Abstract
Study Objectives:
To use time-frequency analysis to characterize developmental changes in the human sleep electroencephalogram (EEG) across early adolescence.
Design:
Sleep EEG was recorded when children were 9/10 years old and 1 to 3 years later after sleeping at home on a fixed schedule for at least one week.
Setting:
A 4-bed sleep laboratory.
Participants:
Fourteen (5 girls) healthy children ages 9/10 (mean = 10.13, SD = ± 0.51) years at initial and 11 to 13 (mean = 12.28, SD = ± 0.62) years at follow-up.
Interventions:
N/A.
Measurements and Results:
All-night polysomnography was performed at each assessment and sleep stages were scored with Rechtschaffen and Kales criteria. Slow wave sleep minutes decreased from the initial to the follow-up session by 29%, while minutes of stage 2 increased by 17%. NREM and REM sleep EEG spectra from two central and two occipital leads were examined for developmental changes. All-night analyses showed a significant decrease of EEG power from the initial to follow-up session across a range of frequencies during NREM and REM sleep. This decline occurred across leads and states in the delta/theta bands (3.8 – 7 Hz). Time-frequency analyses indicated that this effect was consistent across the night. The decline in power with age was most pronounced in the left central and right occipital leads. The frequency of greatest power in the sigma band (11 – 16 Hz) was significantly higher at follow-up.
Conclusions:
This longitudinal analysis highlights asymmetrical frequency-specific declines in sleep EEG spectral power with early adolescent maturation, which may reflect early signs of the cortical synaptic pruning in the healthy adolescent.
Citation:
Tarokh L; Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. SLEEP 2010;33(6):801-809.
Keywords: Sleep EEG, development, adolescence, time-frequency analysis
A CASCADE OF BIOLOGICAL CHANGES ACCOMPANIES THE TRANSITION FROM CHILDHOOD TO ADOLESCENCE. THIS TRANSFORMATION IS AMONG THE most significant neurodevelopmental changes occurring after the perinatal period.1 The transition is marked by major brain reorganization that typically begins between the ages of 9 and 10 years and continues until the end of adolescence, about ages 18 to 20 years. These changes are reflected in a number of neural measures (e.g., waking cerebral blood glucose metabolism as measured by positron emission tomography2). Furthermore, cortical grey matter as measured by magnetic resonance imaging (MRI) in humans3–5 declines during this developmental period. The decreases in cortical grey matter and cerebral blood glucose metabolism are thought to indicate a decline in the number of active synapses during adolescence. Indeed, Huttenlocher and Dabholkar6 showed that dramatic synaptic pruning occurs in the healthy human adolescent cortex. These findings have been corroborated in the macaque monkey.7
EEG amplitude derives from synchronous synaptic activity across the cortex; a reduction in the number of synapses results in dampened EEG amplitude. Therefore, synaptic pruning across adolescence is reflected in the amplitude of the EEG signal. Several cross-sectional studies have shown a negative correlation between waking EEG amplitude over all frequency bands and age across adolescence.8–10 A recent cross-sectional study by Whitford and colleagues11 used resting waking EEG and MRI measures in the same participants to quantify neuroanatomical and neurophysiological differences in healthy participants across the span of ages 10 to 30 years. These authors report a negative correlation between log of age and gray matter volume, as well as with overall waking EEG power. The greatest change in these measures occurred across the second decade and reached an asymptote in the mid-twenties. The authors attribute the reduction in EEG power to the loss of grey matter and elimination of active synapses.
If the elimination of active synapses results in a reduction of EEG power during adolescence, the phenomenon should be state nonspecific. Several cross-sectional studies show that sleep EEG power also declines across adolescence. One cross-sectional study comparing the sleep EEG of prepubertal (ages 9.6 to 12.9) and mature adolescents (ages 11.8 to 15.9 years) found that more mature participants had less EEG power across a wide range of frequencies during NREM and REM sleep.12 This effect was statistically significant at frequencies below 7 Hz for both REM and NREM sleep, in the sigma (11.8-12.6 Hz) and beta (16.2 – 16.8 Hz) bands for NREM, and at all frequencies below 15 Hz except for the alpha range (8.6 – 9.4 Hz) for REM sleep. The differences in EEG spectral power were accompanied by increased time spent in stage 2 sleep and less time spent in stage 4 sleep in the more mature adolescents, which is in line with previous cross-sectional13 and longitudinal14 studies. A second cross-sectional study performed by Gaudreau and colleagues15 examined EEG power in discrete frequency bands in children (6 – 10 years), adolescents (14 – 16 years), young adults (19 – 29) years, and middle age adults (36 – 60 years) across the first five hours of the night. These authors reported an inverse association between age group and NREM sleep EEG power in all the frequency bands examined: delta (0.75 – 4.5 Hz), theta (4.0 – 7.75 Hz), alpha (8.0 – 12.0 Hz), sigma (12.25 – 15 Hz), and beta (15.25 – 31.0 Hz). Compared to the other groups, the power difference in all frequency bands between the young adults and middle-aged adults was least pronounced.
The above studies were cross-sectional, and large individual variability in EEG power limits interpretation of the results. A longitudinal study performed by Feinberg and colleagues16 examined the sleep EEG of thirty-one 9 year olds and thirty-eight 12 year olds at 6-month intervals for two years. These authors reported that delta power (0.3 to 3 Hz) did not differ significantly between the ages of 9 and 11 years, yet they showed a 25% decline in delta power between the ages of 12 and 14. Furthermore, the decline in delta power began earlier in girls than boys by an average of one year, but once initiated, the decay rate of delta power was similar in boys and girls.16 Despite this sex difference, the authors attributed the decline in power to age, and not pubertal stage, height, weight, body mass index (BMI), or sleep schedules. In this study, only sleep EEG power in the delta band (0.3 to 3 Hz) was examined, thus leaving open the question whether similar changes in power occurred in other frequency bands.
