Abstract
Study Objectives:
Idiopathic rapid eye movement sleep behavior disorder (iRBD)—a parasomnia characterized by dream enactments—is a risk marker for the development of Parkinson disease (PD) and other α-synucleinopathies. The pathophysiology of iRBD is likely due to dysfunction of brainstem nuclei that regulate REM sleep. Diffusion tensor imaging (DTI) is a method for studying microstructural brain tissue integrity in vivo. We investigated whether DTI detects microstructural abnormalities in the brain of patients with iRBD—compared with age-matched control subjects—as an in vivo potential indicator for changes related to “preclinical (premotor)” neuropathology in PD.
Design:
N/A
Patients:
Patients with iRBD (n = 12) and age-matched healthy control subjects (n = 12) were studied.
Interventions:
At a 1.5T MRI maschine, whole-head DTI scans of fractional anisotropy, axial diffusivity (a potential marker of neuronal loss), and radial diffusivity (a potential marker of glial pathology) were analyzed using track-based spatial statistics, and 2 types of group analysis tools (FreeSurfer and FSL).
Measurements and Results:
We found significant microstructural changes in the white matter of the brainstem (P < 0.0001), the right substantia nigra, the olfactory region, the left temporal lobe, the fornix, the internal capsule, the corona radiata, and the right visual stream of the patients with iRBD.
Conclusions:
Changes were identified in regions known to be involved in REM-sleep regulation and/or to exhibit neurodegenerative pathology in iRBD and/or early PD. The study findings suggest that iRBD-related microstructural abnormalities can be detected in vivo with DTI, a widely available MRI technique.
Citation:
Unger MM; Belke M; Menzler K; Heverhagen JT; Keil B; Stiasny-Kolster K; Rosenow F; Diederich NJ; Mayer G; Mäoller JC; Oertel WH; Knake S. Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions. SLEEP 2010;33(6):767-773.
Keywords: RBD, MRI, Parkinson's disease, TBSS, DTI, Radial diffusivity
RAPID EYE MOVEMENT (REM) SLEEP BEHAVIOR DISORDER (RBD) IS A REM-PARASOMNIA THAT CLINICALLY PRESENTS WITH NOCTURNAL SPEECH AND motor activity reflecting the dream content of the patient during REM sleep.1,2 Idiopathic RBD (iRBD), i.e., the occurrence of RBD in the absence of other medical (neurologic) conditions, is considered to represent a clinical risk marker, with the—at present—highest positive predictive value for the subsequent development of an α-synucleinopathy, i.e., for Parkinson disease (PD), dementia with Lewy bodies, or multiple system atrophy.3 In the context of evolving PD, iRBD is thought to be an early clinical correlate of a slowly progressing neurodegenerative process in the brainstem. Identification of such a neurodegenerative process with a routinely available technique such as magnetic resonance imaging (MRI) could be of value for the preclinical (premotor) detection of PD and other α-synucleinopathies.
During physiologic REM sleep, the activity of spinal α-motor neurons is suppressed by a complex interaction of superordinate brainstem nuclei (REM-on and REM-off areas, including the sublaterodorsal nucleus4). In RBD, this interaction between REM-on and REM-off areas is considered to be disturbed, leading to a disinhibition of spinal α-motor neurons and, subsequently, to movements during the dream phase. The sublaterodorsal nucleus is presumably one major efferent pathway for mediating muscle atonia during REM sleep,5 pointing to the brainstem as a potential area to be investigated for structural changes in iRBD.
Until now, structural brain changes have not been described in patients with iRBD. Functional imaging studies have reported normal or conflicting findings: a single-photon emission computed tomography (SPECT) study showed that decreased blood flow in the pons and in the superior frontal lobes of patients with iRBD,6 whereas other studies have confirmed the decreased perfusion in the frontal cortices, they have also found an increased perfusion in the pons, putamina, and right hippocampus.7 SPECT studies using ligands to visualize the presynaptic dopamine transporter have reported that tracer uptake in the striatum is frequently reduced in patients with iRBD.8–11 The latter finding must be interpreted in the context of iRBD as a putative “preclinical (premotor)” stage of the α-synucleinopathy PD, rather than as a finding that directly relates to the disruptive nocturnal behavior of RBD-related pathophysiology. The results of previous magnetic resonance spectroscopy studies are conflicting, reporting either no metabolic changes12 or a pathologic choline/creatine ratio in the brainstem.13 Recently, transcranial sonography has revealed hyperechogenicity in the substantia nigra in a group of patients with iRBD,11,14 a sonographic marker that is seen in 90% of all patients with PD. In summary, in vivo imaging studies have not consistently demonstrated disease-specific structural abnormalities in patients with iRBD.
