Abstract
Daily rhythms of behavior are controlled by a circuit of circadian pacemaking neurons. In Drosophila, 150 pacemakers participate in this network, and recent observations suggest the network is divisible into M and E oscillators which normally interact and synchronize. Sixteen oscillator neurons (the small and large LNvs) express a neuropeptide called pigment dispersing factor (PDF) whose signaling is often equated with M oscillator output. Given the significance of PDF signaling to numerous aspects of behavioral and molecular rhythms, determining precisely where and how signaling via the PDF receptor (PDFR) occurs is now a central question in the field. Here we show that GAL4-mediated rescue of pdfr phenotypes using a UAS-PDFR transgene is insufficient to provide complete behavioral rescue. In contrast, we describe a ~70 kB PDF receptor (pdfr) transgene which does rescue the entire pdfr circadian behavioral phenotype. The transgene is widely but heterogeneously expressed among pacemakers, and also among a limited number of non-pacemakers. Our results support an important hypothesis: the small LNv cells directly target a subset of the other crucial pacemaker neurons cells. Furthermore, expression of the transgene confirms an autocrine feedback signaling by PDF back to PDF-expressing cells. Finally, the results present an unexpected PDF receptor site: the large LNv cells appear to target a population of non-neuronal cells that resides at the base of the eye.
Keywords: Circadian Biology, Drosophila, PDF, PDF receptor
INTRODUCTION
The circadian timing system governs daily (~24 hr) rhythmic processes via information that stems from a molecular mechanism which is classically modeled as a cell-autonomous oscillator. That model features transcriptional and post-transcriptional elements forming interlocked loops (Dunlap, 1999; King and Takahashi, 2000; Hardin, 2005). Recent studies in Drosophila and in other systems suggest that cellular interactions are critical for the normal operation of the molecular clock within individual pacemakers (Peng et al., 2003; Aton et al., 2005). Furthermore, different pacemaker cells may be functionally divisible by their inherent rhythmic properties and may normally operate within functionally-distinct oscillator sub-groups (de la Iglesia et al., 2000; Grima et al., 2004; Stoleru et al., 2004). Recent analyses of brain circuitry underlying the circadian control of behavior have therefore concentrated on the identification of key brain pacemaker regions and cells, and also on the nature of interactions that support and/or synchronize their molecular oscillations.
In the mammalian suprachiasmatic nucleus (SCN), in vivo and in vitro studies have revealed a critical role for the neuropeptide VIP to synchronize and support rhythmicity by diverse SCN pacemaker (e.g., Piggins et al., 1995; Colwell, 2000; Aton et al., 2005; reviewed by Aton and Herzog, 2005). VIP is produced by approximately 10% of SCN neurons and VIPR2, the critical receptor for circadian VIP actions is widely expressed by SCN neurons (Harmar et al., 2002; Kalamatianos et al., 2004; Aton et al., 2005). In the fly brain, about 150 neurons show robust rhythmic expression of clock genes, such as period and timeless, and they are named according to their distributed locations in the brain; three groups of dorsal neurons (DN1s, DN2s, and DN3s), three groups of lateral neurons (large LNvs, small LNvs, and LNds), and lateral posterior neurons (LPNs). Their anatomical features have been studied and summarized in several recent reviews (Helfrich-Forster, 2005; Taghert and Shafer, 2006; Nitabach and Taghert, 2008; Sheeba et al., 2008). Interactions between these dispersed pacemaker groups was first suggested based on their common projection patterns (Kaneko et al., 2000).
The neuropeptide PDF is a key component of the neuronal connections that synchronize and support molecular oscillations within the circadian pacemaker groups. It is expressed by about 10% of these 150 clock neurons of the brain, by eight large LNvs (l-LNvs) and eight small LNvs (s-LNvs). Large and small LNvs differ from each other in several important ways – for example, l-LNvs project tangentially in the distal medulla of the optic lobe, whereas s-LNvs arborize more discreetly in the dorsal protocerebrum. Under 12:12 h LD cycles (LD), normal flies exhibit bimodal locomotor behavior with a morning peak around dawn and an evening peak around dusk; continued rhythmic activity under constant dark conditions emanates primarily from the evening peak. Loss of PDF or loss of PDF-secreting LNvs results in weak or no morning peak, and a ~two hour advance in the evening peak, whereas under constant dark condition (DD) it results in de-synchrony among the clock neurons and a degree of behavioral high arrhythmicity (Renn et al., 1999a; Blanchardon et al., 2001). Loss of PDF does not perturb the cellular clock but changes its phase and or reduces its strength (Lin et al., 2004; Yoshii et al., 2009).
In the past few years, several studies have produced a novel conception of the fly circadian pacemaker circuit as a coordinated network of two oscillator groups, termed M and E (Grima et al., 2004; Stoleru et al., 2004; Stoleru et al., 2005; Rieger et al., 2006; Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007). While the models differ on how M and E control different parts of Drosophila’s daily behavioral profile, there is good consensus that coordination between distinct oscillator groups is a useful basis to model the fly’s circadian neuro-architecture. Most models agree that M cells control the morning peak, perhaps also the evening peak and the sustained activity rhythm in constant darkness (DD). M cells constitute the PDF neurons (whether these are the small or large, or both is not yet established). The E cell group is variously held to control the evening peak in LD and/or the sustained behavioral rhythm seen in constant light (LL) in some genetic conditions. M cells provide necessary drive to the E cells for their activities in both LD and LL (Murad et al., 2007; Stoleru et al., 2007). E cells include three of six LNd, the 5th small LNv (Grima et al., 2004; Stoleru et al., 2004; Bachleitner et al., 2007; Picot et al., 2007) and six to eight DN1 cells (Murad et al., 2007; Stoleru et al., 2007). More recently it was shown that when molecular rhythmicity is confined to just four (traditionally-named) E cells, there is sufficient circuit plasticity to produce both morning and evening activity peaks under entrainment by dim light cycles (Rieger et al., 2009). That observation suggests the assignments of E and M are in fact not fixed, and that differing pacemaker components can take alternative positions within the hierarchy depending on variables still to be identified. Nevertheless, the contributions of PDF and PDF-secreting neurons to behavioral rhythmicity are substantial, even under conditions when PDF cells are manipulated to lack pacemaking properties (e.g., Murad et al., Stoleru et al., 2007). PDF signaling therefore represents an important synchronizing pathway regardless of which E and M model is favored.
It was widely thought but not proven that PDF signals to non-PDF oscillators and also feeds back to control PDF cell rhythms as well. The receptor for PDF (PDFR, encoded by CG13758), is a G-protein coupled seven-transmembrane receptor (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005). Antibodies generated to detect PDFR (Hyun et al., 2005; Mertens et al., 2005) fail to display genetic specificity (Shafer et al., 2008). Using a FRET-based cyclic nucleotide sensor (called Epac-camps), Shafer et al. (2008) measured receptivity to PDF within the clock network in vivo. That study reported that all groups of clock neurons, with the exception of the large LNvs, respond to PDF with an increase in cyclic nucleotide levels. This suggested that most clock neuronal groups might express PDFR and that PDF signaling can directly connect M and E elements of the circuit. However, the latency of that assay does not preclude indirect actions nor does it preclude the involvement of other receptors. Lear et al. (2009) reported on behavioral consequences of over-expressing a UAS-pdfr transgene – they found it could rescue some aspects of the pdfr phenotype when it was restricted to the pacemaker network, in agreement with results described by Hyun et al. (2005). In addition, they found that a GAL4 transgene inserted just upstream of the pdfr transcription start site produced wide-spread expression throughout the adult brain, including in many different circadian pacemaker neurons. A novel, tethered-PDF transgene strategy was recently introduced to sample which neurons contain endogenous PDF receptivity (Choi et al., 2009): strong behavioral rhythmicity was induced when the membrane-tethered PDF was confined to small groups of cells that feature many pacemaker neurons. That result suggested PDF receptor is normally expressed widely among pacemakers.
While very informative, in fact none of these efforts from our group or others has produced a definitive anatomical visualization of PDFR expression. Therefore, we have approached that unresolved problem by using recombineering methodologies to create flies bearing a ~70 kB transgene that includes the 35 kB pdfr locus (with a 6xMYC epitope tag to express a PDFR as a fusion protein). We show that, unlike expression reconstituted by pdfr-GAL4 or other GAL4 lines which only produce incomplete rescue, a single copy of the pdfr 70 kB trangene affords complete rescue. On the basis of that complete functional validation, we show that PDFR-MYC is expressed in the clock network, and in the visual system, and in non-clock brain cells. The primary targets of the small LNvs appear to be clock neurons, consistent with feed-forward and feed-back synchronization signaling via PDF. By proximity, the primary targets of the large LNvs appear to be a population of unidentified cells near the fenestrated membrane at the base of the retina: this is an unexpected finding and suggests PDF may modulate visual input to the circadian clock through regulation of non-neuronal elements.
MATERIALS AND METHODS
Fly strains
We acquired pdfr mutant flies (pdfr3369 and pdfr5304) from Jaeseob Kim. We engineered pdfr-GAL4 lines with primer sets listed in Table 1 and w1118 fly genomic DNA as a template. All pdfr genomic fragments were sub-cloned into the pPTGAL vector, and injected to embryos of w1118 flies (Model Systems, Duke University). Multiple numbers of transgenic lines were recovered and tested for their expression pattern via crossing to UAS-lacZ flies (Figure 1 and Figure 2).
Table 1.
