treated mice have less suppressor activity
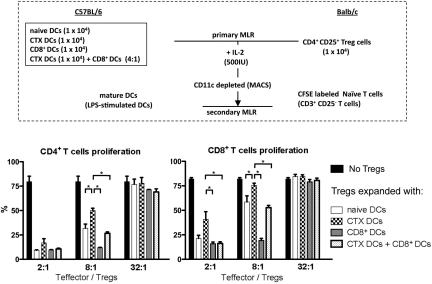
To investigate the effects of different kinds of DCs on the suppressor activity of Tregs, Tregs expanded with different DC subsets (primary MLR) were mixed with 105 CFSE-labeled CD25– CD3+ responder T cells in various ratios. Responder T cells were stimulated with irradiated fully mature LPS-activated DCs (secondary MLR; Figure 4A). When Tregs were expanded with CTX-treated DCs, they were less potent suppressors of CD4+ and CD8+ T-cell proliferation, as shown in the secondary MLR reaction (Figure 4B). Moreover, Tregs expanded with CD8+ DCs alone were significantly more effective at suppressing both CD4+ and CD8+ T-cell proliferation and abrogated the effects on Tregs induced by CTX-treated DCs, restoring function similar to that induced by naive DCs. Typical markers of Tregs (CD25, Foxp3, and glucocorticoid-induced tumor necrosis factor receptor) were retained after the primary MLR, and Tregs expressed similar levels of these markers despite differences in suppressive ability (data not shown). These results favor the hypothesis mentioned in the previous section, which is that Tregs have less suppressor activity when they are expanded with DCs from CTX-treated mice. Interestingly, Tregs expanded with CD8+ DCs have more suppressor activity, and this effect is dominant when CD8+ DCs are admixed with CTX-treated DCs. This suggests that Tregs from CTX-treated mice may be less suppressive in part because of an interaction with DCs and also raises the hypothesis that the depletion of CD8+ DCs in the context of CTX treatment may be important in the immune-enhancing effects of CTX seen in vivo.
Figure 4.
Tregs expanded with DCs from CTX-treated mice have less suppressor activity. Isolated CD4+ CD25+ Tregs from Balb/c mice were cocultured with different DC subsets (DCs from naive mice, DCs from CTX-treated mice, CD8+ DCs, and DCs from CTX-treated mice + CD8+ DCs) from B6 mice (primary MLR). After 7 days, Tregs expanded with different DC subsets were mixed with 105 CFSE-labeled CD25− CD3+ T cells in various ratios (2:1-32:1) and stimulated with irradiated mature DCs activated with LPS (secondary MLR). At 5 days after secondary MLR culture, T-cell proliferation was examined by measurement of CFSE dilution. Results are the mean ± SEM of triplicate wells. Data are representative of 2 independent experiments. *Statistically significant.
Restoration of CD8+ DCs by adoptive transfer inhibits the enhanced antigen-specific T-cell responses seen in vivo after CTX administration
To verify our hypothesis in vivo, we tried to reconstitute the CD8+ DC population by adoptive transfer. When we injected purified CD8+ DCs intravenously or subcutaneously to naive mice or CTX-treated mice, we could not detect any transferred DCs in LNs or spleen (data not shown). However, if CD8+ DCs were injected intradermally in the ear skin, we could detect the adoptively transferred CD8+ DCs in the draining preauricular LNs (supplemental Figure 2). At 24 hours after adoptive transfer, the proportion of CD8+ DCs in the draining preauricular LNs increased by almost 10 times compared with the proportion in nondraining lymph node or without adoptive transfer. Thus, for the next series of experiments, CD8+ DCs were injected into the ear.
