Abstract
Interleukin (IL)-10 is important for regulating inflammation but whether it protects against infection-related deficits in cognitive function is unknown. Therefore, the current study evaluated sickness behavior, hippocampal-dependent matching-to-place performance and several inflammatory cytokines and neurotrophins in wild type (IL-10+/+) and IL-10-deficient (IL-10−/−) mice after i.p. injection of lipopolysaccharide (LPS). Additionally, morphology of dendrites of pyramidal neurons in the dorsal CA1 hippocampus was assessed. Treatment with LPS increased IL-1β, IL-6, and tumor necrosis factor alpha (TNFα) mRNA in all brain areas examined including the hippocampus, in both IL-10+/+ and IL-10−/− mice but the increase was largest in IL-10−/− mice. Plasma IL-1β, IL-6 and TNFα were also higher in IL-10−/− mice compared to IL-10+/+ mice after LPS. Consistent with increased inflammatory cytokines in IL-10−/− mice after LPS treatment, were a more lengthy sickness behavior syndrome and a more prominent reduction in hippocampal levels of nerve growth factor mRNA; brain-derived neurotrophic factor mRNA was reduced similarly in both genotypes after LPS. In a test of hippocampal-dependent learning and memory that required mice to integrate new information with previously learned information and switch strategies to master a task, IL-10−/− mice were found to be less efficient after LPS than were similarly treated wild type mice. LPS did not affect morphology of dendrites of pyramidal neurons in the dorsal CA1 hippocampus in either genotype. Taken together the results are interpreted to suggest that during peripheral infection IL-10 inhibits sickness behavior and tribulations in hippocampal-dependent working memory via its propensity to mitigate inflammation. We conclude that IL-10 is critical for maintaining normal neuro-immune communication during infection.
Keywords: behavior, cytokines, hippocampus, interleukin-10, mice, working memory
Introduction
Interleukin (IL)-10 is a prototypical anti-inflammatory cytokine in that it inhibits production of inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor alpha (TNFα) (Moore et al., 2001; Strle et al., 2001) and promotes the release of other anti-inflammatory cytokines including IL-1 receptor antagonist (IL-1ra) (Howard et al., 1992). In the brain of rats IL-10 has been shown to reduce IL-1β and TNFα after traumatic brain injury and improve neurological recovery (Knoblach and Faden, 1998). Furthermore, infarct volume produced by middle cerebral artery occlusion was exacerbated in IL-10 knockout (IL-10−/−) mice compared to wild types (Grilli et al., 2000), and addition of recombinant murine IL-10 to primary cortical or cerebellar granular neuron cultures from IL-10−/− mice prevented neuronal damage induced by excitotoxicity (Grilli et al., 2000). In the brain IL-10 also appears to inhibit the development and progression of chronic neurodegenerative diseases. For example, the onset of neuroinflammation and progression of prion disease was accelerated in mice deficient in IL-10 (Thackray et al., 2004). Interestingly, individuals with a polymorphism in the IL-10 promoter that results in lower levels of IL-10 have a higher likelihood of developing multiple sclerosis (de Jong et al., 2000) and Alzheimer’s disease (McGeer and McGeer, 1996). Collectively, these findings on brain injury and neurodegenerative disease indicate that IL-10 in the brain is an important counterbalance to the inflammatory response, which is injurious if unchecked.
During a peripheral infection, the immune system conveys a message to the brain and microglial cells respond and produce inflammatory cytokines that induce a behavioral response that is ordinarily adaptive. Excessive production of inflammatory cytokines in the brain, however, can cause behavioral pathology (Dantzer et al., 2008; Sparkman and Johnson, 2008). When lipopolysaccharide (LPS) was administered to mimic a peripheral infection, old mice experienced an exaggerated inflammatory cytokine response in the brain and exhibited signs of behavioral pathology, including prolonged anorexia (Godbout et al., 2005), depressive-like behavior (Godbout et al., 2008), and cognitive disturbances (Chen et al., 2008) that were not seen in younger cohorts. Architectural changes to dorsal CA1 hippocampal neurons have also been reported in young mice given LPS intracerebroventricularly (ICV) (Milatovic et al., 2003) and in older mice given LPS i.p. (Richwine et al., 2008). Other studies provide compelling evidence for a linkage between inflammatory cytokine expression in the brain and learning and memory deficits (Ben Menachem-Zidon et al., 2008) as well as depression (O'Connor et al., 2008). And the expression pattern of genes known to be involved in neuroplasticity, including brain-derived neurotrophic factor (BDNF), Arc, and ephrin receptor, is influenced by inflammatory stimuli (Bonow et al., 2008; Richwine et al., 2008; Rosi et al., 2005). All told, regulating inflammatory cytokines in the brain in the context of a peripheral infection appears important for preserving neuroplasticity and protecting against behavioral disorders.
Whereas the effects of IL-10 in brain injury and some neurodegenerative diseases has received considerable attention, less is known about the role of IL-10 in mediating communication between the peripheral immune system and brain during infection and it is not known if IL-10 has a role in preventing cognitive disorders that are associated with inflammation. In support of an important role for IL-10, results from one study suggested that IL-10 functions as an endogenous antipyretic following peripheral injection of LPS (Leon et al., 1999), and another study found several inflammatory cytokines to be higher in whole brain of IL-10-deficient mice compared to wild type mice after ICV injection of LPS (Agnello et al., 2000). Moreover, ICV injection of IL-10 inhibited the depression in social behavior caused by a peripheral injection of LPS (Bluthe et al., 1999), and recently LPS-induced fatigue and deficits in psychomotor coordination were found to be exacerbated in mice deficient in IL-10 (Krzyszton et al., 2008).
Given the relationship between inflammatory cytokines and cognitive function, the present study was conducted to determine if IL-10 protects against infection-related neurobehavioral complications including cognitive deficits. Specifically, we evaluated sickness behavior, matching-to-place performance and several inflammatory cytokines and neurotrophins in wild type and IL-10-deficient mice after i.p. injection of LPS. Additionally, morphology of dendrites of pyramidal neurons in the dorsal CA1 hippocampus was assessed. Our hypothesis was that inflammatory cytokines in the brain and neurobehavioral deficits in mice challenged with LPS would be increased in the absence of IL-10. It was further hypothesized that increased inflammation would be associated with reduced neurotrophic support and dendritic atrophy of CA1 pyramidal neurons.
