Abstract
We characterized a consanguineous Turkish family suffering from von Willebrand disease (VWD) with significant mucocutaneous and joint bleeding. The relative reduction of large plasma von Willebrand factor (VWF) multimers and the absent VWF triplet structure was consistent with type 2A (phenotype IIC) VWD. Surprisingly, platelet VWF was completely deficient of multimers beyond the VWF protomer, suggesting defective α-granular storage of larger multimers. Patients were nearly unresponsive to desmopressin acetate, consistent with a lack of regulated VWF release from endothelial cell Weibel-Palade bodies, suggesting defective storage also in endothelial cells. We identified an N528S homozygous mutation in the VWF propeptide D2 domain, predicting the introduction of an additional N-glycosylation site at amino acid 526 in close vicinity to a “CGLC” disulphide isomerase consensus sequence. Expression studies in mammalian cells demonstrated that N528S-VWF was neither normally multimerized nor trafficked to storage granules. However, propeptide containing the N528S mutation trafficked normally to storage granules. Our data indicate that the patients' phenotype is the result of defective multimerization, storage, and secretion. In addition, we have identified a potentially novel pathogenic mechanism of VWD, namely a transportation and storage defect of mature VWF due to defective interaction with its transporter, the mutant propeptide.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder and is caused by defects in von Willebrand factor (VWF) function or decreased levels of VWF.1 VWF is a large, adhesive, multimeric plasma glycoprotein that mediates platelet adhesion and aggregation at the site of vascular injury.1 VWF also serves as the carrier protein for coagulation factor VIII (FVIII), protecting it from degradation in plasma. Partial and nearly complete quantitative deficiencies in VWF are classified as types 1 and 3 VWD, respectively.2 Type 2 VWD encompasses qualitative deficiencies and is further subdivided into types 2N, 2M, 2B, and 2A.2 Type 2N variants have markedly reduced capacity to bind FVIII, whereas type 2M variants have decreased platelet-binding function. Type 2B variants are indicated by a loss of high-molecular-weight multimers (HMWMs) due to increased binding to platelets. Type 2A refers to variants of VWF with loss of HMWM and decreased platelet-binding function. The lack of HMWM in type 2A results from either impaired multimer assembly or increased plasma multimer degradation by ADAMTS13 (a disintegrin and metalloprotease with thrombospondin-type 1 motif).2
VWF is synthesized and secreted from endothelial cells and megakaryocytes/platelets.3,4 VWF is synthesized as a large pre-pro-VWF molecule that consists of a 22–amino acid (aa) signal peptide, 741-aa propeptide, and 2050-aa mature VWF protein.5 Intracellularly, VWF is extensively modified including sulfation, glycosylation, carboxy-terminal dimerization, amino-terminal multimerization, and proteolytic cleavage of VWFpp from mature VWF.6,7 Both VWFpp and mature VWF multimers are stored for regulated secretion in Weibel-Palade bodies in endothelial cells and α-granules in platelets.8 Our laboratory and others have shown that VWFpp plays a critical role in the multimerization and regulated storage of VWF.9–11 VWFpp is required for normal VWF multimer assembly and mutations in VWFpp often result in multimerization defects.12,13 Our previous studies have demonstrated that VWF storage is initiated by VWFpp: VWFpp contains the “signal” for trafficking to granules and secondarily cotraffics mature VWF by virtue of a noncovalent association.14,15 However, VWF granular storage and multimerization have also been shown to be independent processes: multimerization is not necessary for granular storage.12,15–17
Here we report the characterization of a Turkish consanguineous family diagnosed with type 2A VWD. Patient studies indicate defective regulated storage of VWF in both platelets and endothelial cells. An N528S mutation was identified in the VWF propeptide. By expressing recombinant N528S-VWF plasmids in mammalian cells, we demonstrate loss of VWF-regulated storage and defective formation of VWF HMWM. In sum, the phenotype observed in patients with an N528S mutation is a result of defective multimerization, regulated storage, and secretion.
Methods
Family history
The study received institutional review board approval from the University Medical Center–Hamburg-Eppendorf and University Medical School Hannover and informed consent was obtained from the patients' parents in accordance with the Declaration of Helsinki. Diagnosis of VWD was made in a Turkish consanguineous family with 3 affected sons and 2 unaffected daughters (Figure 1) after the first bleeding episode of their 4-year-old second child that required a hospital stay. He had a tendency to bruise, and suffered from repeated spontaneous oral cavity bleeding, prolonged bleeding from small wounds, and delayed wound healing. Severe epistaxis at the age of 8 years required blood transfusion. At the age of 12 years, tonsillectomy was complicated by severe bleeding although adequate prophylaxis had been performed. The second boy suffered from severe epistaxis. An episode of severe gingival bleeding required hospital surveillance for 7 days. He also experienced postoperative bleeding after tonsillectomy despite adequate VWF replacement therapy. The third boy also suffered from severe epistaxis and at the age of 6 and 9 years, from joint bleeding of his knee. The 2 daughters never experienced bleeding complications, although the elder girl had a severe finger injury and the younger one was accidentally exposed to rat poison, which normally leads to enhanced bleeding.
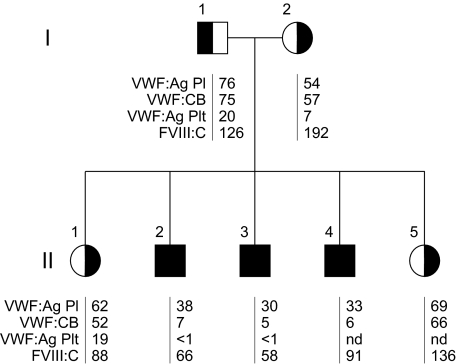
Figure 1.