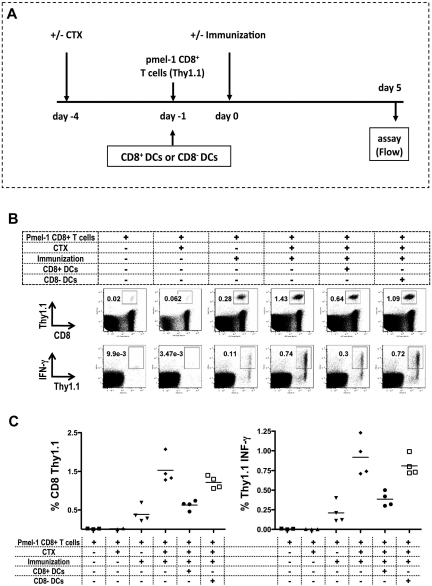
To evaluate the effects of CD8+ or CD8− resident DCs on antigen-specific T-cell priming, we used CD8+ T cells isolated from the pmel-1 mouse, in which 60% to 80% of the T cells express a transgenic TCR specific for gp10025-33, the immunodominant Db epitope of melanosomal matrix protein gp100 expressed on B16 melanoma.20 C57BL/6 Thy1.2 mice received adoptively transferred CD8+ Thy1.1+ pmel-1 cells by intravenous injection followed by peptide (hgp100)–pulsed DC immunization (Figure 5A). We characterized the antigen-specific T-cell response by measuring expansion and IFN-γ secretion from the adoptively transferred T cells. We could detect the adoptively transferred T-cell expansion and IFN-γ secretion from the population when mice were immunized. CTX treatment resulted in a dramatic increase in both the expansion and IFN-γ secretion measured as percentage and absolute number of cells in the draining LN. Adoptive transfer of CD8+ DCs, but not CD8− DCs almost completely abrogated the increases in the absolute number of transferred T cells and percentage of IFN-γ secretion seen with CTX administration (Figure 5B-C). These results indicate that CD8+ DCs in the regional LNs can inhibit the expansion and function of antigen-specific T cells in vivo after CTX administration.
Figure 5.
Reconstitution of CD8+ DCs in LNs inhibits antigen-specific T-cell responses. Mice were treated with CTX on day −4, followed by the adoptive transfer of 105 CD8+ pmel-1 cells from Thy1.1 pmel-1 mice by intravenous injection on day −1. On day 0, mice were immunized with hgp100 peptide–pulsed DCs injected intradermally in the left ear. At 5 days after immunization, draining retroauricular LNs were harvested, and stained for CD8 and Thy1.1 to detect antigen-specific pmel-1 cells (Thy1.1+). To assess IFN-γ production, cells were cocultured with mgp100-pulsed EL-4 cells and then stained for intracellular IFN-γ. All mice received adoptively transferred pmel-1 CD8 T cells. As negative control for immunization, mice were not immunized and treated (▴) or not treated (■) with CTX. As negative control for CTX, immunized mice did (▾) or did not (♦) receive CTX treatment. A group of mice received 106 CD8+ DCs in the left ear (●) or received 106 CD8− DCs in the left ear (□) on day −1. (A) Experiment timeline. (B) Representative plots. (C) The recovery of antigen-specific T cells and percentage of IFN-γ production presented as the mean ± SEM (3-4 mice/group). Data are representative of 3 independent experiments. CD8 pmel-1 recovery and IFN-γ secretion were both statistically significantly enhanced when CTX (♦) or CTX and CD8-DC (□) treatment preceded immunization (P = .05).
