Abstract
T-regulatory (Treg) cells play a major role in cancer by suppressing protective antitumor immune responses. A series of observations (from a single laboratory) suggest that Treg cells are protective in cancer by virtue of their ability to control cancer-associated inflammation in an interleukin (IL)-10–dependent manner. Here, we report that the ability of Treg cells to produce IL-10and control inflammation is lost in the course of progressive disease in a mouse model of hereditary colon cancer. Treg cells that expand in adenomatous polyps no longer produce IL-10and instead switch to production of IL-17. Aberrant Treg cells from polyp-ridden mice promote rather than suppress focal mastocytosis, a critical tumor-promoting inflammatory response. The cells, however, maintain other Treg characteristics, including their inability to produce IL-2 and ability to suppress proliferation of stimulated CD4 T cells. By promoting inflammation and suppressing T-helper functions, these cells act as a double-edged knife propagating tumor growth.
Introduction
T-regulatory (Treg) cells are an obstacle for immune surveillance and immune therapy of cancer. Hallmarks of these cells are expression of the transcriptional factor Foxp3 and the interleukin (IL)-2 receptor α subunit (CD25) together with their inability to produce IL-2 (1). Treg cells suppress CD4 T cells in part through competition for IL-2, render CD8 T cells inactive via cell contact and transforming growth factor (TGF)-β (see ref. 2 for review), and suppress inflammation in part through secretion of IL-10 (3). Initially, differentiation of Treg cells was considered to be incompatible with that of TH17 cells (4). However, recent investigations point to an inherent plasticity of Treg cells. Both share similar homing receptors (5) and are susceptible to convert to the proinflammatory TH17 phenotype when activated in the presence of TGF-β and IL-6 (6, 7). This conversion is normally incompatible with the expression of Foxp3, which is turned off (4, 8). Yet, there is also precedence for Foxp3+IL-17+ T lymphocytes (9).
We recently reported that mast cells are essential hematopoietic components for the development of adenomatous polyps (10). Much is known about the interaction of mast cells with T cells, mostly, however, on how mast cells modulate T-cell behavior and functions. Mast cells express MHC class I and II and costimulatory molecules (11) and are therefore potentially effective antigen-presenting cells. Mast cell–derived lymphotoxin promotes formation of lymph nodes and supports their expansion in the course of an immune response, whereas mast cell–derived tumor necrosis factor (TNF)-α promotes hypertrophy of draining lymph nodes during infection (12), migration of dendritic cells (13), and activation of T cells (14). Mast cells also promote lymph node hypertrophy independently of TNF-α (15). At least one report claims that Treg cells recruit and activate mast cells to mediate regional immune suppression (16). Conversely, a very recent report suggests that Treg cells suppress mast cell degranulation and allergic responses (17).
Earlier investigations using the Min mouse model of polyposis revealed a protective role for naturally occurring Treg cells (nTreg) in bacterial-induced chronic inflammation and cancer (18) and even hereditary colon cancer (19). Yet, many reports show tumor-infiltrating Treg cells to be detrimental to the tumor-bearing host, in part through inhibition of tumor-specific cytotoxic responses. Here, we provide evidence for nTreg suppression of focal mastocytosis in adenomatous polyps and propose this to be a major mechanism by which adoptively transferred Treg cells protect against colon cancer. It is intriguing that the recipient mice have actually elevated levels of Treg cells, which, however, are functionally distinct in that they fail to produce IL-10 and promote rather than suppress mastocytosis. The role of CD4+Foxp3+ cells in cancer is, however, complicated by the plasticity of these cells. On stimulation, a sizable fraction of the polyp-infiltrating population expresses IL-17. We propose that Treg functions are altered early in preneoplasia to endow them with potent proinflammatory and tumor-promoting properties.
Materials and Methods
Chloroacetate staining
Paraffin sections (5 μm), after deparaffinization with xylene (thrice, 5 min each) and rehydration with gradually decreasing solutions of ethanol (100%, 95%, and 70%), were stained with naphthol-AS-D chloroacetate and counterstained with hematoxylin Gill's II.
Preparation of intestinal mononuclear cells
Mononuclear cells (MNC) were prepared by chopping with blades the intestines and incubation of 25 mL suspension in RPMI 1640 with 10 units of collagenase type IV (Worthington Biochemical Corp.) for 20 min at 37°C with agitation. MNCs were collected from the interface of a 40% and 60% discontinuous Percoll gradient, washed, and resuspended in PBS plus 0.2% bovine serum albumin for analysis.
