Abstract
PURPOSE
Paths of inactive lateral rectus (LR) muscles were studied to investigate putative roles of orbital fat and intrinsic muscle stiffness suggested to be alternatives to connective tissue pulleys as determinants of pulling direction.
METHODS
Surface coil orbital magnetic resonance imaging was performed in axial planes in adult humans: seven with chronic unilateral LR paralysis, three with nonparalytic concomitant esotropia of similar angle, and 15 healthy controls. Fixation was controlled using targets placed at a broad range of horizontal positions.
RESULTS
Paralyzed LRs exhibited marked atrophy compared with functional contralateral LRs and LRs of orthotropic and esotropic subjects without LR paralysis. The normal LR exhibited a gradual 18.8° ± 4.5° (mean ± SD) lateral inflection 14.4 ± 2.6 mm posterior to the globe center, bowing the LR away from the orbital center. The paralyzed LR exhibited a significantly (P < 0.002) larger and typically more discrete 29.2° ± 8.8° lateral inflection, similar to that observed in concomitant esotropia in maximal adduction. Average position of this inflection was 11 to 14 mm posterior to the globe center in all three subject groups, but in LR palsy only the inflection of the paralyzed LR—0.17 mm further posterior per degree of abduction (linear fit, R = 0.85)—depended on horizontal gaze. The behavior of the paralyzed LR inflection was consistent with LR pulley anatomy.
CONCLUSIONS
Sharper lateral inflection in the flaccid rather than the tense LR seems inconsistent with intrinsic muscle stiffness or diffuse orbital fat pressure but suggests the influence of discrete connective tissue.
The seminal x-ray tomographic observations of Simonsz1 and the magnetic resonance imaging (MRI) observations of Miller2 showed for the first time that extraocular muscles (EOMs) do not follow the shortest paths from their origins in the orbital apex to their insertions on the globe. It has subsequently emerged that connective tissue pulleys surround the rectus and inferior oblique EOMs at their points of perforation of posterior Tenon fascia.3,4 In secondary and tertiary gazes, at the locations of these pulley rings, the paths of the EOMs are inflected away from the shortest paths to their scleral insertions, which have been shifted by ocular rotation.5–8 Because the direction of force exerted on the globe is determined by EOM path direction at the point at which the EOM inserts on the globe, the inflections in the EOM path determine the directions of EOM force and produce a dependence of EOM pulling direction on instantaneous eye position.9–12 Because of systematic variation in EOM paths, pulling directions of rectus EOMs change with eye position so as to implement Listing’s law of ocular torsion,7 a robust kinematic feature of visually guided eye movements that has been shown not to be encoded in the neural commands to the EOMs normally regarded as having torsional actions.13 Conversely, pathologically abnormal paths14–17 or variations in EOM paths with gaze8 are associated with strabismus.
It has been argued that specialized connective tissues, the pulleys, underlie the stability and gaze direction dependence of EOM paths because the sharp inflections in rectus and inferior oblique paths occur at the sites corresponding to the pulley rings. Rectus EOM pulleys, however, consist of more than focal pulley rings. The focal rings, consisting of dense,3,18,19 woven20 collagen reinforced by elastin3 and some adjacent smooth muscle,3,19,21 are embedded in an extensive sleeve with a much longer anteroposterior dimension along the EOM.4,18 The rings are roughly located at the anteroposterior midpoint of extensive tubular sleeves. The classical centrifugal bowing of rectus EOMs away from the orbital center, observed by Simonsz1 and Miller,2 does not correspond in location to the EOM path inflections due to the more anterior pulley rings.22 Given that centrifugal bowing of the rectus EOM path occurs farther posterior than the pulley rings, centrifugal bowing has a less significant potential effect on EOM pulling direction. In addition, given that this effect does not depend geometrically on eye rotational eye position, the kinematic consequences to EOM pulling direction are minimal. As a functional anatomic phenomenon, centrifugal rectus path bowing is nonetheless instructive.
It has long been suggested that some inflection of EOM paths is caused by pressure on compartmentalized orbital fat.2,23,24 Quantitative histologic examination of the human orbit, however, does not suggest that the fat is compartmentalized into distinct “intraconal” and “extraconal” spaces that would seemingly be required to create the centrifugal gradient of fat pressure required to bow the rectus EOMs away from the orbital center.3 More recently, Schutte et al.24 have offered an alternative explanation for rectus EOM path behavior based on constraints caused by generally homogeneous and isotropic orbital fat, discounting the contribution of the pulleys or connective tissues entirely.24 Based on a finite element model of the mechanical properties that lumps together all the nonmuscular orbital tissues as intraconal fat or extraconal fat, Schutte et al.24 suggested that properties of the orbital fat may be responsible for the bowing and stability of rectus EOMs and that specialized connective tissue structures do not even stabilize EOM paths during gaze shifts. If, however, the orbit is effectively filled with only EOMs and “fat” not distinguished by focal mechanical properties, one would not anticipate the existence of abrupt direction changes in EOM paths not associated with substantial changes in EOM properties such as stiffness. It seems reasonable to assume that EOM cross-section would be an indicator of stiffness or resistance to deformation. Consequently, examination of path bowing could be informative about the uniformity of the mechanical properties of tissues surrounding an EOM and the relative mechanical contributions of surrounding orbital connective tissues to EOM paths. In theory, the location of centrifugal inflections in rectus EOM paths could be informative about the mechanical stiffness of the collagenous posterior pulley sleeve relative to EOM tension and the relative contribution of pressure from more distributed and homogeneous tissues such as loose orbital fat.
