Abstract
Acquisition of a metastatic phenotype by breast cancer cells includes alternations of multigenic programs that permit tumor cells to metastasize to distant organs. Here, we report that angiopoietin-2 (Ang2), a known growth factor, is capable of promoting breast cancer cell invasion leading to metastasis. Analysis of 185 primary human breast cancer specimens that include 97 tumors showing lymph node and/or distant metastasis reveals a significant correlation between the expression of Ang2 and E-cadherin, Snail, metastatic potential, tumor grade, and lymph-vascular invasion during breast cancer progression. Using a xenograft model, we show that over-expression of Ang2 in poorly metastatic MCF-7 breast cancer cells suppresses expression of E-cadherin and induces Snail expression and phosphorylation of Akt and glycogen synthase kinase-3β (GSK-3β) promoting metastasis to the lymph nodes and lung. In cell culture, Ang2 promotes cell migration and invasion in Tie2-deficient breast cancer cells through the α5β1 integrin/integrin-linked kinase (ILK)/Akt, GSK-3β/Snail/E-cadherin signaling pathway. Inhibition of ILK and the α5β1 integrin abrogates Ang2 modulation of Akt, GSK-3β, Snail, and E-cadherin and Ang2-stimulated breast cancer cell migration and invasion. Together, these results underscore the significant contribution of Ang2 in cancer progression, not only by stimulating angiogenesis but also by promoting metastasis, and provide a mechanism by which breast cancer cells acquire an enhanced invasive phenotype contributing to metastasis.
Introduction
Metastasis, the spread of cancer cells from primary tumor sites to distant organs, is a complex process that involves induction of cell motility, activation of extracellular matrix (ECM) proteases, intravasation to vessels, travel via the circulatory system, and survival and establishment of secondary tumors in new microenvironment (1). A hallmark of acquisition of cell motility by tumor cells is the functional loss of E-cadherin and expression of mesenchymal proteins, such as α-smooth muscle actin and vimentin, which defines the key steps toward the invasive phase of breast cancers (2). Several transcription factors, such as Snail, a zinc finger transcriptional repressor, are involved in invasive phenotype acquisition through direct repression of E-cadherin expression (3). Snail expression significantly correlates with the metastatic potential of primary breast cancers and established cancer cell lines (4). Integrin-linked kinase (ILK) has been shown to promote the transcription of Snail-enhancing cell motility (5). ILK is a serine/threonine protein kinase that interacts with β1 and β3 integrins and functions as an intracellular adaptor that links integrins to a range of signaling pathways (6). Overexpression of ILK in epithelial cells results in loss of E-cadherin expression, acquisition of an invasive phenotype, and cell transformation (7). ILK stimulates transcription of Snail by phosphorylation of Akt (6). ILK also negatively regulates the activity of glycogen synthase kinase-3β (GSK-3β) through phosphorylation that suppresses Snail phosphorylation and induces nuclear localization and protein stabilization of Snail, thus stimulating Snail activities (4).
It has been shown that ECM proteins promote cell motility through ILK activation of Snail transcription and suppression of E-cadherin (6). Interaction between integrin and ECM proteins also modulates Snail phosphorylation (3). Integrins are a diverse family of glycoproteins that form at least 25 heterodimeric receptors (one α subunit and one β subunit; ref. 8). Integrins are critical for cell invasion and migration, not only for physically mediating the adhesion of invading tumor cells to the ECM but also for transmitting signals that regulate these processes (9). Among the integrin receptors, α3β1, α5β1, αvβ1, and α6β4 integrins are associated with enhanced cell motility and cancer metastasis (10). β1 integrin is involved in E-cadherin–dependent cell adhesion (11) and Src-induced down-regulation of E-cadherin (12). In breast cancers, β1 integrin activates ILK-mediated signaling pathways promoting cancer cell motility and metastasis (6).
Angiopoietins (Ang1 and Ang2) are ligands for an endothelial cell–specific tyrosine kinase receptor, Tie2, and are important regulators of angiogenesis and tumor progression (13). Moreover, accumulating evidence shows that expression of Ang2 by tumor cells is linked with invasive and metastatic phenotypes of gastric, colon, brain, prostate, and breast cancers (14–21). Although Ang2 modulates angiogenesis through interaction with the Tie2 receptor (13), the highly conserved COOH-terminal fibrinogen-like receptor-binding domain of Ang2 as well as other members of the angiopoietin family implies a functional association with the integrin receptors (22, 23). In fact, Ang2 not only enhances cell adhesion in endothelial cell and Tie2-deficient fibroblasts and myocytes but also triggers integrin signaling cascades in these cells (24–26).
In this study, we explore the roles of Ang2 in promoting breast tumor metastasis through a Tie2-independent pathway. We identify a significant correlation between expression of Ang2 and Snail, E-cadherin, and the metastatic potential of clinical breast cancers. We also find that Ang2 induces breast cancer metastasis in vitro and in vivo through a signaling pathway that involves α5β1 integrin, ILK, Akt, GSK-3β, Snail, and E-cadherin. Thus, our results provide a functional link between Ang2 expression and enhanced tumor cell motility and invasion in breast cancer metastasis.
Materials and Methods
Primary human breast cancer specimens and immunohistochemical and statistical analyses
A total of 185 human breast cancer specimens and the clinicopathologic data were obtained from the Health Science Tissue Bank at the University of Pittsburgh Medical Center (Pittsburgh, PA). Among these surgically resected samples, 97 specimens were from patients with cancer metastasis to peripheral lymph nodes with or without distant organ metastasis. The other 88 samples were from patients without detectable cancer cell spread. In addition, 10 metastatic and 10 nonmetastatic frozen biopsies from the same patients were also used for real-time PCR analyses (Supplementary Table S3). The clinicopathologic characteristics of these 185 tumor specimens were examined and verified by a pathologist specialized in breast cancers (E.E.). Importantly, H&E staining of two separate sections of the same tissues from all 20 frozen specimens contained >90% tumor cells within these biopsies. Immunohistochemical staining of tumor specimens and statistics were done as described previously (19). Results of immunohistochemical staining were categorized independently by two experienced researchers according to the number of immunopositive cells in a blinded fashion. The intensities of immunohistochemical staining with the antibody were defined as follows: negative, the reaction was indistinguishable from the background or <5% of tumor cells were stained; low, 5% to 30% of tumor cells were positively stained; and high, >30% of tumor cells were positively stained.
RNA isolation, cDNA synthesis, and quantitative real-time PCR
Total RNA was isolated from snap-frozen nonmetastatic and metastatic human breast cancer samples using the RNeasy Mini kit (Qiagen). The isolated RNA was DNase treated using the RNase-Free DNase Set (Qiagen), and reverse transcription was done with total RNA (500 ng) from each sample in a 100 µL reaction volume with random hexamer priming and SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time PCR for Ang2 expression was done using the fluorogenic 5′-nuclease assay on the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Inc.). The Ang2 primers and probe were designed using ABI Primer Express version 2.0 software to span exon junctions as follows: 5′-CTGAGCAAACGCGGAAGTTAA-3′ ( forward primer), 5′-TGTCGAGAGGGAGTGTTCCAA-3′ (reverse primer), and 5′-(FAM)-TGGAAGCCCAAGTATTAAATCAGACCACGAGA-(BHQ1)-3′ (probe). The relative expression of Ang2 in the metastatic versus the nonmetastatic samples was calculated using a difference in threshold cycle (ΔCt) method with β-glucuronidase (control gene) as the normalizing control gene as described previously (27). The ΔCt was calculated by subtracting the β-glucuronidase Ct from the Ang2 Ct, and the relative Ang2 mRNA expression was determined. The PCRs were done in triplicate two independent times per sample using a probe concentration of 200 nmol/L and primer concentrations of 300 and 100 nmol/L for Ang2 and β-glucuronidase, respectively. The PCR conditions were as follows: a 12-min denaturation phase at 95°C for 1 cycle followed by 40 cycles of a 15-s denaturation phase at 95°C and a 60-s annealing/extension phase at 60°C. The resultant Ct values for each sample and each gene were averaged for subsequent calculation of the ΔCt.
