Abstract
Background
Since the early 1990s, it has been possible to measure electrically evoked compound action potentials (ECAPs) from Nucleus cochlear implant users. Recording the ECAP does not require active participation by the subject, and the recordings are not adversely affected by attention or sleep, making this response an ideal tool for monitoring long-term changes. Previous research from our laboratory (Hughes et al, 2001) has shown that ECAP thresholds and slope of the ECAP growth functions are relatively stable over time. However, this conclusion was based on results obtained from a fairly limited number of study participants, each of whom used the Nucleus CI24M cochlear implant and were followed for less than two years.
Purpose
To evaluate the effect of long-term use of a cochlear implant on ECAP thresholds and slope of the ECAP input/output function for both pediatric and adult cochlear implant recipients.
Research Design
A longitudinal study that describes how ECAP thresholds and growth functions change over a period of 96 mo following initial activation. Changes over time in ECAP threshold and slope of the ECAP growth function were analyzed, and effects of the subject’s age, type of CI (cochlear implant), and stimulating electrode are included in the analysis.
Study Sample
134 Nucleus CI users participated in this study. All were profoundly deaf. This subject pool included 84 individuals (40 adults and 44 children) who used the Nucleus CI24M cochlear implant and 50 individuals (21 adults and 29 children) who used the Nucleus CI24R cochlear implant.
Data Collection and Analysis
Electrodes 5, 10, 15, and 20 were stimulated, and ECAP growth functions were measured for each subject at regular intervals following the initial activation of the device.
Results
Small increases in mean ECAP thresholds were observed for both pediatric and adult CI users between an “early” visit that occurred within 3–6 mo following hookup and a “late” visit that occurred 4.8–6 yr later. For adults, the average increase in ECAP threshold was 3.94 CL (clinical programming units for Nucleus CIs). For children, the average increase was 4.16 CL. These differences, while small, were statistically significant. Slope of the ECAP growth functions measured over the same time interval did not change significantly. On average, pediatric CI users had ECAP thresholds that were 4–5 CL units higher than the adult CI recipients. The most striking outcome from this study, however, was the finding that when compared with postlingually deafened adults, pediatric CI users had ECAP growth functions that were substantially steeper. The differences between the results obtained from children and those obtained from adults were statistically significant and largely independent of device type or stimulating electrode.
Conclusion
Results from this study show ECAP thresholds and growth functions to change very little over a 5–6 yr observation interval suggesting that long-term use of a CI is not likely to have a significant negative impact on the response of the peripheral auditory system. Pediatric CI users were shown to have, on average, higher ECAP thresholds and steeper ECAP growth functions than postlingually deafened adult CI users. This finding suggests potential differences between the two patient populations either in terms of the current fields within the cochlea or the effective distance between the stimulating electrode and the stimulable neural tissue.
Keywords: Auditory evoked potential, cochlear implant, compound action potential, electrical stimulation, neural response telemetry
Cochlear implants are fully implantable biomedical devices designed to last for the lifetime of the recipient. Single-channel cochlear implants were first introduced in the late 1970s. Multichannel cochlear implants followed in the early 1980s. While crude by today’s standards, those early devices offered profoundly hearing-impaired individuals a degree of hearing that was not possible via conventional amplification, and the success of this technology was such that within a few years, cochlear implantation moved from being an experimental procedure to being the treatment of choice for both adults and children with profound, bilateral sensorineural hearing loss.
The Iowa Cochlear Implant Project was first funded in 1985. This NIH-funded grant created a cohort of patients who received their cochlear implants in exchange for participating in a series of research projects intended to provide independent assessment of the range of benefit that a profoundly hearing-impaired individual could expect to receive from a cochlear implant. In the two decades since, the scope of the project and the size of the cohort have expanded significantly. To date more 625 adults and 300 children have received multichannel cochlear implants at the University of Iowa Hospitals and Clinics. Some of those individuals, originally implanted during the 1985–1990 grant period, continue to participate in our research today. Many are now elderly. Children implanted during the original grant period are now young adults. This report describes the effect of long-term use of a cochlear implant on peripheral measures of the response of the auditory nerve to electrical stimulation.
There are many different reasons why benefit from a cochlear implant could change over time. Some studies have shown that trauma associated with insertion of the intracochlear electrode array and/or the use of inappropriate levels of electrical stimulation can lead to loss of spiral ganglion cells (Zappia et al, 1991; Marsh et al. 1992; Leake and Rebscher, 2004). It would seem possible, therefore, particularly when coupled with the degenerative changes that accompany the aging process, that long-term use of a CI (cochlear implant) may result in loss of sensitivity and/or lower overall levels of performance with the device. On the other hand, there is considerable evidence that electrical stimulation can have protective effects on neural structures (e.g., Hartshorn et al, 1991; Leake et al, 1999). Moreover, in pediatric cochlear implant recipients, it appears that electrical stimulation at an early enough age might actually drive and/or modulate development of the auditory pathways at a cortical or precortical level (Sharma et al, 2002). These observations suggest that long-term use of a CI may be generally beneficial and could potentially lead to improved levels of performance over time.