In physiological situations, centrifugal EOM path bowing is a gradual and poorly localized phenomenon. The relative contributions of orbital fat and pulley tissues may be difficult to discriminate in the normal orbit in the absence of perturbations such as large ocular rotations. Different types of perturbation to normal anatomy are, therefore, instructive. The current report used MRI to characterize the more pronounced and localized centrifugal inflection of the paths of paralyzed, atrophic lateral rectus (LR) EOMs in humans with denervation myopathy and correlated path changes with the known anatomy of the pulley connective tissue system. A further control consisted of subjects with large-angle, nonparalytic esotropia in maximal adduction, which produces maximum physiological LR relaxation in the absence of denervation.
METHODS
Subjects
Seven adults (four men, three women; age range, 20–54 years) with chronic unilateral LR paralysis, three subjects with large-angle esotropia in the absence of paralysis, and 15 healthy control volunteers agreed to undergo clinical examination and MRI after giving written informed consent to a protocol that conformed to the Declaration of Helsinki and that was approved by the local institutional review board. Subjects underwent examination of corrected visual acuity, ocular motility, eyelid structure and function, binocular alignment, anterior segment anatomy, and ophthalmoscopy. All control subjects had normal ocular versions, normal binocular alignment, and normal stereo threshold of at least 40 arcsec by the Titmus method. All subjects with LR paralysis (P1–P7) had limited abduction of the affected eye, slowing of abducting saccades in the affected eye, and esotropia (ET) that increased with effort to look toward the affected side. Subjects with nonparalytic esotropia (ET1–ET3) had deviations of 55Δ to 60Δ in central gaze that were quantitatively similar to deviations of subjects with LR paralysis, did not vary significantly with horizontal gaze direction, and were associated with normal horizontal versions and saccades. Clinical features of subjects with LR paralysis and concomitant ET are listed in Table 1. Most subjects with LR paralysis eventually underwent strabismus surgery, including transposition of the insertions of the vertical rectus EOMs temporally to the lateral rectus insertion, with scleral posterior fixation to augment the temporal shift of the pulleys of the transposed vertical rectus EOMs. Two of three subjects with concomitant ET underwent unilateral or bilateral strabismus surgery consisting of appropriate combinations of LR resection and MR resection. After MRI, subject ET1 underwent strabismus surgery that left his vision orthotropic in all eye positions, and he then attained a stereopsis of 40 arcsec by the Titmus method.
TABLE 1.
Clinical Features of Subjects with Esotropia
| Subject | Age (y) |
Sex | Palsy Laterality |
Central Gaze Esotropia (Δ) |
Etiology | Duration (mo) |
Surgery |
|---|---|---|---|---|---|---|---|
| P1 | 50 | F | Left | 70 | Meningioma | 10 | None |
| P2 | 33 | M | Left | 65 | Chordoma | 2 | None |
| P3 | 46 | M | Left | 60 | Chordoma | 2 | None |
| P4 | 54 | M | Right | 50 | Meningioma | 7 | None |
| P5 | 32 | F | Left | 40 | Idiopathic | 36 | None |
| P6 | 20 | M | Right | 65 | Basilar skull fracture |
8 | None |
| P7 | 51 | F | Left | 50 | Complicated migraine |
120 | Vertical rectus transposition* |
| ET1 | 28 | M | N/A | 60 | Decompensated esophoria |
6 | None |
| ET2 | 54 | M | N/A | 60 | Congenital | 648 | Medial rectus recession |
| ET3 | 42 | F | N/A | 55 | Congenital | 500 | Medial rectus recession |
Subjects P1 to P7 have LR palsy. Subjects ET1 to ET3 have nonparalytic ET. MRI was performed before and after strabismus surgery in subject P7, after strabismus surgery in ET2 and ET3, and before surgery in all other subjects.
Vertical rectus transposition shifted insertions of the superior and inferior rectus muscles to abut the LR insertion on the sclera and were augmented with scleral sutures 8 mm posteriorly to shift further the vertical rectus pulleys temporally
MRI was performed in all subjects with a 1.5-T scanner (Signa; General Electric, Milwaukee, WI) and custom surface coils, as described elsewhere in detail.5,6,25–27 Imaging was performed using T1 weighting or T2 fast spin-echo sequences. During scanning, fixation was controlled using small lighted targets at various horizontal eccentricities that ranged, among subjects with LR paralysis, from 36° adduction to 29° abduction, with subjects P4 and P7 imaged in multiple horizontal gaze positions. Scanning in multiple gaze positions was possible only in a second imaging session in subject P7, after vertical rectus transposition surgery, whereas in subjects ET2 and ET3 scanning was performed only after the types of medial rectus recession surgery listed in Table 1. Subjects with concomitant ET were imaged with one eye fixating centrally and one eye highly adducted; this placed the nonfixating eye in presumably maximal adduction that fully straightened the optic nerve. Quasicoronal images perpendicular to the long axis of the orbit were obtained at 2-mm thickness in a matrix of 256 × 256 over a field of view of 6 to 8 cm. Axial images of 2- or 3-mm thickness in a matrix of 256 × 192 or 256 × 256 were obtained over a field of view of 10 to 12 cm (Figs. 1 and 2.).
FIGURE 1.