Materials and Methods
Animals
Two month old male congenic IL-10 knockout (IL-10−/−) (C.129P2(B6)-Il10tm1Cgn/J) mice and wild type C57BL/6 (IL-10+/+) controls were purchased from The Jackson Laboratory (Ben Harbor, Maine). Because The Jackson Laboratory maintains the IL-10−/− strain homozygously, the standard inbred background strain must be used as controls. The congenic IL-10−/− strain, which was produced by backcrossing 10 generations, is estimated to be 99.9% similar to the background inbred strain at all unlinked gene loci. Upon arrival mice were housed individually under a reverse 12 hour light-dark cycle at 23°C with ad libitum access to food and water for one month before experiments were initiated. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Experiment 1. Behavioral and inflammatory effects of LPS in IL-10−/− mice
Three-month-old male IL-10+/+ and IL-10−/− mice were injected peripherally with saline or Escherichia coli LPS (10 µg, serotype 0127:B8, Sigma). Thus, the four treatments comprised the 2 × 2 factorial combination of genotype (IL-10+/+ and IL-10−/−) and treatment (saline and LPS). A pilot study with wild type mice showed that this dose of LPS was sufficient to increase plasma inflammatory cytokines and induce transient sickness behavior as assessed by depression in social investigation (unpublished data). Home cage locomotor activity was determined before injection and 2, 4, 8, 24, 48, and 72 h afterwards. Food pellets were weighed prior to assessment of baseline locomotor activity and again 24, 48, and 72 h after injection to estimate food intake. Immediately after behavioral testing at 4 and 24 h, a subset of mice from each treatment group was killed by CO2 inhalation and decapitation; all remaining mice were killed after testing at 72 h. The brain was quickly dissected and the hippocampus, cortex, cerebellum, and hypothalamus were placed in RNAlater and stored at −80° C until measuring inflammatory cytokine (IL-1β, IL-6, and TNFα) and neurotrophin (NGF and BDNF) mRNA by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Blood was collected into heparinized tubes and immediately centrifuged at 2000 × g for 15 min at 4° C. Plasma was collected and stored at −80° C until assaying for corticosterone and cytokines.
Locomotor activity
Mice were maintained in their home cage, which provided a floor area of 26 × 20 cm, and video recorded for a three-minute period at the indicated times. Total distance moved (cm) and rearing frequency were determined from the video records using EthoVision computer software (Noldus Information Technology, Wageningen, Netherlands).
Plasma corticosterone and cytokines
Total plasma corticosterone concentration was determined using a radioimmunoassay kit (MP Biomedicals, LLC, Orangeburd, NY). Samples were assayed according to the manufacturer’s instructions and sensitivity of the assay was 7.7 ng/dl. Plasma samples were also assayed for IL-1β, TNFα, and IL-6 using a multiplex bead-based immunoassay kit combined with a Cytokine Reagent kit as described by manufacturer (Bio-Rad, Hercules, CA). Plasma IL-10 was assayed using a quantikine mouse immunoassay kit as described by manufacturer (R & D Systems, Minneapolis, MN). Assays were sensitive to <3 pg/ml cytokine; the inter- and intra-assay coefficients of variation were <8%.
Inflammatory cytokine and neurotrophin mRNA
Total RNA was isolated from brain using the Tri Reagent protocol (Sigma, St. Louis, MO). RNA samples were subjected to a DNAse I digestion procedure and then reverse transcribed to cDNA using a RT Retroscript kit (Ambion, Austin, TX). Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Godbout et al., 2005). In brief, cDNA was amplified by PCR where a target cDNA (IL-1β, Mm00434228_m1; TNFα, Mm00443258_m1; IL-6, Mm00446190_m1; NGF, Mm0043039_m1; BDNF, Mm00432069_m1) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5’ fluorescent reporter dye (6-FAM) and a 3’ quencher dye (NFQ). PCR reactions were performed at the following conditions: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold change.
Experiment 2. Effects of LPS on spatial learning in IL-10−/− mice
A version of the Morris water maze was used to evaluate hippocampus-dependent learning and memory. A circular tank 100 cm in diameter and 30 cm deep filled with water (24 °C) to a depth of 25 cm was used. A transparent round platform 10 cm in diameter was constructed from Plexiglas and positioned approximately 0.5 cm below the surface of the water in one of four quadrants. Numerous visual cues surrounded the tank. A video camera mounted to the ceiling directly above the center of the maze was used in conjunction with the EthoVision animal tracking system.
To begin each trial a mouse was pseudorandomly placed in the water in one of four preset locations 2 cm from the edge of the tank facing the wall. Mice were allowed to swim freely a maximum of 60 s or until the platform was located. After the mouse reached the platform it was required to remain there for 30 s. If the platform was not located during the 60 s, mice were guided to the platform and allowed to remain there for 30 s. Mice were placed on the platform for 30 s preceding the start of each session of testing. Performance parameters that were determined included swim speed, latency to the platform, and distance swam. After completion of three consecutive trials, mice were returned to their home cage and kept under a heat lamp for 10 min.
Three-month-old IL-10+/+ and IL-10−/− mice were trained in a five-day acquisition phase with a static platform position in order to evaluate place learning for reference memory (Morris, 1984). After a two day rest period mice were injected i.p. with saline or LPS (10 µg). They were tested 4 h and 24 h after injection utilizing the same in-trial structure as described above, but in order to evaluate the effects of LPS on working memory performance, a matching-to-place paradigm was utilized wherein the position of the platform was relocated to novel positions before the start of each test session. For an animal to efficiently complete the task, it must effectively utilize the unique within trial information (i.e., novel platform location [working memory]) with the consolidated memories of relation of the spatial cues (reference memory). Swim speed, latency to the platform, and distance swam were determined.
Experiment 3. Effects of LPS on CA1 pyramidal neurons in IL-10−/− mice
Three-month-old IL-10+/+ and IL-10−/− mice were injected i.p. with saline or LPS (10 µg) and killed 72 h later by CO2 inhalation and decapitation. Whole brain was quickly removed and processed for Golgi-Cox staining. Handling of mice before and after injections was kept to a minimum because stress and environmental enrichment affect dendritic arborization (Greenough et al., 1973; Moser et al., 1994; Silva-Gomez et al., 2003; Woolley et al., 1990).