Adoptive transfer of CD8+ DCs after CTX treatment inhibits the effects of CTX on concomitant immunity
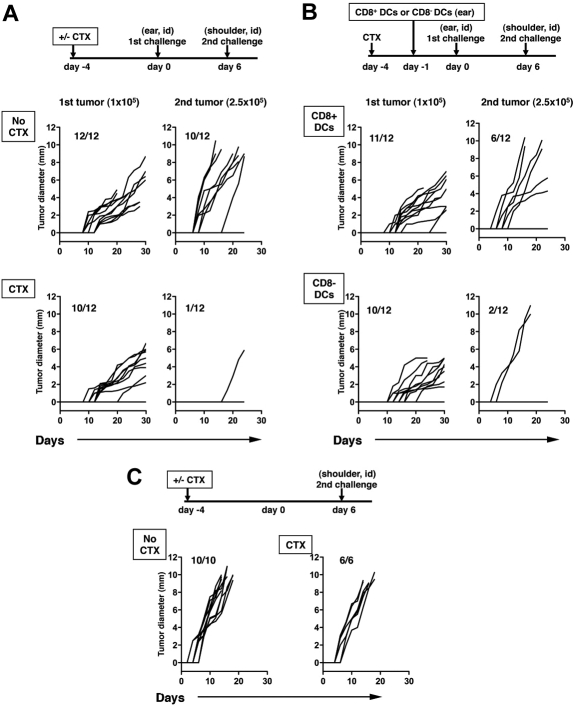
The inoculation of a tumor can induce an immune response capable of rejecting a second challenge with a similar tumor at a distant site, a phenomenon known as concomitant tumor immunity.32 The immune response, which is measured as rejection of the second tumor challenge, is mediated by CD8+ T cells primed by the growth of the primary tumor. We have previously shown that B16 melanoma, a poorly immunogenic tumor, does not spontaneously elicit concomitant immunity because Tregs prevent CD8+ T cell–mediated rejection of the challenge B16 tumors. However, CTX treatment or Treg depletion with anti-CD4 antibody allows rejection to occur.33 Because our results suggested that CD8+ DCs, which are depleted by CTX in vivo, also decrease the potency of antigen presentation by DCs and suppression by Tregs, we asked what effects CD8+ DCs have on concomitant immunity to B16 after CTX treatment using the experimental schema depicted in Figure 6A. Consistent with our previous data, B16 tumors do not induce concomitant immunity after primary tumor inoculation, and 80% to 90% of mice develop the second tumors. After CTX treatment, concomitant immunity is unmasked with 90% of the mice rejecting the second tumor challenge (Figure 6A). Adoptive transfer of CD8+ DCs significantly inhibited concomitant immunity induced by CTX treatment and only 50% of mice were able to reject the second tumor challenge. The adoptive transfer of CD8− DCs had little effect on concomitant immunity induced by CTX treatment (Figure 6B). Interestingly, these results correlate with the antigen-specific responses shown in Figure 5. In the absence of primary tumor challenge, all mice developed tumor growth regardless of whether they received CTX treatment (Figure 6C). These results show that CD8+ DCs decrease the effects of CTX and suggest that transient depletion of this DC subset by CTX treatment has an important role in the immune-enhancing properties of CTX in vivo.
Figure 6.
Local reconstitution of CD8+ DCs inhibits systemic concomitant immunity. Mice were inoculated with B16 cells (105) in the left ear on day 0, and reinoculated with B16 (2.5 × 105) in the right shoulder on day 6. Mice were either untreated (no CTX) of treated with CTX (CTX) 4 days before the primary tumor challenge (A). Mice were treated with CTX as described for panel A and received 106 CD8+ DCs in the left ear 1 day before the primary tumor challenge or were treated with CTX and received 106 CD8− DCs in the left ear using the same timeline (B). The left graphs represent growth of primary tumors, the right graphs depict growth of challenge secondary tumors. P values for the primary tumor were not statistically significant except no CTX and CTX + CD8− DCs (P = .002). P values comparing growth curves between no CTX with CTX, CTX + CD8+ DCs, and CTX + CD8− CCs were < .001, .031, and < .001, respectively. The P value comparing CTX versus CTX + CD8+ DCs was .004. The growth of the second challenge tumor inoculum in naive mice without primary tumor challenge (with or without CTX treatment; C). Data from 1 of 2 independent experiments with similar results are shown.