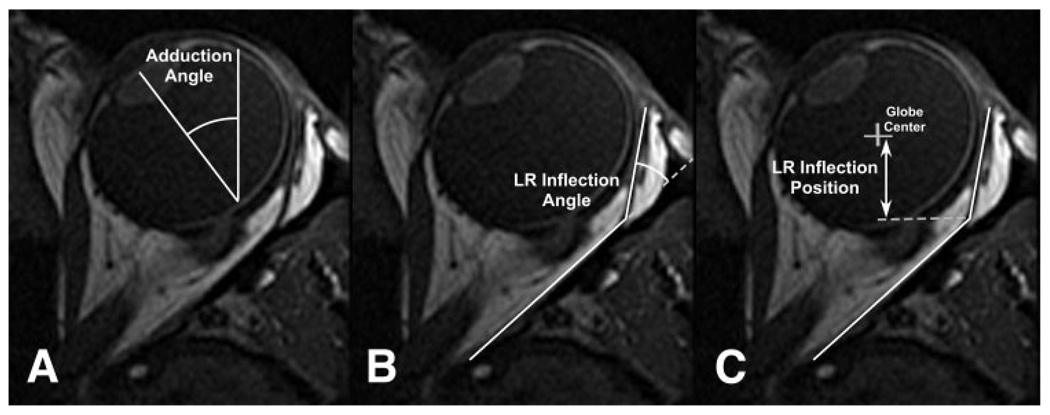
Method of determination of (A) adduction angle, (B) LR inflection angle, and (C) LR inflection position posterior to globe center from T1-weighted axial MRI (Fig. 2) of subject P1, who had left LR palsy.
FIGURE 2.
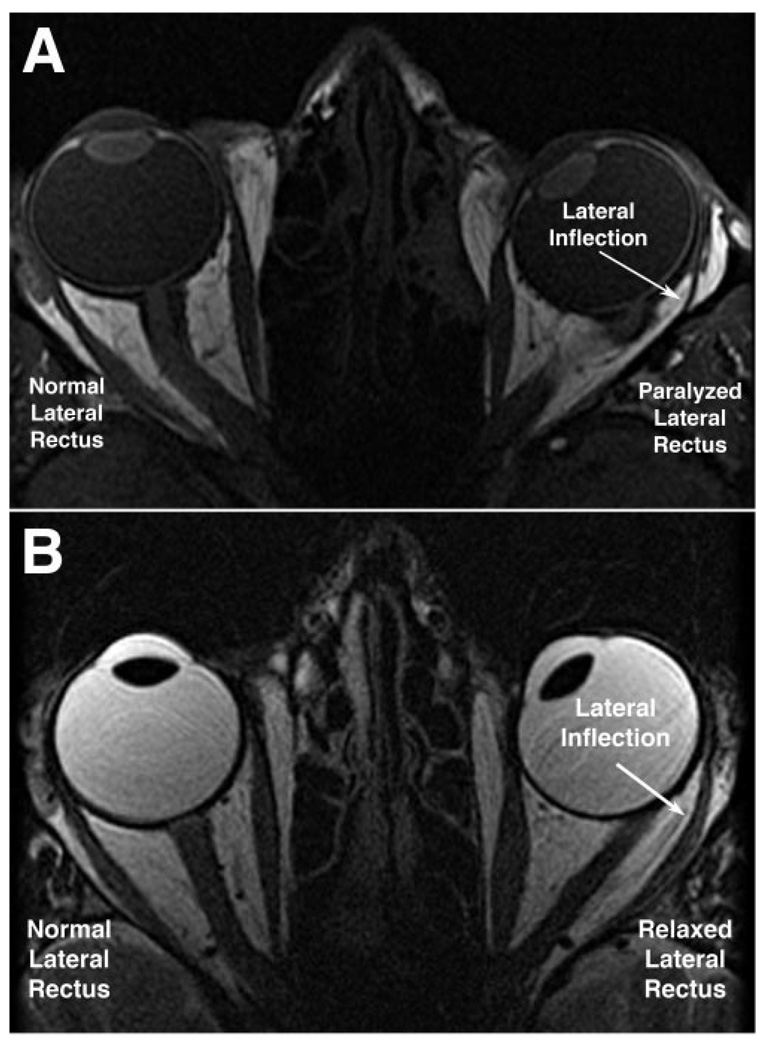
Axial MRI showing LR paths during right eye fixation of a central target. (A) T1-weighted image in subject P1 shows lateral inflection and atrophy of paralyzed left LR muscle during central fixation by the normal right eye. Note left ET, and note that the thinned posterior belly of the left LR is immediately adjacent to the orbital wall. (B) T2-weighted image in subject ET1 exhibiting left ET, similar in magnitude to that of subject P1 with paralytic ET. Note that the relaxed, nonparalyzed LR is thicker and less markedly inflected than the paralyzed LR.
Image Analysis
Spatially calibrated digital magnetic resonance images were quantified using the National Institutes of Health programs Image 1.63 and ImageJ 1.33µ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Eye position was determined in scanner coordinates based on a line perpendicular to the iris plane passing as nearly as possible through the pupillary and lens centers, which were always colocated, in the axial image plane also containing the optic nerve (Fig. 1A). This line intersected the retinochoroid at a location consistent with the foveola. Head orientation was determined in scanner coordinates based on midline symmetric structures in the nasal cavity and brain. Eye position in the head was taken as the difference between eye-in-scanner and head-in-scanner. We have elsewhere shown this method to agree to within 1°, with geometric requirements for binocular fusion during distance viewing and convergence to 23°.28 Lateral rectus inflection position was determined by hand drawing using the digital angle measurement tool of ImageJ and was weighted toward the very anterior and very posterior straight segments of the muscle path (Fig. 1B). The anteroposterior location of the LR inflection was taken relative to the globe center (Fig. 1C).
Statistical Analysis
Grouped data were compared by t-test using a 0.02 level of significance because of multiple comparisons. Given that it was impossible to match gaze positions exactly among the three subject groups, data distributed throughout overlapping ranges of eye positions were compared by linear regression.