Pedigree and laboratory data of a family diagnosed with von Willebrand disease. Affected patients are represented by black symbols; unaffected persons, by half-filled symbols; squares, males; and circles, females. The laboratory test results are listed as follows: VWF:Ag (U/dL), VWF:CB (U/dL), platelet (plt) VWF:Ag (U/1011 plts), and FVIII:C (U/dL); nd indicates not determined. Affected patients (black symbols) were found to have a homozygous N528S VWF mutation, whereas unaffected (half-filled symbols) were heterozygous for the mutation. Affected, N528S VWF homozygous patients had low VWF:Ag with substantially reduced VWF:CB and platelet VWF:Ag.
The parents had only mild or no bleeding symptoms. The mother suffered from nose bleeds in her childhood, but later in life neither menorrhagia nor postpartum bleeding occurred. The father never experienced any bleeding complications.
Isolation of genomic DNA and sequencing
High-molecular-weight genomic DNA was prepared from leukocyte buffy coats by standard techniques and was used for the amplification of VWF coding exons 2 through 52 by polymerase chain reaction as previously described.18 Polymerase chain reaction products were sequenced by the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit on an ABI 310 (Applied Biosystems). The candidate mutation was confirmed by sequencing both strands.
Construction of full-length VWF expression plasmids
VWF mutagenesis and recombinant expression of the mutation identified in the patients were performed as described previously.18 Site-directed mutagenesis of full-length VWF cDNA was carried out using the QuikChange kit (Stratagene). The mutagenesis primer carrying the respective base-exchange at a central position was 40 bp in length. The expression vector pRC.CMV containing full-length mutant and wild-type (wt) VWF cDNA was used to transform Top10 supercompetent cells (Invitrogen). Plasmids were purified by the Endofree Plasmid Maxi Kit (QIAGEN).
The human VWF propeptide (VWFpp), propeptide-deleted VWF (Δpro), and myc-his–tagged wild-type VWF expression vectors were constructed as previously described.15,19 Briefly, pCIneo-VWFpp expression vectors contain signal peptide and VWFpp sequence followed by a stop codon with mature VWF sequence deleted. The pCIneo-Δpro expression vector contains signal peptide followed directly by mature VWF sequence. The wild-type VWF-myc-his contains a myc-his tag on the C-terminus of VWF. The N528S mutation was subcloned from pRC.CMV.VWF-N528S into the pCIneo-VWFpp expression vector using the XbaI and BglII restrictions sites in VWFpp to create pCIneo-VWFpp-N528S.
Antibodies
Anti-VWF antibodies AVW-1, AVW-5, and 105.4 and anti-VWFpp monoclonal antibodies 239.1-239.11, 242.4, and 242.5 were produced by our laboratory. The following polyclonal antibodies were purchased: rabbit anti–human VWF and rabbit anti-ACTH (DAKO), rabbit anti-CD62P (BD Biosciences), and rabbit anti-myc (Affinity BioReagents). Nonimmune mouse and rabbit immunoglobulin G (IgG) were purchased from Jackson ImmunoResearch Laboratories and used as isotype controls. Secondary antibodies used for immunofluorescence detection included goat anti–rabbit and anti–mouse IgG (H+L) (F(Ab′)2) fragments conjugated with either Alexa Fluor 488 or Alexa Fluor 568 (Molecular Probes).
Mammalian cell culture and transfection
293 EBNA cells (2 × 106; Invitrogen) were transiently transfected with 4 μg of mutant or wt full-length VWF cDNA by liposomal transfer (Lipofectamine 2000; Invitrogen). The cells were grown for 72 hours (24 hours in Dulbecco modified Eagle medium [Invitrogen] with 10% [vol/vol] fetal bovine serum and 48 hours in serum-free Iscove modified Dulbecco medium [Sigma-Aldrich] + 2% [vol/vol] Ultroser G [Invitrogen]). VWF secreted in the medium was concentrated in Centricon tubes to one-tenth of the original volume prior to subsequent analysis. Human embryonic kidney cells (HEK293T; D. Ginsburg, University of Michigan), HEK293 cells (CRL 1573; ATCC), mouse pituitary tumor cells (AtT-20/D16v-F2, CRL 1795; ATCC), and K9-VWD–aortic endothelial cells (AECs) were cultured and transfected as described.20,21 HEK293 and 293T cells were transiently transfected using TransIT-293 Transfection Reagent (Mirus Bio) according to the manufacturer's instructions. K9-VWD-AECs were nucleofected as described.21 Negative controls were transfected with expression vector lacking insert. After 72 hours, conditioned media were harvested from the cells, centrifuged to remove debris, and frozen at −80°C for further analysis. Transfected cells were then fixed for intracellular staining.
Multimer analysis
Multimer analysis of patients' plasma and platelet VWF and recombinant VWF in the conditioned medium of transfected cells was performed by sodium dodecyl sulfate (SDS)–agarose gel electrophoresis with luminescent visualization as described.22 The luminescent blot was stored on electronic media by photoimaging (FluorChem 8000; Alpha Innotech Corp). Recombinant VWF in the conditioned medium of transfected HEK293T cells was analyzed by electrophoresis through a 2% high-gelling temperature (HGT(P)) agarose resolving gel (Lonza) containing 1% SDS. VWF was transferred to Immobilin-FL (Millipore) by electroblotting at 100 V for 1 hour in 25mM tris(hydroxymethyl)aminomethane, 200mM glycine, 20% methanol, and 0.03% SDS (wt/vol) SDS. Membranes were blocked with Odyssey Blocking Buffer (LI-COR). For coexpression studies, membranes were incubated with AvW-5 and anti–c-Myc (Affinity BioReagents), followed by incubation with IRDye 680–conjugated goat anti–mouse IgG and IRDye 800–conjugated goat anti–rabbit IgG (1:20 000; LI-COR).
