Introduction
Invasive fungal infections (IFIs) in oncology and transplant populations have been associated with significant morbidity and mortality. Research in this area remains in flux; as epidemiologic patterns shift, more is being learned about optimal treatment, as well as the unique risks that predispose these special populations to such potentially devastating infections. Here, we seek to highlight recent advances and important factors to consider when treating transplant and oncology patients with IFIs.
Epidemiology of Invasive Fungal Infections
Despite high associated morbidity and mortality, the epidemiology of IFIs in high risk populations has not previously been well defined. Incidence estimates have been primarily based on single-center, retrospective studies [1-3]. The Transplant Associated Infections Surveillance Program (TRANSNET), a network of 23 transplant centers in the United States (U.S.), prospectively studied the epidemiology of IFIs among solid organ and stem cell transplant populations over a five year period (March 2001 to March 2006) and provided the first true approximation of the burden of fungal disease among transplant populations in the U.S. Based on TRANSNET data, the overall incidence of IFIs in the hematopoietic stem cell transplant (HSCT) population was 3.4%, somewhat lower than previous estimates (D.P. Kontoyiannis, unpublished data, July 2009). In addition, invasive aspergillosis (IA) surpassed invasive candidiasis (IC) as the most common IFI encountered in the HSCT population: Aspergillus accounted for 43% of infections and Candida accounted for 28%, followed by other or unspecified moulds including Fusarium and Scedosporium (16%), and finally, Zygomycetes (8%). Pneumocystosis, endemic fungal infections, and cryptococcosis were rarely encountered in the HSCT population. Consistent with prior reports [4-7], mortality was high and one-year survival was low for HSCT patients with IFI. Fusarium infections and IA were associated with the lowest one year survival (6% and 25%, respectively); however, survival among patients with zygomycosis (28%) and IC (34%) was not substantially better.
Among solid organ transplant (SOT) recipients, Candida infections were significantly more common than Aspergillus infections. This held true for all solid organ groups except lung transplant recipients. In lung transplant recipients, Aspergillus was the most common fungal pathogen, and when coupled with other moulds, invasive mould infections were responsible for 70% of IFIs (P.G. Pappas, unpublished data, July 2009). This distribution has been shown in other studies of SOT recipients as well [8, 9]. Less common overall but seen more frequently than in the HSCT population were infections due to Cryptococcus and endemic fungi, causing 8% and 5% of IFIs, respectively. Zygomycetes were responsible for 2% of infections (P.G. Pappas, unpublished data, July 2009). The mortality assocated with IFIs in the SOT population is high, but lower overall than in HSCT and oncology patients.
There are no recent, multicenter studies describing the incidence and clinical outcome of IFIs among the general oncology population and it is difficult to obtain an accurate estimate of the frequency of fungal infections in this population from the published literature as most reports do not provide sufficient information regarding the patients' underlying disease. In general, compared with patients with solid tumors, patients with hematologic malignancies are at increased risk for fungal disease and response to IFI treatment is lower [10]. A 1992 international autopsy survey of patients with cancer identified fungal infections in 25% of patients with leukemia, 12% with lymphoma, and 5% with solid tumors. Overall, Candida was the most common fungal pathogen, responsible for 58% of fungal infections, while 30% of fungal infections were caused by Aspergillus [11]. A more recent single center survey of autopsies performed on patients with hematologic malignancy confirmed the increased risk for IFI among patients with leukemia. Further, consistent with trends among transplant populations, the prevalence of IFI remained high and constant throughout the study period (1989-2003); although the rate of IC decreased, the prevalence of invasive mold infections increased [12].
Types of Invasive Fungal Infections
Aspergillus
Aspergillus fumigatus is the most frequent species of Aspergillus causing clinical disease, perhaps due to specific virulence factors unique to the organism [13]. However, other species, most commonly A. flavus, A. terreus, and A. niger, are also implicated in invasive infections in humans. A. terreus has been associated with amphotercin B resistance and a higher mortality [14] than other Aspergillus species although the data to support this claim was primarily gleaned from patients treated with amphotericin B as initial therapy and prior to use of triazoles as first-line treatment for IA [15].
In immunocompromised hosts, Aspergillus most commonly presents as invasive pulmonary aspergillosis, often with subsequent dissemination [16-18]. In lung transplant recipients, Aspergillus may also cause tracheobronchitis and bronchial anastomotic infection. Pulmonary infections can present with fever, hemoptysis, cough, dyspnea, drop in pulmonary function, pleuritic chest pain, respiratory failure, and altered mental status [19], however, and very importantly, the immunosuppressed patient may have few or only subtle clinical signs and symptoms present early in the course of infection. Further clouding the picture, the distinction between colonization and infection with Aspergillus can be difficult. For example, Aspergillus can be recovered from the lower respiratory tract of many patients post lung transplant, but based on a review of the literature, progression from colonization to infection in lung-transplant recipients is rare [20]. In contrast, recovery of Aspergillus from lower respiratory tract specimens in patients with hematologic malignancy or undergoing HSCT has a high positive predictive value for invasive disease [21].
Candida
The overall decrease in Candida infections and the shift from C. albicans to non-albicans Candida as the most common infecting Candida species over the past two decades are notable. Data from Brazil collected between 1997 and 2003 documented that 79% of episodes of candidemia in patients with hematological malignancies and 52% in those with solid tumors were caused by non-albicans Candida (P = 0.034) [22]. Similarly, between 2001 and 2007 at MD Anderson Cancer Center, non-albicans Candida species were responsible for 75% of IC cases occurring in patients with hematologic malignancy or undergoing HSCT [23]. The routine use of azole prophylaxis in high risk cancer populations has certainly contributed to the decreased incidence of IC in these populations [24, 25] and likely accounts in part for the increasing frequency of non-albicans Candida infections [23, 26, 27]. While C. albicans remains the most frequently isolated Candida species among SOT recipients, a shift towards more non-albicans Candida infections seems to be occurring in this population as well [28].
Infections due to Candida can manifest as candidemia, peritonitis, empyema, endopthalmitis, esophagitis, and urinary tract or anastomotic infections. In lung transplant recipients, Candida can also cause tracheobronchitis [29]. Presenting clinical signs may be fever, leukocytosis, and less commonly, hypothermia [30].
Hyaline Hyphomycetes
The “other” moulds responsible for IFIs in immunosuppressed patients are a heterogeneous group of organisms. Over thirty non-Aspergillus hyalohyphomycetes have been implicated in human disease including, species of Acremonium, Fusarium, Paecilomyces, and Scedosporium [31]. These organisms are typically opportunistic, causing invasive disease following environmental exposures. Several of the non-Aspergillus hyalohyphomycetes are unique in their capability to sporulate in vivo which permits recovery of the organisms from the bloodstream and dissemination to other organs, particularly skin [32].