Mast cell progenitor assay and Treg anti-inflammatory activity assay
Briefly, 10,000 MNCs in 100 μL of medium [RPMI 1640 complete with 20 ng/mL IL-3, 10 ng/mL stem cell factor (SCF), and 106/mL irradiated spleen cells] per well were spread in the first row of a 96-well plate followed by a 1:2 serial dilutions in the subsequent rows. In the case of Treg anti-inflammatory assay, 10,000 per well Treg cells were added in each well of the first row and diluted 1:2 in the subsequent rows. Incubation time was 10 to 15 d at 37°C in the presence of 5% CO2, after which colonies of mast cells were scored.
T-cell proliferation/inhibition assay
Total MNCs were isolated from the spleen and intestine as described above. CD4 and Treg cells were isolated using positive selection via MACS cell separation. Assay was performed in 200 μL RPMI 1640 complete in 96-well plates, containing precoated anti-CD3 (10 μg/mL; BD Biosciences) and soluble anti-CD28 (5 μg/mL; eBioscience), 200,000 irradiated (3,000 rad) splenocytes, and, when indicated, 50 units/mL IL-2. T cells were added at specified ratios with an initial number of 20,000 cells and incubated at 37°C in 5% CO2 for 72 h followed by addition of 1 μCi [3H]thymidine for 18 h. Incorporated radioactivity was measured with a scintillation counter.
MNC staining and flow cytometry analysis
All staining reactions were preceded by a 10-min incubation with a blockade mixture made of 2.4G2 supernatant (Fc block) and 10% rat and mouse sera (Jackson ImmunoResearch Laboratories). Dead cells were systematically excluded by 4′,6-diamidino-2-phenylindole staining thereafter for 20 min on ice. For intracellular Foxp3 (Biolegend), IL-2, IL-10, and IL-17 stainings, BD Bioscience fixation and permeabilization reagents were used. CD4-PerCP (L3T4), CD25-bio-APC Cy7 (7D4), IL-2-PE (JES6-5H4), IL-10-PE (JES5-16E3), and IL-17-PE (TC11-18H10) were from BD Biosciences. Enumeration of cells, acquisition, and cell sorting were performed by using FACSAria, FACSCanto, and MoFlo instruments. Single-cell data analyses used the FlowJo software (Tree Star).
TUNEL and bromodeoxyuridine staining
Cryosections (10 μm) were stained with ApopTag red fluorescent in situ detection kit (Qbiogene) to detect apoptosis, and mitotic cells were revealed by bromodeoxyuridine staining. Cell numbers were calculated with the NIH software ImageJ (nuclei counter, and color threshold plug-ins).
ELISA and multiplex ELISA
ELISA was performed according to the manufacturer's instructions (eBioscience). Multiplex ELISA was performed according to the manufacturer's instructions using a customized set of analyte detection from Millipore. The Luminex 100 was used to acquire the results that are analyzed with the software of the machine.
Light microscopic data images
Microscopic images were collected with a Leica DCC camera. For multicolored images, the ImageJ plug-in “threshold color” was used to identify cells of the color of interest, which were then counted with the plug-in “nuclei counting.”
Statistical analysis
The statistical analyses were performed with the use of the Prism 4 software. ANOVA one-way nonparametric with Bonferroni post hoc test and 99% confidence intervals or unpaired one-tailed t tests with Welch's correction were used.