Confocal immunofluorescence microscopy
The intracellular localization of VWF, VWFpp, and adrenocorticotropin (ACTH) was examined by immunofluorescence antibody staining and confocal laser scanning microscopy in the Imaging Core of the Medical College of Wisconsin using a Leica TCS SP2 confocal laser imaging system as previously described.20 Transfected cells grown on 2-well chamber slides were fixed using 3.7% (vol/vol) buffered formalin, permeabilized in 1% Triton X-100 (in 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 300mM sucrose, 50mM NaCl, 3mM MgCl2·6 H2O, pH 7.0), and then blocked with 2% normal goat serum in Hanks balanced salt solution (HBSS). Cells were incubated at 4°C overnight in 2 to 5 μg/mL primary antibodies (see “Antibodies”) diluted in HBSS containing 1% BSA. Cells were washed 3 times and then incubated in secondary antibodies diluted 1:1000 in HBSS containing 1% BSA for 30 minutes at room temperature. Cells were washed 3 times with HBSS and mounted using Vectashield (Vector Laboratories).
Results
Characterization of a family with von Willebrand disease
We had the opportunity to characterize a Turkish consanguineous family diagnosed with VWD. The pedigree of this family is shown in Figure 1. Three of the children (II-2, II-3, II-4) suffered from significant mucocutaneous bleeding and in one case even from joint bleeds (a detailed bleeding history can be found in “Methods”). These patients had decreased plasma VWF:antigen (Ag) levels (< 40 U/dL), markedly reduced binding of plasma VWF to collagen (< 10 U/dL), significantly decreased platelet VWF:Ag levels (< 1 U/1011 platelets), but only slightly reduced or normal levels of FVIII:C (Figure 1). Unaffected family members had VWF:Ag, VWF:collagen binding (CB), and platelet VWF:Ag levels in the low-normal range. FVIII:C was normal or even elevated. Whereas unaffected family members had a ratio of VWF:CB/VWF:Ag near 1.0, the ratio was substantially reduced in the affected patients (to less than 0.2), indicating functionally defective VWF. In the unaffected persons, a desmopressin acetate test resulted in a significant increase of VWF:Ag, VWF:ristocetin cofactor (RCo), VWF:CB, and FVIII:C (data not shown), whereas the affected sons were poor responders for VWF parameters (Figure 2). However, although the VWF response was poor, FVIII:C did increase in response to desmopressin acetate (Figure 2).
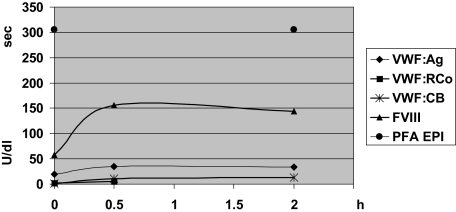
Figure 2.
Desmopressin acetate test of patient II-3. VWF laboratory parameters were determined before (t = 0) and after administration of desmopressin acetate. Almost no response of VWF parameters was observed, except for FVIII:C.
Plasma and platelet VWF multimer structure
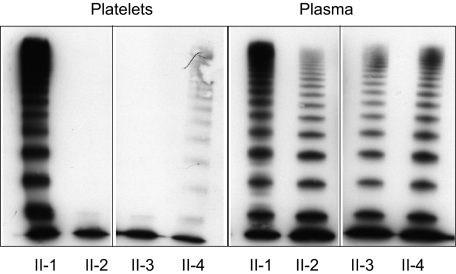
Plasma and platelet VWF multimers of affected and unaffected family members were examined. Plasma VWF multimers (Figure 3) in the affected family members (II-2, II-3, and II-4) showed a relative reduction of medium-sized multimers and HMWMs. The VWF protomer band appeared to be enhanced, and VWF satellite bands were nearly absent compared with normal plasma VWF multimers. This multimer structure is consistent with type 2A and the historical subclassification of type IIC VWD. Unaffected family members (II-1) did not appear to have any apparent multimer abnormalities. Platelet VWF multimers are expected to be composed of a full spectrum of molecular weight subunits with the absence of proteolytic satellite bands. A normal distribution of platelet VWF multimers was observed in one unaffected family member, II-1 (Figure 3). However, the affected patients (II-2, II-3), in addition to having substantially decreased platelet VWF:Ag, had a complete deficiency of platelet VWF multimers beyond the smallest protomer. Patient II-4, however, showed only a severely decreased concentration of large VWF multimers.
Figure 3.
Plasma and platelet VWF multimers in unaffected and affected family members. Plasma and platelet VWF multimers were assessed by SDS-agarose gel electrophoresis and Western blotting. Vertical line(s) have been inserted to indicate a repositioned gel lane. Lane 1 in both frames: platelet and plasma VWF of unaffected heterozygous family member, II-1. Lanes 2-4: affected family members II-2, II-3, and II-4 with a homozygous N528S VWF mutation. Affected family members have an abnormal plasma multimer distribution with a relative decrease of large multimers and pronounced oligomers and either a complete loss (II-2, II-3) or a severely decreased concentration (II-4) of large platelet VWF multimers.
Identification of a mutation in the VWF gene
Sequencing of the VWF gene revealed an 1583A>G mutation in exon 14, predicting the substitution of asparagine to serine at amino acid 528 in the D2 domain of the VWF propeptide (VWFpp). The affected patients (II-2, II-3, and II-4) were found to be homozygous for the mutation, whereas other family members (I-1, I-2, II-1, and II-5) were heterozygous. In sum, affected family members had modestly decreased plasma VWF:Ag, markedly reduced platelet VWF:Ag, abnormal multimer structure, and a homozygous N528S mutation in the VWF propeptide.