Diffusion tensor imaging (DTI) is a novel MRI technique that enables noninvasive in vivo visualization of brain white-matter microstructure. DTI has been applied successfully to several neurodegenerative disorders, providing insights into the pathophysiology of the respective disorders.15–18 Most DTI studies have focused on fractional anisotropy (FA) as an unspecific summary measure of brain-tissue integrity. More recently, 2 components of the diffusion signal, namely axial diffusivity (AD) and radial diffusivity (RD), have been used to describe different aspects of mechanisms of degeneration. In the current study, the microstructural properties of the brains of patients with iRBD and age-matched healthy volunteers were examined using an automated whole-head analysis of the DTI measures of FA, RD, and AD.
METHODS
The study protocol was reviewed and approved by the local institutional review board. All enrolled subjects provided written informed consent to participate in this study.
Subjects
Twelve patients with iRBD and 12 healthy age-matched volunteers were included in the study. All enrolled patients with iRBD had a videopolysomnographically confirmed diagnosis of iRBD according to the revised version of the International Classification of Sleep Disorders.19 Control subjects had no history of neurologic, psychiatric, or systemic illness and no family history of neurodegenerative disorders. All patients underwent a thorough neurologic examination, including Mini-Mental State Examination (MMSE) testing and a threshold-discrimination-identification olfactory function test with Sniffin' Sticks (Burghart Medizintechnik, Wedel, Germany). Control subjects were age matched, but not sex matched, because previous studies revealed age as being the most important variable influencing DTI results.20
MRI Acquisition
Microstructural brain-tissue integrity was assessed using DTI measures of FA and diffusivity (AD and RD21–23). The DTI scans were collected on a Siemens 1.5-T Sonata MRI scanner (Siemens Medical Solutions, Erlangen, Germany), using a circularly polarized head array coil. A single-shot echo planar sequence with a twice-refocused spin echo pulse, optimized to minimize eddy-current–induced image distortions,24 was performed with the following parameters: TR/TE = 10600/104 ms, TI = −1 ms, flip angle = 90°, b = 1000 s/mm2, 256 × 256 mm FOW, 128 × 128 × 60 matrix, and voxel size = 2 × 2 × 2.4 mm. Five T2 b0 images and 30 DWI b1000 images were collected during 1 scan. To minimize motion artifacts, the subjects' heads were firmly fixed in the head coil. All images were determined to be free of motion or ghosting or high-frequency or wrap-around artifacts at the time of image acquisition.
DTI Preprocessing and Analysis
Image preprocessing was performed as has been previously described25: diffusion volumes were motion corrected and averaged using FLIRT (FMRIB's Linear Image Registration Tool; http://www.fmrib.ox.ac.uk/analysis/research/flirt/)26 with mutual information cost function to register each direction to the minimally eddy-current–distorted T2-weighted b0 DTI volume that had no diffusion weighting. Eigenvalues (λ1, λ2, λ3) and eigenvectors of the diffusion tensor matrix for each voxel were computed from the DTI volumes for each subject on a voxel-by-voxel basis using conventional reconstruction methods.27,28 The tools are included in the FreeSurfer package (FreeSurfer version 4.2.0; http://surfer.nmr.mgh.harvard.edu/).
FA and Diffusivity Map Calculation
The primary measure acquired from the DTI data was the FA, a scalar metric unit describing the white-matter microstructure. FA is dependent on the orientational coherence of the diffusion compartments within a voxel.29 FA was calculated using the standard previously defined formula.30 We additionally calculated measures of AD (λ1) and RD ([λ2 +λ3]/2).21–23
T2 b0 images were obtained using the same parameters as those used for the diffusion sensitive images except without any diffusion weighting. Those images were analyzed to determine whether changes other than those in tissue microstructure contributed to the observed effects, such as technical artifacts or individual large-scale signal changes such as white-matter signal abnormalities (e.g., hyperintensities).
Nonlinear Registration and Tract-based Spatial Statistics
Voxelwise statistical analysis of the FA data was carried out using tract-based spatial statistics (TBSS; tract-based spatial statistics31), which is part of the FSL data-analysis suite (FSL 4.1.2; http://www.fmrib.ox.ac.uk/fsl/).32 First, the T2 b0 images were brain extracted using the BET-tool of the FSL stream. Those extracted brains were used to mask the FA images. All subjects' masked FA data were then aligned into a common space using the nonlinear registration tool FNIRT,33 which uses a b-spline representation of the registration warp field.34 The common space was given by the automatically selected subject that was most representative of the whole group. This subject was affinely registered to the T1 template in MNI 152 standard space. Next, a mean FA image was created and thinned to create a mean FA skeleton, which represents the centers of all tracts the group has in common. A threshold of FA greater than 0.2 was applied to the skeleton to include only major fiber bundles. Each subject's aligned FA data was then projected onto this skeleton. Data along the skeleton was smoothed utilizing an anatomic constraint to limit the smoothing to neighboring data within adjacent voxels along the skeleton. For smoothing, the neighboring voxels within a cube of 6-mm edge length were used to calculate the mean. The smoothing step was performed using Matlab (Matlab 7.6.0.324, MathWorks, Aachen, Germany). All analyses were masked to display regions only with FA values of greater than 0.2 as an additional procedure to avoid examination of regions that are likely comprised of multiple tissue types or fiber orientations. The exact transformations derived for the anisotropy maps were applied to the AD and RD volumes for matched processing of all image volumes.