Primer sets used to generate pdfr-GAL4 lines
| Name | Sequence/ Usage | |
|---|---|---|
| 1 | 13758GAL4-1 | GCGCAGATCTCAATGGCAGTTGCCGTTCCCTTT |
| forward primer of pdfr(A)- and pdfr(F)- GAL4s | ||
| 2 | 13758GAL4-2 | GCGCGGATCCGGGTTGAAATCAATTGGGCAATTGC |
| reverse primer of pdfr(A)-, pdfr(B)-, and pdfr(D)- GAL4s | ||
| 3 | 13758GAL4-3 | GCGCAGATCTCCGACTAGTTTAGCCCACACTC |
| forward primer of pdfr(B)- and pdfr(C)- GAL4s | ||
| 4 | 13758GAL4-4 | GCGCGGATCCGCAACGACGTCAACATTGGCCTAG |
| reverse primer of pdfr(F)-GAL4 | ||
| 5 | 13758GAL4-5 | GCGCAGATCTGAATGAATACGTCGCAATTGTGG |
| forward primer of pdfr(D)-GAL4 | ||
| 6 | 13758GAL4-6 | GCGCGGATCCCTTCCTGATGTCTGTTAAACTATG |
| reverse primer of pdfr(C)-GAL4 | ||
| 7 | pPTGAL-S | TAGCTCCTGATCCTCTTGGCCCAT |
| pPTGAL vector primer used for sequencing | ||
| 8 | pPTGAL-AS | CGATAGAACACAGTAGCTTCATC |
| pPTGAL vector primer used for sequencing |
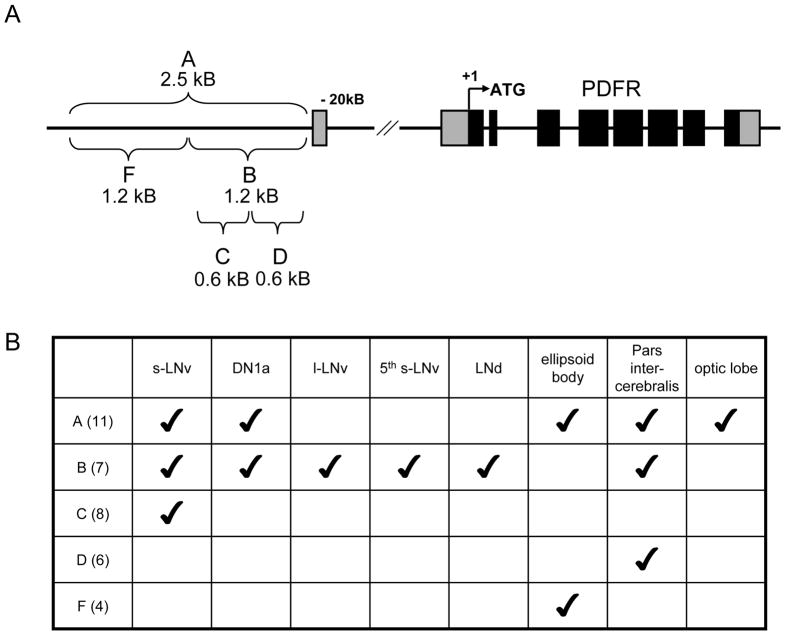
Figure 1. pdfr-GAL4 lines diagram and summary of expression patterns.
Five different pdfr-GAL4 constructs were created, all of which contain a portion of the promoter, upstream of the first exon. (A) Schematic drawing of captured promoter region in each GAL4 construct. (B) Summary table of expression patterns of each GAL4 construct. The first row lists brain regions examined for β–GALACTOSIDASE expression driven by GAL4 lines, whereas the first column lists five pdfr-GAL4 lines and number of independent transgenic fly lines examined for each construct.
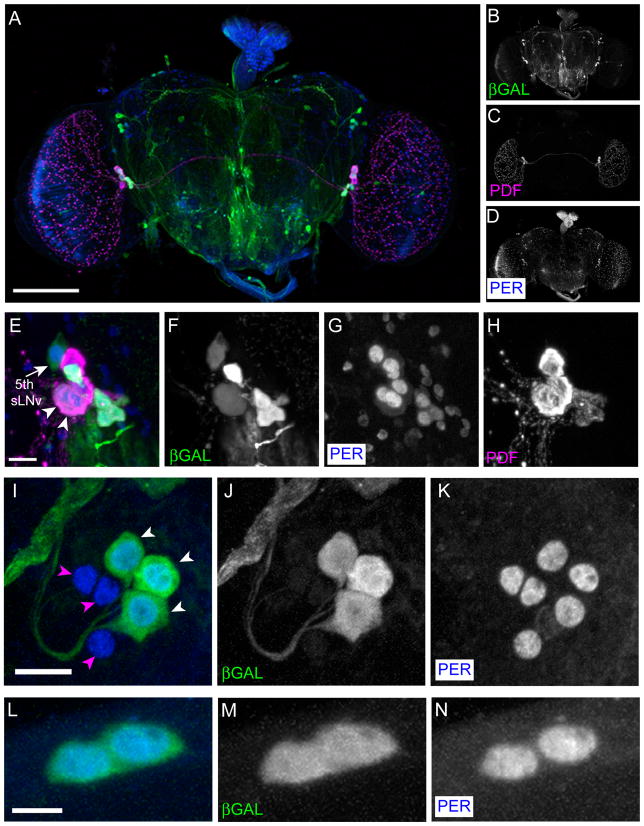
Figure 2. pdfr(B)-GAL4-2 expression pattern.
pdfr(B)-GAL4-2;UAS-lacZ were reared at 29 °C and stained with anti-β-GAL (green), anti-PDF (Magenta), and anti-PER (blue). (A–D) A Z-series projection of the entire brain. (A) Merged image of (B) (β-GAL, green), (C) (PDF, magenta), and (D) (PER, blue). Scale bar, 100 μm. (E–H) LNvs express pdfr(B)-GAL4-2. (E) Merged image of (F) (β-GAL, green), (G) (PER, blue), and (H) (PDF, magenta). Among the LNvs, four PDF-expressing s-LNvs strongly express β-GAL, the 5th s-LNv (arrow) and two of four l-LNvs (arrowheads) weakly express it. Scale bar, 10 μm. (I–K) LNds express pdfr(B)-GAL4-2. (I) Merged image of (J) (β-GAL, green) and (K) (PER, blue). Three LNds out of six show very strong pdfr(B)-GAL4-2 expression (white arrowheads), whereas the others show none (magenta arrowheads). Scale bar, 10 μm. (L-N) Both DN1a cells express pdfr(B)-GAL4-2. (L) Merged image of (M) (β-GAL, green) and (N) (PER, blue). Scale bar, 5 μm.
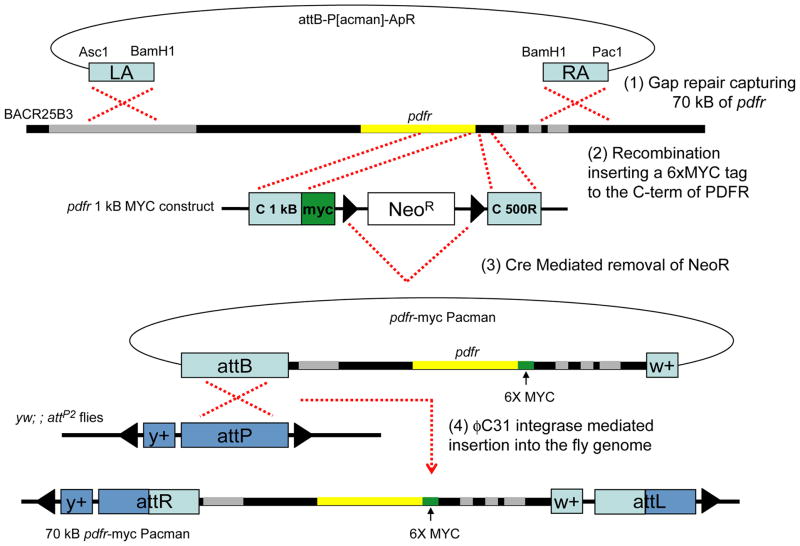
To build the 70 kB pdfr-myc construct and transgenic flies we obtained general recombineering procedures from: http://recombineering.ncifcrf.gov/ and specific procedures for the use of P[acman] from: http://flypush.imgen.bcm.tmc.edu/lab/pacman.html (Venken et al., 2006). All primer sets are listed in Table 2. 70 kB pdfr-myc flies were engineered with four steps of DNA manipulation and fly transgenesis as illustrated in Figure 4. Step (1), to capture 70 kB of genomic DNA including pdfr gene, 500 bp of left (LA) and right (RA) homologous arms amplified and subcloned into attB-P[acman]-ApR. Linearized attB-P[acman]-ApR with LA and RA were transformed into SW102 bacterial cells containing BACR25B3 clone using electroporation and selected by Ampicillin resistance. Single colonies were tested with PCR, sequencing, and restriction enzyme digestion analysis. Step (2), to insert the 6X MYC epitope tag at the C-terminal of the protein, we constructed an 1 kB sequence encoding the C-term into UAST-myc vector, and then transferred this C-term and myc into a neomycin resistance cassette that already contained ~500 bp of pdfr 3’UTR sequence following the NeoR sequence. Homologous recombination occurred between the P[acman] 70 kB PDFR and the myc NeoR cassette and we selected for Neomycin resistance. Step (3), the NeoR cassette has two flanking loxP sites, which later were used to remove the NeoR cassette with Cre recombinase expression induced by Arabinose in EL350 bacterial cells, leaving one loxP site along with ~20 neighboring nucleotides. The P[acman] PDFR-MYC was transferred to the EPI300 bacterial strain, where it was amplified for injection (CopyControl Induction Solution, Epicentre Biotechnologies, Cat No. CCIS125). Step (4), the construct was injected into embryos of nos-φC31 integrase; ; attP2/+ flies (Genetivision Inc, Houston, Texas). P0 flies were crossed to w1118, and F1 transformants subsequently crossed to yw to confirm both transgenesis markers. The line is maintained in a w1118 background. P[acman] pdfr-myc70 flies are homozygous viable.
Table 2.