Feinberg17 has shown that the ontogenetic curves for cortical metabolic rate, synaptic density, and power in the delta band overlap across the first three decades of life in humans, and he has hypothesized that these processes are biologically related and mark a major brain reorganization taking place during human adolescence. The aim of the present study is to add to the knowledge base on adolescent brain development by using spectral analysis to characterize longitudinal changes that occur during early adolescent development over (1) the course of the night (2) from several brain regions and (3) EEG frequencies up to 16 Hz. We hypothesize that, as in other studies, we will see a decline in SWS and an increase in stage 2 sleep with maturation. Additionally, we expect that these changes will be accompanied by a generalized developmental reduction in the amplitude of the sleep EEG signal across frequencies over the entire night.
METHODS
Participants
Participants were recruited using flyers, mailings to previous participants, and radio and newspaper advertisements. Children with a current or chronic illness, evidence of learning disability or sleep disorder were excluded from the study. Children with a personal or family history of posttraumatic stress disorder (PTSD), major depressive/dysthymic disorder, schizophrenia, attention deficit hyperactivity disorder, alcohol use disorder, nicotine dependence, marijuana use disorder, other substance use disorder or a parental history of alcohol abuse or dependence, as assessed by DSM-IV criteria applied to structured parental interviews, were also excluded from the study.18 Additional exclusion criteria included children with a pattern of insufficient sleep or excessive daytime sleepiness as indicated by sleeping less than 9 hours nightly and/or taking two or more naps per week.
Tanner19 staging, a measure of pubertal status, was performed by a physician during a brief physical examination at each assessment. Tanner stage is based on the attainment of external primary and secondary sex characteristics (e.g., genital development in boys, breast development in girls, and pubic hair growth in boys and girls). Tanner stage 1 is characterized by child-like appearance; Tanner stage 5, indicates attainment of adult like appearance of secondary sex characteristics. Pubic hair staging is considered more reliable than the other measures19 and was used in this study.
Data presented here are from 14 participants who were 9 or 10 years old at the time of the initial recording and 11 to 13 years old at the time of the follow-up recording. The ages, Tanner stages, sex and body mass index (BMI) of all participants are reported in Table 1. BMI is thought to be inversely correlated with total sleep time in children and adolescents,20,21 therefore we examined whether BMI was significantly associated with total sleep time, minutes of slow wave, stage 2 or REM sleep. No significant correlations were found at either the initial or the follow-up session.
Table 1.
Participant characteristics
| Subject | Age 1 | Age 2 | T1 | T2 | BMI 1 | BMI 2 | S1 | S2 | Sex |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 10.3 | 12.8 | 4 | 5 | 19.5 | 21.9 | 12.4 | 13 | F |
| S2 | 10.6 | 12.9 | 3 | 5 | 20.6 | 25.6 | 12.6 | 12.8 | F |
| S3 | 10.4 | 13 | 1 | 3 | 17.8 | 21.1 | 12.6 | 13 | M |
| S4 | 10.6 | 13.2 | 1 | * | 17.8 | 19.1 | 12.8 | 13 | F |
| S5 | 9.6 | 12 | 1 | 2 | 16.1 | 16.6 | 12.8 | 13 | M |
| S6 | 9.6 | 11.9 | 2 | 5 | 18.8 | 23.6 | 12.6 | 12.6 | F |
| S7 | 10.2 | 12.3 | 1 | 5 | 23.0 | 22.1 | 12.6 | 12.8 | M |
| S8 | 10.7 | 13.1 | 2 | 5 | 30.8 | 38.0 | 12.8 | 13.2 | M |
| S9 | 9.4 | 11.8 | 1 | 2 | 17.1 | 17.2 | 12 | 12.2 | M |
| S10 | 10.5 | 12.4 | 2 | 3 | 23.8 | 26.5 | 13 | 13.2 | F |
| S11 | 9.9 | 11.8 | 1 | 2 | 17.6 | 17.2 | 12 | 12.2 | M |
| S12 | 9.8 | 11.8 | 1 | * | 16.8 | 17.2 | 12.4 | 12.4 | M |
| S13 | 10.9 | 13.2 | 1 | 2 | 16.8 | 19.0 | 13.4 | 13.8 | M |
| S14 | 9.4 | 11.2 | 1 | 2 | 18.3 | 19.2 | 12.2 | 12.4 | M |
| Mean | 10.13 | 12.38 | NA | NA | 19.6 | 21.7 | 12.6 | 12.8 | NA |
| Std | 0.51 | 0.65 | NA | NA | 4.0 | 5.6 | 0.4 | 0.4 | NA |
Individual and mean data for all participants.