There are several ways to assess response to electrical stimulation in cochlear implant users. Many investigators have reported changes in word or sentence understanding over time in cochlear implant users (e.g., Tyler et al, 2000; Parkinson et al, 2002; Ruffin et al, 2007; Krueger et al, 2008; Wang et al, 2008). Others have reported changes in MAP levels or behavioral detection thresholds for simple stimuli (Shapiro and Waltzman, 1995; Hughes et al, 2001; Henkin et al, 2006; Zwolan et al, 2008). While both metrics are important indicators of success with a CI, interpretation of why change may or may not be observed over time for a specific group of CI users is complicated. Not only does learning and hearing history impact performance measures but attention, motivation, and maturation also can influence results. It is possible to avoid some of these confounding effects by using electrophysiologic recording techniques to assess changes in sensitivity to electrical stimulation over time. While there are a number of different electrically evoked auditory potentials that can be measured from cochlear implant recipients, one of the most popular is the electrically evoked compound action potential (ECAP). This response is a measure of the synchronous firing of a large group of auditory nerve fibers to the presentation of a brief electrical stimulus. Because it is generated peripherally, the ECAP is not affected by age, attention, or learning. Additionally, the amplitude, threshold, and slope of the ECAP growth function are all metrics that have been shown to be closely related to peripheral neural survival (Smith and Simmons, 1983; Hall, 1990; Miller et al, 1994). The introduction of cochlear implants with neural response telemetry (NRT) capabilities in the early 1990s made it possible to record these responses from a wider range of cochlear implant recipients than ever before.
Previous investigators have used the NRT system to record changes in ECAP thresholds over time (Hughes et al, 2001; Lai et al, 2004; Thai-van et al, 2001). Thai-van et al (2001) measured ECAP thresholds in 23 pediatric CI users over a period of 12 mo post implant. Unfortunately, not every child was tested at each recording session, and the data are reported relative to the levels used to program the speech processor of the CI rather than in absolute current levels. The range over which the ECAP thresholds were recorded, however, does not appear to change substantially over the first year of CI use. Lai et al (2004) report data obtained from a larger group of adult CI users (N = 49), each of whom was tested twice over a period of about 4 yr following surgery. Subjects ranged in age from 2 to 77 yr of age at the time of testing. The number of electrodes tested and included in the analysis of their results was not clearly specified and varied across subjects; however, Lai et al (2004) report good test-retest stability, and cross-subject analyses showed ECAP thresholds to be relatively stable across time. The results, as reported, do not allow comparison between adult and pediatric CI recipients. Additionally, no measures of slope of the ECAP growth function are provided, and the study design does not allow within-subject comparisons of ECAP threshold over time.
We previously published a study comparing ECAP thresholds and electrode impedance values recorded over a 2 yr time interval for a group of 31 adult and 21 pediatric CI users (Hughes et al, 2001). All of the subjects participating in that early study used the CI24M cochlear implant (the original version of the Nucleus cochlear implant that had a straight, fully banded intracochlear electrode array). Results from the Hughes et al, 2001, study showed that after about 3 mo of CI use, ECAP threshold and slope measures were stable for both adult and pediatric CI recipients. One particularly striking result that is reported in the Hughes et al (2001) study is that ECAP growth functions recorded from pediatric CI users were significantly steeper than similar measures obtained from adult CI recipients. It was suggested that these results were consistent with a theory that children may have stronger fibrous tissue reactions than adults, resulting in different current flow patterns within the cochlea and an effectively greater difference between the electrode contact and the stimulable neural tissue. This early study is limited in that it only follows patients for a relatively short period of time postimplant; it is not designed in such a way as to allow the effect of stimulating electrode on the results to be investigated; and the number of study participants included in each test session varied significantly. Moreover, these data were obtained from individuals who used the Nucleus CI24M cochlear implant. This device was later replaced by the Nucleus CI24R cochlear implant that included a different intracochlear electrode design (the CI24R includes a half banded, contoured electrode array designed to lie slightly closer to the modiolus of the cochlea).
The purpose of this study was to expand on our previous work. For some study participants, ECAP threshold and slope data were collected for periods as long as 8 yr post hook up. In this study we specifically sought to determine (1) if prolonged use of a cochlear implant would lead to a decline in neural response as evidenced by increased ECAP thresholds and shallower growth functions, (2) whether changes in neural response to electrical stimulation were device dependent, (3) whether ECAP threshold and slope data differed for pediatric and adult populations, and (4) how the position of the stimulating electrode within the cochlea influenced the results.
METHOD
Participants
This study reports data obtained from 139 ears of 134 cochlear implant users. This group included 61 adults and 73 children. Forty adults and 29 children used the Nucleus CI24M cochlear implant. Five of the adult CI24M cochlear implant users were implanted bilaterally, and data from both ears is included in the reported results. The remaining subjects (21 adults and 44 children) used the Nucleus CI24R device. All of the adult CI users were postlingually deafened. Of the pediatric CI recipients, 48 were thought to be deaf since birth, and 11 were deafened within the first 24 mo of life. The remaining pediatric CI recipients had progressive hearing loss and were implanted after two years of age. In each case, surgery was uneventful, and while performance with their CI varied substantially across subjects, all were classified by their programming audiologists as “successful” users.
These 134 CI users were selected to participate in this study because ECAP growth functions had been recorded at multiple visits for at least three of the four stimulating electrodes (5, 10, 15, and 20) over a time period in excess of 3.5 yr. Table 1 describes pertinent demographic information about this group of subjects.
Table 1.