Recently, a shift towards more non-Aspergillus mould infections has been noticed in SOT recipients. In a prospective multicenter study, 53 invasive mould infections were reported from liver and heart transplant recipients. Pathogens included Aspergillus species in 70%, non-Aspergillus hyalohyphomycetes in 9%, phaeohyphomycetes in 9%, Zygomycetes in 6%, and other or unidentified moulds in 6% of patients. Dissemination was significantly more likely with infection due to a non-Aspergillus mould compared with Aspergillus [17].
Zygomycetes
Zygomycetes cause devastating invasive disease in a variety of different hosts. In one review of 929 reported cases of zygomycosis, 36% were seen in patients with diabetes mellitus, 7% in SOT recipients, and 5% in bone marrow transplant recipients. Among the bone marrow transplant group, just over half (52%) had pulmonary zygomycosis with 16% having infection in the sinuses. Outcome from zygomycosis varied based on the underlying condition, site of infection, and use of antifungal therapy. For patients with underlying malignancy, overall mortality was 66% [33]. Other studies cite mortality rates up to 80% among those with hematologic malignancies [34]. Unfortunately, the incidence of zygomycosis appears to be increasing in oncology centers and in HSCT populations specifically, possibly related to the use of voriconazole prophylaxis [35-39].
Pneumocystis jiroveci
The risk of Pneumocystis jiroveci infection (previously P. carinii) in HSCT and SOT recipients can be as high as 5-15% without prophylaxis [40, 41]. In the era of routine P. jiroveci prophylaxis, transplant recipients that develop infection typically do so after stopping their prophylactic regimen [42]. Similarly, patients with cancer that develop Pneumocystis infection typically do so in the absence of prophylaxis [43]. Pneumocystis has a worldwide distribution and the organism that infects humans has been recognized as unique and distinct from that infecting animals [44]; humans appear to acquire Pneumocystis only from other humans but active pneumonia does not seem to be required for transmission to occur. Serologic data indicate that most humans are infected with Pneumocystis within the first 2 to 4 years of life [45]. Immunocompromised patients develop disease as a consequence of re-infection with a new strain or possibly, from reactivation of latent infection. However, it is thought that most cases of P. jiroveci pneumonia develop following acquisition of a new stain shortly before clinical symptoms manifest [46].
Particular attention was given to P. jiroveci infection in SOT recipients in the 1980's given to high rates of infection in heart-lung transplant recipients [47, 48]. However, in the era of routine prophylaxis for at least 6 months following the transplant procedure in all solid organ groups [41], Pneumocystis infections in the SOT population are rare. In one retrospective review of 32,757 kidney recipients transplanted between 2000-2004, the cumulative incidence was 0.4%. Patients receiving sirolimus as part of their immunosuppressive regimen had an increased risk of developing P. jiroveci pneumonia which was associated with increased risk of both graft loss and death [49]. The underlying mechanism by which sirolimus predisposes to P. jiroveci infection is as yet undefined; however, it may ultimately be linked with sirolimus' ability to cause interstitial pneumonia, a known side effect of the drug.
Cryptococcus
Cryptococcus neoformans and Cryptococcus gatti [50] represent the main pathogenic species in the genus Cryptococcus [51]. While cryptococcosis has been most commonly encountered in the HIV infected population [52], a multicenter study reporting 306 cases of cryptococcosis in non-HIV-infected patients found 0.7% of total cases occurred in HSCT recipients, 18% in SOT recipients, 9% in patients with hematologic malignancies, and 9% in patients with other malignancies [53]. Other U.S. studies have found similarly low rates of cryptococcal infection in the HSCT population [1, 5, 54], most likely owing to use of routine fluconazole prophylaxis following HSCT. The overall mortality for cryptococcosis in the non-HIV population was 30%, attributable mortality 12%, and hematologic malignancy as an underlying diagnosis was associated with decreased survival [53].
Cryptococcus infection most commonly involves the lungs and central nervous system, but cutaneous infection and disseminated disease also occur. In one study, heart transplant patients were more likely than other solid organ groups to develop cryptococcosis, but kidney transplant recipients were most likely to have disseminated disease. This study also showed that serum cryptococcal antigen was not always helpful in identifying isolated pulmonary Cryptococcus infection; 82% of patients with cryptococcal pneumonia had a negative serum cryptococcal antigen [55].
Endemic fungi
Endemic fungi, including Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitus, are present in the soil in certain geographic regions and inhalation of conidia leads to systemic infection [56]. Disease may manifest after primary exposure or through reactivation of a latent focus when there is a decrease in cell-mediated immunity. Pulmonary involvement is common but clinical symptoms are non-specific and may be subacute in onset.
Although endemic mycoses are rarely encountered in cancer and transplant populations, immunosuppression (defined as hematologic malignancy or treatment with immunosuppressive medications) has been identified as a risk for developing histoplasmosis. Further, among immunosuppressed patients with histoplasmosis, 74% had fatal or disseminated infections compared with 7% of patients who were not immunosuppressed [57]. Histoplasmosis is the most frequent endemic mycosis reported in the SOT population [58, 59] and it has been transmitted to SOT recipients via the transplanted allograft [60]. Information regarding B. dermatitidis in transplant populations remains limited to individual case reports and small case series [61]. The largest series included 11 cases in SOT recipients; infection occurred a median of 26 months post SOT and rejection did not precede any case [62]. B. dermatitidis pneumonia was frequently complicated by acute respiratory distress syndrome and accordingly, high mortality (67%) [63]. Even in endemic regions, C. immitus infection is rarely encountered in the HSCT population [64] and most descriptions are in SOT recipients [65]. Unfortunately, as with the other endemic mycoses in the immunosuppressed population, dissemination is common, mortality is high (up to 72%), and infection can be transmitted via donated organs [66].