The mutation N528S is predicted to create a new N-glycosylation site at amino acid N526 using a program for the prediction of N-glycosylation sites in human proteins provided by the NetNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc; Center for Biological Sequence Analysis), with a score of 0.7 and a threshold cutoff at 0.5. N526 is located in close vicinity of the “CGLC” disulphide isomerase consensus sequence in the VWFpp D2 domain (C521-C524) that has been implicated in the multimerization process of VWF.11
Abnormal VWF-regulated secretion
VWF is synthesized in endothelial cells and platelets where it is stored for regulated release in Weibel-Palade bodies and α-granules, respectively. The substantially reduced platelet VWF:Ag (Figure 1) and deficiency of platelet multimers in affected patients (Figure 3) suggested defective VWF storage in platelet α-granules. In addition, 2 N528S-VWF homozygous patients tested were found to be unresponsive to desmopressin acetate administration and released very little VWF:Ag after administration (Figure 2). The VWF released in response to desmopressin acetate administration is presumed to result from endothelial cell Weibel-Palade body exocytosis.23 The lack of desmopressin acetate response in the homozygous patients suggests defective regulated storage of VWF in endothelial cell Weibel-Palade bodies, as well as platelet α-granules.
Expression of recombinant N528S-VWF in mammalian cells
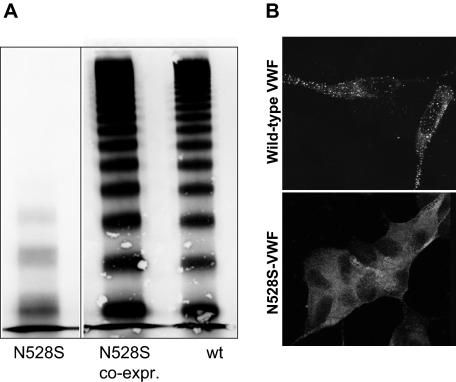
To characterize the effect of the N528S VWF mutation on VWF processing and regulated storage, the mutation was introduced into a full-length VWF expression vector and a VWFpp expression vector. The N528S full-length VWF was transiently expressed in 293 EBNA cells and conditioned medium harvested after 72 hours. The secretion of “homozygous” N528S-VWF was substantially reduced, approximately 7.5% of wild-type VWF (data not shown). In addition, the multimer pattern lacked both HMWM and medium-sized multimers, thereby mimicking the situation in platelet lysates of the homozygous patients (Figures 3,4A). Interestingly, plasma VWF of the homozygous patients showed less reduction in HMWM than the recombinant mutant VWF, suggesting some differences in processing and secretion of VWF by heterologous cells. A similar discrepancy has been observed by other laboratories carrying out VWF expression studies in mammalian cells.17,24,25 These differences could result from differences in glycosylation/carbohydrate processing or a lack of necessary intracellular chaperones. Coexpression of N528S with wt VWF restored the normal multimer pattern, which was indistinguishable from wt VWF, again analogous to the heterozygous unaffected family members and in accordance with recessive inheritance (Figures 3,4A).
Figure 4.
Multimer distribution and intracellular localization of recombinant N528S-VWF. (A) Mutant N528S-VWF (lane 1) alone and coexpressed with wt VWF (lane 2) in 293 EBNA cells in the conditioned medium was assessed by SDS-agarose electrophoresis and Western blotting. Vertical line(s) have been inserted to indicate a repositioned gel lane. Recombinant wt VWF multimers are shown for comparison (lane 3). N528S-VWF multimers lack HMWM completely, whereas N528S coexpressed with wt VWF could not be distinguished from wt VWF alone. (B) AtT-20 cells were transiently transfected with (top panel) wild-type VWF or (bottom panel) N528S-VWF. Cells were immunostained for VWF and examined by confocal microscopy. N528S-VWF was not trafficked to storage granules.
To examine intracellular trafficking, the variant VWF was also expressed in AtT-20 cells. This cell line has been used extensively to investigate VWF-regulated storage.10,15,17,26 AtT-20 cells were transiently transfected, immunofluorescently stained for VWF, and examined by confocal scanning laser microscopy (Figure 4B). In addition to the reduced secretion noted in the previous paragraph, N528S VWF also appeared to have decreased transfection efficiency (data not shown). Whereas wild-type VWF demonstrated the expected granular VWF staining pattern, the N528S-VWF variant showed only diffuse staining. No VWF granules were observed in N528S-VWF–expressing cells. The N528S VWF variant is not normally multimerized and does not appear to be trafficked to storage granules in AtT-20 cells.
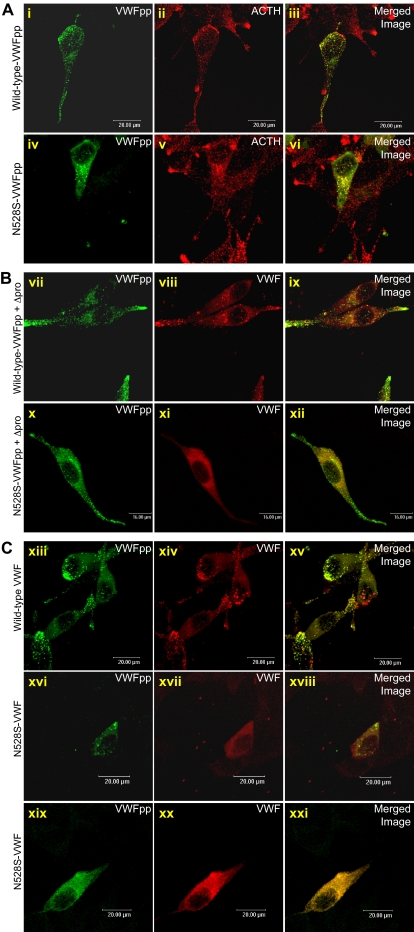
Previous studies in our laboratory (S.L.H.) have demonstrated that VWF-regulated storage is VWFpp dependent: VWFpp contains the necessary signal for trafficking to storage granules, and mature VWF multimers are cotrafficked by virtue of a noncovalent association with VWFpp.15 To determine whether the loss of N528S-VWF storage resulted from a defective VWFpp “storage signal,” N528S-VWFpp was expressed independently. Transfected cells were immunofluorescently stained for VWFpp and adrenocorticotropin (ACTH), the endogenous hormone synthesized and stored by AtT-20 cells. Both N528S-VWFpp and wild-type VWFpp colocalized with ACTH granules (Figure 5A), indicating normal trafficking of the N528S-VWFpp. These data suggest that the loss of VWF-regulated storage is not due to a defect in the VWFpp storage signal.