Golgi-Cox staining
Whole brain was removed and processed as described by Richwine et al. (Richwine et al., 2008). Briefly, whole brain was submerged in Golgi-Cox solution for 10 days, at which time test slices were taken to determine if neurons were well filled. The brain was then coronally blocked at the optic chiasm and dehydrated. Brains were embedded in 12% celloiden and 150 µm thick coronal sections were cut on a microtome. Slices were developed according to Glaser and Van der Loos (Glaser and Van der Loos, 1981) and mounted on glass slides. Brains were coded prior to sectioning to ensure that experimenter was blind to treatments.
Dendrite analysis
A Zeiss Axio Imager microscope (Zeiss, Thornwood, NY) and a computer-based system (Neurolucida; MicroBrightField, Williston, VT) were used to generate three-dimensional neuron tracings that were subsequently visualized and analyzed using NeuroExplorer (MicroBrightField, Williston, VT). For each brain (n=6), 8–10 basal and apical dendritic trees (i.e., a total of 48–60 trees per treatment group) were traced in the dorsal CA1 region of the hippocampus at 40× magnification. In order for a neuron to be selected the following four criteria were met: 1) neuron was in the area of interest, 2) neuron was distinct from other neurons to allow for identification of dendrites, 3) neuron was not truncated, and 4) neuron exhibited dark, well-filled staining throughout (Gould et al., 1990). For Sholl Analyses in NeuroExplorer, concentric spheres that increased in radius (20 µm) were layered around the cell body until dendrites were completely encompassed. Total dendrite length was measured by determining where within the concentric spheres it terminated, and complexity of the tree was determined by the number of nodes (i.e., branching points) and the number of times dendrites intersected spheres.
Spine density was determined using established procedures (Markham et al., 2005; Woolley et al., 1990). In brief, to estimate the density of spines on dendrites, counts were made on 10 µm-long sections of terminating oblique apical branches in the stratum radiatum. To ensure that the number of hidden spines was proportional across all segments counted and among treatment groups, counts were taken in a single plane on sections of dendrite that were approximately 2 µm in diameter. All dendritic protrusions were considered spines and were counted. While double headed spines were rare, they were counted as two separate spines. For each animal (n=6), dendritic spines on 8–10 neurons were counted in triplicate (i.e., 3 counts per neuron) at 100× magnification under oil immersion; therefore, a total of 144–180 segments were counted per treatment group. Data are expressed as the number of spines per 10 µm of dendrite.
Statistics
Data analysis was done using Statistical Analysis Systems software (SAS Institute Inc., Cary, NC). Behavior data (i.e., locomotor and rearing frequency) were subjected to a three-way (genotype × treatment × time) repeated measures ANOVA in which post-injection testing time (4 h & 24 h) was the within-subjects factor (i.e., repeated measure) and genotype (IL-10+/+ or IL-10−/−) and treatment (saline or LPS) were between-subjects factors. Plasma cytokine data from saline-injected animals were below detectable limits; thus, plasma cytokine data only from LPS-injected animals were subjected to a one-way ANOVA to determine the main effect of genotype. All other data were subjected to a two-way ANOVA to determine the main effects of genotype (IL-10+/+ or IL-10−/−) and treatment (saline or LPS), and the genotype × treatment interaction. Brain cytokine mRNA data exhibited unequal variance, which was corrected using a log transformation. The data presented in Table 1 are non-transformed means ± standard error values, but the associated significance values were derived from log transformed data. Overall, F-protected t tests were used to determine differences between treatment means. All data are presented as means ± standard error.
Table 1.