RESULTS
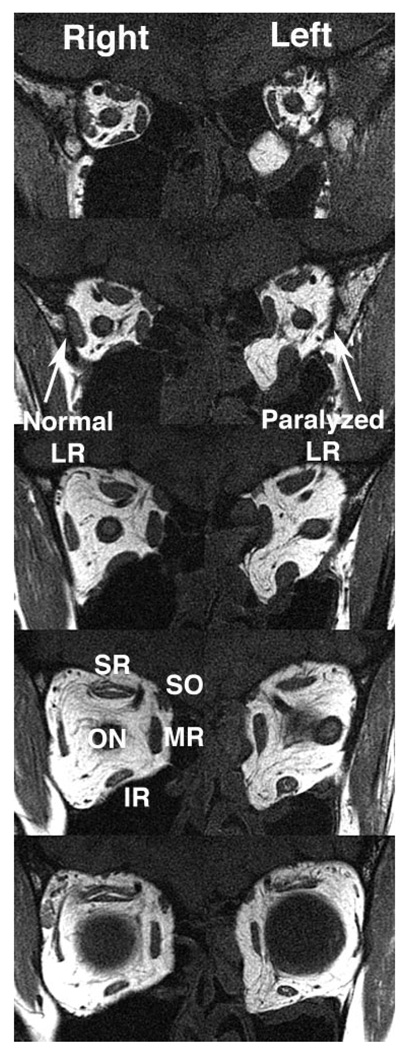
Consistent with previous reports,25,29 the deep bellies of all paralyzed LR muscles exhibited atrophy compared with the fellow normal LR muscles of the same subjects. This deep atrophy was obvious in axial (Fig. 2A) and in quasicoronal images (Fig. 3), though the most anterior LR tendon did not exhibit atrophy. None of the 15 healthy subjects exhibited LR atrophy or size asymmetry. Also striking in axial and quasicoronal MRI planes was that the anterior part of the paralyzed LR belly was immediately adjacent to the orbital wall, lacking the buffer of fat between the muscle belly and the orbital wall observed in mid and anterior normal fellow orbits (Figs. 2, 3) and all normal control orbits. Although subject P1 had shape distortion of the involved orbit because of an old orbital fracture, there were no consistent differences from normal in orbital volume associated with LR paralysis.
FIGURE 3.
Quasicoronal MRI in subject P1 with left LR paralysis. Images are 2-mm thick and are displayed from posterior (above) to anterior (below), with a 2-mm gap between images. The paralyzed left LR exhibits a smaller posterior cross-section than the normal right LR, and the middle three image planes of the left LR lies immediately adjacent to the orbital wall, without the buffer of bright fat evident around the right LR. In the most anterior image plane (bottom) near the globe, the left LR separates from the orbital wall, indicating that the lateral inflection in LR path lies between the fourth and fifth image panels.
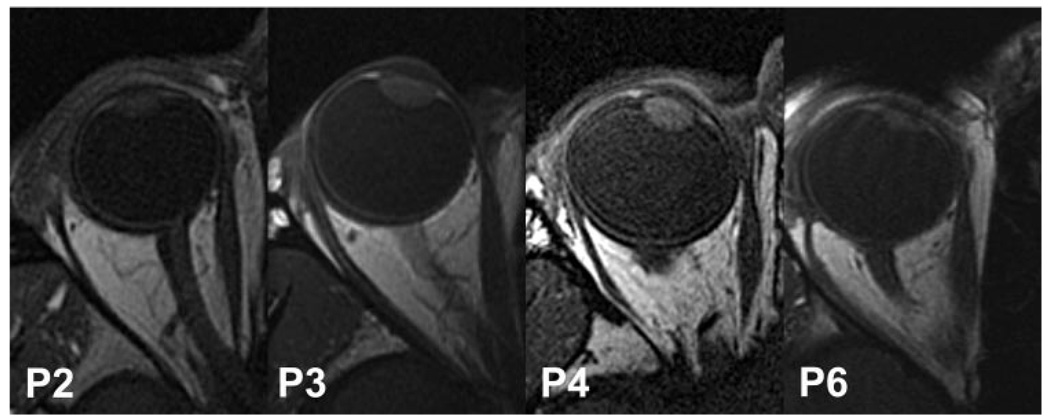
There was some variation in the appearance and paths of paralyzed LR muscles. Figure 4 illustrates this range in subjects P2, P3, P4, and P6. Although in each of these subjects the mid-belly of the paralyzed LR appears intimately applied to the lateral orbital wall, this phenomenon extended particularly anteriorly in P2 and P6 and less so in P3 and P4.
FIGURE 4.
T1-weighted axial MRI of the affected eyes of four subjects with LR paralysis, illustrating the spectrum of lateral inflection of the LR path. Inflection was sharper in subjects P6 and P2 than in subjects P3 and P4. For clarity, all images have been depicted as right eyes, using digital reflection as necessary.