Timing of Invasive Fungal Infections
IFI Timeline: HSCT
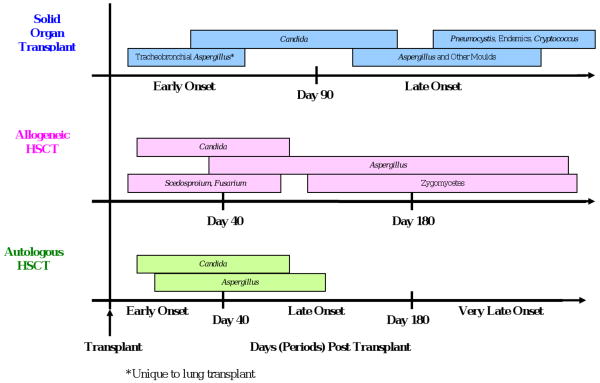
Time to development of IFI after transplantation varies according to type of fungal infection, type of transplant, and the use/duration of antifungal prophylaxis. Per Figure 1, the timeline for IFIs following HSCT is typically broken into 3 periods, early onset (≤40 days post HSCT), late onset (41-180 days post HSCT), and very late onset (>180 days post HSCT). In the TRANSNET cohort, 66% of Candida infections among autologous HSCT recipients occurred within the first 30 days (D.P. Kontoyiannis MD, unpublished data, July 2009). Similarly, in a single center study of 655 allogenic HSCT recipients transplanted between 1994 and 1997 and receiving routine fluconazole prophylaxis, the median time to development of candidemia was day 28 post transplant [25]. A recent, multicenter report of IFIs occurring between 2004 and 2007 reported the median timing of IC after HSCT to be 77days; IC tended to occur earlier after autologous HSCT (median 28 days) compared with allogeneic HSCT (median 108 days) [67]. In general, early onset IC following HSCT is influenced by the presence of neutropenia and mucosal injury (mucositis) while later onset is more often seen in allogeneic HSCT recipients owing to the development of graft versus host disease (GVHD) and the need for chronic central venous catheters.
Figure 1. Timing of invasive fungal infections based on transplant type.
Aspergillus and other mould infections tend to occur later after HSCT. In a single center study of allogeneic HSCT recipients transplanted between 1993 and 1998, 30% of IA diagnoses (N=187) were early, 53% late, and 17% very late onset following the procedure [68]. In the more recent TRANSNET cohort, 50% of IA cases among autologous HSCT recipients were early onset and 24% occurred >120 days post while 22% of cases among allogeneic HSCT recipients were early onset and 47% occurred more than 120 days after transplant (D. P. Kontoyiannis, MD, unpublished data, July 2009). In general, IA occurs more frequently and is encountered later after allogeneic HSCT compared with autologous HSCT. Late IA has been associated with a higher mortality, possibly because of increased fungal burden accompanying a delay in diagnosis as well as the cumulative burden of immunosuppression in patients with chronic/refractory GVHD [69].
The timing of non-Aspergillus mould infections such as Zygomycetes, Fusarium, and Scedosporium, appears to be organism-specific. One large study of over 5500 HSCT recipients showed that the majority (56%) of Zygomycete infections occurred greater than 90 days after transplant and GVHD was associated with Zygomycete infection. On the other hand, Scedosporium infections were more likely to occur within the first 30 days post-transplant [5]. Similarly, nearly half (46%) of patients with fusariosis were neutropenic at the time of diagnosis and the median time from transplant to diagnosis was 64 days [6].
IFI Timeline: SOT
The timeline for infections following SOT has traditionally been divided into three phases: the first month, months 2 through 6, and more than 6 months after the transplant procedure [70]. Recent data regarding the epidemiology of IFIs following SOT suggest the timing of fungal infections may no longer fall succinctly into these risk windows.
Historically, infections due to Candida occurred early post SOT, typically during the transplant hospitalization [9, 71]. However, TRANSNET data showed a somewhat later time to onset, with median time to diagnosis of IC of 103 days (P.G. Pappas MD, unpublished data, July 2009). In addition, a recent Australian study of candidemia in SOT recipients found that 54% of infections developed greater than 6 months after transplant, the majority of these in renal transplant recipients. Notably, nearly all these patients were hospitalized at the time of diagnosis due to complications from various bacterial infections and had been receiving broad-spectrum antibiotics [72].
Most Aspergillus infections historically occurred within the first year following SOT [20, 73, 74]. Tracheobronchial and/or anastomotic Aspergillus infections typically occurred within the first 90 days post transplant compared with invasive pulmonary aspergillosis which tended to occur later [73, 74]. The majority of experts agree that the risk for IA is substantially high enough immediately following lung transplant to warrant antifungal prophylaxis and American Society of Transplantation guidelines recommend continuing prophylaxis following lung transplantation at least until bronchial anastomosis remodeling is complete [75]. A 2006 international survey of lung transplant centers revealed that 69% (30/43) used universal antifungal prophylaxis during the immediate post transplant period as the anastomosis was healing, most commonly an aerosolized formulation of amphotericin B alone or in combination with itraconazole. The median duration of prophylaxis with aerosolized amphotericin B and itraconazole was 30 and 90 days, respectively [76]. In the current era of routine prophylaxis in high risk organ transplant recipients, nearly one-half of Aspergillus infections in SOT recipients are late-occurring (>90 days after SOT) and, as in the HSCT population, late onset IA has been associated with a higher mortality compared with early onset infection [77].
Cryptococcus and the endemic mycoses tend to occur even later in the post-transplant period [70]. In one study of SOT recipients with cryptococcosis, the median time to diagnosis in lung, heart, and kidney transplant recipients was 210, 450, and 630 days, respectively [55]. In the TRANSNET cohort, median time to diagnosis of cryptococcosis was 575 days. Similarly, the median time to diagnosis of the endemic mycoses was 343 days (P.G. Pappas MD, unpublished data, July 2009) and P. jiroveci infections are most often seen after routine prophylaxis is stopped, typically more than 180 days after transplant [70, 78] [49].
Risk Factors Developing Invasive Fungal Infections
Unique Risks for IFIs in HSCT Recipients
Many factors impact a patient's individual risk for fungal disease, including those associated with the host, the transplanted graft, and complications of the procedure. The influence of each factor fluctuates throughout the post transplant course, creating a dynamic timeline. Host (e.g., older age) and transplant variables (e.g., human leukocyte antigen mis-match) tend to influence IFI risk early while complications of the transplant procedure (e.g., GVHD and cytomegalovirus [CMV] disease) tend to predominate later [1, 2, 5, 68]. Certain biological factors such as malnutrition, iron overload, diabetes mellitus, and cytopenias are influential throughout the post transplant course [79]. Risk factors specific to early onset IA have been identified as aplastic anemia, myelodysplastic syndrome, cord-blood transplantation, delayed neutrophil engraftment, and CMV disease. Risks for late onset IA were multiple myeloma, neutropenia, GVHD, and CMV disease [68]. Iron overload has been demonstrated to be a risk factor for severe bacterial infections in autologous HSCT recipients [80] and associated with IA and Zygomycete infections as well [81]. Diabetes mellitus, voriconazole prophylaxis, and malnutrition have also been identified as risks for zygomycosis [39].
Clearly, only a subset of at risk patients will actually develop IFI. This has lead to a growing interest in host genetic differences that may contribute to the individual's risk of developing IFI. Recently, studies in HSCT populations have shown that polymorphisms in Toll-like receptor 4 [82] and genetic variations within the plasminogen allele may influence susceptibility to IA after transplant [83]. More research into host genetic influence on the risk of fungal disease following transplant is needed.