Primer sets used to generate pdfr-myc flies
| name | sequence | usage | |
|---|---|---|---|
| 9 | pdfr-LA-AscI-F | AGGCGCGCCGTTTCCTGGCAAGTTTGCGC | subclone the left homologous arm into P[acman] |
| 10 | pdfr-LA-BamHI-R | CGCGGATCCGATGCAATGCATTTGTTACTGC | |
| 11 | pdfr-RA2-BamH1-F | CGCGGATCCGATCGTGATTTGAGAAGAGCTC | subclone the right homologous arm into P[acman] |
| 12 | pdfr-RA2-Pac1-R | ACCTTAATTAACATGATTACGAGAGAGATGGG | |
| 13 | pacman MCS-F | TTTAAACCTCGAGCGGTCCGTTATC | confirm both homologous arm subclones |
| 14 | pacman MCS-R | CTAAAGGGAACAAAAGCTGGGTAC | |
| 15 | pdfr-5′ check-R | GACACTGCGTTGTTGTCATCTAATTG | confirm step (1) gap repair |
| 16 | pdfr-3′ check-F2 | GTCTGTAGTGAAAGAATGCGC | |
| 17 | pdfr-LA-seq-F | GCATGGCCTGATCACTACAG | |
| 18 | pdfr-RA2- seq-R | GATTAACACAGAATGACAGAGG | |
| 19 | 1KB 3′ pdfr F | ATTTGCGGCCGCCGCTTTAATCACCTATTCACCC | subclone Cterm 1 kB into pUASTmyc vector |
| 20 | 1kB 3′ pdfr R | ATTTGCGGCCGCTTCTGCTCTGACAACTCAAATACAA | |
| 21 | pdfr C+myc F | GGCTAGCCGCTTTAATCACCTATTCACC | subclone 1 kB+myc into 3loxPNeoR vector |
| 22 | pdfr C+myc R | CTCTAGAGGCTAGAGAGGCCTTG | |
| 23 | pdfr C 500R F | ACCTTAATTAAACCAAAACCTAGCCTAACTAAT | subclone C term 500 bp the right homolgous arm |
| 24 | pdfr C 500R R | CCTCGAGTGAATGTATTTGCGTGTCCAG | |
| 25 | Neo F | AATATGTATCCGCTCATGAGAC | check step (2) homologous recombination |
| 26 | Neo R | CACCGTGCGTTTTATTCTGTC | |
| 27 | Neo 5′ R | GTCTCATGAGCGGATACATATT | |
| 28 | Neo 3′ F | GACAGAATAAAACGCACGGTG | |
| 29 | myc F | GCGGCCGCGGCTCGACTCCCATCGA | check step (2) homologous recombination and check step (3) removal of NeoR |
| 30 | pdfr LJ | GATCATTCGCCTGACCTTGTA | |
| 31 | pdfr LLB | TACTGTGGTGGTGGGCTACA | |
| 32 | attP-F | CTTCACGTTTTCCCAGGTCAGAAG | check step (4) recombination between attB/attP |
| 33 | attP-R | GTCGCGCTCGCGCGACTGACGGTC | |
| 34 | attB-F | GTCGACGATGTAGGTCACGGTC | |
| 35 | attB-R | TCGACATGCCCGCCGTGACCGTC |
Figure 4. Diagram of 70 kB pdfr-myc P[acman].
Four steps used to generate the 70 kB pdfr-myc P[acman] flies. Step (1). Gap repair between linearized P[acman] and BACR25B3 at pdfr locus. Step (2). Recombination and insertion of a 6xMYC tag and Neomycin resisitance selection marker at the C terminus of pdfr. Step (3). Cre recombinase mediated removal of NeoR cassette. Step (4). φC31-mediated integration of 70 kB pdfr-myc P[acman] at attP2 site (681A-B2, Groth et al., 2004).
Behavioral Analyses
All locomotor activity experiments were conducted with 1–2 days-old male flies at 25°C. We used w1118 as control flies for pdfr3369 and pdfr5304 as originally reported (Hyun et al., 2005). Locomotor activities were monitored for 6 days under a 12h:12h LD cycle, and for 9 days under DD condition. To analyze the presence or absence of a morning peak, we modified a measurement introduced by Harrasingh et al. (2007). Using the Brandies Rhythm Package, we normalized each fly’s activity and computed group (=genotype) ratios over Days 4–6 of entrainment: the ratio describes the average activity in the three hours preceding the lights-on cue (ZT22-24) divided by the average activity in the six hours preceding that cue (ZT19-24). This measure focuses on anticipation and avoids any direct influence of the light. To analyze rhythmicity under constant conditions, we normalized the activity of flies from DD day 3 to day 9 and used χ2-periodogram analysis with a 95% confidence cut-off, as well as SNR analysis (Levine et al., 2002). Arrhythmic flies were defined by a power value < 10 and width value < 1, and period outside the range, 18 to 30 hours. One way ANOVA measures were followed by the Tukey-Kramer Multiple Comparisons Test and employed Graphpad Instat software.
Immunocytochemistry
Immunocytochemistry was performed in wholemount or upon sections and male flies were used throughout. For wholemounts, brains were dissected in Ca2+-free fly saline and fixed for 1 hour at room temperature in 4 % (w/v) paraformaldehyde, 7 % (v/v) picric acid in PBS (pH 7.4). The tissues were incubated in primary antibodies (sources, dilutions and specificity tests listed in Table 3) for ~48 hr at 4 °C, and in 1:1,000 diluted secondary antibodies for one overnight at 4°C. Rabbit anti-MYC antibody and mouse anti-MYC antibody were used to identify PDFR-MYC expressing cells and rat anti-PER, rabbit anti-βPDH, guinea pig anti-proPDF, and mouse anti-PDF were used to mark the clock neurons. Diluted anti-MYC antibodies (1:100) were first pre-incubated for 24 hr at 4oC with agitation with fixed brains that had been dissected from flies that lacked the MYC-tagged transgene. For pdfr-GAL4 expression pattern studies, GAL4 flies were crossed to UAS-lacZ, and stained with mouse anti-βGal antibody (Promega, Madison, WI). We used secondary antibodies that were conjugated to Alexa 488, Alexa 568, or Alexa 633 (Molecular Probes, Eugene, OR).
Table 3.
Properties of the primary antibodies used in this study.
| Antigen | Immunogen | Manufacturer, species, mono vs poluclonal, catalog no. | Dilution used |
|---|---|---|---|
| MYC | amino acids 410–419 (EQKLISEEDL) of human myc conjugated to KLH. | Bethyl, Rabbit, polyclonal, Cat no. A190-105A | 1:400 |
| MYC | amino acids 410–419 (EQKLISEEDL) of human myc conjugated to KLH. | New England Biolabs, mouse, monoclonal, Cat no. 2276 | 1:1,000 |
| PERIOD | recombinant Drosophila PER (C-terminal 1,108 amino acids) | M. Rosbash, Brandies Univ. Liu et al.(1992), Rat, polyclonal | 1:1,000 |
| βPDH | Full length peptide of Uca Pugilator βPDH | Dircksen et al.(1987), Rabbit, polyclonal | 1:2,000 |
| pro-PDF | amino acids of 65–79 of Drosophila pre-pro-PDF (H-YPLILENSLGPSVPI- OH) conjugated to BSA | Renn et al.(1999), GuineaPig, polyclonal | 1:1,000 |
| amidated PDF peptide, NSELINSLLSLPKNMNDA- NH2 | DSHB, Cyran et al. (2005) | 1:1,000 | |
| REPO | Recombinant Drosophila REPO (amino acids 218–612) fused to 6xhistidine | DSHB, Alfonso and Jones. (2002) | 1:10 |
| β–Galactosidase | 650–926 amino acids of E. coli β–Galactosidase | Promega, mouse, monoclonal, Cat no. Z378A | 1:1,000 |
For sections, heads were frozen in O.C.T. (VWR), and sectioned at 15 to 20 μm thickness. Tissues were immediately fixed with 4 % paraformaldehyde (w/v) for 15 minutes and washed with PBS-Tx (1X PBS containing 0.3 % triton-X100) twice for 15 minutes. Before 1 hour of blocking with 5% normal goat serum in PBS-Tx, tissues were treated with 1 % NaBH4 for 15 minutes to reduce the background by reducing free aldehyde group (Nässel, 1996). Primary antibodies were mouse anti-MYC, guinea pig anti-PDF, and mouse anti-REPO, and Alexa 488-and 568-conjugated secondary antibodies (1:200) were used. Tissues were incubated with primary antibodies at 4°C overnight, and following three PBS-Tx washes, they were incubated with secondary antibodies for two hours at room temperature or overnight at 4°C. Images from both whole mount and head sections were obtained on an Olympus 500 Fluoview Confocal microscope and the lookup tables were used to set appropriate PMT voltage and laser power. Confocal stacks were projected in Image J and edited for brightness and contrast in Photoshop.
Antibody Characterization
Please see Table 3 for a list of all antibodies used.
The commercial rabbit anti-MYC antibody was generated to the amino acids 410–419 of human myc but its specificity was not tested by the manufacturer. We tested its specificity by comparing signals in the fly stock containing a MYC-tagged receptor protein with a control stock (as described below): the control stock exhibited only weak nuclear staining and no cytoplasmic signals, as found in the experimental stock. The mouse anti-MYC antibody was tested by the manufacturer to specifically recognize Myc-tagged protein on western blot of cultured cells and to stain only the cells expressing Myc-tagged protein.
The PER antibody (kindly provided by M. Rosbash) recognized PER on Western blots and failed to label tissue in per01 mutants (Liu et al., 1992).
The PDH antibody was tested for its specificity by preadsorption of the antiserum with U. pugilatorβ-PDH (Dircksen et al., 1987), which resulted in a preparation that generated no visible staining in the sinus gland of U. pugilator. Also, in Drosophila, that antiserum did not stain the PDF expressing LNv cells in Pdf01 mutants (Renn et al. 1999).
The pro-PDF antibody failed to stain the PDF expressing LNv cells in Pdf01 mutants (Renn et al. 1999).
The mouse monoclonal PDF antibody failed to stain the PDF expressing LNv cells in Pdf01 mutants (Cyran et al. 2002).
The REPO antibody recognizes REPO protein in Drosophila expressed in the nuclei of all glial cells except for the midline glia. It fails to stain in Drosophila tissue lacking glial cells (Alfonso and Jones. 2002).
The β–Galactosidase antibody was tested by the manufacturer for specificity on dot-blots, and it fails to stain in Drosophila tissue without β–Galactosidase expression.
Results
pdfr-GAL4 lines
We began our analysis using a conventional approach of building Gal4 driver lines that could harness the regulatory elements of the gene of interest – here pdfr. The pdfr locus spans ~35 kB and includes a large ~20 kB first intron. To map the expression pattern of PDFR, we made a series of GAL4-based constructs containing 0.5~2.5 kB promoter fragments (Figure 1) and tested whether these transgenic drivers could rescue the behavioral defects of two severe pdfr mutants (pdfr5304 and pdfr3369) that were originally described by Hyun et al.2005. The expression patterns of the GAL4 lines varied according to promoter fragment used, and according to individual transgenic line, as summarized in Figure 1. Many lines harboring transgenes that contain the C fragment of the promoter included PDF-expressing s-LNvs (Figure 1). One of the B fragment transgenic lines, pdfr(B)-GAL4-2, includes s-LNvs and l-LNvs, the 5th s-LNv, three LNds, two DN1as, and many other non-clock neurons as well (Figure 2), and this line was most effective (though not complete) in restoring rhythmic behavior (which is described in the following section). We could not find any individual lines that specifically express only in a subgroup of clock neurons populations, for example, pdfr(C)-GAL4 lines always include expression in numerous glial cells.