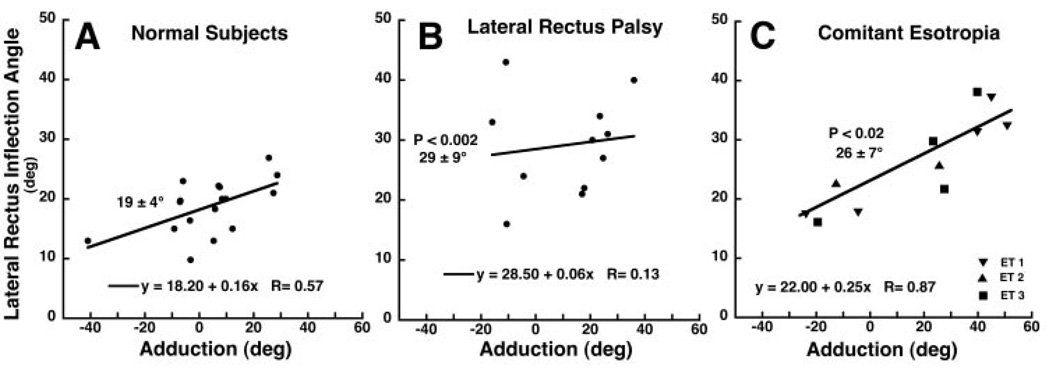
Quantitative analysis confirmed the impression conveyed by Figures 2 and 4. In each subject group, the LR inflection was determined over a wide range of horizontal ductions for scans in which an inflection could be identified and is plotted against horizontal gaze angle in Figure 5. This avoids the problem of varying gaze directions, which are difficult to control in the deviating eyes of subjects with strabismus. In Figure 5, where each point represents data from one MRI scan, linear regressions were taken on the data for each group. The LR muscles of both eyes of subjects with comitant ET contributed data, whereas only the paralyzed LR contributed in the group with LR palsy. In healthy subjects and in subjects with comitant ET, there was a significant linear correlation between horizontal gaze direction and LR inflection angle (P < 0.02). However, in subjects with LR palsy, the regression was not significant (P = 0.696). LR inflection angle was averaged over all determinations for all three subject groups. The LR of healthy control subjects exhibited 18.8° ± 4.5° mean (± SD) lateral inflection, but the paralyzed LR exhibited a significantly larger and markedly more discrete 29.2° × 8.8° (P < 0.002) lateral inflection when all data points were averaged (allowing two subjects to contribute more than one point) and 26.9° ± 10.2° (P < 0.05) when each of the seven subjects contributed only one data point obtained with the eye closer to central gaze. The corresponding mean LR inflection in comitant ET was 26.4° ± 7.9° considering all determinations in these three subjects.
FIGURE 5.
Angle of LR path inflection as a function of horizontal duction in healthy subjects (A), in subjects with LR palsy (B), and in subjects with comitant ET (C). In healthy subjects and subjects with comitant ET, but not in subjects with LR palsy, the inflection angle depended significantly on horizontal eye position (P < 0.02). The inflection angle was significantly larger than normal in subjects with LR palsy and comitant ET but did not differ between groups with strabismus. Each symbol represents one determination in each of 15 healthy subjects, one to four determinations in each of seven subjects with LR palsy, and three to five determinations in each of three subjects with comitant ET. Bold numbers indicate the mean ± SD in degrees of all determinations in each group; these values differed significantly from control values in LR palsy and concomitant ET.
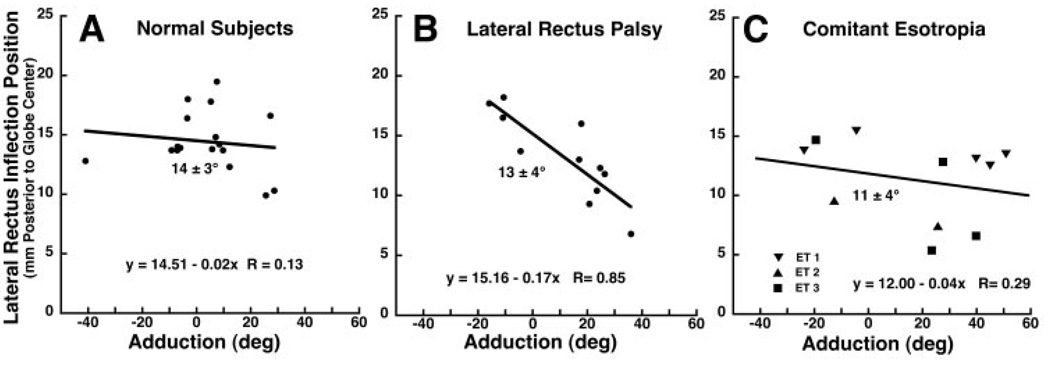
Quantitative analysis was also performed of the posterior position of the LR inflection relative to globe center. Figure 6 plots the anteroposterior position of the lateral inflection against horizontal gaze angle for the three subject groups. The LR muscles of both eyes of subjects with comitant esotropia contributed data, whereas only the paralyzed LR contributed in the group with LR palsy. Each data point represents data from one MRI scan. Linear regression was performed on the data for each group, but it demonstrated no significant correlation between horizontal gaze angle and LR inflection position in healthy subjects (Fig. 6A) and those with comitant esotropia (Fig. 6C). The difference between subjects with normal vision and those with LR palsy in this regard persisted even after exclusion of the data point in subjects with normal vision exceeding 40° abduction (Fig. 6A). For paralyzed LR muscles, however, linear regression demonstrated a significant posterior shift in the inflection from adduction to abduction, 0.17 mm further posterior per degree of abduction over a broad range (linear fit, R = 0.85, P < 0.001, Fig. 6B). For comparison between groups, the posterior location of the inflection was simply averaged over all determinations for subjects and for paralyzed LR muscles. This distance averaged 14.4 ± 2.6 mm in control subjects, 13.2 ± 4.0 mm in subjects with paralyzed LR muscles, and 11.3 ± 3.5 mm in subjects with comitant ET. These values did not differ significantly (P > 0.02).
FIGURE 6.