Change (fold increase) in cytokine mRNA in distinct brain regions of IL-10+/+ (wild type) and IL-10−/− mice 4 and 24 h after a peripheral injection of saline or lipopolysaccharide (LPS). Values are means ± SEM. Brain cytokine mRNA data exhibited unequal variance, which was corrected using a log transformation. Data presented in this table are non-transformed means ± SEM values, but associated significance values were derived from log transformed data.
| Wild type | IL-10−/− | p value | |||||
|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | Geno | LPS | Geno × LPS |
|
| 4 h after injection | |||||||
| Hippocampus | |||||||
| IL-1β | 0.6 ± 3.8 | 9.8 ± 4.6 | 1.2 ± 3.8 | 46.9 ± 3.8 | 0.001 | < 0.001 | 0.029 |
| IL-6 | 0.7 ± 2.1 | 5.5 ± 2.4 | 0.8 ± 2.1 | 17.9 ± 2.1 | 0.002 | < 0.001 | 0.003 |
| TNFα | 0.7 ± 4.1 | 4.2 ± 5.0 | 0.8 ± 4.1 | 25.6 ± 3.8 | < 0.001 | < 0.001 | 0.002 |
| Cortex | |||||||
| IL-1β | 0.8 ± 3.0 | 17.0 ± 3.0 | 2.5 ± 3.0 | 24.6 ± 2.8 | 0.014 | < 0.001 | 0.213 |
| IL-6 | 1.1 ± 1.7 | 5.8 ± 1.8 | 1.0 ± 1.8 | 15.5 ± 1.7 | 0.011 | < 0.001 | 0.014 |
| TNFα | 1.0 ± 4.2 | 26.4 ± 4.5 | 1.6 ± 4.5 | 35.9 ± 4.2 | 0.065 | < 0.001 | 0.796 |
| Cerebellum | |||||||
| IL-1β | 0.8 ± 5.7 | 50.0 ± 5.7 | 2.1 ± 5.7 | 64.8 ± 5.7 | 0.007 | < 0.001 | 0.212 |
| IL-6 | 1.0 ± 11.3 | 56.5 ± 12.2 | 1.2 ± 12.2 | 100.8 ± 11.3 | 0.032 | < 0.001 | 0.087 |
| TNFα | 1.1 ± 5.1 | 28.0 ± 5.5 | 1.4 ± 5.5 | 39.9 ± 5.1 | 0.074 | < 0.001 | 0.768 |
| Hypothalamus | |||||||
| IL-1β | 0.5 ± 8.6 | 31.2 ± 6.1 | 1.0 ± 6.1 | 38.3 ± 5.6 | 0.114 | < 0.001 | 0.678 |
| IL-6 | 1.0 ± 10.5 | 14.4 ± 8.6 | 2.0 ± 8.6 | 45.3 ± 8.0 | 0.033 | < 0.001 | 0.192 |
| TNFα | 1.2 ± 2.9 | 18.1 ± 2.4 | 1.1 ± 2.4 | 21.3 ± 2.2 | 0.673 | < 0.001 | 0.512 |
| 24 h after injection | |||||||
| Hippocampus | |||||||
| IL-1β | 0.7 ± 0.9 | 11.5 ± 0.8 | 1.2 ± 0.9 | 5.5 ± 0.8 | 0.410 | < 0.001 | < 0.001 |
| IL-6 | 0.7 ± 1.4 | 0.4 ± 1.2 | 0.6 ± 1.4 | 3.7 ± 1.2 | 0.332 | 0.989 | 0.214 |
| TNFα | 0.9 ± 1.3 | 8.9 ± 1.1 | 0.8 ± 1.2 | 6.2 ± 1.1 | 0.216 | < 0.001 | 0.500 |
| Cortex | |||||||
| IL-1β | 1.4 ± 0.7 | 5.8 ± 0.7 | 2.5 ± 0.7 | 3.3 ± 0.7 | 0.787 | 0.002 | 0.015 |
| IL-6 | 0.7 ± 0.5 | 0.4 ± 0.5 | 1.0 ± 0.5 | 1.8 ± 0.4 | 0.063 | 0.364 | 0.353 |
| TNFα | 1.2 ± 1.9 | 8.9 ± 1.8 | 1.5 ± 1.8 | 9.5 ± 1.6 | 0.966 | < 0.001 | 0.768 |
| Cerebellum | |||||||
| IL-1β | 1.3 ± 1.1 | 13.9 ± 1.0 | 3.4 ± 1.1 | 8.5 ± 1.1 | 0.734 | < 0.001 | 0.013 |
| IL-6 | 1.4 ± 4.0 | 0.8 ± 3.7 | 0.7 ± 4.0 | 10.2 ± 4.0 | 0.251 | 0.064 | 0.001 |
| TNFα | 1.5 ± 2.8 | 10.8 ± 2.6 | 1.8 ± 2.8 | 10.7 ± 2.8 | 0.832 | < 0.001 | 0.471 |
| Hypothalamus | |||||||
| IL-1β | 0.5 ± 1.3 | 13.4 ± 1.2 | 1.1 ± 1.3 | 4.1 ± 1.2 | 0.220 | < 0.001 | 0.001 |
| IL-6 | 0.9 ± 1.4 | 0.7 ± 1.3 | 0.7 ± 1.4 | 5.0 ± 1.3 | 0.064 | 0.059 | 0.022 |
| TNFα | 0.8 ± 2.4 | 16.3 ± 2.2 | 0.7 ± 2.4 | 11.2 ± 2.2 | 0.707 | < 0.001 | 0.710 |
Results
Sickness behavior
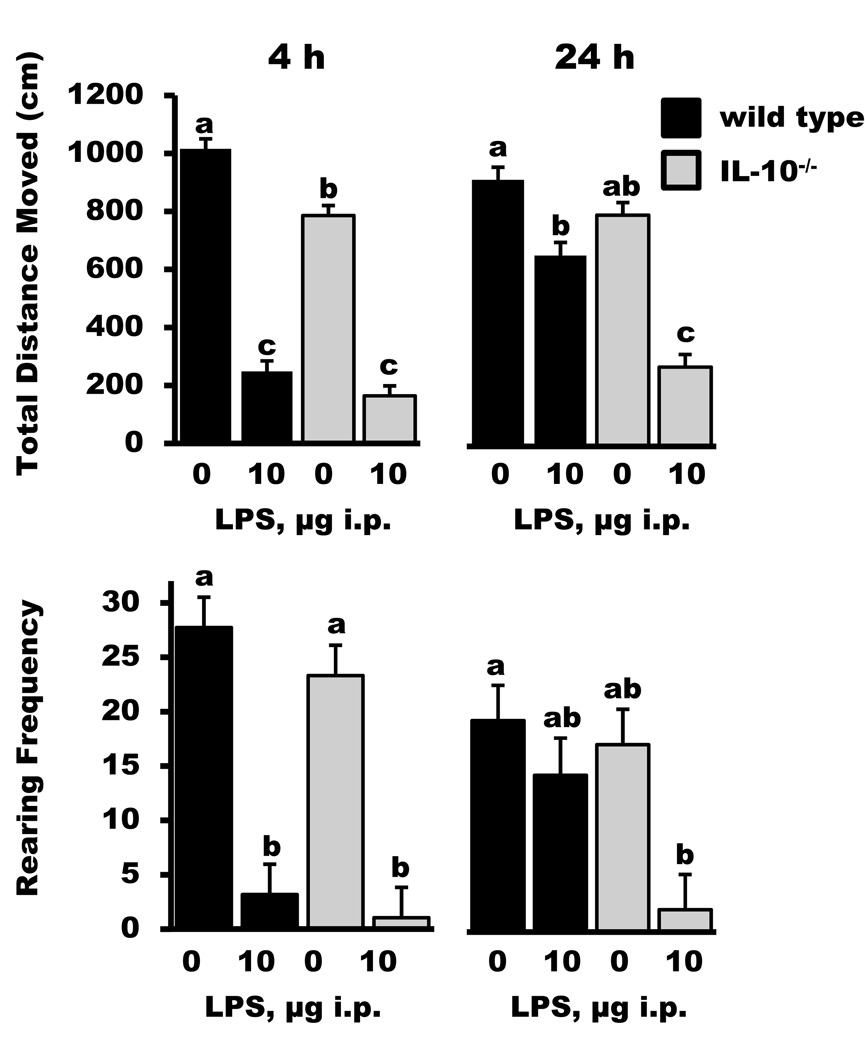
In the first experiment, we sought to determine if IL-10 inhibits LPS-induced deficits in locomotor activity and food intake. Analysis of locomotor behavior revealed both LPS-treated IL-10+/+ and IL-10−/− mice showed significant reductions in distance traveled at the 4 h time point. However, by 24 h IL-10+/+ mice treated with LPS had recovered to approximately 72% of the activity levels expressed by saline-treated IL-10+/+ mice and LPS-treated IL-10−/− mice were still reduced to 35% of their genotype controls (genotype × treatment × time, p < 0.0001; genotype × treatment, treatment × time, genotype, treatment, hour, p < 0.05). Similarly, LPS tended to cause a reduction in rearing frequency at 4 h in both genotypes and by 24 h IL-10+/+ mice had returned to 74% of the saline-treated IL-10+/+ levels and IL-10−/− mice treated with LPS were still reduced to 12% of rearing levels expressed by IL-10−/− mice injected with saline (genotype × treatment × time interaction, p = 0.05; treatment × hour, genotype × time, treatment, time, p < 0.05). By 48 h, all measures of locomotor activity for IL-10−/− mice injected with LPS returned to that of the saline-injected mice (data not shown).