Discussion
CTX, an alkylating agent that cross-links DNA and causes cytotoxicity in various cell types, is a commonly used chemotherapeutic agent for cancer therapy and the treatment of autoimmune disease where until recently it was thought to be immunosuppressive.1,2 However, CTX has pleiotropic effects on the immune system that are both dose and time dependent. In recent years, the immune-enhancing properties of CTX have gotten greater attention. Several mechanisms for these immune-enhancing effects have been proposed, including (1) increased homeostatic expansion of antigen-specific T cells,2 (2) induction of type I IFNs and augmentation of the number of CD44hi effector T cells,8,9 (3) shift in Th2/Th1 cytokine production,34 and (4) elimination of suppressor cell activity.6,35 More recently, CTX has been shown to mobilize early DC precursors that are more capable of inducing CD8+ T-cell responses, but the specific DC subsets involved were not investigated.14 Here we demonstrate that a preferential and transient depletion of CD8+ tissue-resident DCs is a previously unidentified mechanism by which CTX enhances immune responses.
In our study, resident DCs, especially CD8+ DCs, showed the greatest sensitivity to CTX administration. Skin-derived DCs and pDCs were relatively resistant to CTX administration (Figure 1C-E). The cause of this difference in sensitivity requires further investigation, but may be related to the turnover rate of resident DCs in tissues.18 The resulting imbalance in DC subsets after CTX treatment leads to a more potent capacity for antigen presentation and IL-12 secretion in the LN (Figure 2). In our experiments, CD8+ DCs, and not CD8− DCs, inhibited the immune-enhancing effects of CTX both in vitro (Figure 3A-B) and in vivo (Figures 5–6). This supports our conclusion that the transient and relative depletion of resident DCs, specifically those that are CD8+, plays a role in the immune-enhancing effects of CTX.
It has become quite apparent that DCs are critical not only to the initiation of the immune response, but also to the modulation of effector cell responses and Treg functions.16 DCs in peripheral tissues capture antigen and migrate toward secondary lymphoid organs while undergoing maturation, which is characterized by high levels of expression of MHC and T-cell costimulatory molecules and the ability to present antigen captured in the periphery to naive T cells.17 This is important in acute inflammatory challenges and also in the steady state when migratory DCs are able to transport self-antigens captured in peripheral tissues to draining LNs.36–38 Resident DCs, however, are derived from bone marrow precursors within the lymphoid organs without previously trafficking through peripheral tissues.18 In the absence of pathogen encounter, the resident DCs retain an immature status, so they can be distinguished from migratory DCs in the LNs by their lower cell surface expression of MHC class II and costimulatory molecules such as CD80 and CD8639 (Figure 1A-B). Resident CD8+ DCs present self-antigens on MHC class II molecules, and they are the main DC subset that cross-presents antigen on MHC class I molecules.24 Studies examining the function of DC subsets in the initiation of immunity to viral infection have shown that not only migratory DCs, but also “antigen-recipient” resident CD8+ DCs, are involved in priming CD8+ T-cell responses17,23 even when the infection route is in the peripheral tissues.23 Taken together, these studies demonstrate that both migratory DCs and resident CD8+ DCs shape immune responses to both self-antigens and pathogens presented from the peripheral tissues.