Figure 5.
Trafficking of N528S-VWFpp and coexpressed mature VWF in AtT-20 cells. (A) AtT-20 cells were transiently transfected with wild-type VWFpp or N528S-VWFpp. Transfected cells were fixed and dual-stained for VWFpp (i, iv) and ACTH (ii, v) and examined by confocal microscopy. The merged image of the 2 stains is shown in the last column (iii, vi) with colocalization shown in yellow. Both wild-type VWFpp and N528S-VWFpp appear to traffic to endogenous ACTH-containing storage granules. (B) Wild-type VWFpp and N528S-VWFpp were coexpressed with propeptide-deleted VWF (Δpro) in AtT-20 cells. Transfected cells were dual-stained for VWFpp (vii, x) and VWF (viii, xi). The merged image shown in the last column (ix, xii) with colocalization shown in yellow. For wild-type VWFpp coexpressed with Δpro, both VWFpp and VWF were colocalized in granules, whereas for N528S-VWFpp with Δpro, only VWFpp was localized in granules, and VWF demonstrated diffuse staining. (C) Wild-type and N528S-VWF were expressed and cells were dual-stained for VWFpp (xiii, xvi, xix) and VWF (xiv, xvii, xx). The merged image shown in the last column (xv, xviii, xxi) with colocalization shown in yellow. For wild-type VWF, both VWFpp and VWF were colocalized in granules. Cells expressing N528S-VWF showed either granular VWFpp staining with diffuse VWF staining or diffuse staining for both proteins.
To examine the interaction of variant N528S-VWFpp with VWF, AtT-20 cells coexpressing N528S-VWFpp and Δpro were immunostained for VWFpp and VWF. Expression of wild-type VWFpp, and Δpro resulted in colocalized granular storage of both proteins (Figure 5B). In cells expressing N528S-VWFpp and Δpro, granular staining was observed for N528S-VWFpp, whereas coexpressed Δpro showed diffuse staining. The N528S-VWFpp did not traffic mature VWF to storage granules, suggesting an aberrant VWFpp/VWF interaction. AtT-20 cells expressing full-length N528S-VWF were further examined. Whereas most cells demonstrated granular VWFpp and diffuse VWF staining, some cells showed diffuse staining for both proteins (Figure 5C). Defective VWF storage does not appear to result from disrupted VWFpp trafficking.
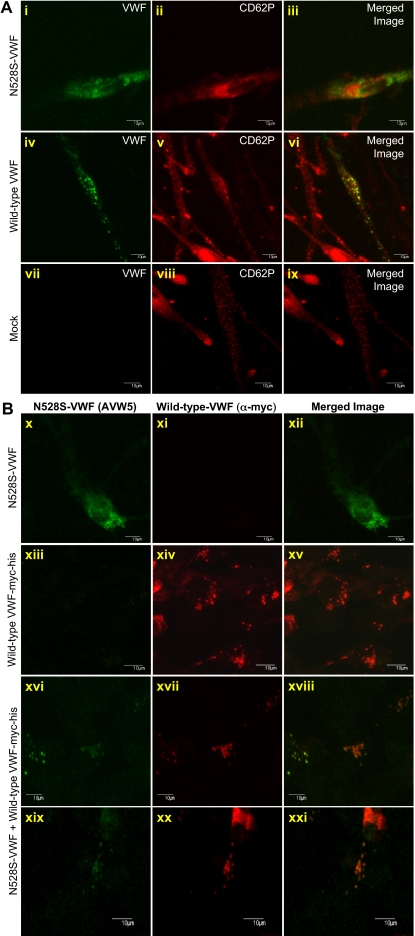
To further examine N528S-VWF–regulated storage and granule biogenesis, wild-type and N528S-VWF were expressed in VWF-null aortic endothelial cells from type 3 VWD dogs (K9-VWD-AECs) and HEK293 cells. The advantage of using these cells is it allows one to examine the ability of VWF to drive the formation of storage granules in the absence of other endogenous secretory granules (eg, ACTH granules in AtT-20 cells).16,21 In K9-VWD-AEC–expressing N528S-VWF only a diffuse staining was observed for VWF, with no evidence of granule formation (Figure 6A). Wild-type VWF formed granules resembling endothelial cell Weibel-Palade bodies and recruited P-selectin (CD62P). Previous studies from our laboratory and others demonstrated that VWF expression in HEK293 cells parallels expression in endothelial cells: neither VWFpp nor mature VWF forms Weibel-Palade bodies independently; expression of both is necessary for granule formation (S.L.H., unpublished data).16,27 HEK293-expressing N528S-VWF demonstrated diffuse staining, similar to that in K9-VWD-AECs and AtT-20 cells, whereas wild-type VWF with a myc-his tag (wild-type VWF-myc-his) formed granules (Figure 6B). N528S-VWF was also coexpressed with wild-type VWF-myc-his in a 1:1 ratio to recapitulate heterozygous expression (Figure 6B). Differential antibody recognition was used to identify each allele with monoclonal anti-VWF antibody AVW-5 recognizing only untagged N528S-VWF and rabbit anti-myc detecting wild-type VWF-myc-his. Heterozygous expression resulted in granular storage of both N528S-VWF and wild-type VWF-myc-his in cells expressing both proteins (Figure 6B).
Figure 6.