Results
nTreg cells protect against polyposis
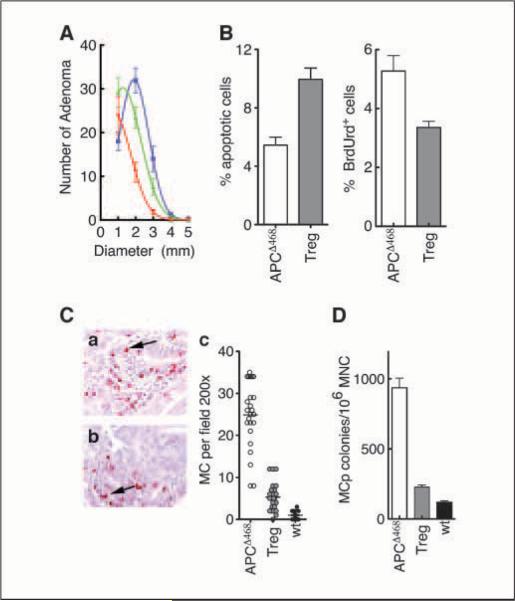
To examine the effect of nTreg cells on polyposis, we performed adoptive transfer of CD4+CD45RBlowCD25high cells from healthy C57BL/6 mice into APCΔ468 mice. As early as 2 days after i.v. transfer of 1 × 106 Treg cells from C57BL/6 mice to polyp-bearing APCΔ468 mice, the cells were readily detectable in the intestine of recipient mice (data not shown). By 3 weeks after adoptive transfer, the recipient mice had fewer polyps of smaller sizes (50 ± 13 polyps of 0.98 ± 0.23 mm mean diameter; mice n = 11) compared with untreated age-matched APCΔ468 mice of 4 months of age (76 ± 6.2 polyps of 1.9 ± 0.0.08 mm mean diameter; mice n = 11). By 6 weeks after transfer, the sizes and numbers of polyps continued to diminish (38 ± 5.1 polyps of 0.21 ± 0.32 mm mean diameter), suggesting active regression of the lesions (P < 0.001, one-way ANOVA with Bonferroni's multiple comparison test; Fig. 1A). The remaining polyps seemed regressive, with 10 ± 0.78% of the total cells displaying apoptotic activity compared with 5.4 ± 0.56% of the total cells in untreated APCΔ468 polyps (n = 11; P = 0.0002, unpaired t test with Welch's correction; Fig. 1B), and had diminished frequencies of mitotic cells (3.4 ± 0.20% versus 5.3 ± 0.52%, respectively; n = 8; P = 0.0072, unpaired t test with Welch's correction; Fig. 1B). These observations are the first independent confirmation of earlier reports suggesting that nTreg cells derived from healthy donors are protective to the host and detrimental to polyp growth, causing regression of the lesions by promoting death and hindering division of aberrant epithelial cells.
Figure 1.
Adoptive transfer of Treg cells in APCΔ468 mice causes polyps to regress and reduces mastocytosis. A, nonlinear regression of the number versus the diameter of polyps remaining after adoptive transfer of nTreg from healthy mice. Blue, control APCΔ468; green, 3 wk after adoptive transfer of 106 Treg cells; red, 6 wkafter adoptive transfer of 106 Treg cells. Adenomatous polyps in mice transferred with Treg cells display increased apoptosis and decreased proliferation. Mice were transferred with 1 × 106 nTreg cells derived from healthy C57BL/6 mice 6 wkbefore analysis. B, frequency of apoptotic nuclei and mitotic cells in polyps of APCΔ468 mice with no transfer (white columns) or after Treg transfer (gray columns). C, chloroacetate esterase staining of mast cells infiltrating the polyps in APCΔ468 (a) and in APCΔ468 6 wkafter adoptive transfer of 106 Treg cells (b). Magnification, ×200. c, mast cells per 200× field. White circles, APCΔ468; gray circles, APCΔ468 6 wkafter adoptive transfer of 106 Treg cells; black circles, wt. D, MCps assay using MNCs prepared from the intestine 6 wkafter adoptive transfer of 106 Treg cells. White column, APCΔ468; gray column, APCΔ468; black column, wt.
nTreg adoptive transfer reduces focal mastocytosis
We have reported that mast cells are crucial hematopoietic components for polyp development (10). Here, we tested the notion that adoptive transfer of nTreg cells into mice with established polyps results in a drop in the frequencies of polyp-infiltrating mast cells and mast cell progenitors (MCp). To this aim, we transferred nTreg cells from healthy donors to polyp-bearing mice as before. Three weeks after transfer, we checked the frequency of resident intestinal mast cells and progenitors. Paraffin sections of intestines from these mice were stained with chloroacetate esterase to quantify the average number of mature mast cells per field. On average, polyps of APCΔ468 mice had a 25-fold more mast cells compared with healthy intestines of wild-type (wt) C57BL/6 mice when counted per 200× field (APCΔ468 = 25 ± 1.6 mast cells, wt = 1.0 ± 016 mast cells, n = 24 fields, n = 6 mice). The nTreg adoptive transfer reduced the mean number of polyp-infiltrating mast cells by at least 4-fold (5.3 ± 069 mast cells, n = 24 fields, n = 6 mice, 99% confidence; P = 0.001, one-way ANOVA with Bonferroni's multiple comparison test; Fig. 1C and D). A comparable drop in the frequency of MCps was observed (Fig. 1D). The average frequency of MCps in the intestine of APCΔ468 mice was 7-fold higher than the frequency of MCps in the intestine of age-matched wt C57BL/6 mice (APCΔ468 = 883 ± 46 colonies/106 MNCs, n = 12; wt = 119 ± 9.4 colonies/106 MNCs, n = 9; Fig.1D). nTreg adoptively transferred APCΔ468 mice showed a 3-fold drop in the frequency of intestine resident MCps compared with untreated APCΔ468 mice, approaching double the number of MCps in wt mice (treated APCΔ468 = 227 ± 15 colonies/106 MNCs, n = 10, 99% confidence; P = 0.0001, one-way ANOVA with Bonferroni's multiple comparison test; Fig. 1D). These observations are consistent with nTreg cell control of tumor-infiltrating mast cells.