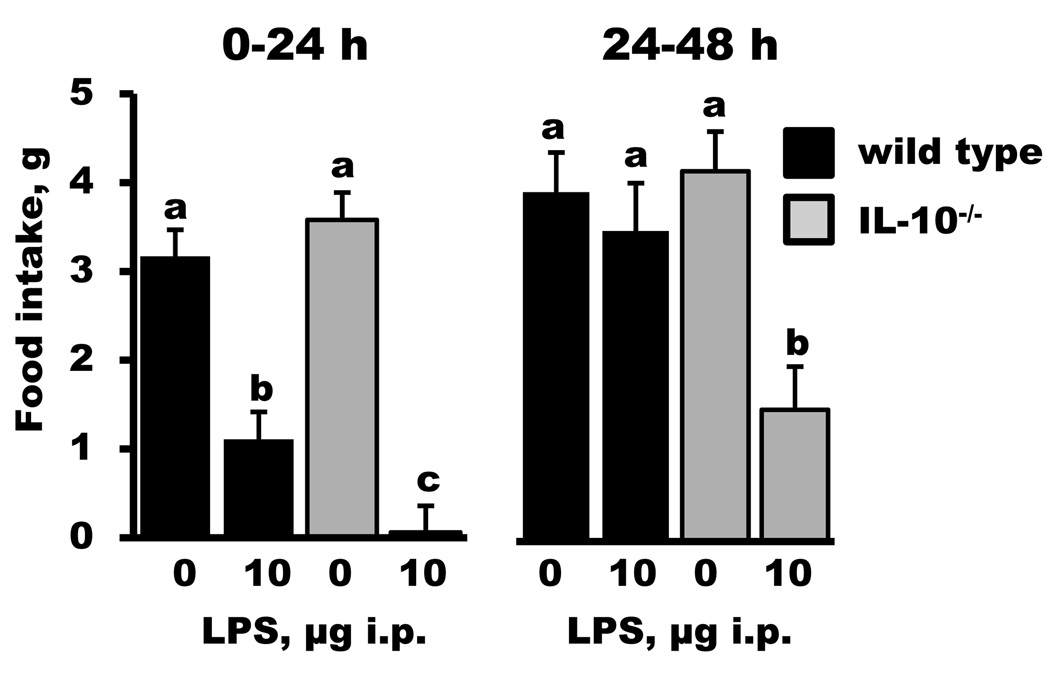
Along with deficits in locomotor activity, LPS decreased food intake in IL-10+/+ and IL-10−/− mice (treatment, p < 0.001); however, the reduction in food intake during the first 24 h was greater in IL-10−/− mice (genotype × treatment, p < 0.05; Figure 2). Additionally the reduction in food intake was still evident at 48 h for IL-10−/− mice injected with LPS (genotype × treatment, p < 0.05; treatment, p < 0.01). By 72 h, appetite in IL-10−/− mice returned to the level of saline-injected mice (data not shown). Together these data indicate that IL-10 serves an important role in mediating the behavioral effects of peripheral LPS.
Figure 2.
Food intake in IL-10+/+ (wild type) and IL-10−/− mice during 24 h periods after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA revealed a significant main effect of treatment (p < 0.01) and a genotype × treatment interaction (p < 0.05) during both post-injection feeding periods. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Inflammatory cytokines and corticosterone
The peripheral innate immune system conveys a message to the brain and induces microglial cells to produce inflammatory cytokines that participate in the sickness behavior syndrome. Therefore, we measured IL-10, IL-1β, IL-6 and TNFα protein in plasma (Figure 3) and IL-1β, IL-6, and TNFα mRNA in discrete brain regions (Table 1) of IL-10+/+ and IL-10−/− mice after peripheral injection of LPS. As anticipated, 4 h after injection of LPS plasma IL-10 was increased in IL-10+/+ mice but was undetectable in IL-10−/− mice. Levels of IL-1β(p < 0.03), TNFα (p < 0.03) and IL-6 (p < 0.01) were significantly increased in LPS-treated IL-10−/− mice compared with LPS-treated-IL-10+/+ mice. At 24 h post-injection, plasma IL-6 and TNFα in LPS-treated IL-10−/− mice returned to baseline, but IL-1β was still elevated (data not shown, p < 0.05). All cytokines measured returned to baseline by 72 h post injection (data not shown).
Figure 3.