Xenograft assays in nude mice
MCF-7 cells were from American Type Culture Collection (ATCC). We generated MCF-7 cell lines that stably express Ang2 and green fluorescent protein (GFP) and LacZ and GFP (control) and observed no alteration in various cell properties of the derived cell lines (see Supplementary Data). In an orthotopic model, 1 × 107 of LacZ/GFP [a fluorescence-activated cell sorting (FACS) cell population of MCF-7/LacZ cells expressing GFP], Ang2#1/GFP or Ang2#52/GFP cells were separately injected into the mammary fat pads of 8-week old ovariectomized female nude mice (Taconic). Two days before the injection, each mouse was supplemented with 17β-estradiol (E2) pellets (0.72 mg/pellet) that were implanted into the right skin on the lateral side on the neck of the animal with a trocar (both from Innovative Research of America). Orthotopic tumor growth was monitored and mice were sacrificed when the tumor size approached 1,000 mm3 (14–18 weeks postinjection). The lung, liver, spleen, kidney, brain, lymph nodes (axillary, inguinal, and cervical), and orthotopic tumors were removed and processed (28). Metastasis in the organs was examined by direct epifluorescent examination of GFP-positive cells using a stereomicroscope (SZX12, Olympus) before embedding and an upright microscope (Olympus BX51) after sectioning followed by H&E staining. Images were captured with SPOT digital cameras (Diagnostic Instrument) equipped on these microscopes.
Immunofluorescent staining
LacZ, Ang#1, and Ang#52 cells were seeded in serum-containing medium in slide chambers without coating overnight. In separate experiments, MCF-7 and T47D cells (ATCC) were seeded in serum-containing medium in slide chambers that were precoated with 10 µg/mL of heat-inactivated bovine serum albumin (BSA), poly-lysine, or purified Ang2 overnight. After washing the cells extensively with PBS, the Ang2-stimulated parental MCF-7 and T47D cells were incubated with control or purified Ang2 for 2 h, whereas LacZ, Ang#1, and Ang#52 cells were left untreated. After washing cells with PBS, the cells were fixed with methanol and separately stained with a mouse anti-E-cadherin antibody (1:200; BD Biosciences) followed by secondary antibodies conjugated with Alexa Fluor 488 (Molecular Probes). The nuclei were visualized by Hoechst (Sigma) staining. Images were then captured with a microscope (Olympus BX51) equipped with a SPOT digital camera.
RNA interference
The sequence of ILK small interfering RNA (siRNA; 5′-AAGGACACAUUCUGGAAGGGG-3′) was reported previously (29). The 21-nucleotide synthetic siRNA duplex was prepared by Dharmacon. Breast cancer cells were transfected with the ILK siRNA or a 21-nucleotide scrambled RNA duplex as a control using Oligofectamine (Invitrogen). The transfected cells were analyzed 48 h after siRNA transfection by Western blotting with the anti-ILK antibody and subjected to different assays as specified.
Western blot
Whole-cell lysates of various cells were analyzed by Western blot assays as described previously (30). For inhibition of integrin-meditated signaling pathway, the cells were preincubated with 25 µg/mL of various neutralizing integrin antibodies and the corresponding IgG as controls for 30 min before cell lysis. For detection of phosphorylation of Akt and GSK-3β and expression of Akt, GSK-3β, and Snail, breast cancer cells were treated with Ang2 or poly-lysine for 4 h before cell lysis. For detection of expression of E-cadherin, breast cancer cells were treated with Ang2 or poly-lysine for 48 h before cells were lysed (30). The information of antibodies used in Western blot analyses is described in the Supplementary Data.
In vitro migration and invasion assays
In vitro migration and invasion assays were done as described previously (30). Briefly, serum-starved LacZ, Ang2#1, Ang2#52 cells, and parental MCF-7 and T47D cells were separately suspended at 1 × 106 cells/mL in serum-free DMEM containing 0.5% BSA and preincubated with or without 25 µg/mL of various neutralizing anti-integrin antibodies for 30 min on ice. Fifty microliters of each cell suspension were seeded into upper compartment of the Boyden chambers. For migration assays, the cells were allowed to migrate through the 12-µm pore size membranes precoated with 10 µg/mL Ang2 or poly-lysine for 12 h at 37°C. For invasion assays, the cells were allowed to invade through the growth factor–reduced Matrigel-coated (0.78 mg/mL; BD Biosciences) membranes with additional precoating of 10 µg/mL Ang2 or poly-lysine for 48 h at 37°C. To evaluate the effects of inhibition of ILK on Ang2 stimulation, MCF-7 and T47D cells were separately transfected with siRNA of ILK and subjected to the migration and invasion assays as described above. After the membrane was fixed and stained, nonmigrating and noninvading cells were removed. The number of migrating cells and invading cells was quantified under a microscope (30, 31).
Statistical analysis
The correlation between expression of Ang2 in tumor cells and that of Snail, E-cadherin, and pathologic features in human breast cancer specimens was analyzed using χ2 test (Mann-Whitney U test was also applied only for a data set composed of 2 rows × 3 columns but not for a table of 3 rows × 3 columns, and identical P values as those of χ2 test were obtained in each category). The statistical difference in the incidence of metastasis between the cell types in the mouse xenograft model was assessed using Fisher’s exact test. All analyses were done using StatView version 5.0 software (SAS Institute, Inc.). A P value <0.05 was considered statistically significant.
Other methods
Cell lines, reagents, antibodies, generation of MCF-7 cell lines that stably express LacZ, Ang2, and GFP, protein expression, purification, reverse transcription-PCR (RT-PCR), FACS, immunohistochemical analyses of MCF-7 cell-derived tumors, coimmunoprecipitation, and pull-down assays for integrin and ligand interaction are described in the Supplementary Data (31, 32).