Unique Risks for IFIs in Oncology Patients
In patients with acute leukemia, the risk for IC in published reports varies considerably. Undoubtedly, this is related to the status of leukemia (newly diagnosed, post-remission, relapsed, or refractory to treatment), duration of neutropenia, and the types of anti-neoplastic agents used. Based on a study of cancer patients with candidemia from Brazil between 1997 and 2003, in comparison with patients with solid tumors, neutropenia and corticosteroid use were more frequent in the hematologic malignancy group. Only 22% of patients with solid tumors were neutropenic before candidemia. The presence of ileus and the use of anaerobicides were independent risk factors for candidemia in patients with solid cancers. Further, compared with candidemic patients without cancer, central venous catheters and gastrointestinal surgery were independently associated with candidemia in patients with solid tumor [22].
Unique Risks for IFIs in SOT recipients
Unquestionably, rejection and exogenous immunosuppressive agents, particularly high-dose steroids and antilymphocyte antibody treatment, lead to increased risk for IFIs in the SOT population [84]. However, within organ transplant groups, the risk for IFI is strongly influenced by medical and surgical factors including technical complexity. For example, prolonged operative time requiring multiple blood transfusions, reperfusion organ injury during transplantation, and/or multiple simultaneous organ transplants have all been associated with the development of fungal infections [85]. One study associated prolonged ischemia time with the development of IA in lung transplant recipients [86]. Liver transplant recipients have been shown to be at higher risk for IFIs if there is fulminant hepatic failure, a need to undergo re-transplantation, or renal failure. Unique risks for renal transplant recipients include diabetes mellitus or need for prolonged hemodiaylsis prior to transplant [87]. Factors predisposing to IFI, primarily IC, in pancreas transplant recipients include older donor age, enteric (versus bladder) drainage, pancreas after kidney transplant (vsersus pancreas alone), the development of post transplant pancreatitis, retransplantation, and preoperative peritoneal dialysis [88].
Infection with certain viruses following SOT has also been associated with the development of IFIs. The most frequently implicated virus is CMV. In a prospective study of liver transplant recipients, 36% of patients with CMV disease developed IFIs within the first year post transplant compared with 8% of those without CMV disease [89]. Further supporting the association is that CMV prophylaxis appears to result in fewer IFIs.
Management
Management of IFIs involves several components and is pathogen-specific. Pharmacologic treatment requires consideration of first and second line therapies, potential drug interactions, and the value of combination therapies. The role of immunomodulation, reversal of neutropenia, and surgery also needs to be considered.
Aspergillus
Treatment of IA has evolved over the past decade, but few randomized controlled trials comparing various agents exist. The therapy of choice had historically been amphotercin B deoxycholate, its administration complicated by infusion reactions and renal dysfunction [90]. A randomized controlled trial documented superiority and decreased toxicity of voriconazole over amphotericin B deoxycholate. This landmark study also noted a 12 week survival advantage for patients treated with voriconazole [91]. As a result, voriconazole is now considered the drug of choice for IA [92].
Complications of voriconazole therapy, as with other azoles, are mainly due to its drug interactions which are particularly pertinent in transplant populations. Importantly, concomitant administration of cyclosporine, tacrolimus, or sirolimus with any azole requires pre-emptive dose adjustments of the immunosuppressants and subsequent close monitoring [93]. Voriconazole is metabolized through the cytochrome p450 system and polymorphisms in the CYP2C19 gene can result in widely variable rates of drug metabolism [94]. In addition, response appears to be lower among patients with IA and low mean voriconazole plasma levels (<0.25 μg/ml). Because of these issues, voriconazole levels should be monitored during therapy [95].
The appropriate choice for therapy in the setting of voriconazole intolerance or failure is a subject of debate. Current Infectious Diseases Society of America (IDSA) guidelines for treatment of IA include echinocandins (caspofungin and micafungin) as an option for salvage therapy, along with lipid formulations of amphotericin B, itraconazole, and posaconazole [92]. Posaconazole, another triazole with activity against moulds, is available in oral formulation only and demonstrates moderate variability in absorption. In a salvage study for IA in patients previously treated with amphotericin products, favorable response was observed in 42% [96] and among SOT recipients specifically, 58% had successful outcomes on treatment. As with voriconazole, drug interactions can be frequently seen with posaconazole, absorption is variable, and therapeutic drug level monitoring is encouraged. Treatment-related adverse events included nausea, vomiting, and elevated liver function tests (the latter occurring in <3% of patients) [97]. Visual disturbances and certain rashes experienced with voriconazole are not seen with posaconazole treatment. Thus, in some patients intolerant to voriconazole, posaconazole is an acceptable alternative. However, whether failure to respond to voriconazole should prompt the switch to a different antifungal class is a different issue. Research has shown that mutations in the Aspergillus cyp51A gene produces clinically significant resistance to the triazoles and different mutations confer unique patterns of azole activity [98]. For example, some mutations lead to high minimal inhibitory concentrations (MICs) for both itraconazole and posaconazole, but not voriconazole and ravuconazole, while others result in high MICs for all 4 drugs [99]. Thus, in cases of voriconazole failure, susceptibility testing is recommended before switching to another triazole.
Echinocandins, which act by inhibiting the synthesis of beta-D-glucan in the cell wall, are generally very well tolerated and offer an appealing option for treatment if intolerance to or failure of voriconazole develops. Caspofungin was studied alone or in combination in 90 patients with IA refractory to or intolerant of other licensed therapy. Favorable response was achieved in 45% and only two patients discontinued drug owing to adverse events [100]. Micafungin, in contrast to caspofungin, does not have a formal indication as salvage treatment for IA, but it has been studied for this use. In an open-label, multi-center study of micafungin in the treatment of IA, an overall favorable response rate of 36% was reported [101]. The main drawback to echinocandin therapy is the relatively narrow spectrum of activity and lack of an oral preparation.
Clearly, there is a need for better outcomes in IA. While it appears that combination antifungal therapy as primary therapy for IA may confer some benefit, this has not yet been rigorously tested in a controlled trial and the decision regarding what combination to use is based primarily on in vitro data, retrospective cohort outcomes, and animal data [102]. Only one, small, prospective randomized trial of combination anti-Aspergillus therapy has been published to date. This study included only 30 patients with hematologic malignancy and IA. Patients were randomized to caspofungin plus liposomal amphotericin B (3 mg/kg/day) versus monotherapy with high dose liposomal amphotericin B (10 mg/kg/day). The combination therapy group had a 66% (10/15) favorable response which was statistically superior to the 27% (4/15) clinical response in the monotherapy group. However 12 week survival was not statistically different and there was significantly more nephrotoxicity in patients treated with the high dose monotherapy. Thus, it is unclear whether the superiority of combination therapy was due to the lower dose of liposomal amphotericin B or the addition of caspofungin [103]. Another study compared 40 SOT recipients with IA who received caspofungin plus voriconazole as primary therapy to a historical cohort of 47 SOTs treated with a lipid formulation of amphotericin B. Survival at 90 days, the primary endpoint, was not significantly different between the two groups [104].