Age 1, Age in years at the Initial recording session; Age 2, Age in years at the Follow-Up recording session; T1, Tanner stage at the Initial recording session; T2, Tanner stage at the Follow-Up recording session; BMI1, Body Mass Index at the Initial recording session; BMI2, Body Mass Index at the Follow-up recording session; S1, Frequency of the peak in the sigma band at the Initial session measured in Hz; S2, Frequency of the peak in the sigma band at the Follow-Up session measured in Hz; F, Female; M, Male;
Missing Data
The average age of the participants at the time of the initial recording was 10.1 (SD = ± 0.5) years and 12.4 (SD = ± 0.6) years at follow-up. Eleven participants were white, one was black, one was Hispanic, and one was Asian. Nine boys and five girls participated in this study. All participants, except one, were right handed, as assessed by the Edinburgh Handedness Inventory.22 The Lifespan Institutional Review Board for the Protection of Human Subjects approved all procedures, and informed consent was obtained from the participants and their parents, and participants received monetary compensation.
Procedures
Before the sleep lab assessments, participants slept on a schedule of at least 10-hours time in bed for at least one week, using the participants' school day rise time to anchor the scheduled sleep. Scheduled time in bed was the same at both assessments (see Table 2), although the precise bed and rise times may have varied. Compliance to the sleep schedule was confirmed using sleep diaries, continuous wrist actigraphy, and daily phone calls to the lab's time-stamped answering machine at rise times and bedtimes. Participants slept in the lab on two separate occasions during the school year, initially when they were 9 or 10 years old (referred to as the Initial recording throughout this paper) and again 11 to 31 months (mean = 26 months) later (referred to as the Follow-up recording throughout this paper). On study days, participants were well slept, healthy, and taking no medications. Participants slept in individual darkened bedrooms while polysomnography (PSG) was recorded for two nights on each occasion. The first night included a screen for sleep-related breathing abnormalities and periodic limb movements using oral/nasal thermocouples and leg electromyogram (EMG), respectively. No sleep disorders were detected. The data from the second night are reported here for all participants with the exception of two for whom the first night was used due to technical difficulties on the second night.
Table 2.
Sleep stage variables
| Sleep Variable | Initial |
Follow-Up |
P-Values |
||||
|---|---|---|---|---|---|---|---|
| Girls | Boys | Girls | Boys | Sex | Time | Sex X Time | |
| Stage 1 | 38 (14) | 31 (12) | 30 (8) | 48 (13) | 0.28 | 0.41 | 0.02 |
| Stage 2 | 186 (44) | 217 (45) | 259 (46) | 244 (25) | 0.60 | 0.004 | 0.15 |
| SWS | 230 (53) | 216 (36) | 168 (69) | 151 (44) | 0.42 | 0.003 | 0.94 |
| REM | 105 (28) | 105 (25) | 109 (22) | 116 (24) | 0.87 | 0.85 | 0.28 |
| WASO | 21 (23) | 12 (10) | 11 (14) | 14 (15) | 0.63 | 0.56 | 0.32 |
| Sleep Latency | 16 (8) | 13 (9) | 18 (2) | 17 (22) | 0.71 | 0.61 | 0.87 |
| REM Latency | 117 (47) | 155 (34) | 109 (21) | 128 (63) | 0.13 | 0.33 | 0.61 |
| Total Sleep Time | 559 (22) | 569 (17) | 567 (13) | 559 (26) | 0.87 | 0.85 | 0.28 |
| Total Recording Time | 600 (8) | 601 (7) | 600 (1) | 600 (1) | 0.88 | 0.64 | 0.85 |
Means (standard deviations) in minutes and P-values of sleep stage variables.
SWS, slow wave sleep; WASO, wake after sleep onset; Sex, main effect of sex (male versus female); Time, main effect of recording session (Initial versus Follow-Up); Sex X Time, interaction of Sex and Time.
Polysomnography (PSG) Recording
Right and left electrooculgram (EOG), EMG (mentalis, submentalis), and electrocardiogram (ECG) along with two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) EEG electrodes placed according to the international 10-20 system23 were recorded. The recordings were performed on two separate systems. The initial recordings of 12 participants were performed using the Albert Grass Heritage System (Astromed, Grass, West Warwick, RI) with GAMMA software. The signals were collected and stored at a sampling frequency of 100 Hz, filtered with Grass Model 8 amplifiers (high-pass EEG filter, 0.3 Hz; low-pass EEG filter, 35 Hz; notch filter 60 Hz) and digitized online (12 bit AD converter; Butterworth filter, −12 dB/octave; low pass-filter, −6dB at 35 Hz, time constant 1.0). Two participants were recorded on the TWin system (Astromed, Grass, West Warwick, RI) using TWin AS40 bedside amplifiers, from which the signals were collected digitally with a sampling frequency of 400 Hz and filtered offline (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz; notch filter 60 Hz). All follow-up recordings were performed on the TWin system. Electrode impedance values were below 10 KΩ. A known signal was input into both systems simultaneously to assess whether signals from the two systems were comparable. Signals from the two systems were in good agreement from 0.2 to 16 Hz; however, small discrepancies emerged at higher frequencies. Therefore, spectral power above 16 Hz was not examined. Furthermore, the different sampling frequencies of the two systems affect the maximum frequency that can be analyzed in the signal. According to the Nyquest theorem, the highest frequency that can be analyzed is ½ the sampling rate; because we only examine frequencies under 16 Hz, we are well within this range.