Attempts at rescue of pdfr behavioral phenotypes with various GAL4 lines
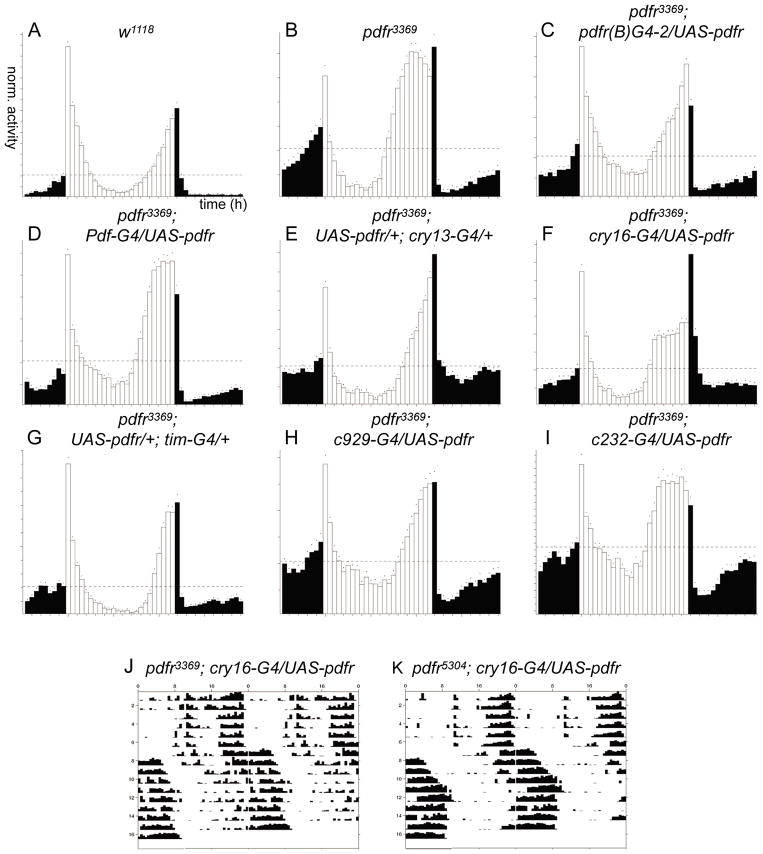
Flies deficient in Pdf (Pdf01) entrain to a light dark cycle, but with two aberrant features – they lack an activity peak that would normally anticipate the lights-on transition (the morning peak) and they display an advanced phase of the activity peak that anticipates the lights-off transition (the evening peak) (Renn et al., 1999a). Both pdfr deletion mutant alleles, pdfr5304 and pdfr3369, were originally described as displaying a phenocopy of the Pdf mutant behavioral syndrome (Hyun et al., 2005) and similarly described by Lear et al. (2009). Hyun et al. (2005) measured the morning activity by considering activity immediately following the light-on signal and concluded that the pdfr mutants phenocopy pdf mutants. However we find that only the advanced evening peak phase is a reproducible feature of these same pdfr mutant flies. On average, flies bearing the smaller deletion allele (pdfr3369) maintained a morning peak in most experiments, and even flies with the larger deletion (pdfr5304) showed it once (in four separate experiments; each experiment tested >30 flies) (Figure 3 for pdfr3369 and Figure 5 for pdfr5304). A morning peak also appeared in male progeny of pdfr5304 in certain control crosses such as (pdfr5304 x cry13 GAL4) or (pdfr5304 x UAS-pdfr-16) (data not shown), suggesting the absence of the morning peak was not stably-associated with either of the pdfr mutations. To quantify this effect, we employed an anticipation index (described in Methods) and compared the “morning behavior” of different genotypes. The two pdfr mutations scored an average of 0.63 +/− 0.08 (pdfr3369) and 0.55 +/− 0.06 (pdfr5304) while w1118 produced a score of 0.62 +/− 0.06; in contrast, Pdf01 - there exists a broad consensus that this strain lacks a morning peak - scored 0.43+/− 0.04. The two pdfr mutants scored similar to the control strain and statistically different from Pdf01 (Table 4). Therefore, to measure potential rescue of pdfr mutant phenotypes, we have restricted consideration to the phase of the evening peak under entraining conditions, and to the percentage and strength of rhythmicity in constant dark conditions during days 3–9 (DD3-9). UAS- pdfr-16 (Mertens et al., 2005) driven by pdfr(B)-GAL4-2 fully rescued the evening peak LD phenotype of pdfr mutants, but only partially rescued the DD3-9 phenotype (Figure 3 and Table 5). Similar partial rescue were observed with other pdfr-GAL4 drivers (i.e. pdfr(A)-GAL4-2, pdfr(A)-GAL4-7) that we tested (data not shown).
Figure 3. Analysis pdfr mutant behavior rescue using various GAL4s.
Circadian behavior was monitored following partial restoration of PDFR expression by various GAL4 lines driving UAS-pdfr-16. (A–I) 12 h:12 h LD behavior group analysis of w1118, han3369, and han3369 tested with UAS-pdfr driven by various GAL4 lines. (J–K) Representative actograms of single flies with cry16-GAL4-driven rescue.
Figure 5. The 70 kB pdfr-myc transgene rescues pdfr behavioral defects.
Representative examples of circadian behavior of three independent experiments. (A–D) Averaged activity for 6 days in a 12 h:12 h LD cycle. (A) w1118 control flies. (B) pdfr5304, a large deletion mutant of pdfr. (C) pdfr5304; ; attP2, pdfr5304 with an empty docking site. (D) pdfr5304; ; pdfr-myc/+, pdfr5304 with a single copy of pdfr-myc. (E–H) Representative actograms of single flies of each genotype for 6 days in LD and 9 days in DD. Wild-type control w1118 exhibits normal morning and evening peaks under LD and a free-running rhythm with a 23.5 hr period (A, E). A larger pdfr deletion (pdfr5304) produced an advanced evening peak and weak or no morning peak; under DD, ~50 % of pdfr5304 became arrhythmic and ~50% displayed short, weak rhythms of ~22 hr period (B, F). pdfr5304; ; attP2 – control for the rescue experiment: pdfr5304 mutant flies containing the attP2 docking site that lacks any pdfr sequences. This control construct cannot rescue the behavioral defects of either pdfr3369 (data not shown) or pdfr5304 (C, G). pdfr5304; ; pdfr-myc/+: 70 kB pdfr-myc transgene in pdfr5304 background. Both LD and DD behavioral defects of both pdfr mutants were rescued by the 70 kB pdfr-myc transgene (D, H) (compiled data is presented in Table 6).
Table 4.
Analysis of Morning Activity –Tukey-Kramer Multiple Comparisons Test, following a one way ANOVA (P< 0.0002)
| Comparison | Difference | q | P value |
|---|---|---|---|
| w1118 vs pdf01 | 0.1870 | 7.166 | *** P<0.001 |
| w1118 vs han3369 | 0.006467 | 0.26 | ns P>0.05 |
| w1118 vs han5304 | 0.07003 | 2.684 | ns P>0.05 |
| pdf01 vs han3369 | 0.1924 | 7.414 | *** P<0.001 |
| pdf01 vs han5304 | 0.1169 | 4.291 | * P<0.05 |
| han3369 vs han5304 | 0.0765 | 2.932 | ns P>0.05 |
Table 5.
Behavioral rescue of pdfr mutants using various GAL4 lines under constant dark condition (DD3-9).