Group Analysis
Two data-analysis approaches (FreeSurfer and FSL) were used for group analysis.
FreeSurfer approach
The resulting skeletonized images were fed into voxelwise cross-subject statistics. For the group analysis, we used the tool mri_glmfit of the FreeSurfer stream. The data was fit into a generalized linear model, and an unpaired t test was performed. The resulting data were corrected for multiple comparisons by a permutation-based approach.35 Therefore, 12.000 simulations were performed under the null hypothesis; this approach was based on the AFNI null-z simulator (AlphaSim; http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim). Last, the data were clustered; in this step, several connected voxels were searched and merged into 1 cluster. For the clustering, we considered only voxels with a minimum significance of P < 0.01. To display the results, all figures were made with the exact same parameters, showing clusters with a significance of P < 0.01; all regions were dilated for better visualization using the dilM function of the FSL tool fslmaths. (Figure 1) The correction for multiple testing was performed with the mri_glmfit-sim tool of the FreeSurfer package. Results were summarized in tables showing the localization of the clusters and their significances (Tables 2-4)
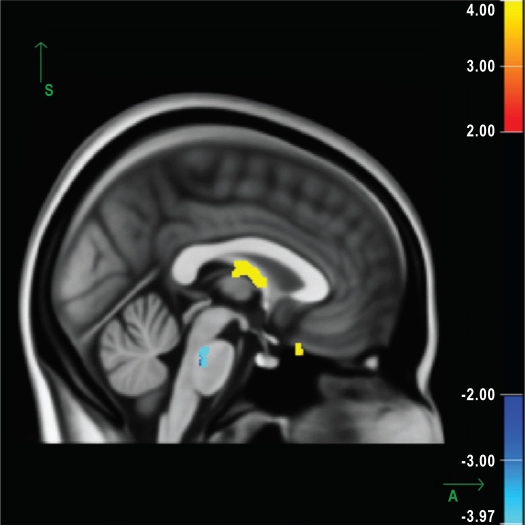
Figure 1.
Sagittal view of the average b0 image, calculated from all study subjects (or representing the average of all subjects in the study), showing the white-matter skeleton all subjects have in common. Significant increases in radial diffusivity can be observed in the fornix and the olfactory region (P < 0.0001). A significant decrease in the axial diffusivity can be seen in the pons (P < 0.0001). Results are displayed as the negative decadic logarithm of the P value (P = 10-x). The increase in radial diffusivity is shown in red-yellow; the decrease in axial diffusivity, in blue-light blue.
Table 2.
Regions with significant changes in fractional anisotropy
| Region | Size, mm3 | Maximum log-transformed P, 10-x | Corrected overall P value | X | Y | Z |
|---|---|---|---|---|---|---|
| Right visual stream | 483 | (−) 4.58 | < 0. 0001 | 12 | -93 | 12 |
| Fornix | 158 | (−) 4.39 | < 0. 0001 | 3 | −9 | 13 |
| Left superior temporal lobe | 333 | (−) 4.16 | < 0. 0001 | −60 | −20 | 4 |
| Olfactory region | ||||||
| Right | 38 | 2.11 | < 0. 0001 | 8 | 32 | −18 |
| Left | 73 | 2.4 | < 0. 0001 | −5 | 39 | −23 |
| Internal capsule | ||||||
| Right | 37 | 2.05 | < 0. 0001 | 19 | 9 | −8 |
| Left | 199 | 3.74 | < 0. 0001 | −15 | 13 | −5 |
The results of the statistical tests were corrected for multiple testing, and regions with clusters of several connected voxels (with a minimum significance of P < 0.01) were searched. X, Y, and Z refer to the corresponding MNI-atlas coordinates.
Table 4.
Regions with significant changes in radial diffusivity
| Region | Size, mm3 | Maximum log-transformed P, 10−x | Corrected overall P value | X | Y | Z |
|---|---|---|---|---|---|---|
| Right visual stream | 979 | 4.08 | < 0. 0001 | 36 | −79 | −4 |
| Fornix | 165 | 2.86 | < 0. 0001 | 4 | −11 | 13 |
| Left superior temporal lobe | 103 | 2.58 | < 0. 0001 | −60 | −18 | 3 |
The results of the statistical tests were corrected for multiple testing, and regions with clusters of several connected voxels (with a minimum significance of P < 0.01) were searched. X, Y, and Z refer to the corresponding MNI-atlas coordinates.