Definition of the Sleep Cycles
A modified version of the criteria of Feinberg and Floyd24 was used to define sleep cycles. According to these criteria, a NREM sleep episode is required to last 15 minutes, begins with stage 2, and ends with the occurrence of a REM sleep epoch. Furthermore, the definition of Feinberg and Floyd requires that a REM episode, with the exception of the first, last a minimum of 5 minutes. We have modified these criteria such that a REM episode of any length signals the end of a NREM cycle. An accurate assessment of the sleep EEG spectra requires a minimum of three minutes, which resulted in the exclusion of one participant's first REM episode during the initial session. A further modification was required because children often “skip” their first REM episode. Because a skipped first REM episode affects calculation of the first and second NREM cycles, we applied the criteria of Jenni and Carskadon.12 According to these criteria, the first REM episode is considered skipped when a continuous episode of stage 1, 2, awake or movement time lasting at least 12 minutes is followed by stage 3 or 4 (slow wave sleep; SWS) sleep. When this criterion is applied, the first NREM cycle ends with the epoch preceding the interval of non-SWS, and the second NREM cycle begins with the first epoch of SWS following this interval, ending with the onset of REM sleep. Applying these criteria, 11 of the 14 participants skipped their first REM episode on the initial recording session, while only 3 skipped their first REM episode on the follow-up recording session. In order to examine whether this approach biased our analyses, we performed an ANOVA comparing the duration of NREM cycles at the initial and follow-up sessions for the first four NREM cycles. No significant main effects or interactions were found, indicating that the duration of NREM episodes did not change from the initial to the follow-up recording session or over the course of the night.
Power Spectral Analysis
Sleep data were visually scored in 30-second epochs according to the criteria of Rechtschaffen and Kales.25 Inter- and intra-rater reliability was at least 86%, and epochs with artifacts (e.g., eye blinks, movement artifact, eye movements or signal noise) were visually identified and rejected. Power spectra (0.6 to 16 Hz) were calculated on each 30-second artifact-free epoch at all EEG electrodes (C3/A2, C4/A1, O2/A1, and O1/A2) using a fast Fourier transform (Matlab, The MathWorks Inc., Natick, MA). A Hanning window was applied to the data before the transform, and averages of six 5-second epochs were calculated. The resulting frequency resolution was 0.2 Hz, and the lowest two frequency bins, 0.2 and 0.4 Hz, were discarded due to the sensitivity of these bins to noise.26 Spectra were calculated separately for NREM sleep (stages 2, 3, and 4) and REM sleep.
Time-frequency analyses were performed to examine how the sleep EEG signal evolves over the course of the night. In this context, time-frequency analysis refers to tracking the frequency content of the signal over time instead of averaging epochs across the entire night. To account for NREM and REM episodes of different durations across participants, each NREM episode was subdivided into 10 equal intervals, and each REM episode was subdivided into 3 equal intervals as per Jenni and Carskadon.12 In this way, all participants had an equal number of intervals over which to average and perform statistical testing. All participants, except one, had at least five NREM and four REM episodes, and therefore, only these cycles were further analyzed. In describing the data, we define the following frequency bands based on previous literature12: delta (0.6 to 4.6 Hz), theta (4.8 to 7 Hz), alpha (7.2 to 11.4 Hz), and sigma (11.6 to 16.0 Hz).
Statistics
Comparisons of sleep stage variables at the two assessments were made using a two (sex: male versus female) by two (recording session: initial versus follow-up) repeated measures ANOVA with alpha set to 0.05.
A bootstrap test was used to evaluate EEG power changes in the all-night spectra in addition to the time-frequency spectra across the two sessions. The bootstrap method makes no assumptions regarding the distribution of the data set; its application to sleep EEG data has been described in Tarokh and Carskadon.27 Briefly, the principle underlying the bootstrap is to generate a random sample, called the bootstrap distribution, by randomly sampling (with replacement) from the original pool of data. In this study, the data pool comprised EEG power at the initial and follow-up session of all participants. The data pool was randomly divided into two sets, S1 and S2, each made up of the data from 14 subjects. Each set, S1 and S2, was averaged resulting in two randomly-generated averaged spectra. The data were randomly assigned and averaged in this manner 1,500 times resulting in two bootstrap distributions at each frequency and electrode. We demonstrate this concept at electrode C3/A2 and frequency 4.6 Hz during NREM sleep in a supplementary figure that can be found online only at www.journalsleep.org. Subtracting S1 from S2 created a difference distribution consisting of 1500 values at each frequency and electrode. The average difference between the initial and final session in the observed data was compared to the distribution of differences. If the difference between the observed data was greater than the top 5% of the difference distribution, the difference was deemed statistically significant. Separate bootstrap tests were performed at each frequency and electrode for the all-night spectra; individual bootstrap tests were performed at each frequency, electrode, and time point for the time-frequency data. The bootstrap assumes the independence of the two samples, and as the two sessions are not independent the bootstrap provides a conservative estimate of significance.
The large number of statistical tests raises the issue of multiple comparisons. Such traditional approaches to correct for multiple comparisons as the Bonferroni correction are too conservative for EEG data, because they are based on the assumption that each sample is independent. Further, Maris and Oostenveld28 have shown that such non-parametric tests as the bootstrap applied to EEG and MEG data control the false alarm rate. Nonetheless, we address the issue of multiple comparisons in two ways: first, we report statistically significant effects only when they are present at the same frequency for multiple electrodes or at adjacent frequency bins; second, we report P-values so that the magnitude of the statistical significance is apparent.
A number of factors may contribute to the changes in power uncovered by the bootstrap analysis. These factors may include the age of the participants at the time of the initial recordings, Tanner stage, sex, change in age or Tanner stage between assessments or a combination of these variables. To test for the effect of these factors on power we performed a backward stepwise regression using SPSS 17.0 with change in Tanner stage and age between assessments, Tanner stage at the initial session, age at the initial session and sex as predictors. Rather than performing a regression at every frequency bin, to minimize the number of statistical test and thus retain power, we used a composite measure of the change in mean power across frequencies (0.6 to 16 Hz) from the initial to the follow-up session at channel C3/A2 during NREM sleep.