| GAL4 elements | N | AR% | Per-tau | pwr | wid | SNR | ACT-Day | ACT-Night | ACT-Cycle | |
|---|---|---|---|---|---|---|---|---|---|---|
| w1118 | 178 | 14.0 | 23.9 | 60.8 | 5.4 | 0.90 | 14.1 | 6.5 | 10.3 | |
| pdfr3369 | 175 | 78.3 | 22.9 | 18.0 | 2.2 | 0.42 | 12.3 | 12.6 | 12.4 | |
| pdfr5304 | 145 | 59.3 | 22.7 | 25.1 | 3.6 | 0.46 | 9.5 | 12.0 | 10.7 | |
| pdfr3369; UAS-pdfr16/+ | ||||||||||
| none | 117 | 85.5 | 23.3 | 16.2 | 2.8 | 0.37 | 13.6 | 12.6 | 13.1 | |
| pdfr(B)-GAL4 | 92 | 55.4 | 23.9 | 21.9 | 3.3 | 0.47 | 17.9 | 13.8 | 15.8 | |
| Pdf-GAL4 | 30 | 93.3 | 22.8 | 14.7 | 2.5 | 0.29 | 11.5 | 12.7 | 12.1 | |
| cry13-GAL4 | 31 | 38.7 | 23.7 | 26.6 | 3.6 | 0.45 | 23.9 | 15.0 | 19.5 | |
| cry16-GAL4 | 61 | 27.9 | 24.5 | 45.9 | 4.8 | 0.94 | 13.9 | 18.1 | 16.1 | |
| tim-GAL4 | 55 | 52.7 | 23.7 | 32.8 | 3.5 | 0.63 | 14.7 | 9.7 | 12.2 | |
| tim-(uas)-GAL4 | 43 | 44.2 | 23.4 | 39.5 | 4.6 | 0.67 | 15.2 | 10.7 | 12.9 | |
| c929 | 38 | 92.1 | 23.5 | 16.2 | 2.5 | 0.43 | 14.4 | 13.5 | 14.0 | |
| c232 | 20 | 95.0 | 25.0 | 11.3 | 2.0 | 0.38 | 12.2 | 11.1 | 11.6 | |
| pdfr5304; UAS-pdfr16/+ | ||||||||||
| none | 93 | 65.6 | 23.1 | 39.3 | 4.5 | 0.58 | 14.8 | 14.9 | 14.9 | |
| pdfr(B)-GAL4 | 95 | 53.7 | 23.8 | 28.5 | 4.0 | 0.58 | 20.7 | 15.5 | 18.1 | |
| cry13-GAL4 | 12 | 41.7 | 25.0 | 29.4 | 3.6 | 0.45 | 24.9 | 15.6 | 20.3 | |
| cry16-GAL4 | 59 | 13.6 | 24.7 | 92.3 | 7.4 | 2.26 | 14.0 | 28.6 | 21.3 | |
| tim-GAL4 | 26 | 46.2 | 23.5 | 52.5 | 5.0 | 1.01 | 17.3 | 14.2 | 15.7 | |
| tim-(uas)-GAL4 | 45 | 33.3 | 23.5 | 35.8 | 4.0 | 0.68 | 16.7 | 11.8 | 14.2 | |
| c929 | 31 | 96.8 | 23.0 | 4.7 | 2.0 | 0.27 | 16.1 | 17.1 | 16.6 | |
|
Control groups | ||||||||||
| pdfr3369 | ||||||||||
| pdfr(B)-GAL4 | 62 | 87.1 | 22.6 | 14.6 | 2.1 | 0.33 | 13.0 | 12.1 | 12.6 | |
| cry13-GAL4 | 8 | 100.0 | 10.8 | 10.8 | 10.8 | |||||
| tim-(uas)-GAL4 | 16 | 43.8 | 23.4 | 25.9 | 4.0 | 0.62 | 17.3 | 12.9 | 15.1 | |
| pdfr5304 | ||||||||||
| pdfr(B)-GAL4 | 54 | 85.2 | 23.6 | 15.8 | 1.9 | 0.37 | 15.0 | 14.8 | 14.9 | |
| cry13-GAL4 | 16 | 62.5 | 22.8 | 19.3 | 3.2 | 0.55 | 12.5 | 14.6 | 13.5 | |
| cry16-GAL4 | 32 | 93.8 | 24.5 | 26.0 | 3.0 | 0.50 | 15.3 | 13.5 | 14.4 | |
| tim-(uas)-GAL4 | 16 | 31.3 | 23.7 | 26.7 | 3.9 | 0.48 | 11.9 | 8.9 | 10.4 | |
Because the expression pattern of many pdfr-GAL4 lines included clock neurons, we went on to assay similar, broadly-acting GAL4 drivers, as well as GAL4 drivers that are more specific for certain brain areas or defined cell classes. These included Pdf(BMRJ)-GAL4, cry13-GAL4, cry16-GAL4, tim-(uas)-GAL4, and tim-GAL4 for clock neurons, c232-GAL4 marking a subset of ellipsoid body (EB) neurons, and c929–GAL4 marking a subset of peptidergic neurons (Renn et al., 1999a; Emery et al., 2000; Zhao et al., 2003; Blau and Young, 1999; Kaneko and Hall, 2000; Renn et al., 1999b; Park et al., 2008). In the adult brain, Pdf-GAL4 is limited to the large and small LNvs. cry13-GAL4 includes some of the ~150 clock neurons and certain ellipsoid body neurons, but does not include all the clock neurons. cry16-GAL4, tim-GAL4, and tim-(uas)-GAL4 are widely-expressed in clock neurons and also in numerous non-clock cells as well (Kaneko and Hall, 2000; Choi et al., 2009; Kilman and Allada, 2009). The evening peak phenotype of pdfr mutant flies under cycling conditions was restored with cry13-GAL4, tim-GAL4, tim-(uas)-GAL4, and c929-GAL4, while Pdf-GAL4, cry16-GAL4 and c232-GAL4 did not provide rescue of the LD phenotype (Figure 3). However under constant conditions (DD3-9), none of these GAL4s could restore rhythmic behavior to wild-type levels (Table 5). Most of the GAL4 lines produced only a partial rescue: many had normal periods, but they also exhibited marginal reductions in the percentage of arrhythmic flies, and marginal increases in power and SNR. Interestingly, cry16-GAL4 generated a new phenotype: PDFR rescue driven by cry16-GAL4 restored strong rhythmic behavior in DD3-9 rhythmic behavior, but the phase was delayed by six to ten hours. In approximately 50% of the rhythmic flies this delay happened instantaneously, while in others it took three to four days to shift and then free-run with a period of ~23.7 h (Figure 3). Zhao et al. (2003) reported that cry16-GAL4 itself has long period phenotype, and we observed the same with cry16-GAL4 alone. The phase shift and free-running with six hours of delay happened only when we reconstituted PDFR expression using cry16-GAL4. Although cry16-GAL4 mediated rescue was the strongest for DD3-9 behavior, because of its broad expression pattern beyond clock neurons, and because of its failure to restore proper phase in LD, we could not conclude from these results where PDFR expression is required for its normal behavioral functions. Therefore, to determine with precision, where and when PDF signaling must occur in the circadian neural circuit, we felt there remained substantial value in defining critical and authentic PDFR-expressing cells using the gene’s own regulatory elements.
Building a more accurate reporter of PDFR, pdfr-myc P[acman]
Because the pdfr gene extends across a large genomic interval, and to overcome the size limitations of P element-mediated transgenesis, we created a pdfr transgenic stock by recombineering using the recently-introduced P[acman] vector and the φC31-mediated method of Drosophila transgenesis (Venken et al., 2006). As described in the Methods section and summarized in Figure 4, these flies possess a transgene that harbors ~70 kB of the pdfr inserted at a defined genomic location on the IIIrd chromosome, within which the pdfr open reading frame is fused with a 6xMYC epitope tag at its C-terminus. All experiments described below were controlled by observations made on flies containing the empty P[acman] vector transduced to the same genomic locus, or on flies containing the empty docking site, attP2.
The 70 kB pdfr-myc transgene completely rescues circadian behavior defects of PDFR mutants
First we asked if the 70 kB pdfr-myc construct can restore normal circadian behaviors in the two pdfr mutant backgrounds. Strikingly, a single copy of pdfr-myc rescued both LD and DD defects of both pdfr mutants (Figure 5 and Table 6). Figure 5A-D shows that LD group averages for control flies and pdfr mutant flies rescued by pdfr-myc, among which the rescued flies display a normal phase in evening peak; Figure 5E-H shows representative actograms of single flies for 6 days in LD and 9 days in DD. In short, both pdfr mutants displayed complete restoration of normal behaviors with a single copy of the transgene, and this suggests that the 70 kB pdfr-myc can generate most if not all the attributes of endogenous pdfr function.
Table 6.
Summary of behavior rescue of pdfr mutants by pdfr-myc under constant dark conditions (DD3-9).
| N | AR% | Per-tau | pwr | wid | SNR | ACT-Day | ACT-Nit | ACT-Cyc | |
|---|---|---|---|---|---|---|---|---|---|
| w1118 | 85 | 15.3 | 23.6 | 70.3 | 5.8 | 1.09 | 12.0 | 4.3 | 8.1 |
| pdfr3369 | 87 | 89.7 | 22.9 | 15.5 | 2.1 | 0.35 | 11.6 | 12.5 | 12.1 |
| pdfr5304 | 87 | 50.6 | 22.5 | 26.8 | 3.8 | 0.49 | 11.0 | 13.2 | 12.1 |
| w1118; ; pdfr-myc/+ | 88 | 3.4 | 23.6 | 73.6 | 5.9 | 0.99 | 21.5 | 7.5 | 14.5 |
| pdfr3369; ; pdfr-myc/+ | 89 | 9.0 | 23.8 | 50.3 | 5.3 | 0.80 | 20.1 | 8.1 | 14.1 |
| pdfr5304; ; pdfr-myc/+ | 89 | 12.4 | 23.8 | 58.6 | 5.3 | 0.79 | 18.1 | 8.2 | 13.1 |
| w1118; ; attP2/+ | 58 | 12.1 | 23.3 | 47.6 | 4.5 | 0.67 | 24.5 | 13.4 | 18.9 |
| pdfr3369; ; attP2/+ | 87 | 77.0 | 23.0 | 24.7 | 3.7 | 0.49 | 15.6 | 16.1 | 15.8 |
| pdfr5304; ; attP2/+ | 89 | 75.3 | 22.6 | 18.3 | 2.8 | 0.37 | 11.2 | 14 | 12.6 |
The expression patterns of PDF and PDFR-MYC
We reasoned that the functional attributes of the 70 kB pdfr-myc transgene validated its use as an anatomical reagent to evaluate PDFR expression. We wondered if PDFR-MYC-expressing cells would localize in close conjunction to sites of PDF expression/release. The proximity of neuropeptide receptors to sites of release for cognate peptides varies according to the system studied. In the extreme (e.g., Ruocco et al., 2001), certain receptors are found far removed from the suspected source of peptide ligands – a situation described by the model of volume transmission (Nicholson and Sykova, 1998). In the case of PDF signaling, previous genetic studies (Helfrich-Forster, 1998) correlated the topographic precision of small LNv projections in the dorsal protocerebrum with the strength of circadian rhythmic behavior. That evidence supported the hypothesis that PDF must be released within close proximity of its nominal targets to have normal physiological effect. We therefore examined PDFR-MYC expression within the context of the three principal PDF release sites: these are produced by the three main PDF-producing cell types in the adult brain (Helfrich-Forster, 1997). Figure 6A and 6B show schematic drawings of PDF neurons and their projections. The first PDF-expressing cell type is the small LNv which projects to the dorsal protocerebrum in the vicinity of other circadian pacemaker (DN) cell bodies and their processes (Park et al., 2000; Helfrich-Forster, 2005). The second cell type is the large LNv which produces a large tangential arbor in the distal medulla that runs perpendicular to the incoming retinotopic projections of photoreceptors; large LNv also project contra-laterally in the posterior optic tract (POT). Finally, a transient pair of tritocerebral neurons (PDF-tri) arborizes along the median bundle and ventral to the eosophageal foramen: PDF-tri neurons express PDF until the first adult day, when they appear to undergo programmed cell death (Renn et al., 1999a).
Figure 6. PDF and PDFR-MYC expression.