A phase III prospective, randomized, double blind trial comparing voriconazole monotherapy with combination voriconazole plus anidulafungin as primary therapy for IA is currently enrolling and should help definitively conclude the efficacy of azole-echinocandin combination therapy for this disease. Until such data is available, combination therapy should be reserved for patients in whom voriconazole monotherapy has failed or is contraindicated and for high-risk patients with unusual or resistant isolates.
Candida
Several randomized control trials comparing various antifungals have been performed over the years and are summarized in Table 1. In 2009, the IDSA revised it's guidelines on the treatment of Candida infections, reflecting new data on the use of echinocandins and the increasing prevalence of non-albicans Candida species. For non-neutropenic adults with candidemia, fluconazole or an echinocandin is recommended as initial therapy. For candidemia in neutropenic patients, initial therapy with a lipid formulation of amphotericin B or an echinocandin is recommended, unless the patient has had limited prior azole exposure, in which case initial therapy with fluconazole is appropriate. Once the infecting pathogen has been identified, treatment can be further tailored. For C. glabrata, treatment with an echinocandin is recommended unless the isolate has been confirmed susceptible to fluconazole or voriconazole, in which case, transition to either drug is appropriate. For C. krusei, which is intrinsically resistant to fluconazole, therapy with a lipid formulation of amphotericin B, voriconazole, or an echinocandin is recommended [105].
Table 1. Major prospective randomized controlled trials of invasive candidiasis.
| Author, Year | Comparators | Number Enrolled* | Proportion Candidemic (%) | C. albicans Infections (%) | End of Therapy Success (%) | Significance (P value) | Comments | |
|---|---|---|---|---|---|---|---|---|
| Rex 1994 [122] | Fluconazole | 113 | 100 | 70 | 72 | 0.17 | Non-neutropenic population; Less toxicity with fluconazole. |
|
| Amphotericin B | 111 | 100 | 63 | 80 | ||||
| Rex 2003[123] | Fluconazole plus Placebo | 107 | 100 | 68 | 56 | 0.043 | Higher APACHE II scores in fluconazole arm; Mortality not improved with combination; Higher nephrotoxicity with combination. |
|
| Fluconazole plus Amphotericin B | 112 | 100 | 68 | 69 | ||||
| Kullberg 2005[124] | Voriconazole | 248 | 100 | 43 | 70 | 0.42 | Non-blinded, non-neutropenic population; More renal toxicity & SAEs with AmB. |
|
| Amphotericin B followed by Fluconazole | 122 | 100 | 51 | 74 | ||||
| Mora-Duarte 2002[125] | Caspofungin | 109 | 83 | 36 | 73 | 0.09 | No difference in mortality; More drug related adverse events with AmB. |
|
| Amphotericin B | 115 | 79 | 54 | 62 | ||||
| Kuse 2007[126] | Micafungin | 264 | 83 | 39 | 74 | NS | 12% of study population neutropenic | |
| LAMB | 267 | 84 | 43 | 70 | ||||
| Reboli 2007[127] | Anidulafungin | 127 | 91 | 64 | 76 | NS | 3% of study population neutropenic; Microbiologic response higher with anidulafungin. |
|
| Fluconazole | 118 | 87 | 59 | 60 | ||||
| Pappas 2007[128] | Caspofungin | 188 | 86 | 44 | 72 | NS | 9% of study population neutropenic | |
| Micafungin 100mg | 191 | 85 | 48 | 76 | ||||
| Micafungin 150 mg | 199 | 84 | 51 | 71 | ||||
NS, not significant; LAMB, liposomal amphotericin B; AmB, amphotericin B; SAEs, serious adverse events
Modified intent to treat population
Zygomycetes
Treatment of invasive zygomycosis has evolved to some extent; perhaps most importantly, lipid formulations of amphotericin B have replaced amphotericin B deoxycholate as the cornerstone of primary therapy [106]. Further, prompt initiation of amphotericin B-based therapy (i.e., initiating treatment within 6 days of diagnosis) has been shown to significantly improve outcome [107]. Although it cannot be recommended as primary therapy for zygomycosis on the basis of available data, posaconazole has been increasingly studied as a therapeutic alternative. In one retrospective review of patients who had intolerance to or progression of infection on an amphotericin B-based regimen, 66% had a complete or partial response to posaconazole [108]. Importantly, the Zygomycetes include many pathogenic moulds and the minimal inhibitory concentration of posaconazole varies considerably between these organisms [109].
Most recently, echinocandins have been shown in vitro to exhibit immunomodulatory activity as well as synergistic activity in combination with amphotericin B against the Zygomycetes [110, 111]. Clinical data supporting the addition of an echinocandin to an amphotericin B based regimen is limited to a retrospective review of 34 diabetic patients with rhino-orbital-cerebral zygomycosis [112]. Treatment was successful for all evaluable patients (n=6) who received amphotericin B-caspofungin combination therapy compared with 41% (14/34) in patients treated with amphotericin B monotherapy (p=0.19). Whether the addition of an echinocandin offers a significant advantage to the patient awaits further clinical study.
Other Moulds
Although correlation between in vitro antifungal susceptibility testing of moulds and clinical outcomes is limited, information regarding intrinsic patterns of resistance for the various non-Asperigllus hyalohyphomycetes has emerged [31]. Unfortunately, many of these moulds are intrinsically resistant to available antifungal agents. Susceptibility to amphotericin B and triazoles is variable for Fusarium and the echinocandins offer no activity against this pathogen. Currently, most experts consider voriconazole as first line therapy for Fusarium [93]. Species of Scedosporium are considered intrinsically resistant to polyene antifungals and as with Fusarium, third generation triazoles are considered first line therapy for S. apiospermum [113], however, S. prolificans is intrinsically resistant to all antifungal agents. Data to support the use of combination antifungal therapy for the management of the hyalohyphomycoses are currently limited to those obtained in vitro and case reports.
Pneumocystis jiroveci
Trimethoprim-sulfamethoxazole (TMP/SMX) remains the treatment of choice for P. jiroveci pneumonia. Oral administration is appropriate for those able to take medication by mouth, given good bioavailability of the TMP/SMX. One of the most problematic side effects of TMP/SMX in the transplant population is cytopenia; all cell lines can be affected and patients must be monitored for this side effect. Duration of therapy for PCP is generally accepted to be 14-21 days. Although data have shown that adding prednisone to the treatment regimen accelerates clinical improvement and improves survival in HIV-infected patients with moderate or severe P. jiroveci infection, no randomized data are available in cancer or transplant patients to support this practice. With that said, assuming the patient was not already on corticosteroids at the time symptomatic infection developed, most clinicians presume efficacy based on data from the HIV literature and would consider adding corticosteroids in transplant and other non-HIV patients with severe disease. If allergic to or intolerant of TMP/SMX, atovaquone, dapsone, or pentamidine have been used as alternative agents [114].