Anteroposterior position of the lateral inflection in LR path as a function of horizontal duction angle. Each symbol represents one determination in each of 15 healthy subjects, one to four determinations in each of seven subjects with LR palsy, and three to five determinations in each of three subjects with comitant ET. Although linear regression demonstrated no significant effect of gaze angle on position of the LR inflection in healthy subjects (A) or subjects with comitant ET (C), the inflection in the paralyzed LR (B) shifted significantly posteriorly in adduction and anteriorly in adduction (P < 0.001). Position of the inflection, indicated on each plot as mean ± SD of all determinations in each group, did not differ significantly (P > 0.02).
DISCUSSION
These images consistently confirm that the axial plane LR muscle path, even in central gaze, is neither a straight path nor the shortest path from origin to scleral insertion. Instead, the LR path is inflected toward the orbital wall a distance averaging some 11 to 14 mm posterior to globe center. This lateral inflection, seen in axial MRI, is posterior to the transverse inflection in LR path produced by the LR pulley, which lies 10 mm posterior to the globe center in central gaze in the coordinate system used here.7 The normal LR belly consistently appears separated from the anterior periorbita by a buffer of fat that has been presumed to confine the EOM. The present data, however, are interpreted differently from both these foregoing presumptions. Although the internal rigidity of EOMs has not been measured, whatever rigidity normally exists would also oppose lateral LR path inflection.
In contrast to the robust cross-sections of normal LR muscles, paralyzed LR muscles develop no active tension and exhibit striking denervation atrophy.25 The paralyzed LR is not only flaccid, it also must be presumed to have less internal rigidity than the normal LR because it is smaller than normal. In this study, MRI consistently demonstrated the paralyzed LR to be thin and pressed against the periorbita in its posterior course (Figs. 1–3). This implies that the posterior orbital fat does not confine and prohibit the LR from contact with the periorbital; rather, it suggests that the orbital fat may flow in to fill the void between the LR and the periorbita that is produced by a different mechanical constraint. In the present study, MRI showed that the anterior portion of the paralyzed LR, probably also the thinnest because of normal transition from muscle fibers to terminal tendon, was sharply inflected so that it was not directly apposed to the periorbita beginning approximately 13 mm posterior to the globe center. Rather than a diffuse effect of homogeneous orbital fat, the sharper inflection of the paralyzed LR must be caused by inhomogeneity, the focal effect of other connective tissues. It is proposed that these connective tissues are in actuality the LR pulley sleeve.
The path behavior of the innervated but highly relaxed LR in comitant ET is similar in some respects to that of the paralyzed LR. As illustrated in Figure 2, for a large angle of ET similar to LR palsy, the highly relaxed but nonparalytic LR also exhibits a substantial lateral path inflection (Fig. 2B). Although neurogenic atrophy of the paralyzed LR may make this inflection more conspicuous, quantitative properties of the inflection are similar to the situation of extreme LR relaxation without atrophy. One difference is that the anteroposterior location of the inflection of the paralyzed LR shifts more with duction than does the normal LR or the LR in concomitant ET. This may reflect the presumably lower elastic tension in the paralyzed LR than in a relaxed but innervated LR.
Biomechanical data are lacking for most orbital tissues, including EOMs. Direct mechanical measurements of many mechanical properties are not likely to be possible in human tissues. Although the distributions of connective tissues in the pulley system have been measured,3 these data at best provide only relative, not absolute, indicators of tissue stiffness. The current observations concerning LR path inflection provide additional relative measures.
Schutte et al.24 have used a finite element model (FEM) to support their proposal that orbital “fat,” rather than the connective tissue pulley system, stabilizes EOM paths. This FEM assumed that all materials except EOMs were homogeneous and isotropic and that, in addition to the globe and EOMs, the orbit contained only an incompressible material denoted as fat. For computational convenience, the fat was represented by a variably coarse mesh of tetrahedral volume elements. Although the optic nerve and connective tissues considered by others to be the pulley system3,4,9 –12,16 –22,29 were lumped into the isotropic mechanical properties of fat, the fat was considered to have an abrupt discontinuity at the border of a hypothetical muscle cone, defined by lines connecting the centers of the rectus EOMs. Although histologic examination provides no anatomic justification for the existence of a muscle cone or any reason to suspect differences in fat lying inside or outside it,3 Schutte et al.24 assumed a Young’s modulus (ratio of stress to strain in the linear region) of 300 Pa for the intraconal fat, abruptly increasing to 1000 Pa for the extraconal fat. A Young’s modulus of 300 Pa resembles the behavior of a 10% solution of gelatin in water,30 so the FEM assumes that intraconal fat is nearly liquid but that the EOMs themselves are externally encased in extraconal fat that is more than threefold more resistant to shear. The Schutte et al.24 FEM also assumed a Young’s modulus of 40 kPa for human EOMs. In contrast, unpublished data from my laboratory indicate a Young’s modulus of approximately 700 kPa for bovine EOMs (Yoo L, unpublished observations, 2008). It is thus evident that the Schutte et al.24 FEM actually makes a strong assumption about a major discontinuity in the Young’s modulus of the orbital fat at the intraconal-extraconal junction while also assuming a low Young’s modulus for EOMs.