Results
Ang2 expression is correlated with Snail, E-cadherin, and pathologic features of human breast cancer specimens
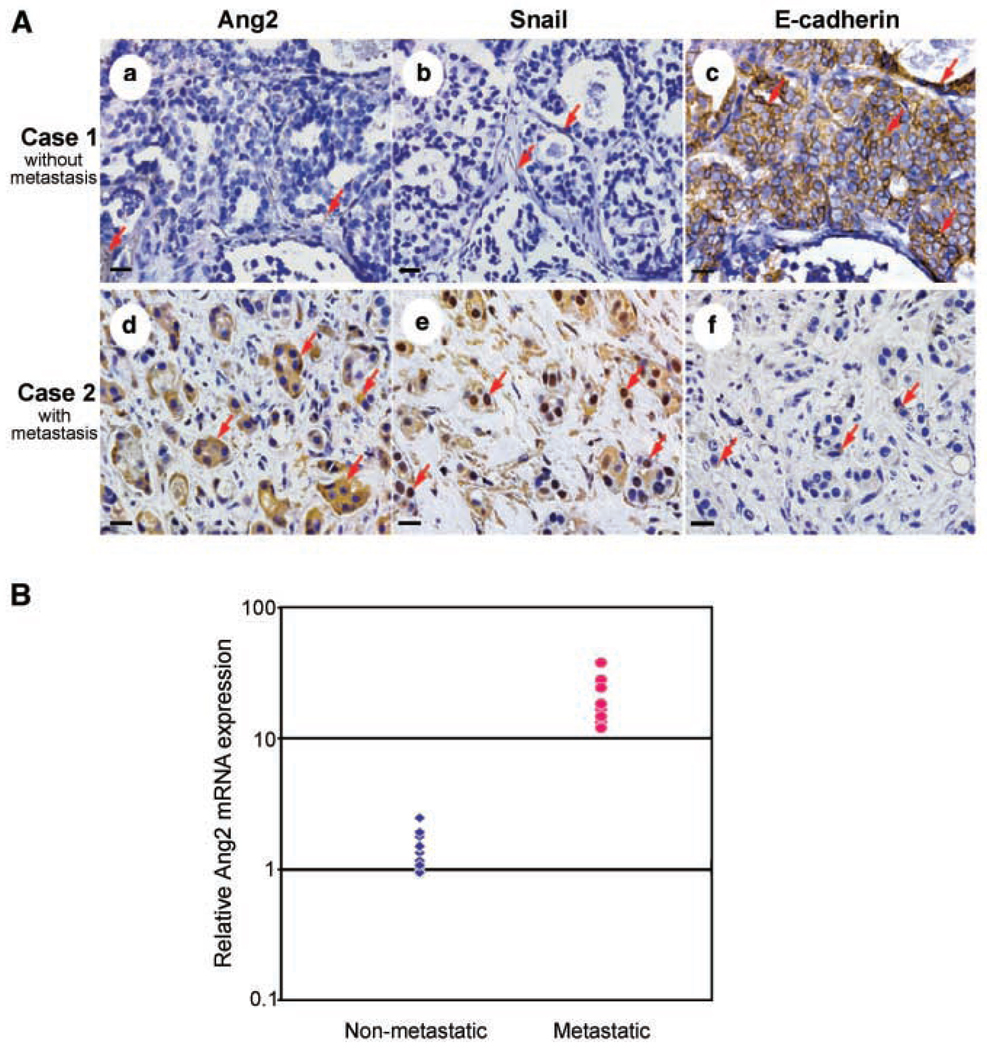
We first compared expression levels of Ang2 and two important modulators of cell invasion, Snail and E-cadherin (3), in a total of 185 human breast tumors by immunohistochemical staining using antibodies specific for Ang2, Snail, and E-cadherin. As shown in Fig. 1, up-regulation of Ang2 (Fig. 1A, d) significantly correlated with increased expression of nuclear localized Snail (Fig. 1A, e) and down-regulation of E-cadherin (Fig. 1A, f). Interestingly, Ang2 expression significantly correlated with lymph node metastasis, tumor grade, and lymph-vascular invasion (summarized in Supplementary Table S1). In addition, both increased expression of Snail and decreased expression of E-cadherin also significantly correlated with lymph node metastasis and lymph-vascular invasion (Supplementary Tables S2 and S3), whereas tumor grade significantly correlated with decreased expression of E-cadherin (Supplementary Table S3) but not Snail (Supplementary Table S2). Finally, using quantitative real-time PCR, we analyzed the expression of Ang2 mRNA in 20 frozen tumor specimens, including 10 tumors showing lymph node and/or distant metastasis (immunohistochemical staining high intensities for Ang2 and Snail and negative for E-cadherin; Supplementary Tables S1 and S4) and 10 nonmetastatic samples (immunohistochemical staining negative for Ang2 and Snail and positive for E-cadherin; Supplementary Tables S1 and S4). Ang2 mRNA expression was significantly higher in the metastatic tumors versus the nonmetastatic samples by at least 10-fold (Fig. 1B).
Figure 1.
Expression of Ang2 correlates with Snail and E-cadherin expression and metastatic potential in primary human breast cancer specimens. A, human breast cancer specimens were stained for Ang2 (a and b), Snail (b and e), and E-cadherin (c and f). Arrows, positive staining of Ang2 (a and d), Snail (b and e, especially in the nuclei), or E-cadherin (c, dominantly on the cell membrane; f , barely positive in the cytoplasm) in the tumor cells. Ang2 proteins were detected mostly in tumor cell cytoplasm. Representative stains from a total of 185 tumor samples. Each specimen was independently analyzed at least twice with similar results. Bar, 20 µm. B, expression of Ang2 mRNA correlates with the metastatic potential of primary human breast cancer specimens. Quantitative real-time PCR was done on cDNA derived from total RNA isolated from nonmetastatic and metastatic snap-frozen human breast cancer specimens. The relative expression of Ang2 in the metastatic versus the nonmetastatic samples was calculated using a difference in threshold cycle (ΔCt) method with β-glucuronidase as the normalizing control gene. PCRs were done in triplicate and each specimen was independently analyzed twice with similar results.
Overexpression of Ang2 promotes metastasis and primary tumor growth of MCF-7 breast cancers in mice
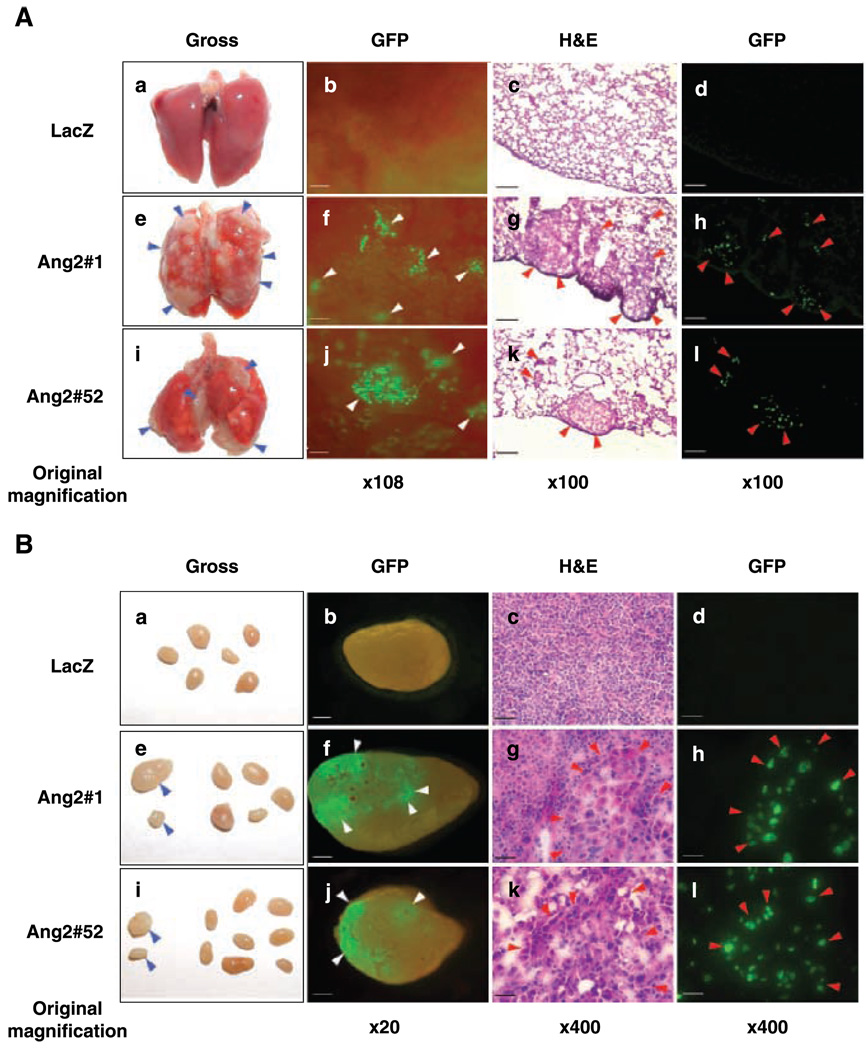
To show the role of Ang2 expression in breast cancer progression, we determined whether overexpression of Ang2 by MCF-7 cells that are poorly metastatic and estrogen dependent is able to stimulate metastasis of breast tumor xenografts in mice. We stably transfected MCF-7 cells with cDNAs encoding LacZ or Ang2 and GFP sequentially (designated as LacZ, Ang2#1, or Ang2#52 hereafter; Supplementary Fig. S1A) and could not detect Tie2 or Tie1 expression by RT-PCR analyses in the various MCF-7 and T47D cells (Supplementary Fig. S1B; data not shown). Then, we injected various MCF-7 cells into mammary fat pads of ovariectomized mice supplemented with E2 pellets (an orthotopic model). As shown in Fig. 2, 14 to 18 weeks postinjection, mice that received control LacZ/GFP cells showed no detectable metastasis in the lung (Fig. 2A, a–d) and all other examined organs (data not shown). Lymph node metastasis was also