Cryptococcosis
Treatment recommendations for cryptococcal disease in the transplant population are based, in large part, on data extrapolated from clinical trials in other hosts and expert opinion. Current IDSA guidelines recommend amphotericin B plus flucytosine for 2 weeks, followed by fluconazole orally at 400-800 mg for up to 10 weeks, followed by a decreased dose of fluconazole (200 mg) for 6-12 months [115] for CNS or other severe disease. There is some data to suggest that in SOT recipients with isolated pulmonary cryptococcosis, prolonged treatment with oral fluconazole is sufficient and induction therapy with amphotericin B may not be necessary [116].
Several management issues unique to the transplant population need to be considered. Owing to concomitant use of calcineurin inhibitors, lipid formulations of amphotericin B are preferred. In addition, flucytosine levels need to be monitored closely to avoid toxicity and side effects [117]. A gradual decrease in corticosteroids is another common management strategy; however, development of immune reconstitution syndrome (IRIS) in this setting has been seen and may be difficult to distinguish from manifestations of the cryptococcal infection itself.
Other management strategies
Reducing immunosuppression requires a delicate balance between improving outcome from infection versus inducing rejection of the graft or an accelerated inflammatory reaction. As noted, rapid reduction of immunosuppressive therapy in conjunction with initiation of antifungal therapy in SOT recipients may lead to the development of IRIS, the clinical manifestations of which mimic worsening disease [118]. Reversal of neutropenia is another oft-used strategy in managing IFIs. The updated 2008 IDSA guidelines for treatment of IA include considering the use of a granulocyte-macrophage colony stimulating factor in those with prolonged neutropenia [92]. Granulocyte infusions may also be used as a bridge to recovery from neutropenia but data to support this practice is scant. In one study of neutropenic patients with hematologic malignancies and IFI refractory to treatment with amphotericin B, 15 patients received granulocyte transfusions from related donors and 8 of the 15 had favorable outcomes [119].
Surgery
The role of surgery in the treatment of IFIs can be paramount, but its utility depends on the type of IFI present. The IDSA recommends that surgery be considered in patients with IA who have a solitary lung lesion prior to chemotherapy or HSCT, those with hemoptysis from a lung lesion, disease that invades the chest wall, or situations in which the infection involves the pericardium or great vessels [92]. For zygomycosis in particular, treatment often requires surgical intervention in addition to pharmacologic therapy [120]. In one review of 86 cases of pulmonary zygomycosis reported in the literature, mortality was higher (55%) in those not receiving adjuvant sugery compared with those who did (27%) [121]. Finally, for infections with highly resistant fungi, particularly for localized infection, surgical debridement and debulking should be considered.
Conclusion
Clearly, recent shifts in the epidemiology of IFIs among transplant and oncology populations has brought with it new recommendations on treatment; however, it has brought with it new controversies as well. New pharmacologic therapies are being studied, both alone and in combination, and guidelines for management of several IFIs have been changed accordingly. More information is being discovered about unique genetic factors that put some transplant recipients at greater risk for fungal infection than others. The role of immunomodulation continues to be investigated; as always, the delicate balance of maintaining some immune integrity while assuring protection of the graft remains critical. Despite advances in the field, further studies are needed. For transplant and oncology patients, the diagnosis and management of IFIs remains a challenge and improving outcomes depends on continued progress in all of these arenas.
Acknowledgments
This work was supported by Grant No. NIAID K24 AI072522 (BD Alexander) from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dimitrios P. Kontoyiannis, Email: dkontoyi@mdanderson.org.
Barbara D. Alexander, Email: alexa011@mc.duke.edu.
References
- 1.Martino R, et al. Invasive fungal infections after allogeneic peripheral blood stem cell transplantation: incidence and risk factors in 395 patients. Br J Haematol. 2002;116(2):475–82. doi: 10.1046/j.1365-2141.2002.03259.x. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda T, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102(3):827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 3.Singh N. Antifungal prophylaxis for solid organ transplant recipients: seeking clarity amidst controversy. Clin Infect Dis. 2000;31(2):545–53. doi: 10.1086/313943. [DOI] [PubMed] [Google Scholar]
- 4.Gudlaugsson O, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37(9):1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38(9):1237–42. doi: 10.1086/383319. [DOI] [PubMed] [Google Scholar]
- 7.Husain S, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis. 2005;40(1):89–99. doi: 10.1086/426445. [DOI] [PubMed] [Google Scholar]
- 8.Pugliese F, et al. Incidence of fungal infections in a solid organ recipients dedicated intensive care unit. Transplant Proc. 2007;39(6):2005–7. doi: 10.1016/j.transproceed.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 9.Grossi P, et al. Prevalence and outcome of invasive fungal infections in 1,963 thoracic organ transplant recipients: a multicenter retrospective study. Italian Study Group of Fungal Infections in Thoracic Organ Transplant Recipients. Transplantation. 2000;70(1):112–6. [PubMed] [Google Scholar]
- 10.DiNubile MJ, et al. Invasive candidiasis in cancer patients: observations from a randomized clinical trial. J Infect. 2005;50(5):443–9. doi: 10.1016/j.jinf.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Bodey G, et al. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11(2):99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 12.Chamilos G, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989-2003). Haematologica. 2006;91(7):986–9. [PubMed] [Google Scholar]
- 13.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):323–326. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lass-Florl CKG, Kropshofer G, et al. In vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Steinbach WJ, et al. In vitro analyses, animal models, and 60 clinical cases of invasive Aspergillus terreus infection. Antimicrob Agents Chemother. 2004;48(9):3217–25. doi: 10.1128/AAC.48.9.3217-3225.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz P, et al. Risk factors of invasive aspergillosis after heart transplantation: protective role of oral itraconazole prophylaxis. Am J Transplant. 2004;4(4):636–43. doi: 10.1111/j.1600-6143.2004.00390.x. [DOI] [PubMed] [Google Scholar]
- 17.Husain S, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37(2):221–9. doi: 10.1086/375822. [DOI] [PubMed] [Google Scholar]
- 18.Minari A, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4(4):195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 19.Marr KA, Patterson T, Denning D. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect Dis Clin North Am. 2002;16(4):878–83. doi: 10.1016/s0891-5520(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 20.Mehrad B, et al. Spectrum of Aspergillus infection in lung transplant recipients: case series and review of the literature. Chest. 2001;119(1):169–75. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 21.Perfect JR, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33(11):1824–33. doi: 10.1086/323900. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualotto AC, et al. Candidaemia and cancer: patients are not all the same. BMC Infect Dis. 2006;6:50. doi: 10.1186/1471-2334-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sipsas NV, et al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009 doi: 10.1002/cncr.24507. [DOI] [PubMed] [Google Scholar]