Demographic Characteristics of All Study Participants
| Adults |
Children |
|||
|---|---|---|---|---|
| CI24M | CI24R | CI24M | CI24R | |
| Number of subjects | 40 | 21 | 44 | 29 |
| Number of ears | 45 | 21 | 44 | 29 |
| Gender | Female = 23 | Female = 12 | Female = 16 | Female = 13 |
| Male = 17 | Male = 9 | Male = 28 | Male = 16 | |
| Age al implant | 53 yr | 57 yr | 50 mo | 54 mo |
| Duration of deafness | 9.3 yr | 8.4 yr | 36 mo | 36 mo |
As with any clinical study that includes multiple data collection sessions spanned over years, not every subject was tested at each visit. In order to minimize the impact of unequal subjects numbers on the results, we selected a subset of 68CI users (73 ears total) for whom ECAP growth functions were available on all four stimulating electrodes at both an “early” visit between 3 to 6 mo (mean = 3.8 mo) following the initial stimulation and at a “late” visit that occurred between 5 and 7.5 yr (mean = 5.8 yr) after the initial device activation. Table 2 describes pertinent demographic information about this smaller subject.
Table 2.
| Adult |
Children |
|||||
|---|---|---|---|---|---|---|
| CI24M | CI24R | CI24M | CI24R | |||
| Postlingual | Postlingual | Congenital | Progressive | Congenital | Progressive | |
| Number of subjects | 21 | 10 | 12 | 7 | 13 | 5 |
| Number of ears | 26 | 10 | 12 | 7 | 13 | 5 |
| Gender | Female = 11 | Female = 5 | Female = 7 | Female = 1 | Female = 6 | Female = 1 |
| Male = 10 | Male = 5 | Male = 5 | Male = 6 | Male = 7 | Male = 4 | |
| Age at implant | 54 yr (36–73 yr) | 58 yr (34–78 yr) | Mean = 47 mo: Median = 35 mo (19–142 mo) | Mean = 68 mo; Median = 42 mo (17–185 mo) | Mean = 37 mo; Median = 18 mo (11–183 mo) | Mean = 52 mo; Median = 41 mo (14–134 mo) |
| Duration of deafness | 12.0 yr | 5.3 yr | 47 mo | 21 mo (3–65 mo) | 37 mo | 11 mo (4–16 mo) |
Procedure
Prior to testing, each adult study participant was asked to listen to the stimuli as the level was slowly increased from the point where it was just audible to a point where it was near the maximum acceptable loudness level. Levels used to measure the ECAP were limited to that range. Some children were too young to reliably estimate loudness. These children were tested using an ascending approach, and the child was observed carefully for any signs of discomfort during testing. Typically subjects were seated in a reclining chair and asked to relax, sleep, read, or watch captioned TV. Younger children were often tested while sitting on a parent's lap or playing quietly. The time required to obtain ECAP growth functions from a set of four electrodes varied but rarely exceeded 30 min regardless of the subject's age, duration of deafness, length of CI use, or device type.
Stimulation, Recording, and Analysis Parameters
All of the ECAP responses included in this study were collected using the NRT capabilities of the Nucleus CI. The software used for recording this neural response evolved over time. Between 1995 and early 2008, NRT software versions 1.4 to 3.1 were used. More recently, Custom Sound EP has been used to record ECAP growth functions.
This study reports data collected from individuals who use two different versions of the Nucleus CI (the Nucleus CI24M and CI24R CIs). Both devices have 22 intracochlear electrodes and 2 extracochlear grounds. The major difference between the CI24M and CI24R cochlear implants was that the CI24M device has a straight, fully banded electrode array while the CI24R, or Contour, device has somewhat smaller electrode contacts that are mounted on a silastic carrier that has memory allowing it to curl as it is inserted into the cochlea. This design presumably allows for closer proximity of the stimulating electrodes to the modiolus.
For each subject ECAP growth functions were recorded for four different stimulating electrodes spaced across the intracochlear electrode array (electrodes 5, 10, 15, and 20). In both the CI24M and CI24R cochlear implants, electrode 1 is the most basal of the intracochlear electrodes, and electrode 22 is the most apical. The stimulus was a 25 μsec/phase, cathodic leading, biphasic current pulse with an 8μsec interphase gap presented in a monopolar stimulation mode at a rate of 80 Hz. Typically, an electrode located two electrodes apical relative to the stimulating electrode was used for recording. Stimulus artifact was minimized using the two-pulse subtraction technique described in detail in previous publications (Abbas et al, 1999; Miller et al, 2000). This procedure involves presentation of two biphasic current pulses, a masker and a probe, that are separated by a short interstimulus interval. In order to record an ECAP growth function, the amplitude of the second pulse in the two-pulse sequence (the probe) is systematically varied. A neural response to the probe pulse is recorded but is often contaminated by stimulus artifact. In order to minimize the impact of stimulus artifact on these recordings, a second set of recordings are obtained in which the probe pulse is preceded by a masker pulse that is of equal or greater amplitude than the probe. If the interpulse interval is sufficiently brief, neurons responding to the masker will be refractory and unable to respond at the time the probe is presented. Recordings made in this condition are then subtracted from similar measures obtained using the probe alone condition. This technique has been referred to as the “subtraction method” and is widely used to record ECAP measures with Cochlear’s NRT software. In this study, the masker level was fixed at or near the maximum level that the subject felt was still comfortable, and the probe level was varied, or the two pulses were linked and varied together. In the later case, the masker pulse was always at least 5 CL (clinical programming units for Nucleus CIs) higher than the probe. Previous data from our laboratory has shown that these two techniques yield similar results (Hughes et al, 2001). In all cases, the masker-probe interval was fixed at 500 μsec.