absent in all mice (Fig. 2B, a–d) except for one mouse with a small cluster of LacZ/GFP cells in the lymph nodes (Supplementary Table S5). In contrast, multiple lung metastases were detected in 12 of 13 (92%) mice that received Ang2#1 cells (Fig. 2A, e–h; Supplementary Table S5) and 5 of 8 (62.5%) mice that received Ang2#52 cells (Fig. 2A, i–l ; Supplementary Table S5). Metastatic lesions on the surface of the lung were grossly visible in more than half of the cases with metastasis (Fig. 2A, e and i, arrowheads). Metastasis in the lymph nodes was found in 10 of 13 (77%) mice that received Ang2#1 cells (Fig. 2B, e–h; Supplementary Table S5) and 4 of 8 (50%) mice that received Ang2#52 cells (Fig. 2B, i–l; Supplementary Table S5). Liver metastasis was also found in two (15%) mice that received Ang2#1 cells (Supplementary Table S5). It is noteworthy that the metastatic potential of Ang2-expressing cells correlates with Ang2 expression levels in these cells (Supplementary Fig. S1A). Ang2#1 displayed high incidence of metastasis, Ang2#52 had lower metastatic potential (Supplementary Table S5), whereas Ang2#4 did not cause metastasis in mice (data not shown). Experiments were also done using Ang2#5 cell clone in mice and Ang2 stimulation of tumor metastasis was found to be of similar frequency to that of Ang2#1 cell clone. In addition, we also found that Ang2 overexpression moderately affects the growth, angiogenesis, but not lymphangiogenesis of orthotopic MCF-7 tumors in the mice that displayed a metastatic phenotype (data not shown).
Figure 2.
Overexpression of Ang2 by MCF-7 breast cancer cells promotes tumor metastasis in mice. MCF-7 parental cells were obtained from ATCC. Various MCF-7 cell lines were generated to stably express Ang2 or LacZ and GFP with no observable alterations in cell properties (see Materials and Methods in the Supplementary Data). A and B, tumor metastasis was examined by gross observation (a, e, and i) and epifluorescent observation of GFP (b, f , and j). Cryosections were further examined by epifluorescence (d, h, and l) followed by H&E staining (c, g, and k). Mice that received MCF-7/Ang2/GFP cells (clone 1 or 52) showed metastasis in the lung and lymph nodes (e–l in A and B) with high incidence. Grossly visible metastases (blue arrowheads) in the lung (e and i in A) and lymph nodes (e and i in B) were identified as green foci (f and j, white arrowheads) and micrometastatic cells expressing GFP (h and l, red arrowheads) and by H&E staining (g and k , red arrowheads). Bars, 120 µm (b, c, d, f, g, h, j, k, and l in A), 600 µm (b, f , and j in B), and 30 µm (c, d, g, h, k, and l in B). The experiments in (A) and (B) were done three independent times and similar results were obtained. Additionally, these experiments were also done twice using Ang2#4 (low expression of Ang2) and Ang2#5 (high expression of Ang2; Supplementary Fig. S1A) in mice. High incidence of lymph node and lung metastasis was found in mice that received Ang2#5 cells, whereas no tumor metastasis was found in mice that received Ang2#4 cells.
Ang2 modulates E-cadherin expression in breast cancer cells
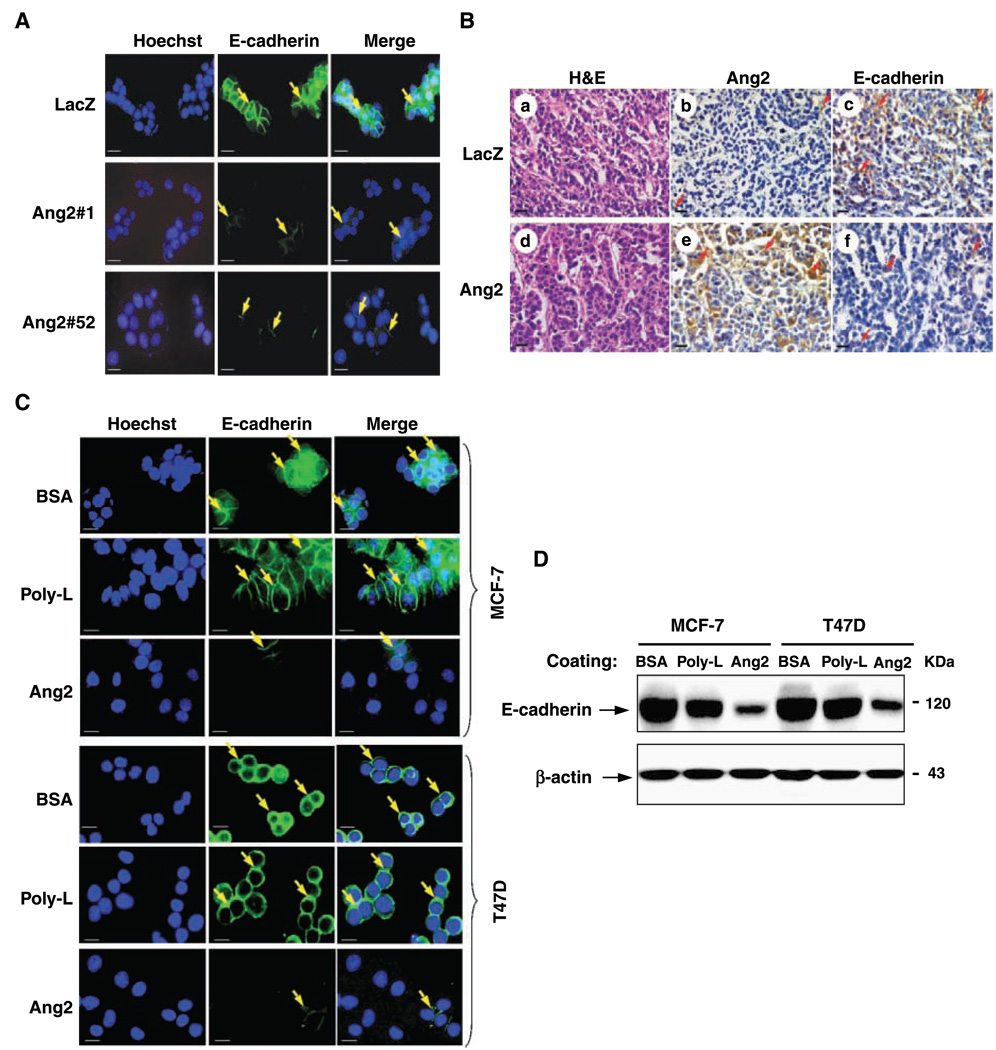
Because loss of E-cadherin expression is linked with acquisition of breast cancer cell invasion (2), we sought to determine whether Ang2-stimulated MCF-7 tumor metastasis is associated with modulation of E-cadherin expression in breast cancer cell lines that were stimulated by Ang2. As shown in Fig. 3A, LacZ control cells formed compact cell clusters with strong E-cadherin expression along the cell-cell contacts. However, Ang2#1 and Ang2#52 cells had diminished E-cadherin expression. In various orthotopic MCF-7 tumors, Ang2 was expressed at low levels in LacZ tumors (Fig. 3B, b), whereas high levels of E-cadherin were found in the cytoplasm and the cell-cell contacts (Fig. 3B, c). In contrast, Ang2-overexpressing tumors (Fig. 3B, e) showed a significant decrease in E-cadherin expression (Fig. 3B, f).