- 24.Goodman JL, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326(13):845–51. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 25.Marr KA, et al. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181(1):309–16. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 26.Van Burik JHLW, Myerson D, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine. 1998;(77):246–54. doi: 10.1097/00005792-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Wingard JR. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20(1):115–25. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 28.Horn DL, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 29.Palmer SM, et al. Candidal anastomotic infection in lung transplant recipients: successful treatment with a combination of systemic and inhaled antifungal agents. J Heart Lung Transplant. 1998;17(10):1029–33. [PubMed] [Google Scholar]
- 30.Fraser VJ, et al. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992;15(3):414–21. doi: 10.1093/clind/15.3.414. [DOI] [PubMed] [Google Scholar]
- 31.Alexander BDSW. Hyalohyphomycosis. In: Kauffman CA, Mandell GL, editors. Atlas of Fungal Infections. 2nd. Philadelphia: Current Medicine Group, Inc.; 2006. pp. 253–266. [Google Scholar]
- 32.Schell WA. New aspects of emerging fungal pathogens. A multifaceted challenge. Clin Lab Med. 1995;15(2):365–87. [PubMed] [Google Scholar]
- 33.Roden MM, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 34.Kontoyiannis DP, et al. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis. 2000;30(6):851–6. doi: 10.1086/313803. [DOI] [PubMed] [Google Scholar]
- 35.Trifilio SM, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007;39(7):425–9. doi: 10.1038/sj.bmt.1705614. [DOI] [PubMed] [Google Scholar]
- 36.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004;350(9):950–2. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 37.Siwek GT, et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39(4):584–7. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]
- 38.Imhof A, et al. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39(5):743–6. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- 39.Kontoyiannis DP, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191(8):1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 40.Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34(8):1098–107. doi: 10.1086/339548. [DOI] [PubMed] [Google Scholar]
- 41.Pneumocystis jiroveci (formerly Pneumocystis carinii) Am J Transplant. 2004;4 10:135–41. doi: 10.1111/j.1600-6135.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 42.De Castro N, et al. Occurrence of Pneumocystis jiroveci pneumonia after allogeneic stem cell transplantation: a 6-year retrospective study. Bone Marrow Transplant. 2005;36(10):879–83. doi: 10.1038/sj.bmt.1705149. [DOI] [PubMed] [Google Scholar]
- 43.Torres HA, et al. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jiroveci pneumonia in cancer patients. Eur J Clin Microbiol Infect Dis. 2006;25(6):382–8. doi: 10.1007/s10096-006-0149-4. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs JA, et al. Identification of antigens and antibodies specific for Pneumocystis carinii. J Immunol. 1988;140(6):2023–31. [PubMed] [Google Scholar]
- 45.Meuwissen JH, et al. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977;136(1):43–9. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- 46.Stringer JR. Pneumocystis. Int J Med Microbiol. 2002;292(5-6):391–404. doi: 10.1078/1438-4221-00222. [DOI] [PubMed] [Google Scholar]
- 47.Dummer JS, et al. Infections in heart-lung transplant recipients. Transplantation. 1986;41(6):725–9. doi: 10.1097/00007890-198606000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Gryzan S, et al. Unexpectedly high incidence of Pneumocystis carinii infection after lung-heart transplantation. Implications for lung defense and allograft survival. Am Rev Respir Dis. 1988;137(6):1268–74. doi: 10.1164/ajrccm/137.6.1268. [DOI] [PubMed] [Google Scholar]
- 49.Neff RT, et al. Analysis of USRDS: incidence and risk factors for Pneumocystis jiroveci pneumonia. Transplantation. 2009;88(1):135–41. doi: 10.1097/TP.0b013e3181aad256. [DOI] [PubMed] [Google Scholar]
- 50.MacDougall L, et al. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis. 2007;13(1):42–50. doi: 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20(3):507–44. v–vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Mirza SA, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis. 2003;36(6):789–94. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 53.Pappas PG, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33(5):690–9. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 54.Baddley JW, et al. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32(9):1319–24. doi: 10.1086/319985. [DOI] [PubMed] [Google Scholar]
- 55.Vilchez R, et al. Longitudinal study of cryptococcosis in adult solid-organ transplant recipients. Transpl Int. 2003;16(5):336–40. doi: 10.1007/s00147-002-0541-7. [DOI] [PubMed] [Google Scholar]
- 56.Kauffman CA. Endemic mycoses: blastomycosis, histoplasmosis, and sporotrichosis. Infect Dis Clin North Am. 2006;20(3):645–62. vii. doi: 10.1016/j.idc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Wheat LJ, et al. Risk factors for disseminated or fatal histoplasmosis. Analysis of a large urban outbreak. Ann Intern Med. 1982;96(2):159–63. doi: 10.7326/0003-4819-96-2-159. [DOI] [PubMed] [Google Scholar]
- 58.Peddi VR, Hariharan S, First MR. Disseminated histoplasmosis in renal allograft recipients. Clin Transplant. 1996;10(2):160–5. [PubMed] [Google Scholar]
- 59.Freifeld AG, et al. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl Infect Dis. 2005;7(3-4):109–15. doi: 10.1111/j.1467-8365.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 60.Limaye AP, et al. Transmission of Histoplasma capsulatum by organ transplantation. N Engl J Med. 2000;343(16):1163–6. doi: 10.1056/NEJM200010193431605. [DOI] [PubMed] [Google Scholar]
- 61.Serody JS, et al. Blastomycosis in transplant recipients: report of a case and review. Clin Infect Dis. 1993;16(1):54–8. doi: 10.1093/clinids/16.1.54. [DOI] [PubMed] [Google Scholar]
- 62.Bradsher RW, Chapman SW, Pappas PG. Blastomycosis. Infect Dis Clin North Am. 2003;17(1):21–40. vii. doi: 10.1016/s0891-5520(02)00038-7. [DOI] [PubMed] [Google Scholar]
- 63.Gauthier GM, et al. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis. 2007;9(4):310–7. doi: 10.1111/j.1399-3062.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 64.Glenn TJ, Blair JE, Adams RH. Coccidioidomycosis in hematopoietic stem cell transplant recipients. Med Mycol. 2005;43(8):705–10. doi: 10.1080/13693780500147840. [DOI] [PubMed] [Google Scholar]
- 65.Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis. 2001;33(9):1536–44. doi: 10.1086/323463. [DOI] [PubMed] [Google Scholar]
- 66.Wright PW, et al. Donor-related coccidioidomycosis in organ transplant recipients. Clin Infect Dis. 2003;37(9):1265–9. doi: 10.1086/378741. [DOI] [PubMed] [Google Scholar]
- 67.Neofytos D, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48(3):265–73. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 68.Marr KA, et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100(13):4358–66. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 69.Upton A, et al. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 70.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338(24):1741–51. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 71.Patel R. Infections in recipients of kidney transplants. Infect Dis Clin North Am. 2001;15(3):901–52. xi. doi: 10.1016/s0891-5520(05)70178-1. [DOI] [PubMed] [Google Scholar]