In most instances, the gain of the recording amplifier was set to 60 dB, and the interval between presentation of the stimulus and initiation of the recording was at least 60 μsec. In cases where stimulus artifact was problematic, the recording delay, recording electrode, and gain were systematically changed following a protocol outlined previously (Abbas et al, 1999). At clearly supra-threshold levels, ECAPs were recorded using 50 sweeps. At stimulation levels near threshold, the number of sweeps was increased to 100. Amplitude of the ECAP was determined offline using custom designed, MATLAB software. Standard peak picking techniques were used to assess the difference in amplitude between the negative peak of the ECAP and the following positive peak. ECAP thresholds were determined by visually examining the recorded responses and identifying the lowest stimulation level where an N1 peak could be positively identified in the recorded waveform.
Figure 1 shows ECAP waveforms and growth functions recorded from both an adult CI24M cochlear implant user (M33) at 3 mo post hook up and again for the same electrode and stimulation levels almost 8 yr later. Also shown is data from a pediatric CI24R cochlear implant user (CM12) recorded 2 mo after his initial stimulation and again for the same electrode and stimulation levels almost 7 yr later. Similar graphs were generated for each electrode for each subject who participated in this study. The growth curves were fit using a linear model, and the slope of each of the ECAP growth functions was calculated.
Figure 1.
ECAP waveforms recorded from two different CI users are shown. M33 is a postlingually deafened adult. ECAP growth functions were recorded at 3 and 96 mo posthookup. CM 12 is a child with congenital, progressive hearing loss implanted at 42 mo of age. ECAPs were recorded at 2 and 85 mo posthookup. Stimulation levels for both visits are shown. The response marked with the asterisk was identified as threshold. The panel on the right shows ECAP amplitude plotted as a function of the current level of the probe for both visits for these two study participants.
Each subject was tested at multiple intervals over a period of up to eight years postimplant. Trends in the mean data, plotted across as a function of time with results from each of the stimulating electrodes pooled, are shown in Figures 2 and 3. The effect of stimulating electrode position on the ECAP growth functions is separated out in Figures 4 and 5.
Figure 2.
The two panels on the left show changes in ECAP threshold and slope over time for study participants who use the CI24M cochlear implant. The two panels on the right show changes in ECAP threshold and slope over time for study participants who use the CI24R cochlear implant. Data recorded from children are shown with open symbols and dashed lines. Data recorded from adults are shown with filled symbols and solid lines. The error bars indicate ± 1 SE around the mean. Stimulation level is specified in clinical programming units (CL).
Figure 3.
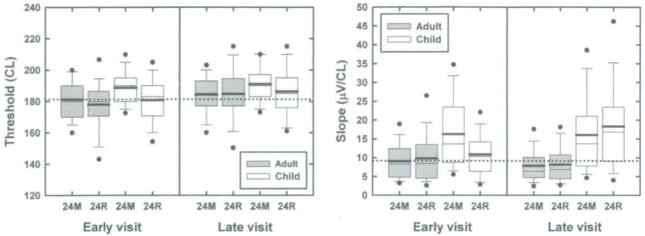
Box plots showing distribution of the ECAP threshold and slope data for subjects tested at both the early and late intervals. The labels on the abscissa indicate the device type. Filled bars indicate results obtained from adult study recipients. Open bars indicate results obtained from pediatric CI users. Dots indicate the 5th and 95th percentiles. Whiskers indicate the 10th and 90th percentiles. The upper and lower boundaries of the box indicate the 25th and 75th percentiles. The mean and median are shown with the thick and thin lines, respectively, located near the center of the individual boxes. Stimulation level is specified in clinical programming units (CL).
Figure 4.
These graphs show mean ECAP thresholds plotted as a function of the stimulating electrode. Data from children are shown with open symbols and dashed lines. Data from adults are shown with filled symbols and solid lines. The circles are used to indicate data obtained from study participants who used the Nucleus CI24M cochlear implant. Squares are used to show results obtained from Nucleus CI24R cochlear implant users. Error bars indicate ±1 SE around the mean. Stimulation level is specified in clinical programming units (CL).
Figure 5.
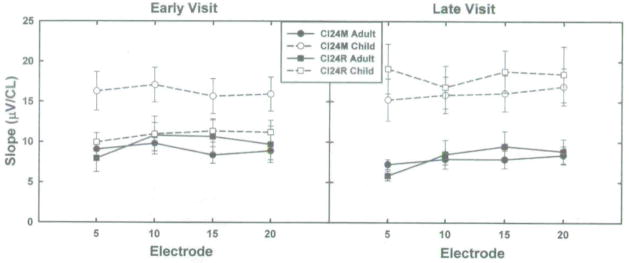
These graphs show slope of the ECAP growth functions plotted as a function of the stimulating electrode. Data from children are shown with open symbols and dashed lines. Data from adults are shown with filled symbols and solid lines. Circles are used to indicate data obtained from study participants who used the Nucleus CI24M cochlear implant. Squares are used to show results obtained from Nucleus CI24R cochlear implant users. Error bars indicate ± 1 SE around the mean. Stimulation level is specified in clinical programming units (CL).