Figure 3.
Ang2 stimulation induces loss of E-cadherin expression in breast cancer cells. MCF-7 and T47D cells used in Fig. 3 to Fig 6 were obtained from ATCC. A, immunofluorescent staining of MCF-7/LacZ control and MCF-7/Ang2 cells (clones 1 and 52) using an anti-E-cadherin antibody (green) and Hoechst (blue for nuclear staining). MCF-7/Ang2 cells showed decreased expression of E-cadherin in the cell membrane leading to loss of cell-cell contact (arrows in MCF-7/Ang2 cells). Bar, 15 µm. B, immunohistochemical analyses of orthotopic tumors established by LacZ/GFP (a–c) or Ang2#1 cells (d and f) using H&E (a and c), anti-Ang2 (b and e), or anti-E-cadherin antibodies (c and f). Arrows, Ang2 (b and e) or E-cadherin (c and f) staining. Three to five serial-cut paraffin sections from five or more individual tumor samples of each group were independently analyzed. Bars, 20 µm. C, immunofluorescent staining of MCF-7 and T47D cells adhered to exogenous Ang2, heat-inactivated BSA, or poly-lysine (Poly-L). Down-regulation of E-cadherin in the cell membrane and loss of cell-cell contact (arrows) were observed in Ang2-treated cells. Bar, 15 µm. D, Western blot analyses of E-cadherin expression in exogenous Ang2-treated breast cancer cells. MCF-7 and T47D cells were treated with heat-inactivated BSA, Ang2, or poly-lysine for 48 h. β-Actin was used as a loading control. The experiments in (A) to (D) were done two independent times with similar results.
We then assessed whether stimulation by exogenous Ang2 on MCF-7 and T47D parental breast cancer cells modulates expression of E-cadherin in vitro. As shown in Fig. 3C, when MCF-7 and T47D cells were treated with exogenous Ang2, loss of E-cadherin expression along the cell-cell contacts, as well as in the cytoplasm, was evident. The levels of decreased E-cadherin expression in cells treated with Ang2 were similar to that of Ang2-expressing cells. In contrast, cells treated with heat-inactivated BSA or poly-lysine (an inert substrate for integrin receptor activation; ref. 33) had strong E-cadherin staining along the cell-cell border. Next, loss of E-cadherin expression in Ang2-stimulated cells was further examined by Western blot analyses. A decreased expression of E-cadherin was detected in MCF-7 and T47D cells stimulated with Ang2 compared with stimulation of heat-inactivated BSA or poly-lysine (Fig. 3D). Interestingly, when we reseeded these Ang2-stimulated cells onto poly-lysine–coated surfaces, E-cadherin expression was restored (Supplementary Fig. S2).
Ang2 activates the ILK/Akt and GSK-3β/Snail pathway in breast cancer cells
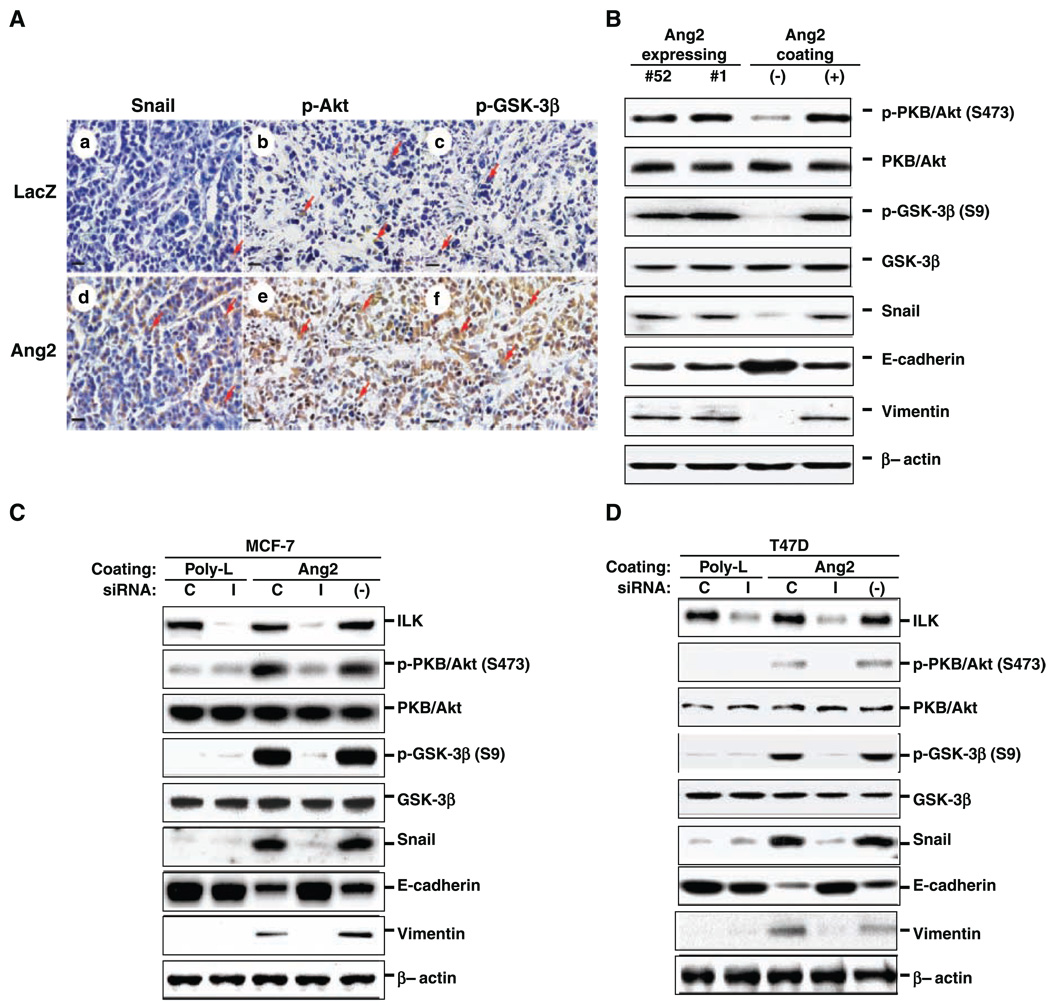
Next, we examined Snail expression in various MCF-7 orthotopic tumors by immunohistochemistry. As shown in Fig. 4A, up-regulated Snail expression was found mostly in the nuclei of Ang2-expressing tumors (Fig. 4A, d) but not in the LacZ controls (Fig. 4A, a), suggesting that nuclear localized Snail may be responsible for decreased E-cadherin expression in these tumors. GSK-3β and Akt are two major regulators modulating Snail expression in tumor cells (4). Activation of Akt by phosphorylation at Ser473 stimulates transcription of Snail (6), whereas inactivation of GSK-3β by phosphorylation at Ser9 leads to enhanced nuclear localization and protein stabilization of Snail (34). Therefore, we assessed whether phosphorylation of Akt (pSer473-Akt, activated form) and GSK-3β (pSer9-GSK-3β, inactivated form) were modulated in various MCF-7 orthotopic tumors. As shown in Fig. 4A, the phosphorylation of Akt and GSK-3β was enhanced in the Ang2-expressing tumors compared with the controls (Fig. 4A, compare e and f with b and c).