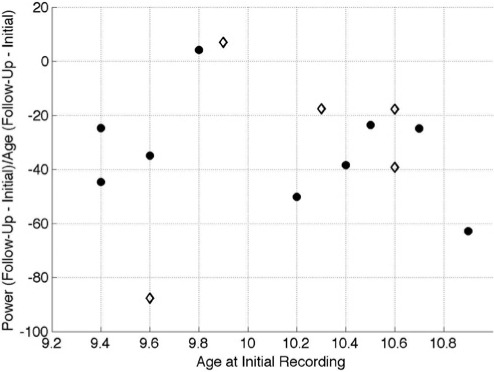
In order to give the reader an appreciation of the individual variability in the data, for each participant we visually examined the rate of change in power (PΔ) against the age of the participant (in years) at the time of the initial recording session. PΔis defined as:
In equation 1, PI and PF are average all-night NREM sleep power from 0.6 to 16 Hz (measured in μV2) at the initial and follow-up session, respectively; and AI and AF are age (measured in decimal years) at the initial and follow-up session, respectively. Figure 1 illustrates PΔ for channel C3/A2 against age at the initial recording session.
Figure 1.
Rate of power decline as a function of age at the initial recording session. Each point on this plot corresponds to an individual's data. On the Y-axis is the rate of change in power at electrode C3/A2 during NREM sleep. Change in power is defined as the average power (measured in μV2) during NREM sleep at all frequencies between 0.6 to 16 Hz at the follow-up recording minus the power at the initial recording divided by age at the follow-up recording minus age at the initial recording (measured in years). On the X-axis is the age of the participants at the initial recording session (measured in years). Boys are plotted as black circles and girls as diamonds.
RESULTS
Sleep stage variables
Sleep stage data are summarized in Table 2. Recording time was identical across nights, and conditions according to protocol; total sleep time remained consistent as well. On the other hand, statistically significant developmental changes of sleep stages included a decline of SWS and an increase in stage 2 from initial to follow-up. The average changes (computed within individuals) were a 29% decline in SWS and a 17 % increase in stage 2. According to Cohen's29 criteria, these were medium effect sizes (0.55 for SWS; 0.47 for stage 2 sleep). Stage 1 sleep manifested a significant interaction of sex and recording session: at the follow-up recording session, boys showed more stage 1 sleep, while girls showed less as compared to the initial recording session. Although statically significant, this was a small effect (effect size = 0.22).
Absolute All-night spectra
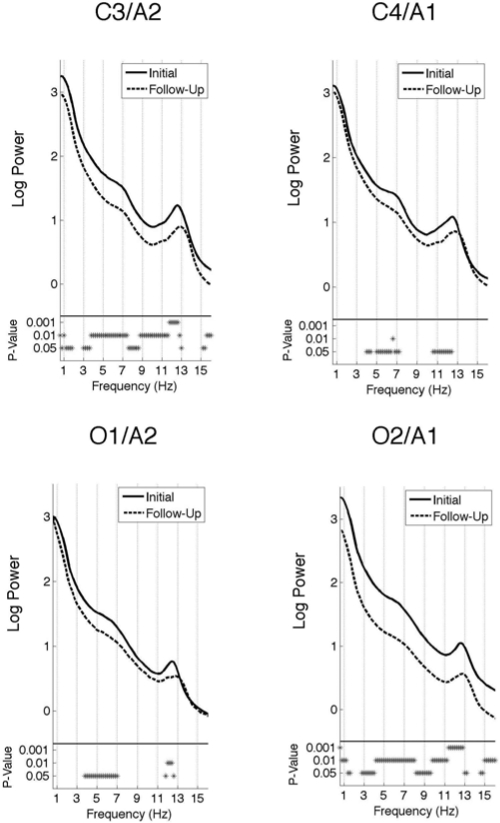
Log values of the absolute power for the all-night spectra at each electrode position (C3/A2, C4/A1, O1/A2, and O2/A1) in NREM and REM sleep are plotted in Figures 2 and 3, respectively. NREM sleep was marked by a slight peak in the theta range (5 – 8 Hz) for all derivations. Power in this band was significantly greater during the initial session compared to the follow-up session at all electrode positions. A more prominent spectral power peak was seen in the sigma range (11 – 16 Hz) across all electrodes during NREM sleep. As with the theta peak, power was significantly greater for all electrode positions at the initial than the follow-up session but only for the lower range of sigma frequency (11 – 12.8 Hz). In addition, power was significantly greater at the initial session than at follow-up at all frequencies in the left central and right occipital electrode, with the exception of a narrow band in the delta (1.6 – 2.8 Hz) and spindle (13.2 – 14.6 Hz) ranges.
Figure 2.
All-night spectra: Subject average NREM sleep spectra of the log power at two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) electrodes. The spectra at the initial recording session is plotted as a solid line and the follow-up session as a dashed line. The frequency resolution of these plots is 0.2 Hz. The P-values are indicated by asterisks below the spectra.