FSL standard approach
For comparison of identified regions, data were also analyzed using the established standard FSL approach for group statistics. The skeletonized images resulting from the TBSS procedure were processed using the randomize GLM tool of the FSL stream. For this procedure, we followed the standard instructions for performing a GLM test: First, a design matrix and a contrast matrix were created with the FSL-tool design_ttest2, then the tool randomize from the FSL stream was applied with the standard parameters for the TBSS-analysis. The correction for multiple testing was performed using the FSL tool randomize using the same parameters as for mri_glmfit-sim. Again, 12.000 permutations were performed, and, afterward, the results were clustered. To display the results, all figures were calculated with the exact same parameters as in FreeSurfer, showing voxels with a significance of P < 0.01. All regions were dilated for better visualization, using the dilM function of the FSL tool fslmaths.
RESULTS
The main demographic and clinical data of all enrolled subjects are summarized in Table 1. We investigated 12 patients with iRBD and 12 age-matched control subjects using whole-brain DTI. Accordingly, in the neurologic examination, none of the patients with iRBD fulfilled the UK Brain Bank Criteria for the diagnosis of PD.36
Table 1.
Demographics and diagnostic findings among 12 subjects with iRBD and 12 control subjects
| Demographics | Subjects with iRBD | Control subjects |
|---|---|---|
| Men | 11 | 3 |
| Age, y | 59 ± 10.5 (38-71) | 56.8 ± 10.6 (42-74) |
| Clinical scores | ||
| MMSE | 29.2 ± 0.8 (26-30) | NA |
| TDI sum score | 23 ± 9.4 (8-35) | NA |
| Imaging study results | ||
| FP-CIT SPECT tracer uptakea | ||
| Normal | 3 | NA |
| Reduced or asymmetric | 4 | NA |
Data are shown as mean + SD (range) or number. MMSE refers to Mini-Mental Status Examination; TDI, the threshold-discrimination-identification in the Sniffin' Stick Test (a TDI sum score ≥ 30 is defined as normosmia).64
Data are from 7 subjects with idiopathic rapid eye movement sleep behavior disorder (iRBD) who agreed to undergo 123I-N-Ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)-nortropane single photon emission tomography (FP-CIT SPECT) scanning.
FA Results
Significant FA increases (corrected P < 0.0001) were found in the internal capsule bilaterally (along the anterior thalamic radiation) and in the olfactory region, whereas significant decreases were found in the fornix, the right visual stream (V1,V2), and the left superior temporal lobe. Figure 1, supplementary Figure S1 (supplementary figures are available online only at www.journalsleep.org), and Table 2 show the regions with the main changes in FA.
AD and RD Results
AD describes the principal eigenvector (λ1) and is assumed to contribute information regarding the integrity of axons37 or changes in extraaxonal/extracellular space.38 Regional reductions in AD seem to represent regions with altered axonal integrity.37 Significant decreases (corrected P < 0.0001) in the axial diffusivity were observed in the brainstem, i.e., in the pons and the right substantia nigra. Changes also occurred in the corona radiata bilaterally. Changes in AD are shown in Figure 1, supplementary Figure S2, and Table 3.
Table 3.
Regions with significant changes in axial diffusivity
| Region | Size, mm3 | Maximum log-transformed P, 10−x | Corrected overall P value | X | Y | Z |
|---|---|---|---|---|---|---|
| Pons | 425 | 3.37 | < 0. 0001 | 2 | −29 | −30 |
| Corona radiata | ||||||
| Right | 382 | 4.42 | < 0. 0001 | 13 | 6 | 54 |
| Left | 153 | 2.89 | < 0. 0001 | −6 | 38 | 45 |
| Substantia nigra | 9 | 1.98 | < 0. 0001 | 14 | −17 | −14 |
The results of the statistical tests were corrected for multiple testing, and regions with clusters of several connected voxels (with a minimum significance of P < 0.01) were searched. X, Y, and Z refer to the corresponding MNI-atlas coordinates.
RD describes an average of the eigenvectors perpendicular to the principal direction ([λ2 + λ3]/2).22,23,39,40 Regional increases in RD are considered to be associated with changes in myelination or glial cell morphology.22,23,40 In our study, patients with iRBD had significantly increased RD (corrected P < 0.0001) in the fornix, the right visual stream (V1,V2), and the left superior temporal lobe. as shown in supplementary Figure S3 and Table 4.
Comparison Between the 2 Data-Analysis Methods
Two approaches for data analysis were used for group comparisons: the tool Randomize of the FSL data analysis suite and the approach using the mri_glmfit of the FreeSurfer package identified the same pattern of microstructural tissue alteration, as shown in the supplementary Figure S4.