Figure 4.
Ang2 stimulates the ILK/Akt, GSK-3β/Snail/E-cadherin pathway. A, immunohistochemical analyses of identical orthotopic tumors established by LacZ/GFP (a–c) or Ang2#1 (d–f) using anti-Snail antibody (a and d, paraffin-embedded tissue sections), anti-p-Akt (Ser473) antibody (b and e, frozen tissue sections), and anti-p-GSK-3β (Ser9) antibody (c and f, frozen tissue sections). Arrows, positive staining of Snail especially in nuclei of tumor cells (a and d), p-Akt in cytoplasm (b and e), or p-GSK-3β in cytoplasm (c and f). Three to five serial-cut sections from five or more individual tumor samples of each group were independently analyzed. Bar, 20 µm. B, Western blot analyses of Ang2-expressing cells or LacZ control cells treated with Ang2 using anti-p-Akt (S473), anti-p-GSK-3β (S9), anti-Snail, anti-E-cadherin, and anti-vimentin antibodies. The membranes were reprobed with anti-Akt, anti-GSK-3β, or anti-β-actin antibodies as loading controls. C and D, Western blot analyses of parental MCF-7 (C) and T47D (D) cells transfected with siRNA for ILK (I), control siRNA (C), or no transfection (−) followed by stimulation with Ang2 or poly-lysine. The cell lysates were then analyzed as described in (B) except an anti-ILK antibody was used. The results from (A) to (D) are representative of three independently repeated experiments.
Next, we determined whether Ang2 stimulation of MCF-7 cells activates Akt and GSK-3β/Snail signaling pathway regulators critical for breast cancer cell invasion (2). As shown in Fig. 4B, both Ang2-expression and exogenous Ang2 stimulation increased phosphorylation of Akt at Ser473 and GSK-3β at Ser9, enhanced intracellular Snail protein level, and attenuated E-cadherin expression in MCF-7 cells. Vimentin, a mesenchymal marker, was also induced in cells stimulated by Ang2 but was undetectable in nonstimulated MCF-7 cells.
ILK activation promotes phosphorylation of Akt on Ser473 and GSK-3β on Ser9 and the activation of integrin and ILK stimulates cell invasion (6). Therefore, we assessed whether activation of the ILK/Akt and GSK-3β pathway is essential to modulate Ang2-regulated expression of Snail and E-cadherin in breast cancer cells. As shown in Fig. 4C and D, siRNA suppression of ILK (29) attenuated Ang2-stimulated phosphorylation of Akt and GSK-3β in MCF-7 (Fig. 4C) and T47D (Fig. 4D) cells and resulted in restoration of E-cadherin expression and inhibition of Snail and vimentin expression. In these experiments, we only used the inert ligand, poly-lysine, as a negative control for integrin/ILK stimulation (33) because BSA has minimal effects on integrin signaling (31).
Ang2 associates with β1 integrin in Tie2-deficient MCF-7 cells
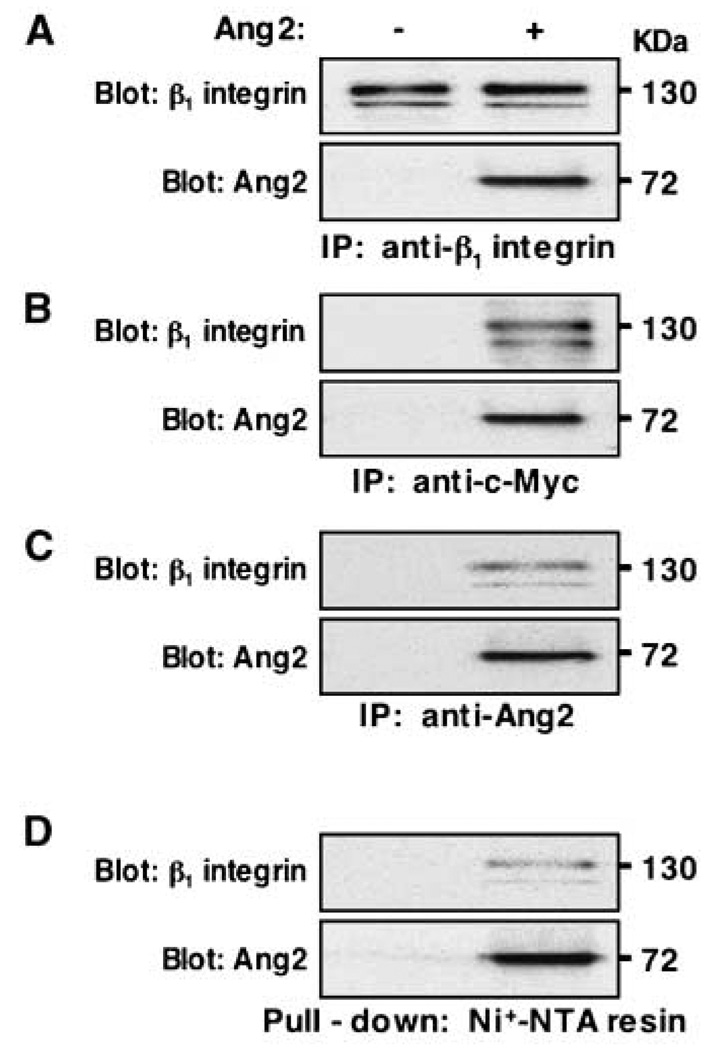
ILK exerts its function as an intracellular signaling adaptor through interaction with the cytoplasmic domains of β1 and β3 integrin subunits (6). Moreover, integrin mediates cellular signaling events by forming αβ heterodimeric integrin receptors (8). Therefore, we examined the expression profile of various α and β integrin subunits that have been implicated in cancer metastasis in breast cancer cells (10). We found that β1 was abundantly expressed, but β3 was absent in both MCF-7 and T47D cells. The subunits, α2, α3, α5, α6, αv but not α1 and α4, were also expressed in these breast cancer cell lines (Supplementary Table S6; refs. 35, 36). These data led us to focus on the involvement of β1 and α subunits that have been shown to associate with β1. Next, we did coimmunoprecipitation assays and found that equal amounts of β1 integrin in immunoprecipitated complexes from both groups, but only immunoprecipitated complexes from the MCF-7 cells stimulated with exogenous Ang2, had Ang2 protein present (Fig. 5A). In reciprocal experiments, only the immunoprecipitates from MCF-7 cells treated with c-Myc–tagged Ang2 contained β1 integrin (Fig. 5B and C). In a pull-down assay, Ang2-associated β1 integrin was pulled down with His-tagged Ang2 by Ni+-NTA beads, and both β1 integrin and Ang2 were detected only in the cells incubated with Ang2 but were undetectable in the cells treated with control (Fig. 5D).
Figure 5.
Ang2 associates with β1 integrin in Tie2-deficient MCF-7 cells. Coimmunoprecipitation (IP) and pull-down assays. Exogenous Ang2 was encoded by a c-Myc and His-tagged pSecTagB expression vector that is described in the Supplementary Data and expressed in MCF-7 Ang2#1 cells. The associated Ang2 with β1 integrin on various cells was assessed by immunoprecipitation separately with anti-β1 (A), anti-c-Myc (B), or anti-Ang2 (C) antibodies followed by Western blot using anti-β1 (A–C, top) or anti-Ang2 (A–C, bottom) antibodies. D, a pull-down assay. The Ang2-associated β1 integrin was pulled down by its His tag of Ang2 using Ni+-NTA beads followed by Western blot using anti-β1 or anti-Ang2 antibodies. Similar results were also found when the identical experiments were done using T47D cells (data not shown). Results are representative of three independently repeated experiments.