RESULTS
Figure 2 shows changes in mean ECAP thresholds and slope of the ECAP growth functions over time for all 134 subjects who participated in this study. The two graphs on the left side of Figure 2 show mean ECAP threshold and slope data obtained from 69 CI24M cochlear implant users (40 adults and 29 children). The graphs on the right side of Figure 2 include mean ECAP threshold and slope data for 65 CI24R cochlear implant users (21 adults and 44 children). All four graphs show data from adults plotted using filled symbols and solid lines while open symbols and dashed lines are used to show results obtained from pediatric CI users. This figure includes data from up to four different stimulating electrodes tested at multiple intervals over a time period that exceeded 3.5 yr. Not all subjects were seen for electrophysiological testing at each test interval, and data were not always available from each subject for all four of the stimulating electrodes. Still, several observations can be made by examining this figure. First, regardless of device type (CI24M vs. CI24R) or subject age (adult vs. child), mean ECAP threshold and slope functions appear relatively stable across time. That is particularly true after the first year of CI use. If long-term use of a CI was damaging to the peripheral neural tissue, one may have expected to see thresholds increasing and slopes decreasing over time. No such trends are evident in the data. Second, there are some notable differences in mean data obtained from children relative to similar measures recorded from postlingually deafened adults. Specifically, while pediatric CI recipients seem to have ECAP thresholds that approximate or exceed adult ECAP thresholds, their ECAP growth functions are almost twice as steep as those measured in adult CI users.
While descriptive, the results shown in Figure 2 are difficult to analyze statistically due to the variance in sample size at each test interval. In order to address that problem, a subset of the total data pool was selected for further analysis. This smaller data set included results obtained only from individuals for whom we had data from all four stimulating electrodes at both an “early” visit, which took place between 3 and 6 mo (mean = 3.8 mo) postconnection, and a second visit (the “late” visit) that occurred about 4.5 to 6 yr later (mean = 5.8 yr). Figures 3–5 show results collected from this smaller subject group.
In Figure 3, box plots are used to represent the distribution of results obtained from this more selected subject pool. The panel on the left side of Figure 3 shows the impact that device type (CI24M vs. CI24R), subject age group (child vs. adults), and visit (early vs. late) had on ECAP threshold. The panel on the right shows how these same variables influence slope of the ECAP growth function. In this figure, results from the four stimulating electrodes have been combined. Box plots are used to represent the distribution of the data. Dots indicate the 5th and 95th percentiles. Whiskers indicate the 10th and 90th percentiles. The upper and lower boundaries of the rectangular boxes mark the 25th and 75th percentiles. The thick line within the rectangle is the mean; the thinner line is the median. Data obtained from children is shown with the white boxes. Data obtained from adults is shown in gray. In order to facilitate comparison across visits, a dotted line has also been added to each panel. The dotted line in the panel on the left side of Figure 3 is set equal to the mean ECAP threshold recorded from the adult CI24M CI users at the early visit. The dotted line on the right panel of Figure 3 is set at a level equal to the mean slope recorded from adult CI24M CI users at the early visit.
Figures 4 and 5 show the effect of stimulating electrode on the average ECAP threshold and slope data (±1 SE). Recordings obtained from adults are shown with solid lines and filled symbols. Similar measures recorded from children are shown with open symbols and dashed lines. Circles are used to depict results obtained from subjects who use the CI24M device. Squares are used to show results obtained from CI24R cochlear implant users.
In order to investigate the statistical significance of the mean trends observed in Figures 3–5, two linear mixed models were developed (SAS v9.2, Proc Mixed). In one analysis, ECAP threshold was the outcome variable. In the other analysis, slope of the ECAP growth function was the outcome variable. Age (child vs. adult) and device (CI24M vs. CI24R) were treated as fixed effects. Both stimulating electrode (5, 10, 15, and 20) and visit (early vs. late) were repeated factors. Compound symmetric covariance matrices were used to model the within-subject correlation on the repeated factors. All multiple comparisons that were performed used the Tukey-Kramer adjusted p-value.
Threshold Effects
The effects of age, device, and visit on ECAP threshold are shown in the panel on the left side of Figure 3. Electrode effects are shown in Figure 4. In the statistical model used, age, device, visit, and electrode were treated as main effects. All pairwise interactions were examined and eliminated from the model if the p-values was greater than 0.1. The remaining significant interactions were electrode*device (p < 0.0001) and device*visit (p = 0.03).
As shown in Figure 2 as well as in Figures 3 and 4, there is a tendency for ECAP thresholds to be equal to or slightly higher for pediatric as opposed to adult CI recipients. This trend in the mean data is subtle but was marginally significant (p = 0.04). Regardless of the visit (early vs. late), the stimulating electrode (5, 10, 15, or 20), or the device type (CI24M vs. CI24R), children had slightly higher ECAP thresholds than adults.
Additionally, while the mean differences are small, regardless of device type or subject age, ECAP thresholds recorded at the late visit were significantly higher than those recorded at the early visit (p < 0.0001). For adults, the average increase in ECAP threshold between the early and late visits was 3.94 CL. For children, the average increase was 4.16 CL. This trend, while subtle, can be appreciated by comparing the results plotted on the right and left sides of the panel on the left side of Figure 3.