VWF granule biogenesis in HEK293 cells transfected with mutant and wild-type VWF. (A) K9-VWD-AECs were transiently transfected with N528S-VWF, wild-type VWF, or pCIneo (Mock). Cells were immunostained for VWF (green; i, iv, vii) and P-selectin/CD62P (red; ii, v, viii) and intracellular localization examined by confocal microscopy. The merged image is shown in the last column with colocalization shown in yellow (iii, vi, ix). Wild-type VWF formed granules and recruited CD62P, whereas N528S-VWF failed to form granules. (B) HEK293 cells were transiently transfected with N528S-VWF, wild-type VWF-myc-his, or heterozygous N528S-VWF/wild-type VWF-myc-his. Cells were immunostained using monoclonal AVW-5 that does not recognize wild-type VWF-myc-his (green; x, xiii, xvi, xix) and rabbit antimyc that does not recognize the N528S-VWF lacking a myc-his tag (red; xi, xiv, xvii, xx). Intracellular localization was determined by confocal microscopy and the merged image shown in panels xii, xv, xviii, and xxi. When expressed alone, wild-type VWF formed granules, whereas N528S-VWF did not. When coexpressed, both N528S-VWF and wild-type VWF are localized in granules.
Although N528S-VWF had substantially decreased secretion when expressed alone, it did not appear to affect the secretion of wild-type VWF, even at a high ratio of N528S/wild type (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). HEK293 cells cotransfected at various ratios of N528S-VWF/wild-type VWF-myc-his were stained and examined by confocal microscopy. Whereas the expression efficiency of wild-type VWF-myc-his was approximately 29%, N528S-VWF was present in only 0.7% to 1.5% of cells. When N528S-VWF was expressed, it was found mostly in wild-type VWF–expressing cells. Differential antibody recognition was used to visualize multimers of coexpressed N528S-VWF (red) and wild-type VWF-myc-his (green) with colocalization shown in yellow (supplemental Figure 1). The majority of secreted VWF was expressed from the wild-type allele and very little N528S-VWF was detected.
Discussion
In this study, we identified an N528S VWF mutation in a consanguineous family with VWD and characterized the effect of this mutation on VWF processing and secretion. From the data presented in this paper, we can conclude that (1) the homozygous expression of this variant causes decreased secretion, defective multimerization, and loss of regulated storage of VWF, (2) the D2 domain mutation has no effect on VWFpp-regulated storage, and (3) coexpression of wild-type VWF with the variant VWF overcomes the defective processing and secretion of the N528S variant allele, confirming the recessive nature of this mutation. The defective interaction of VWF with its intracellular chaperone, VWFpp, may represent a novel mechanism causing VWD.
Based on the current VWD classification, the affected patients fit the criteria for type 2A VWD.2 Affected patients have low plasma VWF:Ag, greatly reduced VWF:CB, increased VWF dimer band with decreasing intensity of mid-sized multimers to HMWMs, and lack of VWF multimer satellite bands (Figures 1,3). Only the homozygous expression of the N528S VWF mutation appears to produce a bleeding phenotype consistent with recessive inheritance. These parameters are indicative of the historic subclassification of type 2A, subtype IIC.28–31 These patients are likely to have a VWFpp mutation.13,29–32 It is not surprising that VWFpp mutations are associated with VWF multimerization abnormalities, as VWFpp has been shown to play a critical role in facilitating VWF multimerization.33
An unexpected finding from the current study is the defective VWF-regulated storage in affected patients. The substantially reduced levels of platelet VWF:Ag together with the lack of VWF mid-sized multimers to HMWMs in platelet lysates suggests defective α-granular storage. Regulated secretion from endothelial cell Weibel-Palade bodies is also affected as homozygous patients were nearly unresponsive to desmopressin acetate (Figure 2).23 Interestingly, FVIII was released in response to desmopressin acetate. A similar FVIII response has been observed in severe type 1 and type 2A patients with minimal VWF release.34 Studies have suggested that FVIII is stored with VWF in Weibel-Palade bodies.35 It is possible that very little VWF may be necessary to traffic FVIII to storage, or perhaps FVIII is stored independently of VWF.
Expression in AtT-20 cells, HEK293 cells, and K9-VWD-AECs demonstrated a lack of N528S-VWF–regulated storage and granule biogenesis (Figures 5,6). VWF-regulated storage is VWFpp dependent: VWFpp independently traffics to the regulated secretory pathway and secondarily traffics VWF to storage through a noncovalent association.15,20 The mutated propeptide, N528S-VWFpp, trafficked to storage granules indicating that the mutation does not interfere with the VWFpp storage signal (Figure 5). N528S-VWFpp did not facilitate trafficking of coexpressed mature VWF to storage granules, suggesting that a disrupted VWFpp-VWF interaction contributes to the lack of regulated storage. This is also supported by the putative consequences of the mutation, which is predicted to create a new N-glycosylation site at N526. The glycosyl side chain could potentially interfere directly or could alter the structure of the postulated binding region of the propeptide for mature VWF. N526 is located in close vicinity of the CGLC disulphide isomerase consensus sequence (C521-C524) that has been implicated in the multimerization process of VWF at its D3 domain.11 Thus, it could additionally impair VWF multimerization by interference of the new glycosyl side chain with disulphide bonding in the VWF D3 domain. In contrast is the previous study by Mayadas and Wagner in which disruption of the CGLC sequence did not abrogate VWF storage in AtT-20 cells, although multimerization was abolished.11 The potential alteration in glycosylation may have a greater impact on VWF structure that impacts regulated storage.
Taken together, this represents a novel pathogenic mechanism of VWD. The defects that occur early in the processing pathway (eg, intracellular retention in the endoplasmic reticulum [ER]) may be more detrimental to the phenotype than downstream defects such as multimerization and secretory granule formation. Misfolded proteins are recognized and retained in the ER and targeted for degradation.36 If misfolded protein escapes the ER, downstream processing should be unaffected. N528S-VWF demonstrated decreased synthesis and secretion (ER), as well as downstream defects including aberrant multimerization (Golgi) and lack of granule formation (after Golgi). It is well established that VWF multimerization is not required for regulated storage.10,15,37 Several mutations in VWF disrupt multimerization but not regulated storage.12,16,17 The lack of N528S granular storage is rather distinctive in this regard, although regulated storage has yet to be examined for the majority of the many reported mutations.