Plasma cytokine concentrations in IL-10+/+ (wild type) and IL-10−/− mice 4 h after a peripheral injection of lipopolysaccharide (LPS). Bars are means ± SEM. Plasma cytokine concentrations in mice receiving a peripheral saline injection were below the detectable threshold and were therefore excluded from the statistical analysis. One-way ANOVA revealed significant genotype effects for IL-1β (p < 0.05), IL-10 (p < 0.05), TNFα (p < 0.01), and IL-6 (p < 0.001). For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Next, we examined inflammatory cytokine mRNA (IL-1β, IL-6 and TNFα) in discrete brain regions. As reported in Table 1, LPS increased expression of inflammatory cytokines 4 h post injection in the hippocampus, hypothalamus, cortex, and cerebellum (treatment, p < 0.001). Interestingly, in the hippocampus, cytokine levels were significantly increased in LPS-treated IL-10−/− compared to LPS-treated IL-10+/+ mice (treatment × genotype, p < 0.05). Additionally, in the cortex and cerebellum, IL-6 was increased in LPS-treated IL-10−/− compared to LPS-treated IL-10+/+ mice (treatment × genotype, p < 0.05). At 24 h post-injection, IL-1β was still elevated in the four brain areas in IL-10+/+ mice compared to IL-10−/− mice (genotype × treatment, p < 0.05). Additionally, IL-6 levels were still elevated in the cerebellum and hypothalamus in IL-10−/− compared to IL-10+/+ mice (genotype × treatment, p < 0.05). By 72 h all inflammatory cytokines in each brain region had returned to the levels of saline-injected mice (data not shown). Taken together, these data suggest that IL-10 is an important mediator of the inflammatory response after peripheral LPS and the absence of IL-10 results in an amplified inflammatory cytokine response in the periphery and in discrete brain regions.
Plasma corticosterone was also measured 4, 24, and 72 h after injection of LPS or saline (Figure 4). Four hours following LPS, both IL-10+/+ mice and IL-10−/− mice had a similar increase in plasma corticosterone compared to saline-injected mice (treatment, p < 0.05). However, at 24 h corticosterone in IL-10+/+ mice injected with LPS returned to the levels of saline injected mice, while IL-10−/− mice still had elevated circulating levels of corticosterone (genotype × treatment, p < 0.05; genotype, treatment, p < 0.05). By 72 h, plasma corticosterone in LPS treated mice returned to baseline. These data indicate that in the absence of IL-10 activity of the HPA axis is prolonged, most likely because of the heightened inflammatory response to LPS.
Figure 4.
Plasma corticosterone concentrations in IL-10+/+ (wild type) and IL-10−/− mice 4, 24, and 72 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA revealed a significant main effect of treatment at 4 (p < 0.001), 24 (p < 0.05), and 72 h (p < 0.05). A significant main effect of genotype (p < 0.01) and a genotype × treatment interaction (p < 0.001) were observed 24 h post-injection. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Matching-to-place assessment in the water maze
To determine if IL-10 plays a role in protecting against infection-related cognitive deficits, hippocampal-dependent spatial working memory was assessed during the matching-to-place sessions of the water maze task in IL-10+/+ and IL-10−/− mice after injection of LPS. Genotype did not affect distance swam, swim speed or latency to reach the platform during the acquisition phase (days 1–5; data not shown). During the first matching-to-place session (4 h after saline or LPS treatment) there was a significant main effect of treatment in which animals that received LPS had slower swim speeds (data not shown). There were no significant effects on distance swam or latency to reach the platform (data not shown) indicating cognition was not significantly affected 4 h after treatment with LPS. During the second matching-to-place session (24 h after saline or LPS treatment), however, there was a significant genotype × treatment interaction (p < 0.05) for swimming speed where in IL-10−/− animals treated with LPS swam more slowly (Figure 5). Importantly, there was also a significant genotype × treatment interaction (p < 0.05) for distance swam to locate the platform in which LPS-treated IL-10−/− mice swam farther to reach the platform compared to saline-treated IL-10−/− and IL-10+/+ mice and LPS-treated IL-10+/+ mice (Figure 5). Thus, these data suggest that IL-10 is important for protecting against cognitive deficits associated with a peripheral infection.
Figure 5.
Performance of IL-10+/+ (wild type) and IL-10−/− mice in a matching-to-place paradigm 24 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are test session means ± SEM. The main effect of genotype was observed for distance swam (p < 0.01) and latency (p < 0.01), while the main effect of treatment was observed for latency (p < 0.05) and speed (p < 0.001). Significant genotype × treatment interactions (p < 0.05) were observed for all three measures. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Neurotrophins and CA1 pyramidal neuron morphology
Because IL-10 deficiency facilitated the LPS-induced increase in inflammatory cytokines and deficits in working memory we next sought to assess treatment effects on two neurotrophins—NGF and BDNF—that are important substrates for learning and memory and inhibited by inflammatory stimuli. Hippocampal NGF and BDNF mRNA were measured 4, 24, and 72 h after peripheral injection of saline or LPS in IL-10+/+ and IL-10−/− mice (Figure 6). Data were initially subjected to three-way ANOVA but time post injection was not significant, so NGF and BDNF mRNA data were pooled across time. In saline-injected mice, BDNF mRNA was lower in the hippocampus of IL-10−/− mice compared to IL-10+/+ mice (p < 0.01). Following LPS, hippocampal expression of BDNF decreased in both genotypes compared to saline-injected IL-10+/+ mice (p < 0.01), but BDNF expression was markedly lower in mice deficient in IL-10 (p < 0.01). In addition, IL-10−/− mice injected with LPS was the only treatment group to have reduced expression of NGF (genotype × treatment, p < 0.01; genotype, p < 0.01, treatment, p <0.01).
Figure 6.
Hippocampal expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in IL-10+/+ (wild type) and IL-10−/− mice after a peripheral injection of saline or lipopolysaccharide (LPS). Neurotrophin mRNA expression was determined 4, 24, and 72 h post-injection, but no effect of time was evident so data were pooled across time. Bars are means ± SEM. Significant main effects (p < 0.001) of genotype and treatment were observed for both BDNF and NGF. Additionally, a genotype × treatment interaction (p < 0.001) was observed for NGF. For each graph, treatment means with dissimilar letters are significantly different (p <0.05).
In order to determine if the augmented inflammatory cytokine response and decrease in neurotrophins after activation of the peripheral innate immune system in IL-10−/− mice was associated with neuronal atrophy, basal and apical dendritic complexity in the dorsal CA1 region of the hippocampus was assessed 72 h after an injection of saline or LPS (Figure 7). We focused on this region and time point because a previous study with aged mice reported a loss in dendritic complexity in the CA1 region after peripheral LPS, and aged mice have an amplified inflammatory cytokine response similar to what was observed in the present study (Chen et al., 2008; Godbout et al., 2005; Richwine et al., 2008). Two-way ANOVA of the number of intersections revealed a significant main effect of genotype on the basal tree (p < 0.05) which indicated the dendritic complexity in this region was greater in IL-10−/− mice than IL-10+/+ mice (p < 0.05). However, LPS did not influence dendritic branching in either genotype, and no differences in the apical tree were evident. Spine density was also determined on the same basal and apical trees used to measure dendritic arborization. However, neither genotype, treatment nor a genotype × treatment interaction were evident for spine density in basal or apical branches of CA1 pyramidal neurons (data not shown).