Because device interacted significantly with electrode and visit, several additional comparisons were done. As shown in Figure 4, for subjects who used the CI24M device, there was a tendency for lower ECAP thresholds to be recorded from the more apical stimulating electrodes. ECAP thresholds on electrodes 5 and 10 or electrodes 15 and 20 were not significantly different from each other (p = 0.99 for both comparisons). However, ECAP thresholds obtained when electrodes 5 and 10 were stimulated were significantly higher than either electrode 15 or electrode 20 (p < 0.0001 for each comparison). For subjects who use the CI24R cochlear implant, all the means differ significantly from each other at the p < 0.0001 level with the exception of the difference in ECAP thresholds recorded using stimulation of electrodes 5 and electrode 15. Regardless of the visit, like their CI24M counterparts, slightly lower ECAP thresholds are obtained when more apical stimulating electrodes are used. Unlike the CI24M cochlear implant data, however, ECAP thresholds obtained using stimulation of electrode 5 (the most basal electrode tested) were lower than similar measures obtained using stimulation of electrode 10. This may reflect the fact that the CI24R cochlear implant incorporates a “modiolar hugging” design that may result in closer proximity of the stimulating electrodes to the modiolus and lower thresholds.
Slope Effects
Slope data are illustrated in the panel on the right side of Figure 3 and in Figure 5. In the statistical model used to analyze the ECAP slope data, age, device, visit, and electrode were main effects. Interactions included device*visit and age*visit. In contrast to the ECAP threshold data reviewed above—where the mean trends, while significant, were subtle—the slope data shown in the panel on the right in Figures 3 and 5 are robust. Pediatric CI users have significantly steeper growth functions than adult CI users (p < 0.0001). This is true regardless of device type, electrode, or visit. Neither of the interactions that were tested (device*visit or age*visit) were significant at the p = .05 level.
DISCUSSION
Electrically evoked compound action potentials can be recorded easily, quickly, and noninvasively in cochlear implant users of all ages. These evoked potentials provide a relatively direct measure of the response of the auditory nerve to electrical stimulation. Much of the research on clinical applications for the ECAP has focused on predicting the levels needed to program the speech processor of the cochlear implant. However, this is clearly not the only potential application for this technology. While cochlear implants are designed to last a lifetime, malfunctions do occur. It is also unclear whether long-term use of a cochlear implant could be damaging. ECAPs provide an efficient method of monitoring changes over time. They are not influenced by learning effects or attention. Additionally, ECAP threshold and rate of growth of these electrically evoked potentials are known to be related to neural survival (Miller et al, 1994) and as such may provide an early indication of any stimulation-induced changes that may occur.
This study reports changes in ECAP threshold and slope measures for a relatively large group of CI users over a span of more than 5 yr. Animal studies have shown that inappropriate levels of electrical stimulation can lead to degenerative change in the cochlea and at the level of the auditory nerve. If that were the case, one might expect that long-term use of a CI would result in elevated ECAP threshold and a reduction in slope. Examination of Figure 3 shows that for both adult and pediatric CI recipients, mean ECAP thresholds are slightly higher at the late visit than they are at the early visit. This difference, while small and likely not of much significance from a clinical perspective, is statistically significant. Mean slope values recorded from adults at the late visit are slightly lower than those recorded at the early visit, but this trend was not statistically significant. No such trend was apparent in the data recorded from pediatric CI recipients.
This finding—that over the long term, ECAP thresholds and slopes are relatively stable—is generally consistent with previous results published from our laboratory (Hughes et al, 2001) and with the results of other investigators who have monitored changes in ECAP threshold and/or slope for periods of up to approximately two years following surgery (Thai-Van et al, 2001; Henkin et al, 2003; Lai et al, 2004; Henkin et al, 2006; Zwolan et al, 2008).
The most robust result to come out of this study is that ECAP growth functions are much steeper for children than they are for adults. This difference was highly significant regardless of the implant type or stimulating electrode and was obvious in the ECAP measures obtained from CI24M cochlear implant users at both the early and late visits. The trend is less robust but still statistically significant for the CI24R cochlear implant users at the early visit but clearly apparent at the later visit. The finding—that children have considerably steeper ECAP growth functions than adults —is also consistent with data published previously by our group based on results obtained from CI24M cochlear implant users over a period of 2 yr following surgery (Hughes et al, 2001). This study extends those results to include data from a larger group of subjects, who were followed for longer observation intervals and included data from individuals who use the CI24R as well as the CI24M cochlear implant.
Steeper growth functions coupled with lower thresholds may indicate larger populations of surviving neurons (Smith and Simmons, 1983; Hall, 1990; Miller et al, 1994). That was not what was observed in this study. We found that while children tended to have much steeper growth functions than adults, they also had significantly higher ECAP thresholds. This observation—steeper growth functions but higher thresholds—is consistent with a theory that on average the children participating in this study may have a greater distance between the electrode contact and the location where the auditory nerve fiber is activated. Because the cochlea in a child is very nearly fully developed at birth, and because the increased slope values that we measure have been shown to persist well past early childhood, it seems unlikely that the differences observed in the NRT measures for children and adults are due to age-related changes in overall cochlear dimensions. More likely is the possibility raised by Hughes et al (2001) that current fields within the cochlea are different for children than they are for adults. It may be that a child who receives a cochlear implant develops more fibrous tissue within the cochlea than an adult and as a result the current fields within the cochlea are changed such that there is effectively more distance between the stimulating electrode contact and the point on the auditory nerve where stimulation occurs. Hughes et al (2001) note that excessive scarring after surgical procedures is more common in children and adolescents than in adults (Bardach and Hurwitz, 1983; Linares et al, 1972). It may be that a similar reaction could be occurring within the cochlea of pediatric CI users. Consistent with this hypothesis is the fact that Hughes et al (2001) report higher electrode impedance values for pediatric CI24M cochlear implant users than for their adult counterparts.