Ang2-stimulated breast cell motility is mediated through the α5β1 integrin/ILK pathway
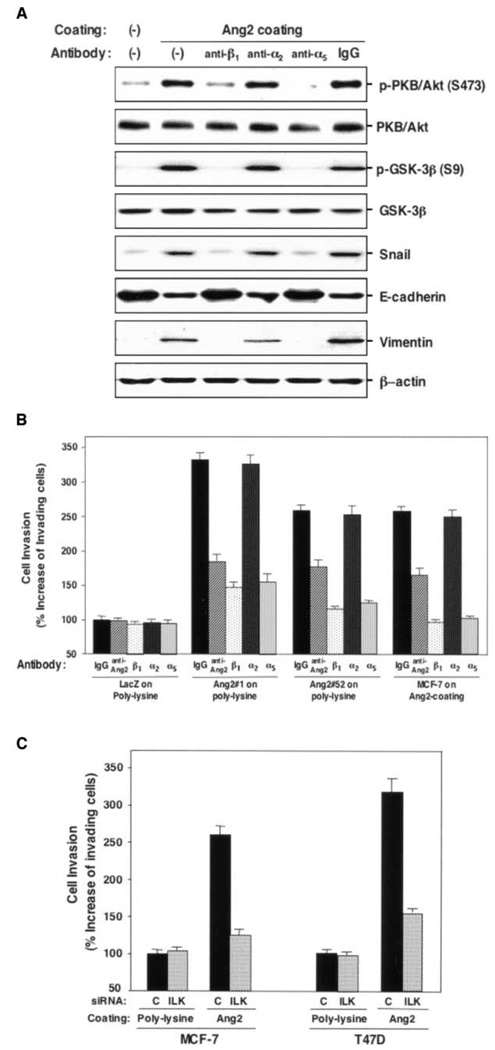
We tested the hypotheses that Ang2 stimulation would increase breast cancer cell motility and inhibition of integrins will suppress Ang2 activation of the ILK/Akt and GSK-3β pathway, cell migration, and invasion. As shown in Fig. 6A, inhibition of β1, α5 but not α2, β4 (data not shown), or IgG suppressed Ang2-induced phosphorylation of Akt and GSK-3β, Ang2-enhanced expression of vimentin and Snail, and Ang2 repression of E-cadherin, suggesting that α5β1 could be the integrin receptor involved in these Ang2-stimulated intracellular signaling processes. Finally, we determined whether functional inhibition of β1 and α5 integrins and ILK attenuates Ang2 stimulation of breast cancer cell motility. As shown in Fig. 6B and Supplementary Fig. S3A, Ang2 expression and exogenous Ang2 stimulation promoted MCF-7 cell invasion (Fig. 6B) and migration (Supplementary Fig. S3A). Consistent with the results shown in Fig. 6A, inhibition of β1 and α5 by neutralizing antibodies diminished Ang2-stimulated cell motility, whereas suppression of α2 had no effect on Ang2 stimulation (Supplementary Fig. S3A). Interestingly, an anti-Ang2 antibody (30) also attenuated Ang2-stimulated cell migration and invasion (Fig. 6B; Supplementary Fig. S3A), corroborating an autocrine effect by Ang2. Additionally, when ILK expression was inhibited by a specific siRNA for ILK in MCF-7 and T47D cells, Ang2-stimulated cell migration and invasion were completely abrogated (Fig. 6C; Supplementary Fig. S3B).
Figure 6.
Ang2-stimulated breast cancer cell invasion is mediated through the α5β1 integrin-mediated pathway. A, Western blot analyses. Inhibition of β1 and α5, but not α2, integrins attenuates Ang2 modulation of Akt, GSK-3β, Snail, E-cadherin, and vimentin. The membranes were reprobed with anti-Akt, anti-GSK-3β, or anti-β-actin antibodies as loading controls. B and C, in vitro cell invasion assays. B, inhibition of β1 and α5, but not α2, integrins suppresses Ang2 stimulation of breast cancer cell invasion. Mouse IgG and an anti-Ang2 (a nonneutralizing) antibody are included as controls. C, inhibition of ILK using siRNA specific for ILK suppresses Ang2 stimulation of breast cancer cell invasion. Results in (A) to (C) are representative of three independent times. Columns, mean percentage increase in the number of invading cells compared with control; bars, SD.
Discussion
In this study, we show that expression of Ang2 by Tie2-deficient breast cancer cells induces tumor metastasis to distant organs in animals by stimulating cell invasion potentially through the α5β1 integrin-mediated pathway. The association of Ang2 with α5β1 integrin results in activation of ILK, Akt, and GSK-3β leading to the stimulation of Snail and vimentin expression and down-regulation of E-cadherin. Our results provide a probable explanation for the prometastatic ability Ang2 in animals and support our finding of a significant correlation of Ang2 up-regulation with metastatic potential in human breast cancers. These data also corroborate recent studies showing that α5β1 integrin is centrally implicated in tumor cell invasion induction of Ha-Ras–transformed epithelial cells (37). However, we cannot rule out whether α3 and αv integrins are also involved in mediating Ang2 effects because inhibition of α3 and αv integrins moderately attenuated Ang2-stimulated cell motility as well as ILK signaling (data not shown). Because α5β1 and α3β1 integrins are involved in cancer cell motility (9), it is plausible that Ang2 promotes breast cancer cell metastasis through association with α5β1 and to a lesser extent α3β1 and αvβ1 integrins. These results suggest a complex interplay between Ang2 and integrin signaling, leading to stimulation of breast cancer cell invasion and facilitation of metastasis.
Emerging evidence has shown that angiopoietins can function as adhesive ligands to stimulate cell adhesion, migration, and survival of endothelial cells, fibroblasts, cardiac and skeletal myocytes, and cancer cells through integrin-mediated pathways independent of Tie2. Ang1 and Ang2 were first shown to stimulate Tie2-independent cell adhesion of endothelial cells and fibroblasts to Ang1- or Ang2-coated surfaces through α5β1 and αvβ5 integrin-mediated activation of extracellular signal-regulated kinase (ERK) and focal adhesion kinase (FAK) signaling (24). Skeletal myocytes lacking Tie2 adhere to Ang1- and Ang2-coated surfaces in a similar manner as to laminin, fibronectin, and vitronectin. The angiopoietin-stimulated skeletal myocyte adhesion is mediated by integrin receptors, such as α5β1, activating ERK, FAK signaling, and promoting cell survival (25). It has been postulated that the interaction of angiopoietin with integrins is likely through the fibrinogen-like receptor-binding domain present in the angiopoietin protein structure (22). This theory has been examined recently showing that a monomeric Ang1 variant (ΔAng), composed only of the fibrinogen-like receptor-binding domain that is also present in Ang2, ligates Tie2 without activating the receptor. Moreover, ΔAng binds to α5β1 integrin with similar affinity compared with Tie2. When endothelial cells were plated on ΔAng-coated surfaces, ΔAng displays similar biological effects as full-length Ang1, such as stimulation of cell adhesion, ERK signaling, and vascular maturation (23). Even in endothelial cells that express the Tie2 receptor, immobilized Ang1 is able to selectively mediate α5β1 integrin outside-in signaling leading to a cross-talk between Tie2 and α5β1and promotion of angiogenesis (26). In glioma cells that lack Tie2 expression, Ang2 induces glioma cell invasion by stimulating matrix metalloproteinase-2 expression through the αvβ1 integrin and FAK pathway (31). In this study, we report that Ang2 associates with α5β1 integrin in Tie2-deficient breast cancer cells. Ang2 activates an integrin-mediated signaling pathway leading to breast cancer cell invasion and metastasis. Inhibition of β1 or α5, but not other integrins, attenuates Ang2 modulation of ILK, Akt, GSK-3β, Snail, E-cadherin, and vimentin and Ang2-stimulated breast cancer cell motility. Furthermore, similar to the association of Ang2 with integrins in skeletal myocytes (25) and PG-MV/vesican with integrin in glioma cells (32), the association of Ang2 with α5β1 integrin was highly calcium and manganese dependent in our system. Taken together, our results establish a critical role of Ang2 in promoting breast cancer metastasis through stimulation of cell motility and invasion mediated by the α5β1 integrin/ILK pathway independent of Tie2.