Figure 7.
Dendritic complexity of pyramidal hippocampal neurons in the dorsal CA1 region. The total number of neuronal intersections were measured by the Sholl Ring method in basal and apical trees in IL-10+/+ (wild type) and IL-10−/− mice 72 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA of the number of intersections revealed a significant main effect of genotype on the basal tree (p < 0.05). No other significant effects were observed.
Discussion
We recently reported that infection-related fatigue and deficits in psychomotor coordination were exacerbated in mice deficient in IL-10 (Krzyszton et al., 2008). The fatigue and psychomotor deficits in IL-10−/− mice were associated with higher plasma inflammatory cytokine levels and increased expression of inflammatory cytokine genes in brain areas important for skilled motor behaviors. The results of the present study are consistent with this earlier report but extend it by showing that during peripheral infection IL-10 also serves to inhibit sickness behavior and tribulations in hippocampal-dependent learning and memory. These findings are significant because infection-related cognitive impairment can interfere with self care behavior, delaying recovery and causing recurring infection. Furthermore, infection is a risk factor for several neurodegenerative diseases (Holmes et al., 2003; Sibley et al., 1985), and a genetic predisposition leading to lower levels of IL-10 is correlated with a higher incidence of Alzheimer’s (McGeer and McGeer, 1996) and multiple sclerosis (de Jong et al., 2000). Thus, IL-10, via its propensity to mitigate inflammation, appears to be critical for maintaining normal neuro-immune communication during infection.
Working memory can be defined as a cognitive construct wherein information is briefly stored and manipulated before it undergoes consolidation processes. Previous studies have demonstrated that LPS can impair memory consolidation (Barrientos et al., 2002; Pugh et al., 1998) and thus impair reference memory formation, and has been shown to not influence cognitive performance in already learned tasks (Aubert et al., 1995; Sparkman et al., 2005). Consistent with these findings, Gibertini (Gibertini, 1996) showed that animals that received i.p. injections of IL-1β demonstrated intact reference memory for a previously trained platform position but were less efficient in locating a novel platform position compared to saline-treated controls. Similarly, the matching-to-place version of the water maze has been shown to be a sensitive measure of hippocampal impairment (Squire, 1992; Whishaw and Tomie, 1997). Previously, we demonstrated that wild type C57BL/6 mice given a higher dose of LPS were impaired in the matching-to-place version of the water maze (Sparkman et al., 2006) as were LPS-treated aged Balb/c mice (Chen et al., 2008).
In the current study mice deficient in IL-10 swam farther to locate the platform when tested 24 h after LPS treatment in a matching-to-place version of the water maze. This suggests that the IL-10−/− mice treated with LPS were less efficient than other treatment groups at integrating information regarding the new platform location with previously learned information and switching strategies to master the task. During tests of spatial working memory, the hippocampus seems to serve an important role in spatial navigation (Morris, 1984), integrating the relational aspects of the environment (Gibertini, 1996) and inhibiting responding to previous but no longer relevant platform locations (Whishaw and Tomie, 1997) possibly resulting in a net reduction in the strength of the working memory trace. The fact that no deficits were apparent at 4 h probably reflects the changing cognitive demands associated with the testing paradigm. As the platform is repositioned for each swim session after the acquisition phase, this can lead to cumulative increases in cognitive load if the conditioning to previous platform locations causes interference and impairs working memory performance. As we do not expect our results to be equivalent to ablation of the hippocampus, it is not surprising that the interaction between LPS and genotype was manifest only when the task difficulty increased. That only mice deficient in IL-10 injected with LPS showed a deficit in working memory is interpreted to suggest that IL-10 is important for preserving certain aspects of hippocampal function during peripheral infection.
The current findings are consistent with the prevailing view that inflammatory cytokines produced in the hippocampus disrupt cognition. For example, increased levels of brain IL-1β impaired hippocampal-dependent memory consolidation (Barrientos et al., 2004; Pugh et al., 1998; Pugh et al., 2001). Moreover, IL-1ra has been shown to prevent memory disturbances caused by infection or stress (Ben Menachem-Zidon et al., 2008; Pugh et al., 1998; Pugh et al., 2001). And mice deficient in IL-6 are refractory to LPS-induced deficits in working memory, in part because the central cytokine compartment is insensitive to signals from the peripheral immune system (Sparkman et al., 2006). In the present study, IL-1β, IL-6 and TNFα mRNAs were markedly higher at 4 h in the hippocampus of IL-10−/− mice injected with LPS compared to similarly treated IL-10+/+ mice. At 24 h, this difference had mostly dissipated and IL-1β mRNA was actually highest in IL-10+/+ mice given LPS. This implies a disconnection between high levels of inflammatory cytokines in the hippocampus (4 h) and the occurrence of deficits in hippocampal function (24 h). Therefore, inflammatory cytokines may affect hippocampal-dependent learning and memory via the induction of other genes important for learning and memory that were not considered herein. For example, a recent microarray study reported a change in the expression of genes involved in learning and memory, synaptic transmission and neuron migration in the hippocampus and cortex of mice 24 h after ICV injection of LPS (Bonow et al., 2008). Whatever the explanation, from the current study and others (Agnello et al., 2000; Krzyszton et al., 2008) it is reasonable to suggest that overall, the brain exposure to elevated inflammatory cytokines after LPS injection was greater in the absence of IL-10. Therefore, IL-10 likely preserves hippocampal function during infection by inhibiting inflammatory cytokine production and the related downstream effects.