- 72.van Hal SJ, et al. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis. 2009;11(2):122–7. doi: 10.1111/j.1399-3062.2009.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Heart Lung Transplant. 2003;22(3):258–66. doi: 10.1016/s1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 74.Sole A, et al. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2005;11(5):359–65. doi: 10.1111/j.1469-0691.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 75.Fungal infections. Am J Transplant. 2004;4 10:110–34. doi: 10.1111/j.1600-6135.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 76.Husain S, et al. Variation in antifungal prophylaxis strategies in lung transplantation. Transpl Infect Dis. 2006;8(4):213–8. doi: 10.1111/j.1399-3062.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 77.Singh N, et al. Late-onset invasive aspergillosis in organ transplant recipients in the current era. Med Mycol. 2006;44(5):445–9. doi: 10.1080/13693780600684494. [DOI] [PubMed] [Google Scholar]
- 78.Zaas AK, Alexander BD. Prevention of Fungal Infections in Lung Transplant Patients. Current Fungal Infection Reports. 2008 [Google Scholar]
- 79.Garcia-Vidal C, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47(8):1041–50. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miceli MH, et al. Iron overload is a major risk factor for severe infection after autologous stem cell transplantation: a study of 367 myeloma patients. Bone Marrow Transplant. 2006;37(9):857–64. doi: 10.1038/sj.bmt.1705340. [DOI] [PubMed] [Google Scholar]
- 81.Maertens J, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24(3):307–12. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 82.Bochud PY, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359(17):1766–77. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaas AK, et al. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet. 2008 Jun 20;4(6):e1000101. doi: 10.1371/journal.pgen.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48(6):772–86. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 85.Gabardi S, et al. Invasive fungal infections and antifungal therapies in solid organ transplant recipients. Transpl Int. 2007;20(12):993–1015. doi: 10.1111/j.1432-2277.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 86.Iversen M, et al. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur J Clin Microbiol Infect Dis. 2007;26(12):879–86. doi: 10.1007/s10096-007-0376-3. [DOI] [PubMed] [Google Scholar]
- 87.Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am. 2003;17(1):113–34. viii. doi: 10.1016/s0891-5520(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 88.Benedetti E, et al. Intra-abdominal fungal infections after pancreatic transplantation: incidence, treatment, and outcome. J Am Coll Surg. 1996;183(4):307–16. [PubMed] [Google Scholar]
- 89.George MJ, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med. 1997;103(2):106–13. doi: 10.1016/s0002-9343(97)80021-6. [DOI] [PubMed] [Google Scholar]
- 90.Bowden R, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2002;35(4):359–66. doi: 10.1086/341401. [DOI] [PubMed] [Google Scholar]
- 91.Herbrecht R, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 92.Walsh TJ, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 93.VFend® [package insert] New York, NY: Pfizer Inc; 2008. [Google Scholar]
- 94.Weiss J, et al. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49(2):196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 95.Denning DW, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 96.Walsh TJ, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 97.Alexander BD, et al. Posaconazole as salvage therapy in patients with invasive fungal infections after solid organ transplant. Transplantation. 2008;86(6):791–6. doi: 10.1097/TP.0b013e3181837585. [DOI] [PubMed] [Google Scholar]
- 98.Pfaller MA, et al. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J Clin Microbiol. 2008;46(8):2568–72. doi: 10.1128/JCM.00535-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez-Tudela JL, et al. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52(7):2468–72. doi: 10.1128/AAC.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maertens J, et al. Multicenter, noncomparative study of caspofungin in combination with other antifungals as salvage therapy in adults with invasive aspergillosis. Cancer. 2006;107(12):2888–97. doi: 10.1002/cncr.22348. [DOI] [PubMed] [Google Scholar]
- 101.Denning DW, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53(5):337–49. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinbach WJ, Stevens DA, Denning DW. Combination and sequential antifungal therapy for invasive aspergillosis: review of published in vitro and in vivo interactions and 6281 clinical cases from 1966 to 2001. Clin Infect Dis. 2003;37 3:S188–224. doi: 10.1086/376524. [DOI] [PubMed] [Google Scholar]
- 103.Caillot D, et al. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: a randomized pilot study (Combistrat trial) Cancer. 2007;110(12):2740–6. doi: 10.1002/cncr.23109. [DOI] [PubMed] [Google Scholar]
- 104.Singh N, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006;81(3):320–6. doi: 10.1097/01.tp.0000202421.94822.f7. [DOI] [PubMed] [Google Scholar]
- 105.Pappas PG, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spellberg B, et al. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–51. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 108.van Burik JA, et al. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61–5. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 109.Almyroudis NG, et al. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007;51(7):2587–90. doi: 10.1128/AAC.00452-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lamaris GA, et al. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis. 2008;198(2):186–92. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perkhofer S, et al. Posaconazole enhances the activity of amphotericin B against hyphae of zygomycetes in vitro. Antimicrob Agents Chemother. 2008;52(7):2636–8. doi: 10.1128/AAC.00492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reed C, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–71. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perfect JR, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003;36(9):1122–31. doi: 10.1086/374557. [DOI] [PubMed] [Google Scholar]
- 114.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. Jama. 2009;301(24):2578–85. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 115.Saag MS, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30(4):710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 116.Singh N, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195(5):756–64. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dromer F, et al. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4(2):e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh N, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40(12):1756–61. doi: 10.1086/430606. [DOI] [PubMed] [Google Scholar]
- 119.Dignani MC, et al. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11(10):1621–30. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- 120.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12):1301–9. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 122.Rex JH, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. Candidemia Study Group and the National Institute. N Engl J Med. 1994;331(20):1325–30. doi: 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 123.Rex JH, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis. 2003;36(10):1221–8. doi: 10.1086/374850. [DOI] [PubMed] [Google Scholar]
- 124.Kullberg BJ, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–42. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 125.Mora-Duarte J, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347(25):2020–9. doi: 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 126.Kuse ER, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369(9572):1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 127.Reboli AC, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356(24):2472–82. doi: 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 128.Pappas PG, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883–93. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]