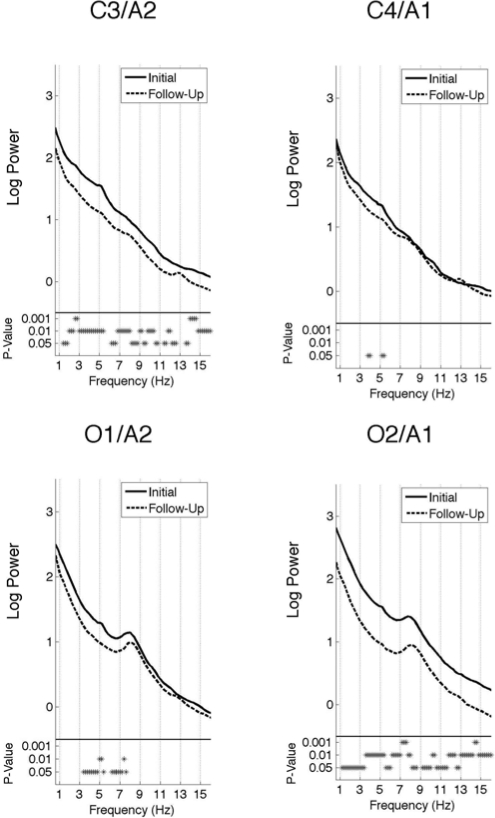
Figure 3.
All-night spectra: Subject average REM sleep spectra of the log power at two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) electrodes. The spectra at the initial recording session is plotted as a solid line and the follow-up session as a dashed line. The frequency resolution of these plots is 0.2 Hz. The P-values are indicated by asterisks below the spectra.
REM sleep (Figure 3) showed a peak in the theta/alpha band (6.5 – 10 Hz) for the occipital, but not the central electrodes. As in NREM sleep, the greatest power differences between the two recording sessions were found in the left central and right occipital electrodes. Spectral EEG power at these channels was significantly greater at the initial compared to the follow-up sessions for every frequency except in the lowest delta (0.8 – 1.2 Hz) range and a narrow portion of the spindle range (12.8 – 13.2 Hz). For O1/A2, power was greater at the initial session for most frequencies between 3.4 and 7.6 Hz. The right central lead (C4/A1) showed the least number of significant differences between the two recording sessions, with only a few bins (3.8 – 4 Hz and 5.2 – 5.4 Hz) exhibiting significantly greater power at the initial than the follow-up session.
In summary, during NREM and REM sleep, power was significantly greater at all electrode positions for many frequencies at the initial compared to the follow-up session. This effect was most pronounced at the left central (C3/A2) and right occipital (O2/A1) electrodes.
Regression Analysis
None of the predictors in the backwards-stepwise regression reached statistical significance at alpha set to 0.05. There was, however, a trend towards significance for the change in Tanner stage between assessments (F = 4.46, P = 0.061).
Sigma Peak
A careful examination of the all-night NREM spectral plots (Figure 2) reveals a shift in the frequency at which the peak in the spindle band occurred. We examined this pattern by determining the frequency at which the peak power occurred at electrode C3/A2 in the spindle range (11 – 15 Hz) at both recording times. The peak sigma frequency was higher in the follow-up than the initial recording in 12 of the 14 participants; in two, peak signal power frequency did not change from initial to follow-up. Higher peak sigma frequency at time 2 was statistically significant at P < 0.001.
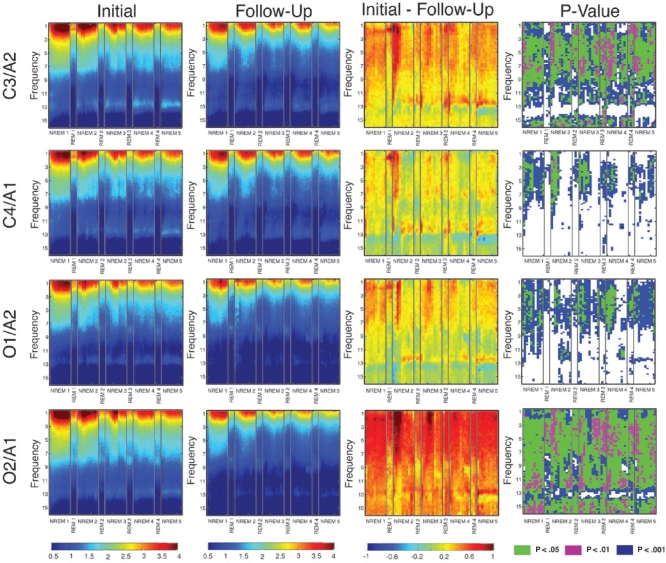
Time-Frequency Analysis
We performed a time-frequency analysis at all four electrode positions to gain a better understanding of how the EEG signal evolves over the course of the night. Because one participant, S6, only had three REM episodes at the initial recording session, the fourth REM cycle at both recording sessions is averaged over 13 rather than 14 participants. We note that the data from the first REM period should be regarded with caution because all but three participants skipped this episode at the initial recording session; therefore, the first REM episode data presented at both recording sessions is the average of just three participants. As shown in the fourth column of Figure 4, power at the initial recording session was significantly greater across the night in most frequency bins for NREM and REM sleep. A notable exception during NREM sleep was the frequency range of 13 to 14 Hz, where few time bins achieved statistical significance across the course of the night. As in the all-night spectra, the greatest power differences between the two assessments occurred at the left central (C3/A2) and right occipital (O2/A1) electrodes. Furthermore, consistent across channels were larger differences at frequencies below 10 Hz at all times across the night. This analysis confirms that developmental differences in power are consistent across the night and that the all-night effects are not driven by a particular portion of the night.
Figure 4.