Brains of w1118; ; pdfr-myc flies were immunostained with anti-MYC (green) and anti-PDH (magenta) antibodies. (A) Schematic drawing of PDF-expressing neurons in the fly brain in frontal view. Dorsal is to the top and ventral to the bottom. Yellow inner boxes depict the region of images presented in panels (C) and (I). (B) Schematic drawing of PDF-expressing neurons in the fly brain in horizontal view. Anterior is to the top and posterior to the bottom. A yellow inner box depicts the region of images represented in panel in (F). (C–E) Z-series projections of dorsal brain imaged from the posterior aspect. (C) Merged image of (D) (MYC, green) and (E) (PDF, magenta). Red asterisks indicate the incidence of non-pacemaker receptor-positive cell bodies. (F–H) Sub-retinal glial cells express PDFR-MYC at the boundary of retina and lamina; and large LNv PDF is localized in distal medulla. (F) Merged image of (G) (MYC, green) and (H) (PDF, magenta). (I–K) Suboesophageal ganglion (SOG) with PDF-tri cells imaged from the anterior aspect. (I) Merged image of (J) (MYC, green) and (K) (PDF, magenta). Arrows, PDF-tri cell bodies. re, retina; la, lamina; me, medulla. Scale bars, 50μm.
Significantly, we found corresponding PDFR-MYC expression in proximity to each of these three PDF-positive cell groups. Figure 6 shows confocal images of PDF and PDFR-MYC double staining in the central brain and visual system. There is strong expression in projections along the dorsal projections from s-LNvs (Figure 6C), and also weaker but noticeable expression in POT from l-LNvs (Figure 6C). The most extensive PDF positive varicosities are localized on the medulla layer. We could not find PDFR-MYC expression in the optic lobe with the whole mount brain staining, in which lamina and retina have been removed. To examine potential sites of PDFR-MYC expression in the visual system, we sectioned fly heads and performed immunocytochemistry with anti-PDF and anti-MYC antibodies. Figures 6F and 7 reveal PDF immunosignals in the medulla and PDFR-MYC signals at the boundary of the lamina and retina. The MYC expression represents a population of cells at or near the fenestrated membrane that lies at the base of the retina. Antibody staining with anti-REPO antibodies to label glial cells mark several layers of sub-retinal cells; PDFR-MYC expression in this region is more limited (narrower) but its precise correspondence to glial cells remains uncertain (Figure 7). Finally, ten or so cell bodies and processes were stained for PDFR-MYC in conjunction with the processes of PDF-tri cells in the region between the tritocerebrum and suboeosophageal ganglion (SOG) (Figure 6I). Thus the most intensely-stained PDFR-MYC cells and projections were found in close correspondence to PDF-expressing processes. In addition, all major PDF-expressing projections were aligned with correspondent putative receptor populations.
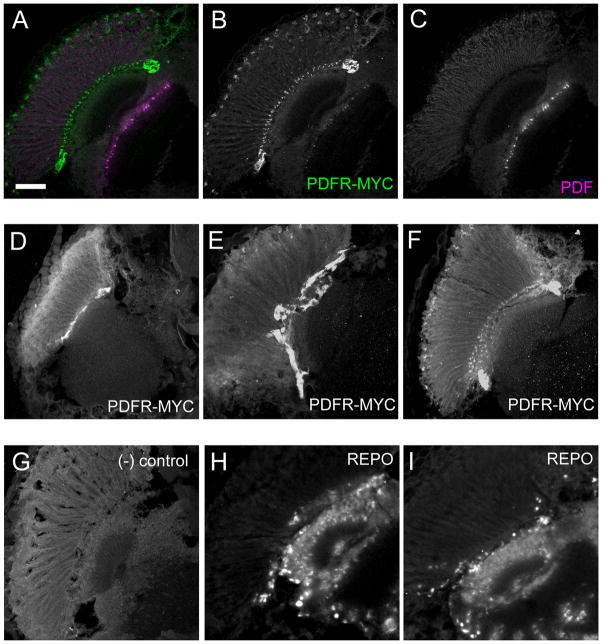
Figure 7. PDFR-MYC in a subset of cells near the fenestrated membrane of the retina.
Adult fly heads sections immunostained showing PDFR-MYC expression near the fenestrated membrane of the retina. (A–C) PDFR-MYC fly heads showing PDF and PDFR-MYC expression. (A) Merged image of (B) (MYC, green) and (C) (PDF, magenta). (D–F) Expression of PDFR-MYC. (G) Negative control: a PDFR-MYC brain stained without incubation in the primary (anti-MYC) antibody. attP2 fly heads with anti-MYC antibody display similar results (not shown). (H–I) anti-REPO staining in w1118 fly heads. REPO is a useful marker for many glial cells: The outer-most layer of REPO-positive cells lies along the fenestrated membrane that separates the retina from the brain: this position is similar to that exhibited by PDFR-MYC positive cells. Scale bar = 50 μm.
PDFR is expressed in the clock network
We evaluated potential PDFR-MYC expression in pacemaker neurons with cellular resolution using anti-PER and anti-PDH counterstains (Figure 8). PDFR-MYC is strongly expressed in a large subset of clock neurons. Approximately six DN1s out of 17 expressed high levels of PDFR-MYC (Figure 8A), whereas four or five DN1s, both DN2s, and ~ten (of ~30) DN3s express low levels of PDFR-MYC (Figure 8A, 8O, and 8R, respectively). Among LN groups, the 5th s-LNv (arrow in Figure 8E) and three of six LNds (Figure 8L) show very strong expression of PDFR-MYC. Two of four l-LNvs also express a moderate level of PDFR-MYC (arrowheads in Figure 8E) and the remaining two express very low or no PDFR-MYC. Occasionally we also found brains in which all four l-LNvs showed low level expression of PDFR-MYC (data not shown). Lower levels of PDFR-MYC expression were detected in s-LNvs (Figure 8I). We could detect PDFR-MYC signal in both the s-LNv cell bodies and the projections (Figure 9).
Figure 8. PDFR expression in PER-expressing clock neurons.
PDFR-MYC expression by identified pacemaker neurons. (A–D) Six of the 17 DN1s express PDFR-MYC at strong levels, whereas three or four of the remaining ones express it at lower levels. (A) Merged image of (B) (PDF, magenta), (C) (PER, blue), and (D) (MYC, green). (E–H) LNvs express PDFR-MYC. (E) Merged image of (F) (PDF, magenta), (G) (PER, blue), and (H) (MYC, green). The 5th s-LNv expresses high levels of PDFR-MYC (arrow), two l-LNvs express PDFR-MYC at intermediate levels (arrowhead), while the others have lower to undetectable levels. s-LNvs are best seen in panel (I). Red asterisks indicate the incidence of non-pacemaker, receptor-positive cell bodies. (I–K) s-LNvs express PDFR-MYC. (I) Merged image of (J) (MYC, green) and (K) (PDF, magenta). Red asterisks indicate the incidence of non-pacemaker, receptor-positive cell bodies. (L–N) Three LNds out of six show very strong PDFR-MYC expression (white arrowheads), whereas the others show no PDFR-MYC (magenta arrowheads). (L) Merged image of (M) (MYC, green) and (N) (PER, blue). Asterisk, non-specific MYC signal near LNd area. (O–Q) Single optical section reveals low-level PDFR-MYC expression by DN2s (arrow). (O) Merged image of (P) (MYC, green) and (Q) (PER, blue). (R–T) DN3s express PDFR-MYC at low levels. (R) Merged image of (S) (MYC, green) and (T) (PER, blue). Scale bars, 10 μm.
Figure 9. PDFR-MYC in the processes of clock neurons.
PDFR-MYC is expressed and localized in the PDF dorsal projections. A single focal plane shows overlap between PDF and PDFR-MYC in s-LNv projections. PDFR-MYC positive projections occupy a wider area than do PDF positive projections, which indicates PDFR-MYC is localized in PDF cells and non-PDF clock neurons. (A) Merged image of (B) and (C). Dorsal is to the top and ventral is to the bottom, and the mid-line is to the left. Scale bar, 20 μm.
In summary, six DN1s, the 5th s-LNv, three LNds express strong PDFR-MYC, while two l-LNvs, four s-LNvs, four or so DN1s, DN2s, and DN3s express PDFR-MYC at lower levels. Very strong PDFR-MYC expression is present on neuronal projections within the dorsal brain neuropil, where most of clock neurons project their axons (Helfrich-Forster et al., 2007). Beyond the clock pacemaker network of neurons, we found expression at lower levels in about 50 or so cells:these are dispersed in the anterior and posterior surfaces of central brain and subeosophageal ganglion. Examples of non-pacemaker receptor-positive cells are shown in Figure 6C, 8E. At present, all such cells remain unidentified.
DISCUSSION
Like the mammalian SCN, the circadian neural circuit in the Drosophila brain features a mixture of diverse neurons that exhibits complex and parallel interactions. In the mammalian SCN, the neuropeptide VIP plays a critical role in synchronizing diverse pacemaker neurons (Harmar et al., 2002; Aton et al., 2005) and its actions have many parallels with that of PDF in Drosophila. The behavioral phenotypes of VIP and VIPR2 knockout mice are similar: both models display entrained activity rhythms in light-dark conditions. In DD however, both mutants display poor rhythmicity: approximately half the animals fail to display sustained rhythmic behavior, while the remainder have weak short-period rhythms. At a cellular level, VIP and PDF both contribute to molecular oscillations and to the synchronization of diverse pacemakers (Peng et al., 2003; Lin et al., 2004; Aton et al., 2005; Vosko et al., 2007).
The contribution of our studies centers on the cellular resolution afforded by Drosophila brain studies to identify specific targets of PDF signaling and the ability to relate that property to current models of oscillator organization. PDF is a key modulator of the Drosophila circadian network and its role as a synchronizer between clock neurons has been demonstrated in several independent studies (e.g., Lin et al., 2004; Lear et al., 2005; Nitabach et al., 2006; Shafer et al., 2008). These studies indicate PDF promotes communication within the clock network, but it has not been established whether this widespread PDF signaling is direct or indirect. Changing rhythmic behavior by application of a tethered transgene PDF to pacemaker neurons (Choi et al., 2009) showed that PDFR expression is normally present among pacemaker neurons, but did not specify where it was required to support its normal functions. Lear et al. (2009) addressed the latter point of cell requirements and found good rescue by selective pacemaker expression of UAS-pdfr. In our hands however, and using measures confined only to periods of constant darkness (DD3-9), such UAS-pdfr rescues were always partial. Now, with detailed mapping of expression resulting from a ~70 kB PDFR-MYC transgene, we establish widespread receptor expression among circadian pacemaker groups, especially among those neurons associated with the originally-defined M and E oscillator functions. pdfr-GAL4 patterns were also widely-exhibited by clock neurons, but with certain differences. For example, one notable difference between the pattern of pdfr-GAL4 expression and that of the PDFR-MYC was the stronger expression of PDFR-MYC in the 5th small LNv. We note also that the PDFR-MYC transgene also permits the operation of any PDFR splicing variants (if these exist) which could permit more efficient rescue.