It may also be that there are other anatomical differences between the pediatric CI users who participated in this study and the adult CI users that could result in increased distance between the electrode contact and the point where the auditory nerve is stimulated. Pediatric CI users were often deaf since birth while the adults all lost their hearing due to a range of different etiologies after the cochlea and auditory nerve fibers were fully developed. One possibility is that children with congenital hearing loss may have fewer and/or shorter dendritic processes than is typical for postlingually deafened adults. If that were the case, it would create a situation where initial site of stimulation on the auditory nerve of pediatric CI users may be near the modiolus of the cochlea while for adults it may be more peripheral, that is, closer to the electrode array. While it is unlikely that imaging or temporal bone studies will effectively resolve this issue, one might expect that surface recordings of the average electrode voltages, psychophysical measures of pitch perception, and/or electrophysiologic measures of channel interaction may differ for pediatric and adult CI users. Additional studies are currently underway in our laboratory to explore these possibilities.
Finally, this study also points to some device-related effects. As shown in Figure 4, ECAP threshold tended to be slightly lower for the more apical stimulating electrodes. This trend was significant for both devices and is consistent with previous reports (e.g., Miller et al, 2008). When compared to the CI24M cochlear implant, the intracochlear electrode array of the CI24R device is contoured so that once inserted into the cochlea, the electrode contacts may lay closer to the modiolus. That effect might be more apparent for electrodes near the base of the cochlea. In this study, the effect of device on ECAP threshold and slope was minimal; however, mean ECAP thresholds were found to be significantly lower on electrode 5 than on electrode 10 only for subjects who used the CI24R cochlear implant (see Figure 4). Electrode 5 is the most basal electrode tested. Future studies could determine the effect of device-dependent differences in electrode position on the ECAP.
SUMMARY AND CONCLUSIONS
ECAP thresholds and growth functions are relatively stable over time for both pediatric CI users and postlingually deafened adults.
Adults show very small but statistically significant increases in ECAP thresholds over time. While on average their growth functions show a tendency to become shallower after prolonged periods of device use, this difference did not reach statistical significance.
Children who receive a CI exhibit higher ECAP thresholds and significantly steeper growth functions than adult CI users. This finding was robust, consistent with previous studies and independent of device type (CI24M vs. CI24R), electrode (5, 10, 15, or 20), or visit (early vs. late).
ECAP thresholds tend to be slightly lower for more apical electrodes regardless of device type (CI24M vs. CI24R). Subjects who used the CI24R cochlear implant, on average, had slightly lower ECAP thresholds on electrode 5 than for electrode 10. This result may have been expected based on differences between the CI24M and CI24R cochlear implants in terms of electrode design.
Acknowledgments
This work was supported in part by grants from the NIH/NIDCD (P50DC00242), NIH/NCRR (RR00059), and the Iowa Lions Sight and Hearing Foundation.
The authors would like to thank the CI users and their families for participating in this long-term study and the following individuals, each of whom helped collect and/or analyze the data included in this report: Michelle Hughes, Suzanne Dunn, Amy Behrens, Tanya Van Voorst, Lisa Stille, Aayesha Dhuldhoya, Hyo Chang Arnold, Sean Sweeney, Li-Kuei Chiou, Julie-Eun Kyung Jeon, and Sheryl Erenberg.
Abbreviations
- CI
cochlear implant
- CI24M
the original version of the Nucleus cochlear implant that had a straight, fully banded intracochlear electrode array
- CI24R
a more recently introduced version of the Nucleus cochlear implant that includes a half banded, contoured electrode array designed to lie slightly closer to the modiolus of the cochlea
- CL
clinical programming units for Nucleus CIs
- ECAP
electrically evoked compound action potential
- NRT
neural response telemetry
Footnotes
A subset of the data included in this report was presented at the 4th International Symposium on Objective Measures in Cochlear Implants, 2005, Hannover, Germany.