Time-frequency plots. Each row corresponds to an EEG channel. The two leftmost columns are subject average plots of EEG spectral power (in μV2), the third column from the left is the difference between log power at the initial minus the follow-up session (in μV2), and the far right is a plot of the P-values. These plots display NREM and REM episodes consecutively on the x-axis and frequency (with a resolution of 0.2 Hz) on the y-axis. Each NREM episode is subdivided into 10 time points and each REM episode is subdivided into 3 times points. One subject had only three REM episodes therefore the last REM episode is averaged over 13 rather than 14 participants.
DISCUSSION
This paper presents longitudinal data of sleep EEG changes during early adolescence from fourteen healthy participants. Although we identify a number of developmental changes during this period, due to the small sample size we may have been underpowered to assess the impact of puberty or other factors on these changes. One notable feature of this study was the control that was imposed on the at-home and in-lab sleep schedules. In particular, time in bed (and consequently time awake during the days) and the recording conditions were constant across these sleep laboratory assessments making it possible to ensure that sleeping conditions (such as lighting and noise levels) did not vary. Hence, the significant findings did not result from alterations to sleep “pressure” or differences in the sleeping environment.
Several cross-sectional studies have reported that the “spindle peak” in the power spectrum increases in frequency from pre- to post-puberty.12,30 To our knowledge, this is the first study showing this shift in a longitudinal data set during early adolescent development. Although we did not measure the duration, incidence or frequency of sleep spindles directly, EEG power in the sigma band has been shown to be closely related to spindles.31 Spindles are generated in the thalamus and transmitted to the cortex via thalamocortical pathways, and the change in dominant sleep related sigma frequency may indicate maturational changes in the thalamocortical systems. This finding is difficult to interpret without knowing the functional significance of sigma peak frequency in the human EEG spectrum.
We found a decline in absolute EEG spectral power for both NREM and REM sleep in the average all night data. Furthermore, our time-frequency analysis revealed that this decline was stable across the night and across states (NREM and REM). Because this power decline is state non-specific and stable across the night, we attribute this decline to the developmentally programmed synaptic pruning that occurs in the healthy human cortex,17 as summarized in the introduction of this paper. Moreover, our results are in line with most waking and sleep EEG studies that show a decline in spectral power with increasing age.11,12,16 We also note that, although the aforementioned studies have age ranges that overlap with the current study, the specific age ranges examined varied and most studies were cross-sectional. One longitudinal study that examined power only in the delta band found no change from age 9 to 11 years in one group, and a significant decline from the age of 12 to 14 in another group.32 The age of our participants at follow up was slightly older than the younger group in that study; however, we found extensive differences in EEG power, including the delta range. While we may have captured the developmental progression missed by Campbell et al.,32 in their younger group, we may also have captured the phenomenon because of our carefully controlled sleep-wake protocol. Because sleep conditions were not controlled in the Campbell study, their participants may have had longer waking episodes at follow up and hence greater sleep pressure that would specifically affect the slower frequencies.33 Some evidence for this speculation is the reduction of nearly an hour of sleep at the follow-up assessments of their 9- and 12-year-old cohorts. Such a difference may produce sleep pressure sufficient to mask a developmental change in the delta frequencies.
The present longitudinal study and previous waking11 and sleep12 cross-sectional studies showed that developmental changes of EEG spectral power are most pronounced at frequencies below 10 Hz. Some have proposed that EEG activity below 10-Hz arises among cortical (pyramidal) neurons that are highly synchronous, whereas higher frequency activity results from asynchronous activity of cortical pyramidal neurons.11 Therefore, we believe that the synaptic pruning hypothesis outlined above also accounts for the larger differences at lower frequencies.
Although we show that EEG power decreases with increasing age, we were unable to pinpoint what factors influence the magnitude of the decline. We did, however, find a trend towards significance for the change in Tanner stage as a predictor of the change in power. This finding is not surprising since Tanner stage is a marker of maturation, and we believe that the decline in power is tied to some maturational process. With a larger sample size we may have been able to demonstrate the influence of this factor and other factors more definitively; however as it stands, future studies with larger sample sizes are needed to evaluate this phenomenon.
While our study was unable to address which parameters determine the rate of synaptic pruning, evidence from the animal literature indicate the contribution of interindividual variability. For example, one study measuring the number of cortical synapses in the macaque monkey visual cortex across a period corresponding to adolescence found large interindividual variability during the synaptic pruning phase, which they attribute to diverse epigenetic events and environmental factors.7 Future studies with a larger sample size will be necessary to address this issue in humans.
An unexpected finding was that the greatest change between the two assessments for NREM and REM sleep were at the left central and right occipital electrodes. Due to concerns that this finding resulted from recording equipment error or from using two different systems, we carefully tested the characteristics of both systems. We used a sine-wave generator to determine the frequency response curve at each electrode for both the Twin and the Gamma systems. The signals from the two systems were comparable across electrodes and frequencies. Furthermore, a calibration signal was sent to all electrodes in both systems prior to each recording to ensure that the systems were correctly calibrated. Although we cannot be certain that our finding did not result from a recording error these steps limit the role of this possibility.
Our finding of regional differences in the developmental trajectory of sleep EEG power is partially in line with previous structural MRI studies that show hemispheric asymmetries with larger anteroinferior frontal cortical and right temporo-occipital volumes in the developing brain. One longitudinal MRI study examined hemispheric asymmetries in children from approximately 11 to 18 years and found similar hemispheric asymmetries in healthy children.34 These investigators report that the left ortbitofrontal and inferiorfrontal gyrus were thicker compared to the right in early childhood, while the reverse was true in the medial occipital region. Across adolescence this pattern reversed and by the end of adolescent development, the asymmetry resembled that of adults. As outlined in the introduction, greater cortical thickness is thought to underlie synaptic density and hence translates into greater EEG power. In our study, we found greater EEG power for the left central and right occipital lobes at the initial session, and these regions also underwent the greatest change by the follow-up session. This developmental progression of hemispheric asymmetry may support the acquisition of cognitive skills or reflect the differential involvement of the two hemispheres during development.