How complete is the PDFR map?
There are two reasons to argue that our map of PDFR expression is largely complete. The first is based on its functional properties – the map derives from a transgene that provides complete behavioral rescue of the circadian rhythm defects observed in pdfr mutant flies. This indicates PDFR-MYC is expressed in all the requisite areas, at proper times and in those amounts, needed to support normal PDF signaling. The second reason is anatomical – there are three principal PDF neurons of the adult brain (the small LNv, large LNv, and PDF-tri) with distinct topographic projections. Significantly, each is paired with its own proximate field of PDFR-MYC-positive cells and/or processes. On these twin functional and anatomical bases, we suggest the pdfr-myc transgene provides an accurate and reasonably complete indication of where and when PDFR must be expressed to support PDF signaling that underlies the circadian pacemaking circuitry. Hyun et al. (2005) rescued the same pdfr5304 mutant LD and DD phenotypes using a per-GAL4 driver and argued that PDFR expression confined to the circadian pacemaker network was therefore sufficient, although the rhythmic flies under constant darkness were only 63% among rescued genotypes, whereas wild-type flies were 90% rhythmic. They speculated the less complete rescue with per-GAL4 may be due to a failure of UAS-transgene expression in all PER-expressing neuron or a failure of PDFR expression in non-PER neurons which might be required for complete rescue of behavior. Our experimental results with various GAL4 drivers were similar in that any rescue experiments using GAL4/UAS system always produced incomplete rescue. Moreover, per-GAL4 expression patterns are typically much larger than just the ~150 principal circadian pacemakers (Kaneko and Hall, 2000).
What do the details of the PDFR-MYC expression map tell us about how PDF signaling supports the circadian pacemaker system?
Small LNv projections
Our observations suggest many neurons expressing PDFR project to the dorsal protocerebrum where the small LNv release PDF. In fact, the most intensely-stained neurons for PDFR-MYC in the entire adult brain were (i) a subset of LNds, (ii) a subset of DN1s, and (iii) the 5th small LNv, all of which project to this exact brain region. The identities of these neurons are highly significant: they represent precisely those circadian pacemakers previously implicated to be critical components of the so-called E oscillator group (recently reviewed by Nitabach and Taghert, 2008). Specially, the three LNd (Grima et al., 2004; Stoleru et al., 2004; Picot et al., 2007) and the 5th small LNv (Rieger et al., 2006; Bachleitner et al., 2007) heavily influence the evening activity peak. Interestingly, this precise four-cell set is what Rieger et al. (2009) showed was sufficient for PERIOD function to produce both morning and evening peaks of anticipatory behavior with cycling dim light. Likewise the ability to drive behavioral rhythms under constant light (under defined genetic conditions) is a property of the same three LNd (Picot et al., 2007) and also six-seven DN1s (Murad et al., 2007; Stoleru et al., 2007). E oscillators are heavily dependent on PDF signalling for re-setting information that influences period and phase, and which supports rhythmic output under constant dark or constant light condition (Lin et al., 2004; Murad et al., 2007; Picot et al., 2007; Stoleru et al., 2007; Choi et al., 2009; Lear et al., 2009). Thus, E neuronal oscillators are responsive to PDF signaling according to genetic analysis and now according to PDFR-MYC mapping studies. Therefore, our studies strongly support the proposition that E cells represent critical direct targets of PDF action.
Also, we found PDFR-MYC expression in the PDF-positive neurons themselves. Previously, Lin et al. (2004) reported that loss of PDF results in a dispersed phase of PER rhythm in s-LNvs, and low amplitude and advanced period in LNds. Likewise, Lear et al. (2005) showed that a mutation of the pdfr gene results in similar phenotypes. In addition, Shafer et al. (2008) observed physiological responses by s-LNv to PDF monitoring levels of cyclic nucleotides, consistent with their normal expression of the PDFR. Now PDFR-MYC expression in s-LNvs fits into this observation, in that PDF and PDFR form autocrine circuits in s-LNvs.
Large LNv projections
The large LNvs project to a distal layer of medulla of the optic lobe and form a widespread tangential projection across the retinotopic inputs from the eye. Analysis of PDFR-MYC expression indicates an absence of any receptor population for the large LNv in the immediate vicinity of the large LNv terminals within the medulla. The closest receptor-expressing cells were found at or near the fenestrated membrane below the base of the retina. We could not provide a precise identification of this population, but in our estimation, the best candidates are fenestrated glia ((FG) lying about 100μm away from the medulla and immediately opposite the fenestrated membrane of the retina (Saint Marie and Carlson, 1983).. FGs form a barrier at the retina-brain border, and ensheath the axon bundles of the photoreceptors coming into the lamina. They contain pigment granules, and their morphology and protein expression profile suggest they might play an active role in trafficking (Kretzschmar and Pflugfelder, 2002). However, we stress that the identification of these receptor positive cells is tentative. Use of better cell-type-specific markers may reveal them to be other glial cells in the sub-retinal region, or non-glial elements of cells within the retina, such as secondary or tertiary pigment cells. When PDF or PDF antiserum is injected into the optic lobe of the cricket, photo-responsiveness changed greatly (Saifullah and Tomioka 2003). PDFR-MYC expression in or near the sub-retinal region represents a good candidate to mediate such actions and hence PDF modulation of photosensitivity may operate by targeting non-neuronal cells. I In insects, glia of the retina and of the first optic neuropil have been implicated in nutritive regulation of photoreceptors (Coles et al., 2008), in neurotransmitter metabolism (Borycz et al., 2002), and in circadian control of neuronal size changes (Pyza and Górska-Andrzejak, 2004). At what level PDF exerts its actions on glia and how this affects circadian photoreception are questions our results have newly-generated.
PDF-tri cell projection
PDF-tri cells form a compact projection ventral to the esophageal foramen and reproducibly disappear (lose PDF immunostaining) on the first day after adult eclosion (Helfrich-Forster, 1997). Over-expression of the anti-apoptotic gene P35 (Zhou et al., 1997) prevented the loss of these cells (Renn et al., 1999a), which consistent with the assumption that they normally undergo programmed cell death soon after eclosion. Speculation regarding the specific functions of these neurons is limited by their truncated developmental history. The projection dorsally via the Median Bundle is consistent with the path of many peptidergic neurons in this deutero-/tritocereberal region to the Pars Intercerebralis. The abrupt nature of their removal suggests a regulatory association with adult eclosion, which has a rhythmic nature controlled in part by PDF (Myers et al., 2003). Alternatively, the projection lies close to the Antennal Mechanosensory and Motor Center, which receives input from Johnston’s Organ (Baker et al., 2007); this proximity may be meaningful as Pdf mutant flies exhibit a defect in geotaxic behaviors (Toma et al., 2003). Interestingly, the PDFR-MYC projections that are coextensive with PDF-tri projections on adult Day 1 persist long after the PDF staining disappears. This suggests PDFR expression may in some cases serve functions beyond reception of the PDF ligand or respond to PDF from other source as a paracrine hormone.
How well does the map of PDFR expression correspond to the map of PDFR activation?
Recently, Shafer et al. (2008) used realtime imaging and a genetic FRET reporter sensitive to cyclic nucleotide levels to show that most circadian pacemaker neurons in the Drosophila brain appear sensitive to PDF, in a manner consistent with direct activation. That study was limited to surveying responses by the 6 major groups of pacemakers – small LNvs, large LNvs, LNds, DN1s, DN2s, and DN3s. Of these only the large LNvs were generally unresponsive (3 of 51 cells positive), although they could be made uniformally responsive by forced mis-expression of a pdfr cDNA transgene. To what extent is this PDFR activation map in register with the one here derived from expression of the pdfr-myc transgene? First we note that most elements of the activation map are also represented in the expression map: we found PDFR-MYC expression in all the same pacemaker cells groups that showed PDF responses physiologically. Hence, the two maps exhibit significantly high concordance.
We emphasize two additional points of comparison, however, because these highlight differences between the two maps – (i) Certain identified neurons are activated by PDF in vivo, but do not express detectable PDFR-MYC. An example of this is found in the LNd: all six LNds appear to respond to PDF within the 1–2 minute delay recorded by the FRET reporter (Shafer et al., 2008), yet only three are PDFR-MYC-positive. Also, (ii) there are PDFR-MYC-expressing neurons (e.g., the large LNv, two of which routinely express PDFR-MYC) that do not appear robustly sensitive to PDF. Can these discrepancies between the two maps provide novel information about neuropeptide signaling in the complex circuitry that underlies behavior, and how might we account for these two discrepant examples?
Receptor (−)/ Response (+)
In the case of the LNd (only a subset express PDFR, but all LNds respond to PDF within 1–2 min (Shafer et al., 2008)), we speculate that the three neurons which are directly-activated signal secondarily to the three PDFR-MYC negative cells in that group. The time course of the activation of cyclic nucleotide levels (1–2 minutes) does not preclude such a mechanism. The coupling mechanism between groups of LNd may include gap junctions, as exhibited by mammalian circadian pacemaker neurons (Colwell, 2000; Long et al., 2005; Schneider and Stengl, 2006), or via autocrine feedback - release of a small molecule transmitter like nitric oxide, as exhibited by vasopressin-releasing magnocellular neurons (Brown et al., 2008). Yoshii et al. (2008) have likewise postulated a similar signaling pathway from the three CRY-positive LNds to the three CRY-negative ones, based on their similar rates of light-induced TIM degradation. PDF signaling may involve several second messenger pathways and this could necessitate the need to preclude direct activation to just a subset of LNd pacemakers. An alternative explanation invokes the existence of a separate receptor for PDF that is expressed by the PDFR-MYC-negative LNds – this possibility cannot be excluded at present. However, the close match in circadian behavioral phenotypes between Pdf and pdfr (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005) suggests it is the primary PDF receptor. Interestingly, another important E cell pacemaker group – the DN1 – also displayed limited receptor expression (six-seven of 17 cells express PDFR-MYC), yet most tested DN1s responded physiologically (Shafer et al., 2008). This suggests there may be a broader principle of intra pacemaker group signaling to be realized by further examination of this apparent mismatch.