DISCUSSION
Until now, no structural abnormalities have been described in patients with iRBD using MRI or other structural imaging techniques. Here, we report focal microstructural brain changes in the white matter of the brain of patients with a videopolysomnographically confirmed diagnosis of iRBD using DTI with a hypothesis-free approach. DTI measures the diffusional motion of water molecules, which is predetermined by the (sub)cellular microenvironment. By this means, DTI can be used as a sensitive measure of microstructural integrity. The diffusion within a single voxel can be expressed as FA, a metric measure ranging from 0 (isotropic) to 1 (anisotropic). The FA is high (close to 1) in regions in which diffusion is restricted to 1 orientation, e.g., along highly organized structures, like bundles of myelinated fibers, whereas the FA is low (close to 0) in less-organized structures, such as regions of cellular damage in which diffusion tends to be equally likely in all directions (i.e., isotropic).
Localization of Microstructural Brain Changes
Alterations in microstructure were found in the iRBD group compared with control subjects in brainstem regions known to regulate REM sleep, as well as in the olfactory region, the thalamic radiation in the anterior limb of the internal capsule, the right substantia nigra, the left superior temporal lobe and the right occipital lobe, and the fornix.
We found a widespread decrease in AD (indicating possible axonal damage) in the brainstem, especially the pons, i.e., regions known to be involved in the regulation of REM sleep.4 Dysfunction of the respective structures is thought to be the pathophysiologic correlate of RBD.5 This assumption is compatible with the results of postmortem studies that describe degeneration of monoaminergic neurons and the presence of Lewy bodies in brainstem structures in patients with RBD.41,42 Furthermore, structural brainstem lesions that affect the sublaterodorsal nucleus (its afferent or efferent pathways and linked nuclei, respectively) have been shown to result in symptomatic RBD.43–46 Finally, experimental lesions in the ventral part of the pons and the ventral mesopontine junction in animal models can provoke an RBD-like phenotype as well.47 The reported morphologic in vivo changes seen in the brainstem of our iRBD population overlap with anatomic regions that are thought to be responsible for mediating muscle atonia during REM sleep.
According to the Braak pathoanatomic model of PD (which is based on a cross-sectional cliniconeuropathologic study),48–50 the olfactory bulb, as well as the medulla oblongata and the pons, exhibit Lewy body pathology prior to the occurrence of Lewy body pathology in mesencephalic neurons in the substantia nigra. Braak staging is not undisputed,51 which is in part due to the fact that Lewy body pathology can also occur in the absence of any neurologic signs. Therefore, Lewy body pathology in the lower brainstem might not unavoidably constitute a preclinical stage of PD. Nevertheless, Braak staging is likely to represent an approximate model for the development of sporadic PD in a large number of cases. The Braak hypothesis is compatible with retrospective clinical and neuropathologic studies12,42 that show that iRBD can represent a premotor stage of an underlying α-synucleinopathy.
Patients with iRBD also frequently show hyposmia,10,52 a fact that is also compatible with the concept of iRBD as a premotor manifestation of PD. In accordance with this clinical symptom, we found a bilateral increase in global FA and a decrease in RD in the olfactory region of patients with iRBD, suggestive of disturbed microstructure and demyelination in this area.22,23,40 Previous DTI studies in patients with PD have not detected abnormalities in the olfactory region. This might be due to methodologic aspects: brain regions adjacent to cerebrospinal fluid (like the olfactory region) are prone to artifacts and registration errors, especially when using linear registration tools. We tried to minimize those artifacts by using the novel technique of TBSS and a nonlinear registration31 (for olfactory bulb, see discussion of methodologic aspects).
Changes in AD were seen also in the right substantia nigra. This finding supports the concept of iRBD as a preclinical stage of PD and is in line with 2 recently published studies reporting comparable FA changes in regions of interest in the substantia nigra of patients with PD.15,18 The fact that we observed significant changes in the substantia nigra on only 1 side might reflect the typical asymmetric onset of PD and the potential transition state from iRBD to PD in some of our patients.
Another brain region exhibiting structural differences (FA changes and changes in RD) between iRBD patients and control subjects in our study is the fornix. FA changes in the fornix have already been described for patients with PD and are thought to be involved in the generation of excessive daytime sleepiness.53 This may be of relevance because excessive daytime sleepiness has also been described in the preclinical phase of PD.54 Furthermore, the results of experimental studies have suggested that the perifornical region is involved in the regulation of REM sleep.55,56 Impaired color discrimination has been described clinically in drug-naïive patients with early-stage PD57 as well as in patients with iRBD.58 Consequently, impaired color discrimination is thought to be an early clinical feature of the disorders that deteriorates over time59 as the neurodegenerative process progresses. Impaired color discrimination in patients with iRBD and PD has been explained by degeneration of retinal neurons, yet there is still a lack of clear evidence concerning the structural neuropathologic correlate of this clinical sign. Our finding of FA changes and changes in RD in the right visual stream (V2 area) raise the question of whether the anatomic correlate for impaired color discrimination might be additionally located in the visual pathway.