The functional differences in antigen presentation between migratory and resident DCs, however, remain unclear. Some studies have shown that presentation of skin-expressed self-peptide by migratory DCs induces a predominantly autoimmune CD8+ T-cell response and that DCs did not induce self-tolerance in the steady state, suggesting that migratory DCs are immunogenic both in the steady state and after pathogen encounter.40,41 On the other hand, among the resident DCs, CD8+ DCs have a greater potential to promote tolerance. Splenic CD8– DCs induced a vigorous proliferative response in CD4+ T cells, whereas CD8+ DCs induced a lesser response that was associated with Fas-dependent T-cell apoptosis in vitro.42 Targeting antigen through DEC-205 to CD8+ DCs in situ results in deletional tolerance in the steady state and leads to peripheral conversion of naive CD4+FoxP3− T cells into Tregs in vivo.28,29,43–45 Even after pathogen encounter, CD8+ DCs can lead to tolerance after IFN-γ stimulation and can induce Tregs.46 CD8 expression alone, however, does not confer tolerogenic properties because CD8+ DCs also have pivotal roles in initiation of immunity and have significant functional plasticity.17,47 Tolerogenic DCs conceivably correspond to a functional subtype or state rather than to a unique DC lineage in vivo, and perhaps CD8+ DCs have a greater tendency to develop tolerogenic properties in various immune environments. Alternatively, subsets within the CD8+ DC population may be tolerogenic, whereas others are more immunogenic.46,48–50 Although, we do not address what determines the tolerogenicity of resident CD8+ DCs, we propose that the balance between “immunogenic” migratory DCs and “immunoinhibitory” resident CD8+ DCs may determine the outcome of the immune responses and that depletion of “immunoinhibitory” CD8+ DCs after CTX treatment can enhance immunity in vivo.
Several studies have specifically focused on the effects of CTX on Tregs.11–13 CD4+ CD25+ Tregs display a greater sensitivity to the cytotoxic effects of CTX, and Tregs isolated from CTX-treated mice exhibited increased levels of apoptosis. We hypothesized that depletion of CD8+ DCs by CTX could alter Treg function. A regulatory feedback loop between Tregs and DCs has been reported, where decreases in resident DC number correlate with decreased Treg number in a Flt3-dependent manner.15 Furthermore, CD8+ and CD8− DCs have differential capabilities to induce the peripheral conversion of Tregs or expand natural Tregs in vivo, respectively.45 Finally, targeting low doses of antigen to the DEC-205 receptor, which is expressed at highest level on CD8+ DCs, can lead to the peripheral conversion of Tregs.45 In an effort to evaluate DC and Treg interactions after CTX treatment, we examined the interaction between DCs from CTX-treated animals and naive Tregs. Tregs expanded with DCs from CTX-treated mice were less suppressive than Tregs expanded with DCs from naive mice (Figure 4), consistent with prior studies that showed decreased suppression by Tregs from mice treated with CTX.11,13 Tregs expanded with CD8+ DCs were more potent suppressors, and the addition of CD8+ DCs to CTX-treated DCs abrogated the overall DC effect on Tregs. Thus, at least in this in vitro setting, DCs from CTX-treated mice enhance immune responses by inhibiting Treg suppressor activity, and this inhibition is induced by the depletion of CD8+ DCs.
In our experiments, CD8+ DCs were adoptively transferred locally, not systemically. Nevertheless, CD8+ DCs transferred locally decreased the effects of CTX not only on antigen-specific immune responses in the draining LNs, but also in systemic concomitant immunity. These results imply that the microenvironment of LNs where antigen-bearing DCs contact antigen-specific T cells can modify immune responses, and thus elimination of immunoinhibitory factors in the LNs can amplify local and systemic antigen-specific immune responses.
In conclusion, we have shown for the first time that CTX has profound and selective effects on CD8+ resident DCs in LNs and spleen, causing an imbalance between different DC subsets. The CTX-sensitive CD8+ DCs showed immunoinhibitory function through enhancement of Treg suppressor activity in vitro. In addition, reconstitution of this subset in LNs by adoptive transfer dramatically decreased the immune-enhancing effects of CTX. From these results, we conclude that the ability of CTX to enhance immunity is related to its capacity to deplete “immunoinhibitory” CD8+ resident DCs. We believe this is a novel and important mechanism that cooperates with other reported immunomodulatory mechanisms attributed to CTX.2,8,9,11–13,34,35
Acknowledgments
The authors thank Dr Ralph Steinman for comments and careful reading of this paper.