The FEM of Schutte et al.24 was limited to ocular ductions of less than 15° due to the use of a thin, low-elasticity interface to approximate sliding of EOM tendons over the sclera that, by computational necessity, prohibited much actual sliding motion. Larger rotations caused the tetrahedron volume elements to turn inside out,24 a situation so grossly unrealistic that FEM behavior for modestly smaller ductions would also be questionable. Sliding between the globe and the posterior Tenon fascia was neglected altogether in the Schutte et al.24 FEM on the assumption that the retrobulbar fat is in direct contact with the globe and has negligible elasticity. These assumptions, which limit or ignore sliding, are neither anatomically nor mechanically realistic but are likely to have resulted in the EOM path stability that Schutte et al.24 claim as the important emergent feature of the FEM. Given that the simulated EOM tendons are artificially prohibited from sliding over the globe in the FEM, their paths are “stuck” to the underlying surface of the globe surface whenever the tendons make globe contact. It is thus true by assumption that the simulated EOM tendons must bend to move with globe rotation. Where they are not in contact with the globe, more posterior portions of the highly elastic simulated EOM bellies are, not surprisingly, constrained by the assumption of relatively high stiffness of the extraconal fat located between them. This behavior arises from unrealistic anatomic assumptions in the FEM and should not be interpreted to negate the influence of actual anatomic structures such as the orbital connective tissues. In reality, the anatomic ocular globe is suspended by the thick concave surface of posterior Tenon fascia, formed of particularly dense collagen and elastin reinforced by smooth muscle.4 Pulleys, composed of dense collagen and elastin encirclements of each of the four rectus EOMs where the EOMs penetrate the posterior Tenon fascia, are suspended from nasal and temporal anchors on the orbital walls.3
It is proposed here that efforts to understand the mechanical behavior of the EOMs and orbital tissues should remain grounded in anatomic realism. Although this mechanical behavior is so complex that detailed models of it must be computationally intensive, computational convenience should not trump anatomic accuracy. The present finding of a lateral inflection in path of the slack LR argues against the assumptions that the orbital tissues consist of isotropic and homogeneous fat. Alternatively, it is suggested that the known structure of lumped orbital connective tissue structures—the pulleys and their suspensions— can account for the present finding of lateral inflection in the path of the slack LR.
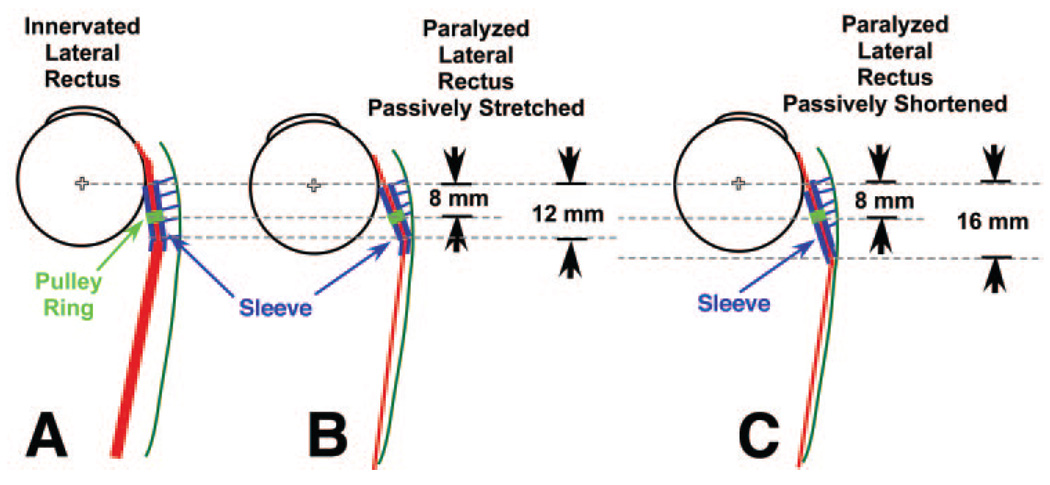
It is also proposed that stiffness of the posterior portion of the LR pulley sleeve is low enough that normal LR tension deflects the posterior mouth of the sleeve medially (Fig. 7A). When the LR is paralyzed and chronically atrophic, or when it is maximally relaxed in concomitant esotropia, LR tension is significantly lower than normal tension in the LR pulley suspension, which then pulls the LR pulley adjacent the orbital wall, displacing the mobile fat (Figs. 7B, 7C). Even so, the passive elastic tension in the slack LR is sufficient to deflect nasally what can be inferred to be the thin and elastic tissue at the posterior apex of the LR pulley sleeve (Fig. 7B). However, when the medial rectus relaxes to permit the eye to abduct slightly into the paralyzed LR’s field of action, passive LR tension decreases because LR path length is reduced (Fig. 7C). The decrease in passive elastic tension in the paralyzed LR allows the thin posterior apex of the LR pulley sleeve to straighten (Fig. 7C), shifting posteriorly the inflection point in LR path, as observed in the present data (Fig. 6B). The posterior shift in LR inflection permits the inference that the stiffness of the posterior apex of the LR pulley sleeve must be greater than the transverse component of the passive elastic tension of a paralyzed LR when the LR is subjected to further relaxation.
FIGURE 7.
Diagrammatic concept of LR path inflection in axial view. (A) Path of LR under normal tension is only slightly inflected laterally because LR tension substantially exceeds tension in suspension of the pulley sleeve. Although the pulley ring (green) is 8 mm posterior to the globe center, the pulley sleeve is continuous anteriorly and posteriorly with the pulley ring. Normal LR tension is sufficient to deform the posterior mouth of the pulley sleeve and to stretch the elastic tissues suspending the pulley from the orbital wall. (B) With the eye in adduction, the thin, paralyzed LR has less radial component of tension than the pulley suspension, which pulls the posterior LR belly into apposition with the periorbita. The paralyzed LR has sufficient tension to deform the posterior mouth of the pulley sleeve so that the lateral path inflection is approximately 12 mm posterior to globe center. (C) Relaxation of the antagonist medial rectus muscle (not shown) permits the eye to abduct slightly because of passive elastic tension in the paralyzed LR. This shortens the LR path and reduces its elastic tension relative to the adducted position. With less passive LR tension, the LR deforms the posterior pulley mouth less anteriorly, shifting the lateral inflection posteriorly.