Despite the heightened levels of inflammatory cytokines and corticosterone in IL-10−/− mice, LPS did not decrease dendritic complexity in CA1 pyramidal neurons 72 h post injection. This was in contrast to a previous study that reported dendritic degeneration after a peripheral injection of LPS in aged mice (Richwine et al., 2008). In aged mice, LPS induces an exaggerated neuroinflammatory response that is suspected to contribute to changes in neuron morphology. The fact that no change in dendrite morphology was observed in the present study suggests that increased inflammatory cytokines alone, at least under the conditions modeled here, are not sufficient to affect dendrite morphology. It is more plausible that other age-related events that are not present in young IL-10−/− mice sensitize neurons to the deleterious effects of infection and inflammation. For instance, increased lipid peroxidation and chronic low-grade inflammation have been reported in the brain of old but otherwise healthy animals (Richwine et al., 2005). Furthermore, neurotrophins and/or their receptors can be decreased by aging (Tapia-Arancibia et al., 2008). In the present study, while IL-10−/− mice had a decrease in steady-state level of BDNF mRNA, NGF expression was not affected by genotype. It is noteworthy that the effect of LPS to lower BDNF expression was greatest in IL-10−/− mice because neurotrophins play an important role in synaptic plasticity in the hippocampus and in other brain regions (McAllister et al., 1996; McAllister et al., 1995). Thus, the effect of LPS on neurotrophin expression was consistent with its effect on working memory even if changes in dendrite morphology were not apparent. It must be noted that a more thorough interrogation of neuron morphology (e.g., different brain areas and different times after injection) may have yielded different results. Consistent with this notion, ICV injection of LPS resulted in a decrease in dendrite length and spine density on CA1 neurons 24 h after injection, but by 72 h the dendrite architecture was nearly restored (Milatovic et al., 2003). Another study also reported a decrease in the expression of ephrin receptor B1 mRNA, which is involved in neurogenesis in the adult hippocampus and in the morphogenesis of neurites and dendritic spines, 24 h after ICV injection of LPS (Bonow et al., 2008). Thus, in the current study assessing spine density and dendritic branching earlier may have revealed changes in neuron morphology. However, the 72 h time point chosen for the current study was based upon our previous observations in aged mice treated with LPS i.p. (Richwine et al., 2008).
As a cautionary note, when using gene knockout models one must consider the potential for developmental compensatory mechanisms. Further, because the congenic IL-10−/− strain is maintained homozygously, using heterozygous littermate controls to account for potential effects of maternal care is problematic. To our best knowledge, however, there have been no reports to indicate maternal differences between the congenic IL-10−/− strain and the background strain. Moreover, in the present study there were few observed differences between saline-treated wild type and IL-10−/− mice, and there appears to be no developmental compensation in the IL-10 knockouts since their inflammatory and behavioral response to LPS is greater (not equal to) than wild type controls. Because the measures were similar for the two genotypes given saline, the likelihood that developmental issues related to maternal care significantly impacted the outcomes relevant to this study, seems small.
In conclusion, the results of the present study suggest IL-10 has an important role in mediating neuro-immune communication during peripheral infection. Therefore, in conditions where IL-10 production is reduced, individuals may be prone to suffer neurobehavioral complications during infection. The findings may be particularly relevant to individuals with a polymorphism in the IL-10 promoter region that influences IL-10 production during infection (Schippers et al., 2005). The findings may also be relevant to geriatric patients as several studies with old mice or rats found brain IL-10 to be reduced (Moore et al., 2005; Ye and Johnson, 2001).
Figure 1.
Locomotor behavior in IL-10+/+ (wild type) and IL-10−/− mice 4 and 24 h after a peripheral injection of saline or lipopolysaccharide (LPS). Bars are means ± SEM. Two-way ANOVA, with post-injection time as a repeated measure, revealed significant main effects (p < 0.001) of genotype and treatment for total distance moved. Only treatment was significant for rearing frequency (p < 0.001). No genotype × treatment interactions were present. For each graph, treatment means with dissimilar letters are significantly different (p < 0.05).
Acknowledgments
This research was funded by National Institutes of Health grants AG016710 and AG023580.
References
- Agnello D, Villa P, Ghezzi P. Increased tumor necrosis factor and interleukin-6 production in the central nervous system of interleukin-10-deficient mice. Brain Res. 2000;869:241–243. doi: 10.1016/s0006-8993(00)02392-1. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bonow RH, Aid S, Zhang Y, Becker KG, Bosetti F. The brain expression of genes involved in inflammatory response, the ribosome, and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharmacogenomics J. 2008 doi: 10.1038/tpj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong BA, Schrijver HM, Huizinga TW, Bollen EL, Polman CH, Uitdehaag BM, Kersbergen MC, Sturk A, Westendorp RG. Innate production of interleukin-10 and tumor necrosis factor affects the risk of multiple sclerosis. Ann Neurol. 2000;48:641–646. doi: 10.1002/1531-8249(200010)48:4<641::aid-ana11>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Gibertini M. IL1 beta impairs relational but not procedural rodent learning in a water maze task. Adv Exp Med Biol. 1996;402:207–217. doi: 10.1007/978-1-4613-0407-4_27. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, Perry VH. Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1109–R1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Kozak W, Rudolph K, Kluger MJ. An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol. 1999;276:R81–R89. doi: 10.1152/ajpregu.1999.276.1.R81. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Anti-inflammatory drugs in the fight against Alzheimer's disease. Ann N Y Acad Sci. 1996;777:213–220. doi: 10.1111/j.1749-6632.1996.tb34421.x. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ. Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem. 2003;87:1518–1526. doi: 10.1046/j.1471-4159.2003.02120.x. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33:573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Pugh RC, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Godbout JP, Berg BM, Chen J, Escobar J, Millard DK, Johnson RW. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19:512–520. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers EF, van't Veer C, van Voorden S, Martina CA, Huizinga TW, le Cessie S, van Dissel JT. IL-10 and toll-like receptor-4 polymorphisms and the in vivo and ex vivo response to endotoxin. Cytokine. 2005;29:215–228. doi: 10.1016/j.cyto.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Martin LA, Calvert WS, Boehm GW. Effects of intraperitoneal lipopolysaccharide on Morris maze performance in year-old and 2-month-old female C57BL/6J mice. Behav Brain Res. 2005;159:145–151. doi: 10.1016/j.bbr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Thackray AM, McKenzie AN, Klein MA, Lauder A, Bujdoso R. Accelerated prion disease in the absence of interleukin-10. J Virol. 2004;78:13697–13707. doi: 10.1128/JVI.78.24.13697-13707.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]