We found microstructural changes in the left anterior thalamic radiation and in the left temporal lobe, which may account for neuropsychological deficits in patients with iRBD. Indeed, disruption of the left anterior thalamic radiation has been shown to result in memory dysfunction60; in addition, the left temporal lobe is involved in memory and verbal processing. The fact that some of our findings were seen only unilaterally might reflect the asymmetry of the neurodegenerative process in PD. However, the clinical impact of these findings remains speculative and needs further validation.
Discussion of Methodologic Aspects
Our study was performed without any a-priori hypothesis (i.e., predefined regions of interest). We used the method of nonlinear registration (TBSS) to control for method-inherent shortcomings of DTI, such as registration and spatial smoothing errors. For technical reasons, we did not find changes in small brain regions like the olfactory bulb. Due to its size and location, the olfactory bulb is prone to susceptibility artifacts, and the large degree of interindividual anatomy in the olfactory bulb will lead to coregistration problems. Using TBSS, the olfactory bulb will not be displayed because only brain tissue with low intraindividual anatomic variability is included.
Two different approaches were used for statistical-group comparisons. Both techniques, FSL and FreeSurfer, identified the same pattern of microstructural tissue alteration, supporting the goal of the FSL and the FreeSurfer teams to make interoperation of FSL and FreeSurfer as easy as possible.
DTI is a technique that can quantify alterations in tissue microstructure regardless of the underlying pathologic mechanisms. FA) is the most commonly used parameter in DTI studies. FA is an unspecific measure of white-matter integrity. Recently, studies have focused on the calculation of RD and AD. Changes in these values might be more specific: previous work in animal models described a correlation between axonal loss and a decrease in AD as well as a correlation between an increase in RD and demyelination.22,23,61,62,63 Recently, using combined radiologic and neuropathologic assessment of unfixed brain tissue that was collected post-mortem, these hypotheses were confirmed in humans with multiple sclerosis.63 Nevertheless, detailed histopathologic validation in healthy humans is still missing.22,23,63
DTI is still a nonspecific measure that does not provide information about the underlying causes for the reported microstructural pathology. Nevertheless, our study hints at new potential neuropathologic correlates of iRBD that need to be further validated in postmortem studies. Likewise, future research is necessary to determine the sensitivity and specificity of this technique as a potential early marker for a neurodegenerative process in iRBD as a predictor for α-synucleinopathies, such as PD. In addition, long-term studies are needed to address the question of whether the identified pathologic signals in FA, AD and RD, change over time and whether these signals might be useful to monitor disease progression.
CONCLUSION
In conclusion, our DTI study describes in vivo specific microanatomic alterations in the brain of patients with the so-called idiopathic form of RBD. Our findings are compatible with the current hypotheses about the location of RBD pathology and its association with PD. The observed changes in the pons are perhaps representative of a structural correlate for the disruptive, dream-enacting behavior during REM sleep. Changes seen in the substantia nigra are in accordance with the assumption that iRBD may represent a preclinical (premotor) stage of PD. Our study suggests that RBD-related neuropathology can be detected in vivo with a widely available MRI technology.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Stiasny-Kolster has been on the advisory boards of Boehringer Ingelheim, Orion, Mundipharmi, and Schwarz Pharma and has participated in speaking engagements for Schwarz Pharma, UCB, Boehringer Ingelheim, and Hoffmann-LaRoche. Dr. Rosenow has received research support from UCB and has been on the advisory boards of UCB, Pfizer, and GlaxoSmithKline. Dr. Mayer has participated in speaking engagaments for UCB, Sanofi-Aventis, GlaxoSmithKline, and Cephalon. Dr. Oertel has received research support from Novartis; has participated in speaking engagements for Desitin, Boehrunger Engelheim, GlaxoSmithKline, TEVA, Orion Pharma, Novartis, Schwarz Pharma, and Neuroscience; and has consulted for Novartis, Orion Pharma, Neuroscience, Solvay Pharmaceuticals International, Desitin, Boehringer Ingelheim, GlaxoSmithKline, Teva, Protosys, and Sysnosia.
ACKNOWLEDGMENTS
This work was supported by the Willy Robert Pitzer Foundation (to SK and WHO), the Föorderverein Neurologie Marburg (to SK), and the BMBF (to WHO, funding code: 01GI0401) and partly supported by a UKGM Research Grant (to SK and WHO). We thank all patients and healthy control subjects who participated in this study.
Annotations:
- RBD
REM sleep behaviour disorder
- MMSE
Mini Mental State Examination
- NA
not applicable
- TDI
threshold-discrimination-identification in Sniffin' Stick Test (a TDI sum score ≥ 30 is defined as normosmia) (Kobal et al., 2000)
- FP-CIT
123I-N-Ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)-nortropane
- SPECT
single photon emission computed tomography
Footnotes
A commentary on this article appears in this issue on page 731.