This work was supported by grants from the National Cancer Institute (R01 CA56821, P01 CA33049, and P01 CA59350), Swim Across America, the Lita Annenberg Hazen Foundation, and Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Cancer Foundation for Research.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.N. and H.U. designed and performed research, analyzed data, and wrote the paper; A.M.L. performed research, analyzed data, and contributed to writing the paper; F.A., G.A.R., D.H.-C., and T.M. performed research and contributed to writing the paper; K.S.P. performed statistical analysis; and J.D.W. and A.N.H. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander M. Lesokhin, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Room Z-1462, New York, NY 10065; e-mail: lesokhia@mskcc.org.
References
- 1.Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52(11):686–692. doi: 10.1007/s00262-003-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61(9):3689–3697. [PubMed] [Google Scholar]
- 4.Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984;27(6):604–606. doi: 10.1007/BF00276978. [DOI] [PubMed] [Google Scholar]
- 5.Yasunami R, Bach JF. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988;18(3):481–484. doi: 10.1002/eji.1830180325. [DOI] [PubMed] [Google Scholar]
- 6.Polak L, Geleick H, Turk JL. Reversal by cyclophosphamide of tolerance in contact sensitization: tolerance induced by prior feeding with DNCB. Immunology. 1975;28(5):939–942. [PMC free article] [PubMed] [Google Scholar]
- 7.Röllinghoff M, Starzinski-Powitz A, Pfizenmaier K, Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiavoni G, Mattei F, Di Pucchio T, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95(6):2024–2030. [PubMed] [Google Scholar]
- 9.Proietti E, Greco G, Garrone B, et al. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101(2):429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bracci L, Moschella F, Sestili P, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13(2; pt 1):644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 11.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 12.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 13.Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6603–6612. doi: 10.4049/jimmunol.177.10.6603. [DOI] [PubMed] [Google Scholar]
- 14.Radojcic V, Bezak KB, Skarica M, et al. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59(1):137–148. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darrasse-Jèze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206(9):1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82(6):1365–1374. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 17.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7(7):543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8(6):578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 19.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29(5):795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour: evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65(1):87–92. [PMC free article] [PubMed] [Google Scholar]
- 22.Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301(5641):1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 23.Belz GT, Smith CM, Kleinert L, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101(23):8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belz GT, Behrens GM, Smith CM, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196(8):1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0307s86. Chapter 3:Unit 3 7. [DOI] [PubMed] [Google Scholar]
- 27.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 28.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kronin V, Fitzmaurice CJ, Caminschi I, Shortman K, Jackson DC, Brown LE. Differential effect of CD8(+) and CD8(-) dendritic cells in the stimulation of secondary CD4(+) T cells. Int Immunol. 2001;13(4):465–473. doi: 10.1093/intimm/13.4.465. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bursuker I, North RJ. Generation and decay of the immune response to a progressive fibrosarcoma: II, failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med. 1984;159(5):1312–1321. doi: 10.1084/jem.159.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200(6):771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matar P, Rozados VR, Gervasoni SI, Scharovsky GO. Th2/Th1 switch induced by a single low dose of cyclophosphamide in a rat metastatic lymphoma model. Cancer Immunol Immunother. 2002;50(11):588–596. doi: 10.1007/s00262-001-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. 1990;71(1):90–95. [PMC free article] [PubMed] [Google Scholar]
- 36.Huang FP, Platt N, Wykes M, et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191(3):435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25(12):655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125(1):116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilson NS, El-Sukkari D, Belz GT, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102(6):2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 40.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123(1):109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 41.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21(3):391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183(4):1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196(8):1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki S, Dudziak D, Heidkamp GF, et al. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orabona C, Puccetti P, Vacca C, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107(7):2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 47.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8(10):1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 48.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6(3):280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 49.Smits HH, de Jong EC, Wierenga EA, Kapsenberg ML. Different faces of regulatory DCs in homeostasis and immunity. Trends Immunol. 2005;26(3):123–129. doi: 10.1016/j.it.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Grohmann U, Bianchi R, Orabona C, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171(5):2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]