Heterozygous expression of wild-type VWF with N528S-VWF overcame the defects in processing, consistent with the recessive inheritance pattern. Most of type 2B, 2M, and 2A mutations are autosomal dominant, whereas other mutations (type 2N and type 3) are autosomal recessive. Type 2A(IIC) mutations, including the N528S mutation, are characterized by recessive inheritance.28 The nature of the interaction of VWF expressed from the “normal allele” with mutant VWF expressed from the variant allele must influence the nature of the phenotype. In type 2B, the heteromultimerization of wild-type and mutant VWF proteins results in the incorporation of subunits with increased platelet GPIb reactivity resulting in a dominant phenotype.38 The formation of a heterodimer between type 1 VWD variant, C1149R-VWF, and wild-type VWF has been shown to result in a dominant-negative phenotype with impaired secretion and intracellular degradation.24,39 In contrast, in type 2A(IIC) VWD variants, the VWF expressed from the wild-type allele may be able to compensate for the inadequacies of the VWF expressed from the variant allele. The mechanism for this compensation could potentially result from differential expression of the 2 alleles with wild-type VWF expressed and secreted more efficiently, or the interaction of VWF proteins along the synthesis pathway with wild-type VWF restoring escape from the ER and rescuing multimerization. N528S-VWF appeared to have decreased expression, suggesting defects even further upstream such as a translational deficit. An additional variable that may contribute to the structure and functional consequences of the N528S VWF mutation is the extent of N-linked glycosylation. The glycosylation may not always be complete, and cell-specific differences in glycosylation may result in variable functional deficiencies of the secreted variant VWF protein.
In summary, we have characterized an N528S-VWF mutation identified in a family with type 2A(IIC) VWD. The homozygous expression of this mutation causes defective secretion, multimerization, and regulated storage of VWF. We identified a potentiallynovel pathogenic mechanism of VWD, namely a transportation and storage defect of mature VWF due to defective binding to its transporter, the mutant propeptide. Heterozygous expression of wild-type VWF and N528S-VWF in vitro and in vivo results in normal secretion and VWF multimerization, confirming the recessive nature of this mutation.
Supplementary Material
Acknowledgments
This work was supported by American Heart Association SDG 0435466N, National Institutes of Health/National Heart, Lung, and Blood Institute HL-081588 and HL-33721 (S.L.H.), and the German National Genome Research Network NGFN2 (Cardiovascular Diseases Network, grant NHK-S17T22; R.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.L.H. designed and performed research, analyzed and interpreted cell biology data, and wrote the paper; U.B. designed research and analyzed the patients' phenotypic data; T.O. performed the expression studies and analyzed data; S.S. performed research and analyzed the VWF multimers; C.W. performed the clinical investigations, analyzed data, and wrote the paper; and R.S. designed and performed research, analyzed and interpreted the molecular data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandra L. Haberichter, Department of Pediatrics-Hematology/Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: shaberic@mcw.edu.
References
- 1.Sadler JE, Mannucci PM, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000;84(2):160–174. [PubMed] [Google Scholar]
- 2.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachman R, Levine R, Jaffe EA. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij CL, Diergaarde PJ, Hart M, Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit [published erratum appears in EMBO J. 1986;5(11):3074]. EMBO J. 1986;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- 7.Vischer UM, Wagner DD. von Willebrand factor proteolytic processing and multimerization precede the formation of Weibel-Palade bodies. Blood. 1994;83(12):3536–3544. [PubMed] [Google Scholar]
- 8.Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70(1):105–110. [PubMed] [Google Scholar]
- 9.Voorberg J, Fontijn R, van Mourik JA, Pannekoek H. Domains involved in multimer assembly of von willebrand factor (vWF): multimerization is independent of dimerization. EMBO J. 1990;9(3):797–803. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner DD, Saffaripour S, Bonfanti R, et al. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- 11.Mayadas TN, Wagner DD. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci U S A. 1992;89(8):3531–3535. doi: 10.1073/pnas.89.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg JB, Haberichter SL, Jozwiak MA, et al. The role of the D1 domain of the von Willebrand factor propeptide in multimerization of VWF. Blood. 2002;100(5):1699–1706. doi: 10.1182/blood-2002-03-0789. [DOI] [PubMed] [Google Scholar]
- 13.Allen S, Abuzenadah AM, Hinks J, et al. A novel von Willebrand disease-causing mutation (Arg273Trp) in the von Willebrand factor propeptide that results in defective multimerization and secretion. Blood. 2000;96(2):560–568. [PubMed] [Google Scholar]
- 14.Haberichter SL, Jozwiak MA, Rosenberg JB, Christopherson PA, Montgomery RR. The von Willebrand factor propeptide (VWFpp) traffics an unrelated protein to storage. Arterioscler Thromb Vasc Biol. 2002;22(6):921–926. doi: 10.1161/01.atv.0000017063.36768.87. [DOI] [PubMed] [Google Scholar]
- 15.Haberichter SL, Fahs SA, Montgomery RR. Von Willebrand factor storage and multimerization: 2 independent intracellular processes. Blood. 2000;96(5):1808–1815. [PubMed] [Google Scholar]