Quantitative modeling of biological materials remains a complex and evolving field, subject to continuing development of even the most fundamental theoretical approaches. 31 Even the types of tissue properties mechanically relevant to modeling remain under fundamental debate, and these are markedly nonlinear with respect to static and dynamic factors. In such a setting, it is prudent support functional conclusions with a wide variety of quantitative and qualitative observations. The present observations concerning the path of the paralyzed or maximally relaxed LR can suggest only bounds and relative values of stiffness of pulley tissues in comparison with EOM tensions. Of course, there is a need for more and better measurements of the biomechanical properties of the EOMs and orbital connective tissues, including fat. While lumping orbital fat with connective tissues has suggested the order of magnitude of average elasticities and viscosities,30 accurate modeling will require parameterization of specific connective tissue components, particularly in the pulleys and their suspensions. Models based on observed parameters will have to conform to the behavior observed here for the paralyzed and relaxed LR.
Acknowledgments
Supported by U.S. Public Health Service, National Eye Institute Grants EY08313 and EY00331. J.D. is Leonard Apt Professor of Ophthalmology.
Footnotes
Disclosure: J.L. Demer, None
References
- 1.Simonsz HJ, Harting F, de Waal BJ, Verbeeten BWJM. Sideways displacement and curved path of recti eye muscles. Arch Ophthalmol. 1985;103:124–128. doi: 10.1001/archopht.1985.01050010130036. [DOI] [PubMed] [Google Scholar]
- 2.Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. doi: 10.1016/0042-6989(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 3.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–2932. [PubMed] [Google Scholar]
- 4.Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 5.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797. [PubMed] [Google Scholar]
- 6.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys inferred from muscle paths. Invest Ophthalmol Vis Sci. 1997;38:227–240. [PubMed] [Google Scholar]
- 7.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 8.Oh SY, Clark RA, Velez F, Rosenbaum AL, Demer JL. Incomitant strabismus associated with instability of rectus pulleys. Invest Ophthalmol Vis Sci. 2002;43:2169–2178. [PubMed] [Google Scholar]
- 9.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 10.Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157. doi: 10.1159/0000100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demer JL. Ocular motility in a time of revolutionary paradigm shift. Invest Ophthalmol Vis Sci. 2006 doi: 10.1111/j.1442-9071.2006.01390.x. e-Letter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 15.Demer JL, Clark RA, Miller JM. Heterotopy of extraocular muscle pulleys causes incomitant strabismus. In: Lennerstrand G, editor. Advances in Strabismology. Buren, Netherlands: Aeolus Press; 1999. pp. 91–94. [Google Scholar]
- 16.Demer JL. Anatomy of strabismus. In: Taylor D, Hoyt C, editors. Pediatric Ophthalmology and Strabismus. 3rd ed. London: Elsevier; 2005. pp. 849–861. [Google Scholar]
- 17.Demer JL. Orbital connective tissues in binocular alignment and strabismus. In: Lennerstrand G, Ygge Y, editors. Advances in Strabismus Research: Basic and Clinical Aspects. London: Portland Press; 2000. pp. 17–32. [Google Scholar]
- 18.Lim KH, Poukens V, Demer JL. Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers. Invest Ophthalmol Vis Sci. 2007;48:3089–3097. doi: 10.1167/iovs.06-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JM, Demer JL, Poukens V, Pavlowski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J Vis. 2003;3:240–251. doi: 10.1167/3.3.5. [DOI] [PubMed] [Google Scholar]
- 20.Porter JD, Poukens V, Baker RS, Demer JL. Structure-function correlations in the human medial rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 1996;37:468–472. [PubMed] [Google Scholar]
- 21.Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. [PubMed] [Google Scholar]
- 22.Miller JM. Understanding and misunderstanding extraocular muscle pulleys. J Vis. 2007;7:1–15. doi: 10.1167/7.11.10. [DOI] [PubMed] [Google Scholar]
- 23.van den Bedem SPW, Schutte S, van der Helm FCT, Simonsz HJ. Mechanical properties and functional importance of pulley bands or ‘faisseaux tendineux’. Vis Res. 2005;45:2710–2714. doi: 10.1016/j.visres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Schutte S, van den Bedem SP, van Keulen F, van der Helm FC, Simonsz HJ. A finite-element analysis model of orbital biomechanics. Vis Res. 2006;46:1724–1731. doi: 10.1016/j.visres.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL. A 12 year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS. 2003;6:337–347. doi: 10.1067/mpa.2002.129040. [DOI] [PubMed] [Google Scholar]
- 26.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. Philadelphia: WB Saunders; 1999. pp. 84–98. [Google Scholar]
- 27.Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci. 1998;39:207–212. [PubMed] [Google Scholar]
- 28.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 29.Demer JL. Extraocular muscles. In: Jaeger EA, Tasman PR, editors. Duane’s Clinical Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1–23. [Google Scholar]
- 30.Schoemaker I, Hoefnagel PP, Mastenbroek TJ, et al. Elasticity, viscosity, and deformation of orbital fat. Invest Ophthalmol Vis Sci. 2006;47:4819–4826. doi: 10.1167/iovs.05-1497. [DOI] [PubMed] [Google Scholar]
- 31.Nasseri S, Bilston LE, Phan-Thien N. Viscoelastic properties of pig kidney in shear, experimental results and modelling. Rheol Acta. 2002;41:180–192. [Google Scholar]