One limitation of this study is the small sample size (n = 14), which limits our ability to reach definitive conclusions regarding our findings. The reader should keep this caveat in mind regarding the findings reported here. Although others have looked at developmental changes in the sleep EEG in this age range,16 our data extend those findings by examining a broader frequency range across four brain regions having controlled the quantity of sleep prior to studying sleep in a controlled laboratory setting. In this paper, we highlight a range of neural maturational changes in the sleep EEG across early adolescent development. Further longitudinal studies are needed to explore the course of these changes into late adolescence and early adulthood.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Peter Achermann, Ronald Seifer, Christine Acebo, Eliza Van Reen, Tracy Rupp, Oskar Jenni, Monique LeBourgeois, Gahan Fallone, and Margaret Borkowski for technical assistance. The authors are grateful to Drs. Elizabeth Forbes and Judith Owens for performing the Tanner staging and William Coon and Henry Arantes for sleep stage scoring. Drs. Peter Monti, Timothy Roehrs, Todd Arnedt, and Robert Swift served as consultants on this project, and we thank them for their contributions. We also thank our research staff, laboratory technicians, and participants. This research was supported by the National Institute on Alcohol Abuse and Alcoholism, grants AA13252 (to MAC) and AA07459-21 (to LT).
Supplementary Material
Bootstrap analysis methodology. The left and middle plots show histograms of the bootstrap distribution obtained by random shuffling of the spectral power data at frequency bin 4.6 Hz from electrode C3/A2 from both nights (initial and follow-up) to create two new distributions of the data, S1 and S2. The plot on the right presents the histogram of the bootstrap distribution of the differences between S1 and S2 for C3/A2 at 4.6 Hz. The asterisk in this plot marks the observed subject average power difference between the initial and follow-up sessions. This power difference is considered statistically significant because it is larger than 5% of the shuffled data.
REFERENCES
- 1.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 2.Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Preventive medicine. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 3.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of neurology. 1994;51:874–87. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 4.Steen RG, Ogg RJ, Reddick WE, Kingsley PB. Age-related changes in the pediatric brain: quantitative MR evidence of maturational changes during adolescence. Ajnr. 1997;18:819–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 6.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matousek M, Petersen I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalography and clinical neurophysiology. 1973;35:603–12. doi: 10.1016/0013-4694(73)90213-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura M, Yamamoto K, Fukuzawa H, et al. Age development and sex differences of various EEG elements in healthy children and adults--quantification by a computerized wave form recognition method. Electroencephalography and clinical neurophysiology. 1985;60:394–406. doi: 10.1016/0013-4694(85)91013-2. [DOI] [PubMed] [Google Scholar]
- 10.Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents I. Analysis of band power. Electroencephalography and clinical neurophysiology. 1988;69:91–9. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- 11.Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM. Brain maturation in adolescence: concurrent changes in neuroanatomy and neurophysiology. Human brain mapping. 2007;28:228–37. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 13.Coble PA, Kupfer DJ, Taska LS, Kane J. EEG sleep of normal healthy children. Part I: Findings using standard measurement methods. Sleep. 1984;7:289–303. doi: 10.1093/sleep/7.4.289. [DOI] [PubMed] [Google Scholar]
- 14.Karacan I, Anch M, Thornby JI, Okawa M, Williams RL. Longitudinal sleep patterns during pubertal growth: four-year follow up. Pediatric research. 1975;9:842–6. doi: 10.1203/00006450-197511000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. Journal of sleep research. 2001;10:165–72. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 16.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. American journal of physiology. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg I, Thode HC, Jr., Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. Journal of theoretical biology. 1990;142:149–61. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 18.Robins L, Cottler L, Bucholz K, Compton W, North C, Rourke K. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) 2000 [Google Scholar]
- 19.Tanner J. Growth at Adolescence. Oxford: Blackwell; 1962. [Google Scholar]
- 20.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95:956–63. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 21.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–8. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroenceph. Clin. Neurophysiol. 1958;10:370–1. [Google Scholar]
- 24.Feinberg I, Floyd TC. Systematic trends across the night in human sleep cycles. Psychophysiology. 1979;16:283–91. doi: 10.1111/j.1469-8986.1979.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 26.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 27.Tarokh L, Carskadon MA. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. Journal of sleep research. 2009 doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of neuroscience methods. 2007;164:177–90. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. 2 ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 30.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep medicine reviews. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 31.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain research. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 32.Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep. 2005;28:637–43. doi: 10.1093/sleep/28.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borbely AA. A two process model of sleep regulation. Human neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 34.Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2009;66:888–96. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bootstrap analysis methodology. The left and middle plots show histograms of the bootstrap distribution obtained by random shuffling of the spectral power data at frequency bin 4.6 Hz from electrode C3/A2 from both nights (initial and follow-up) to create two new distributions of the data, S1 and S2. The plot on the right presents the histogram of the bootstrap distribution of the differences between S1 and S2 for C3/A2 at 4.6 Hz. The asterisk in this plot marks the observed subject average power difference between the initial and follow-up sessions. This power difference is considered statistically significant because it is larger than 5% of the shuffled data.