A significant association between Ang2 up-regulation in tumor cells and cancer invasion/metastasis, and decreased patient survival has been shown in various types of human cancers, including breast cancers (15–21, 38–41). In one study, tumor cell-expressed Ang2 was detected in primary human glioma tissues by an anti-Ang2 antibody and expression was verified in identical tumor cells in sister tissue sections by in situ hybridization analysis using a human Ang2 DNA probe (14). Our analyses of 185 primary breast cancer specimens corroborate these findings. We show that Ang2 expression significantly correlates with Snail up-regulation and E-cadherin down-regulation as well as with lymph node metastasis and tumor grade. Similar to the aforementioned studies, we show that up-regulated Ang2 was primarily found in tumor cells. Quantitative real-time PCR analysis indicates a 10-fold higher expression of Ang2 in the metastatic samples versus nonmetastatic tumors. Although we cannot rule out the contribution of the tumor stroma to Ang2 expression, this is unlikely due to the fact that fresh-frozen breast cancer tissues analyzed by immunohistochemistry contained >90% tumor cells, thus suggesting that the up-regulation of Ang2 was primarily by cancer cells not stromal or endothelia compartments in the metastatic breast cancers. In orthotopic MCF-7 tumors, although the anti-Ang2 antibody that was used in immunohistochemical analyses recognizes both human and mouse Ang2, Ang2 proteins were detected almost exclusively in tumor cells in the Ang2-expressing tumors. Additionally, we also found that Ang2 expression in MCF-7 cells promoted a moderate increase in angiogenesis and tumor growth in metastatic breast tumors. Because MCF-7 tumors express low levels of vascular endothelial growth factor (VEGF; ref. 28), insufficient VEGF within the tumor microenvironment resulted in a moderate increase in tumor vessel growth in Ang2-expressing tumors (42), most likely limiting the contribution of Ang2-stimulated angiogenesis to the acquisition of tumor metastasis. Together, these data show that in both human breast cancer specimens and tumor xenograft models, Ang2 was primarily derived from tumor epithelial cells, thus supporting the hypothesis that Ang2 is involved in breast cancer metastasis through an autocrine pathway independent of Tie2.
In summary, we have presented evidence showing that Ang2 induces breast cancer cell metastasis through the α5β1 intergin/ILK-Akt-GSK-3β-Snail–mediated pathway stimulating breast cancer cell migration and invasion. Our results underscore the contribution of Ang2 in cancer progression, not only by stimulating angiogenesis but also by promoting metastasis. These data provide new insight into the mechanisms underlying cancer metastasis and could establish Ang2 and its effectors as potential targets for breast cancer treatment.
Supplementary Material
Acknowledgments
Grant support: Department of Defense grants DAMD17-01-1-0375 and DAMD-17-02-1-0584 (S-Y. Cheng) and the Hillman Fellows Program at the University of Pittsburgh Cancer Institute (S-Y. Cheng and B. Hu).
We thank C. Wu and T. El-Hefnawy for their advice and assistance.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 3.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 4.Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail b y GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 5.Barbera MJ, Puig I, Dominguez D, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 6.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Keightley SY, Leung-Hagesteijn C, et al. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 10.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 11.Schreider C, Peignon G, Thenet S, Chambaz J, Pincon-Raymond M. Integrin-mediated functional polarization of Caco-2 cells through E-cadherin-actin complexes. J Cell Sci. 2002;115:543–552. doi: 10.1242/jcs.115.3.543. [DOI] [PubMed] [Google Scholar]
- 12.Avizienyte E, Wyke AW, Jones RJ, et al. Srcinduced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 13.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 14.Koga K, Todaka T, Morioka M, et al. Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res. 2001;61:6248–6254. [PubMed] [Google Scholar]
- 15.Etoh T, Inoue H, Tanaka S, et al. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- 16.Tanaka F, Ishikawa S, Yanagihara K, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62:7124–7129. [PubMed] [Google Scholar]
- 17.Ogawa M, Yamamoto H, Nagano H, et al. Hepatic expression of ANG2 RNA in metastatic colorectal cancer. Hepatology. 2004;39:528–539. doi: 10.1002/hep.20048. [DOI] [PubMed] [Google Scholar]
- 18.Ochiumi T, Tanaka S, Oka S, et al. Clinical significance of angiopoietin-2 expression at the deepest invasive tumor site of advanced colorectal carcinoma. Int J Oncol. 2004;24:539–547. [PubMed] [Google Scholar]
- 19.Guo P, Imanishi Y, Cackowski FC, et al. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 γ2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–890. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind AJ, Wikstrom P, Granfors T, et al. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. Prostate. 2005;62:394–399. doi: 10.1002/pros.20163. [DOI] [PubMed] [Google Scholar]
- 21.Sfiligoi C, de Luca A, Cascone I, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466–474. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 22.Davis S, Papadopoulos N, Aldrich TH, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization, and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 23.Weber CC, Cai H, Ehrbar M, et al. Effects of protein and gene transfer of the angiopoietin-1 fibrinogen-like receptor-binding domain on endothelial and vessel organization. J Biol Chem. 2005;280:22445–22453. doi: 10.1074/jbc.M410367200. [DOI] [PubMed] [Google Scholar]
- 24.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 25.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–e24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 26.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo P, Fang Q, Tao HQ, et al. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63:4684–4691. [PubMed] [Google Scholar]
- 29.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 30.Hu B, Guo P, Fang Q, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci U S A. 2003;100:8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Jarzynka MJ, Guo P, et al. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the αvβ1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 2006;66:775–783. doi: 10.1158/0008-5472.CAN-05-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Chen L, Zheng PS, Yang BB. β1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–12301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Asawa T, Takato T, Sakai R. Cooperative roles of Fyn and cortactin in cell migration of metastatic murine melanoma. J Biol Chem. 2003;278:48367–48376. doi: 10.1074/jbc.M308213200. [DOI] [PubMed] [Google Scholar]
- 34.Blanco MJ, Moreno-Bueno G, Sarrio D, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–3246. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 35.Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin αVβ3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Saad S, Bendall LJ, James A, Gottlieb DJ, Bradstock KF. Induction of matrix metalloproteinases MMP-1 and MMP-2 by co-culture of breast cancer cells and bone marrow fibroblasts. Breast Cancer Res Treat. 2000;63:105–115. doi: 10.1023/a:1006437530169. [DOI] [PubMed] [Google Scholar]
- 37.Maschler S, Wirl G, Spring H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad SA, Liu W, Jung YD, et al. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138–1143. doi: 10.1002/1097-0142(20010901)92:5<1138::aid-cncr1431>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 39.Loges S, Heil G, Bruweleit M, et al. Analysis of concerted expression of angiogenic growth factors in acute myeloid leukemia: expression of angiopoietin-2 represents an independent prognostic factor for overall survival. J Clin Oncol. 2005;23:1109–1117. doi: 10.1200/JCO.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 40.Mitsuhashi N, Shimizu H, Ohtsuka M, et al. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105–1113. doi: 10.1053/jhep.2003.50204. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Wu K, Zhang D, et al. Expressions and clinical significances of angiopoietin-1, -2 and Tie2 in human gastric cancer. Biochem Biophys Res Commun. 2005;337:386–393. doi: 10.1016/j.bbrc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 42.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.