Supplementary Material
Regions with significant changes in fractional anisotropy, displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, internal capsule; panel B, fornix; panel C, olfactory cortex; panel D, left temporal cortex; panel E, right visual cortex. Increases in fractional anisotropy are shown in red-yellow; decreases in blue-green.
Regions with significant changes in axial diffusivity, a potential measure of axonal damage. Significant changes are displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, pons; panel B, right substantia nigra. Decreases in axial diffusivity are shown in blue-green.
Regions with significant changes in radial diffusivity, a potential measure of myelin damage. Significant changes are displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, fornix; panel B, left temporal cortex; panel C, right visual cortex. Increases in radial diffusivity are shown in red-yellow.
Sagittal view of the average b0 image and the overlaid white-matter skeleton (in green) showing the changes in radial diffusivity (red-yellow) and axial diffusivity (blue-light blue) using the randomize tools from Freesurfer package (A) and the FSL stream (B), as described in the methods section.
REFERENCES
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder. A treatable parasomnia affecting older adults. JAMA. 1987;257:1786–9. [PubMed] [Google Scholar]
- 3.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 5.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa S, Takeuchi N, Uchimura N, et al. Study of image findings in rapid eye movement sleep behavioural disorder. Psychiatry Clin Neurosci. 2002;56:291–2. doi: 10.1046/j.1440-1819.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazza S, Soucy JP, Gravel P, et al. Assessing whole brain perfusion changes in patients with REM sleep behavior disorder. Neurology. 2006;67:1618–22. doi: 10.1212/01.wnl.0000242879.39415.49. [DOI] [PubMed] [Google Scholar]
- 8.Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123:1155–60. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 9.Eisensehr I, Linke R, Tatsch K, et al. Increased muscle activity during rapid eye movement sleep correlates with decrease of striatal presynaptic dopamine transporters. IPT and IBZM SPECT imaging in subclinical and clinically manifest idiopathic REM sleep behavior disorder, Parkinson's disease, and controls. Sleep. 2003;26:507–12. doi: 10.1093/sleep/26.5.507. [DOI] [PubMed] [Google Scholar]
- 10.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain. 2005;128:126–37. doi: 10.1093/brain/awh322. [DOI] [PubMed] [Google Scholar]
- 11.Unger MM, Moller JC, Stiasny-Kolster K, et al. Assessment of idiopathic rapid-eye-movement sleep behavior disorder by transcranial sonography, olfactory function test, and FP-CIT-SPECT. Mov Disord. 2008;23:596–9. doi: 10.1002/mds.21908. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A, Santamaria J, Pujol J, Moreno A, Deus J, Tolosa E. Brainstem proton magnetic resonance spectroscopy in idiopathic REM sleep behavior disorder. Sleep. 2002;25:867–70. [PubMed] [Google Scholar]
- 13.Miyamoto M, Miyamoto T, Kubo J, Yokota N, Hirata K, Sato T. Brainstem function in rapid eye movement sleep behavior disorder: the evaluation of brainstem function by proton MR spectroscopy (1H-MRS) Psychiatry Clin Neurosci. 2000;54:350–1. doi: 10.1046/j.1440-1819.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Stockner H, Iranzo A, Seppi K, et al. Midbrain hyperechogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2009;24:1906–9. doi: 10.1002/mds.22483. [DOI] [PubMed] [Google Scholar]
- 15.Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. Am J Neuroradiol. 2009;30:1222–6. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knake S, Salat DH, Halgren E, Halko MA, Greve DN, Grant PE. Changes in white matter microstructure in patients with TLE and hippocampal sclerosis. Epileptic Disord. 2009;11:244–50. doi: 10.1684/epd.2009.0272. [DOI] [PubMed] [Google Scholar]
- 17.Rosas HD, Tuch DS, Hevelone ND, et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–25. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 18.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–84. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The International Classification of Sleep Disorders, revised: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 20.Fjell AM, Westlye LT, Greve DN, et al. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage. 2008;42:1654–68. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budde MD, Kim JH, Liang HF, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- 22.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 24.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current–induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 25.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 27.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–54. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 28.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 29.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 30.Basser PJ. New histological and physiological stains derived from diffusion-tensor MR images. Ann N Y Acad Sci. 1997;820:123–38. doi: 10.1111/j.1749-6632.1997.tb46192.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Andersson JLR, JMaSS Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2 from wwwfmriboxacuk/analysis/techrep. 2007 [Google Scholar]
- 34.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18:712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 35.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glenn OA, Henry RG, Berman JI, et al. DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. J Magn Reson Imaging. 2003;18:641–8. doi: 10.1002/jmri.10420. [DOI] [PubMed] [Google Scholar]