References
- Abbas PJ, Brown CJ, Shallop JK, et al. Summary of results using the nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear. 1999;20(1):45–59. doi: 10.1097/00003446-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Bardach J, Hurwitz DJ. Pediatric plastic and reconstructive surgery of the head and neck. In: Bluestone CD, Stool SE, Kenna MA, editors. Pediatric Otolaryngology. 1.3. Philadelphia: WB Saunders; 1983. pp. 805–830. [Google Scholar]
- Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res. 1990;49(1–3):155–168. doi: 10.1016/0378-5955(90)90102-u. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104(3):311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Kaplan-Neeman R, Kronenberg J, Migirov L, Hildesheimer M, Muchiuk C. A longitudinal study of electrical stimulation levels and electrode impedance in children using the Clarion cochlear implant. Acta Otolaryngol. 2006;126(6):581–586. doi: 10.1080/00016480500443391. [DOI] [PubMed] [Google Scholar]
- Henkin Y, Kaplan-Neeman R, Muchnik C, Kronenberg J, Hildesheimer M. Changes over time in electrical stimulation levels and electrode impedance values in children using the Nucleus 24M cochlear implant. Int J Pediatr Otorhinolaryngol. 2003;67:873–880. doi: 10.1016/s0165-5876(03)00131-9. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Vander Werff KR, Brown CJ, et al. A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users. Ear Hear. 2001;22(6):471–486. doi: 10.1097/00003446-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Krueger B, Joseph G, Rost U, Strauss-Schier A, Lenarz T, Buechner A. Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol. 2008;29:509–512. doi: 10.1097/MAO.0b013e318171972f. [DOI] [PubMed] [Google Scholar]
- Lai WK, Aksit M, Akdas F, Dillier N. Longitudinal behaviour of neural response telemetry (NRT) data and clinical implications. Int J Audiol. 2004;43(5):252–263. doi: 10.1080/14992020400050034. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412(4):543–562. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leake PA, Rebscher SJ. Anatomical consideration and long-term effects of electrical stimulation. In: Zeng F-G, Popper AN, Fay R, editors. Cochlear Implants: Auditory Prostheses and Electric Hearing. New York: Springer; 2004. [Google Scholar]
- Linares HA, Kischer CW, Dobrkovsky M, Larson DL. The histotypic organization of the hypertrophic scar in humans. J Invest Dermatol. 1972;59(4):323–331. doi: 10.1111/1523-1747.ep12627386. [DOI] [PubMed] [Google Scholar]
- Marsh MA, Coker NJ, Jenkins HA. Temporal bone histopathology of a patient with a nucleus 22-channel cochlear implant. Am J Otol. 1992;13(3):241–248. [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Brown CJ. An improved method of reducing stimulus artifact in the electrically evoked whole-nerve potential. Ear Hear. 2000;21(4):280–290. doi: 10.1097/00003446-200008000-00003. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. The use of long-duration current pulses to assess nerve survival. Hear Res. 1994;78( 1):11–26. doi: 10.1016/0378-5955(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Miller CA, Brown CJ, Abbas PJ, Chi SL. The clinical application of potentials evoked from the peripheral auditory system. Hear Res. 2008;242(1–2):184–197. doi: 10.1016/j.heares.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Parkinson AJ, Arcaroli J, Staller SJ, Amdt PL, Cosgriff A, Ebinger K. The nucleus 24 contour cochlear implant system: adult clinical trial results. Ear Hear. 2002;23(Suppl):41S–48S. doi: 10.1097/00003446-200202001-00005. [DOI] [PubMed] [Google Scholar]
- Ruffin CV, Tyler RS, Witt SA, Dunn CC, Gantz BJ, Rubinstein JT. Long-term performance of Clarion 1.0 cochlear implant users. Laryngoscope. 2007;117(7):1183–1190. doi: 10.1097/MLG.0b013e318058191a. [DOI] [PubMed] [Google Scholar]
- Shapiro W, Waltzman S. Changes in electrical thresholds over time in young children implanted with the Nucleus cochlear prosthesis. In: Clark GM, Cowan RSC, eds. International Cochlear Implant, Speech and Hearing Symposium—Melbourne. Ann Otol Rhinol Laryngol. 1995;104(Suppl):177–178. [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Smith L, Simmons FB. Estimating eighth nerve survival by electrical stimulation. Ann Otol Rhinol Laryngol. 1983;92(1, pt. 1):19–23. doi: 10.1177/000348948309200105. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Chanal JM, Coudert C, Veuillet E, Truy E, Collet L. Relationship between NRT measurements and behavioral levels in children with the Nucleus 24 cochlear implant may change over time: preliminary report. Int J Pediatr Otorhinolaryngol. 2001;58(2):153–162. doi: 10.1016/s0165-5876(01)00426-8. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Kelsay DM, Teagle HF, Rubinstein JT, Gantz BJ, Christ AM. 7-year speech perception results and the effects of age, residual hearing and preimplant speech perception in pre-lingually deaf children using the Nucleus and Clarion cochlear implants. Adv Otorhinolaryngol. 2000;57:305–310. doi: 10.1159/000059134. [DOI] [PubMed] [Google Scholar]
- Wang N-Y, Eisenberg LS, Johnson KC, et al. CDaCl Investigative Team. Tracking development of speech recognition: longitudinal data from hierarchical assessments in the Childhood Development after Cochlear Implantation Study. Otol Neurotol. 2008;29(2):240–245. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia JJ, Niparko JK, Oviatt DL, Kemink JL, Altschuler RA. Evaluation of the temporal bones of a multichannel cochlear implant patient. Ann Otol Rhinol Laryngol. 1991;100(11):914–921. doi: 10.1177/000348949110001111. [DOI] [PubMed] [Google Scholar]
- Zwolan TA, O’Sullivan MB, Fink NE, Niparko JK CDACI Investigative Team. Electric charge requirements of pediatric cochlear implant recipients enrolled in the Childhood Development After Cochlear Implantation study. Otol Neurotol. 2008;29(2):143–148. doi: 10.1097/MAO.0b013e318161aac7. [DOI] [PubMed] [Google Scholar]