Receptor (+)/ Response (−)
In the case of large LNv that do not respond but contain PDFR-MYC, the simplest way to explain the occurrence of receptor expression in the absence of a strong functional response invokes strain differences – the background of the 70 kB transgene is distinct from that in which the FRET reporter was studied. Alternatively, large LNv pacemakers in all Drosophila strains may express PDFR. At present, we cannot distinguish between these possibilities. We offer three potential molecular explanations to explain why, if a subset of large LNv express PDFR, their physiological responses to PDF may be limited. First, pdfr mRNA could be differentially spliced such that in large LNvs it produces only a non-functional protein in spite of having a proper MYC-tagged C terminus. Second, the receptor may be expressed and functionally intact in these neurons but for reasons unknown, it is primarily located in sub-cellular compartments that are not accessible to exogenous PDF. Finally, large LNvs may normally express functional PDFR but receptor occupancy or receptor desensitization by ambient levels of normally-released PDF may be high, such that additional peptide does not engender additional signaling. Conceivably, each of these three situations could be overcome to produce PDF responses by forcing the large LNvs to express large amounts of a functional and fully spliced pdfr cDNA, as was shown by Shafer et al., (2008). Likewise, each of these three possibilities provides a testable prediction.
In summary, we showed that unlike pdfr-GAL4 lines, the 70 kB pdfr-myc transgene is capable of fully complimenting the circadian behavioral deficiencies of the pdfr mutant flies. Furthermore we showed it is expressed prominently within the clock network. PDFR-MYC is also found in the sub-retinal glial cells of the visual system, and as well in other brain cells which are not pacemakers. Previous studies have indicated PDF likely targets all three canonical components of the circadian system - the pacemaker (Peng et al., 2003; Lin et al., 2004), the “input” (Pyza and Meinertzhagen, 2003), and “output” (Myers et al., 2003). Our results begin to provide cellular identities for these categorical entities (Figure 10) and thus a basis for detailed and direct questions by which to define principles of signaling within a circadian neural network.
Figure 10. PDFR expression in the circadian system.
PDF secreting large and small LNvs are in the center of the pacemaker, signaling directly to the non-PDF clock neurons, to non-neuronal cells in or near the retina, to the output non-clock receptor positive cells, and feeding back to PDF secreting cells.
Acknowledgments
This work is supported by a grant from the NIH (R01MH067122) to P.H.T., a scholarship to S.H.I. from Korea Science and Engineering Foundation (M06-2004-000-10108), and a P30 NIH Neuroscience Blueprint Core Grant (#NS057105) to Washington University.
We thank Dr. Mimi Ernst for the P[acman] control vector transgenic line, and Renate Lewis, Michael Nonet, Dennis Oakley, Matt Thimgan, and Michael Casey for helpful technical advice. We thank members of our laboratory for helpful discussions, and Orie Shafer, Joel Levine, and John Ewer for comments on an earlier draft of the manuscript. We acknowledge support by the Bakewell Imaging Center, by a P30 Neuroscience Core grant NS057105 to Washington University; the work was supported by a National Institutes of Health (NIH) grant R01MH067122 (to PHT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LITERATURE CITED
- Alfonso TB, Jones BW. gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–383. doi: 10.1006/dbio.2002.0740. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right...now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachleitner W, Kempinger L, Wülbeck C, Rieger D, Helfrich-Förster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Beckingham KM, Armstrong JD. Functional dissection of the neural substrates for gravitaxic maze behavior in Drosophila melanogaster. J Comp Neurol. 2007;501:756–764. doi: 10.1002/cne.21257. [DOI] [PubMed] [Google Scholar]
- Blanchardon E, Grima B, Klarsfeld A, Chélot E, Hardin PE, Préat T, Rouyer F. Defining the role of Drosophila lateral neurons in the control of circadian rhythms in motor activity and eclosion by targeted genetic ablation and PERIOD protein overexpression. Eur J Neurosci. 2001;13:871–888. doi: 10.1046/j.0953-816x.2000.01450.x. [DOI] [PubMed] [Google Scholar]
- Blau J, Young MW. Cycling vrille expression is required for a functional Drosophila clock. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Loubani M, Meinertzhagen IA. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci. 2002;22:10549–10557. doi: 10.1523/JNEUROSCI.22-24-10549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Ruan M, Scott V, Tobin VA, Ludwig M. Multi-factorial somato-dendritic regulation of phasic spike discharge in vasopressin neurons. Prog Brain Res. 2008;170:219–228. doi: 10.1016/S0079-6123(08)00419-6. [DOI] [PubMed] [Google Scholar]
- Choi C, Fortin JP, McCarthy E, Oksman L, Kopin AS, Nitabach MN. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JA, Martiel JL, Laskowska K. A glia-neuron alanine/ammonium shuttle is central to energy metabolism in bee retina. J Physiol. 2008;586:2077–2091. doi: 10.1113/jphysiol.2007.148734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Rhythmic coupling among cells in the suprachiasmatic nucleus. J Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::aid-neu6>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyran SA, Yiannoulos G, Buchsbaum AM, Saez L, Young MW, Blau J. The double-time protein kinase regulates the subcellular localization of the Drosophila clock protein period. J Neurosci. 2005;25:5430–5437. doi: 10.1523/JNEUROSCI.0263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Reihm JP. The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: Identification by an antiserum against a synthetic PDH. Cell Tissue Res. 1987;250:377–387. [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Förster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714– 722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27:12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol. 1998;182:435–453. doi: 10.1007/s003590050192. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Neurobiology of the fruit fly’s circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Shafer OT, Wülbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, Bae E, Kim J. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kalló I, Piggins HD, Coen CW. Expression of VIP and/or PACAP receptor mRNA in peptide synthesizing cells within the suprachiasmatic nucleus of the rat and in its efferent target sites. J Comp Neurol. 2004;475:19–35. doi: 10.1002/cne.20168. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Killman VL, Allada R. Genetic analysis of ectopic circadian clock induction in Drosophila. J Biol Rhythms. 2009;24:368–378. doi: 10.1177/0748730409343761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Pflugfelder GO. Glia in development, function, and neurodegeneration of the adult insect brain. Brain Res Bull. 2002;57:121–131. doi: 10.1016/s0361-9230(01)00643-8. [DOI] [PubMed] [Google Scholar]
- King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Ann Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;7:E1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Signal analysis of behavioral and molecular cycles. BMC Neurosci. 2002;3:1. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zwiebel LJ, Hinton D, Benzer S, Hall JC, Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992;12:2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP. Immunocytochemical localization of pigment-dispersing hormone and its coexistence with FMRFamide immunoreactive material in the eye stalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res. 1988;250:365–375. [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila. Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EM, Yu J, Sehgal A. Circadian control of eclosion: interaction between a central and peripheral clock in Drosophila melanogaster. Curr Biol. 2003;13:526–533. doi: 10.1016/s0960-9822(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Nassel DR. Advances in the immunocytochemical localization of neuroactive substances in the insect nervous system. J Neurosci Methods. 1996;69:3–23. doi: 10.1016/S0165-0270(96)00016-7. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Syková E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:E315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyza E, Meinertzhagen IA. The regulation of circadian rhythms in the fly’s visual system: involvement of FMRFamide-like neuropeptides and their relationship to pigment dispersing factor in Musca domestica and Drosophila melanogaster. Neuropeptides. 2003;37:277–289. doi: 10.1016/j.npep.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Pyza E, Górska-Andrzejak J. Involvement of glial cells in rhythmic size changes in neurons of the housefly’s visual system. J Neurobiol. 2004;59:205–215. doi: 10.1002/neu.10307. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. Pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999a;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Renn SC, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, Taghert PH. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol. 1999b;41:189–207. [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Wülbeck C, Rouyer F, Helfrich-Förster C. Period gene expression in four neurons is sufficient for rhythmic activity of Drosophila melanogaster under dim light conditions. J Biol Rhythms. 2009;24:271–282. doi: 10.1177/0748730409338508. [DOI] [PubMed] [Google Scholar]
- Ruocco I, Cuello AC, Shigemoto R, Ribeiro-da-Silva A. Light and electron microscopic study of the distribution of substance P-immunoreactive fibers and neurokinin-1 receptors in the skin of the rat lower lip. J Comp Neurol. 2001;432:466–480. doi: 10.1002/cne.1114. [DOI] [PubMed] [Google Scholar]
- Saifullah AS, Tomioka K. Pigment-dispersing factor sets the night state of the medulla bilateral neurons in the optic lobe of the cricket, Gryllus bimaculatus. J Insect Physiol. 2003;49:231–239. doi: 10.1016/s0022-1910(02)00270-6. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica L. J Neurocytol. 1983;12:213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- Schneider NL, Stengl M. Gap junctions between accessory medulla neurons appear to synchronize circadian clock cells of the cockroach Leucophaea maderae. J Neurophysiol. 2006;95:1996–2002. doi: 10.1152/jn.00835.2005. [DOI] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Kaneko M, Sharma VK, Holmes TC. The Drosophila circadian pacemaker circuit: Pas De Deux or Tarantella? Crit Rev Biochem Mol Biol. 2008;43:37–61. doi: 10.1080/10409230701829128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Nawathean P, Fernández MP, Menet JS, Ceriani MF, Rosbash M. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Shafer OT. Mechanisms of clock output in the Drosophila circadian pacemaker system. J Biol Rhythms. 2006;21:445–457. doi: 10.1177/0748730406293910. [DOI] [PubMed] [Google Scholar]
- Toma DP, White KP, Hirsch J, Greenspan RJ. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat Genet. 2002;31:349–353. doi: 10.1038/ng893. [DOI] [PubMed] [Google Scholar]
- Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. J Neurosci. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kilman VL, Keegan KP, Peng Y, Emery P, Rosbash M, Allada R. Drosophila clock can generate ectopic circadian clocks. Cell. 2003;113:755–766. doi: 10.1016/s0092-8674(03)00400-8. [DOI] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci U S A. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]