- 38.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 39.Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–41. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–40. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 41.Arnulf I, Bonnet AM, Damier P, et al. Hallucinations, REM sleep, and Parkinson's disease: a medical hypothesis. Neurology. 2000;55:281–8. doi: 10.1212/wnl.55.2.281. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama M, Isse K, Tanaka K, et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology. 1995;45:709–12. doi: 10.1212/wnl.45.4.709. [DOI] [PubMed] [Google Scholar]
- 43.Culebras A, Moore JT. Magnetic resonance findings in REM sleep behavior disorder. Neurology. 1989;39:1519–23. doi: 10.1212/wnl.39.11.1519. [DOI] [PubMed] [Google Scholar]
- 44.Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S, Waki R. A discrete pontine ischemic lesion could cause REM sleep behavior disorder. Neurology. 2000;55:894–5. doi: 10.1212/wnl.55.6.894. [DOI] [PubMed] [Google Scholar]
- 45.Tippmann-Peikert M, Boeve BF, Keegan BM. REM sleep behavior disorder initiated by acute brainstem multiple sclerosis. Neurology. 2006;66:1277–9. doi: 10.1212/01.wnl.0000208518.72660.ff. [DOI] [PubMed] [Google Scholar]
- 46.Xi Z, Luning W. REM sleep behavior disorder in a patient with pontine stroke. Sleep Med. 2009;10:143–6. doi: 10.1016/j.sleep.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Lai YY, Hsieh KC, Nguyen D, Peever J, Siegel JM. Neurotoxic lesions at the ventral mesopontine junction change sleep time and muscle activity during sleep: an animal model of motor disorders in sleep. Neuroscience. 2008;154:431–43. doi: 10.1016/j.neuroscience.2008.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–34. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 51.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson's disease. Ann Neurol. 2008;64:485–91. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fantini ML, Postuma RB, Montplaisir J, Ferini-Strambi L. Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder. Brain Res Bull. 2006;70:386–90. doi: 10.1016/j.brainresbull.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Matsui H, Nishinaka K, Oda M, et al. Disruptions of the fornix fiber in Parkinsonian patients with excessive daytime sleepiness. Parkinsonism Relat Disord. 2006;12:319–22. doi: 10.1016/j.parkreldis.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–6. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 55.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–19. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 56.Lu JW, Fenik VB, Branconi JL, Mann GL, Rukhadze I, Kubin L. Disinhibition of perifornical hypothalamic neurones activates noradrenergic neurones and blocks pontine carbachol-induced REM sleep-like episodes in rats. J Physiol. 2007;582:553–67. doi: 10.1113/jphysiol.2007.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buttner T, Kuhn W, Muller T, Patzold T, Heidbrink K, Przuntek H. Distorted color discrimination in ‘de novo’ parkinsonian patients. Neurology. 1995;45:386–7. doi: 10.1212/wnl.45.2.386. [DOI] [PubMed] [Google Scholar]
- 58.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–51. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 59.Diederich NJ, Raman R, Leurgans S, Goetz CG. Progressive worsening of spatial and chromatic processing deficits in Parkinson disease. Arch Neurol. 2002;59:1249–52. doi: 10.1001/archneur.59.8.1249. [DOI] [PubMed] [Google Scholar]
- 60.Stenset V, Grambaite R, Reinvang I, et al. Diaschisis after thalamic stroke: a comparison of metabolic and structural changes in a patient with amnesic syndrome. Acta Neurol Scand Suppl. 2007;187:68–71. doi: 10.1111/j.1600-0404.2007.00851.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Loy DN, Liang HF, Trinkaus K, Schmidt RE, Song SK. Noninvasive diffusion tensor imaging of evolving white matter pathology in a mouse model of acute spinal cord injury. Magn Reson Med. 2007;58:253–60. doi: 10.1002/mrm.21316. [DOI] [PubMed] [Google Scholar]
- 62.Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- 63.Schmierer K, Wheeler-Kingshott CA, Boulby PA, et al. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage. 2007;35:467–77. doi: 10.1016/j.neuroimage.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257:205–11. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions with significant changes in fractional anisotropy, displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, internal capsule; panel B, fornix; panel C, olfactory cortex; panel D, left temporal cortex; panel E, right visual cortex. Increases in fractional anisotropy are shown in red-yellow; decreases in blue-green.
Regions with significant changes in axial diffusivity, a potential measure of axonal damage. Significant changes are displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, pons; panel B, right substantia nigra. Decreases in axial diffusivity are shown in blue-green.
Regions with significant changes in radial diffusivity, a potential measure of myelin damage. Significant changes are displayed as the negative decadic logarithm of the P value (P = 10-x). Panel A, fornix; panel B, left temporal cortex; panel C, right visual cortex. Increases in radial diffusivity are shown in red-yellow.
Sagittal view of the average b0 image and the overlaid white-matter skeleton (in green) showing the changes in radial diffusivity (red-yellow) and axial diffusivity (blue-light blue) using the randomize tools from Freesurfer package (A) and the FSL stream (B), as described in the methods section.