- 16.Michaux G, Hewlett LJ, Messenger SL, et al. Analysis of intracellular storage and regulated secretion of 3 von Willebrand disease-causing variants of von Willebrand factor. Blood. 2003;102(7):2452–2458. doi: 10.1182/blood-2003-02-0599. [DOI] [PubMed] [Google Scholar]
- 17.Hommais A, Stepanian A, Fressinaud E, et al. Mutations C1157F and C1234W of von Willebrand factor cause intracellular retention with defective multimerization and secretion. J Thromb Haemost. 2006;4(1):148–157. doi: 10.1111/j.1538-7836.2005.01652.x. [DOI] [PubMed] [Google Scholar]
- 18.Schneppenheim R, Budde U, Obser T, et al. Expression and characterization of von Willebrand factor dimerization defects in different types of von Willebrand disease. Blood. 2001;97(7):2059–2066. doi: 10.1182/blood.v97.7.2059. [DOI] [PubMed] [Google Scholar]
- 19.Haberichter SL, Allmann AM, Jozwiak MA, Montgomery RR, Gill JC. Genetic alteration of the D2 domain abolishes von Willebrand factor multimerization and trafficking into storage. J Thromb Haemost. 2009;7(4):641–650. doi: 10.1111/j.1538-7836.2009.03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberichter SL, Jacobi P, Montgomery RR. Critical independent regions in the VWF propeptide and mature VWF that enable normal VWF storage. Blood. 2003;101(4):1384–1391. doi: 10.1182/blood-2002-07-2281. [DOI] [PubMed] [Google Scholar]
- 21.Haberichter SL, Merricks EP, Fahs SA, Christopherson PA, Nichols TC, Montgomery RR. Re-establishment of VWF-dependent Weibel-Palade bodies in VWD endothelial cells. Blood. 2005;105(1):145–152. doi: 10.1182/blood-2004-02-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneppenheim R, Plendl H, Budde U. Luminography: an alternative assay for detection of von Willebrand factor multimers. Thromb Haemost. 1988;60(2):133–136. [PubMed] [Google Scholar]
- 23.Kaufmann JE, Oksche A, Wollheim CB, Gunther G, Rosenthal W, Vischer UM. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106(1):107–116. doi: 10.1172/JCI9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eikenboom JC, Matsushita T, Reitsma PH, et al. Dominant type 1 von Willebrand disease caused by mutated cysteine residues in the D3 domain of von Willebrand factor. Blood. 1996;88(7):2433–2441. [PubMed] [Google Scholar]
- 25.Hilbert L, Gaucher C, Mazurier C. Identification of two mutations (Arg611Cys and Arg611His) in the A1 loop of von Willebrand factor (vWF) responsible for type 2 von Willebrand disease with decreased platelet-dependent function of vWF. Blood. 1995;86(3):1010–1018. [PubMed] [Google Scholar]
- 26.Blagoveshchenskaya AD, Hannah MJ, Allen S, Cutler DF. Selective and signal-dependent recruitment of membrane proteins to secretory granules formed by heterologously expressed von Willebrand factor. Mol Biol Cell. 2002;13(5):1582–1593. doi: 10.1091/mbc.01-09-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaux G, Abbitt KB, Collinson LM, Haberichter SL, Norman KE, Cutler DF. The physiological function of von Willebrand's factor depends on its tubular storage in endothelial Weibel-Palade bodies. Dev Cell. 2006;10(2):223–232. doi: 10.1016/j.devcel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Ruggeri ZM, Nilsson IM, Lombardi R, Holmberg L, Zimmerman TS. Aberrant multimeric structure of von Willebrand factor in a new variant of von Willebrand's disease (type IIC). J Clin Invest. 1982;70(5):1124–1127. doi: 10.1172/JCI110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaucher C, Dieval J, Mazurier C. Characterization of von Willebrand factor gene defects in two unrelated patients with type IIC von Willebrand disease. Blood. 1994;84(4):1024–1030. [PubMed] [Google Scholar]
- 30.Holmberg L, Karpman D, Isaksson C, Kristoffersson AC, Lethagen S, Schneppenheim R. Ins405AsnPro mutation in the von Willebrand factor propeptide in recessive type 2A (IIC) von Willebrand's disease. Thromb Haemost. 1998;79:718–722. [PubMed] [Google Scholar]
- 31.Schneppenheim R, Thomas KB, Krey S, et al. Identification of a candidate missense mutation in a family with von Willebrand disease type IIC. Hum Genet. 1995;95(6):681–686. doi: 10.1007/BF00209487. [DOI] [PubMed] [Google Scholar]
- 32.Gaucher C, Uno H, Yamazaki T, Mashiba H, Mazurier C. A new candidate mutation (N528S) within the von Willebrand factor propeptide identified in a Japanese patient with phenotype IIC of von Willebrand disease. Eur J Haematol. 1998;61(2):145–148. doi: 10.1111/j.1600-0609.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 33.Wise RJ, Pittman DD, Handin RI, Kaufman RJ, Orkin SH. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- 34.Federici AB, Mazurier C, Berntorp E, et al. Biologic response to desmopressin in patients with severe type 1 and type 2 von Willebrand disease: results of a multicenter European study. Blood. 2004;103(6):2032–2038. doi: 10.1182/blood-2003-06-2072. [DOI] [PubMed] [Google Scholar]
- 35.Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274(28):19587–19592. doi: 10.1074/jbc.274.28.19587. [DOI] [PubMed] [Google Scholar]
- 36.Hampton RYE. R-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14(4):476–482. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 37.Journet AM, Saffaripour S, Wagner DD. Requirement for both D domains of the propolypeptide in von Willebrand factor multimerization and storage. Thromb Haemost. 1993;70(6):1053–1057. [PubMed] [Google Scholar]
- 38.Cooney KA, Ginsburg D. Comparative analysis of type 2b von Willebrand disease mutations: implications for the mechanism of von Willebrand factor binding to platelets. Blood. 1996;87(6):2322–2328. [PubMed] [Google Scholar]
- 39.Bodó I, Katsumi A, Tuley EA, Eikenboom JC, Dong Z, Sadler JE. Type 1 von Willebrand disease mutation Cys1149Arg causes intracellular retention and degradation of heterodimers: a possible general mechanism for dominant mutations of oligomeric proteins. Blood. 2001;98(10):2973–2979. doi: 10.1182/blood.v98.10.2973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.