APCΔ468 polyps are enriched with CD4+CD25+Foxp3+ cells
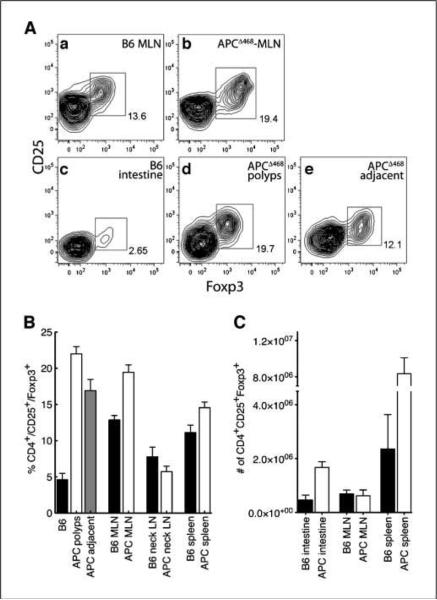
Because adoptive transfer of a limited number of nTreg cells into APCΔ468 mice hindered polyposis and reduced mast cell numbers in the intestine, we expected the recipient mice to be deficient either in the number or function of endogenous Treg cells. To investigate this, we isolated CD4+ lymphocytes either from pools of microdissected polyps or from the entire small bowel and stained for cell surface expression of CD25 and intracellular Foxp3. Surprisingly, CD4+CD25+Foxp3+ cells were significantly more abundant in the polyp-ridden APCΔ468 mice compared with age-matched healthy mice.
CD4+CD25+Foxp3+ T-cell frequencies were elevated in the mesenteric lymph nodes (MLN) of APCΔ468 mice compared with age-matched healthy mice [19 ± 1.0%, n = 7, compared with 13 ± 0.62%, n =7; P = 0.0002, unpaired t test with Welch's correction; Fig. 2A (a and b) and B]. The difference was significantly greater when comparing adenomatous polyps with the healthy small intestine [22 ± 0.98%, n = 11, compared with 4.6 ± 0.9%, n =7; P < 0.0001, unpaired t test with Welch's correction; Fig. 2A (c–e) and B]. Treg frequency dropped in tissue adjacent to the polyps relative to the polyps [17 ± 1.6%, n = 11; P = 0.0069, unpaired t test with Welch's correction; Fig. 2A (d and e) and B] but was still significantly higher than in healthy intestine (P < 0.0001). The larger spleens of polyp-ridden APCΔ468 mice contained elevated frequencies and markedly higher numbers of CD4+CD25+Foxp3+ T cells compared with the spleens of wt mice (8.3 × 106 ± 1.8 × 106 cells compared with 2.4 × 106 ± 1.8 × 106 cells; P = 0.01, unpaired t test with Welch's correction; Fig. 2B and C). In contrast, we found similar frequencies of Treg cells in peripheral lymph nodes that did not drain the intestine, such as the cervical lymph nodes (Fig. 2B, neck LN).
Figure 2.
Frequencies and numbers of CD4+CD25+Foxp3+ cells are elevated in APCΔ468 mice. A, contour fluorescence-activated cell sorting (FACS) plots of CD4+CD25+Foxp3+ cells prepared from the MLN of (a) control C57BL/6 and (b) age-matched APCΔ468 mice or intestine of (c) control C57BL/6 and (d) microdissected intestinal polyps of APCΔ468 mice or (e) tissue marginal to polyps of APCΔ468 mice. B, frequencies of CD4+CD25+Foxp3+ cells in C57BL/6 (black columns) and APCΔ468 (white columns) mice. Lymph nodes from the neck, spleen, and MLN were analyzed from healthy intestine (black columns), microdissected polyps (white columns), and tissue marginal to polyps (gray column). C, total numbers of CD4+CD25+Foxp3+ cells in the spleen, MLN, and intestine of control C57BL/6 (black columns) and APCΔ468 (white columns) mice. B6 mice (n = 7) and APCΔ468 mice (n = 11).
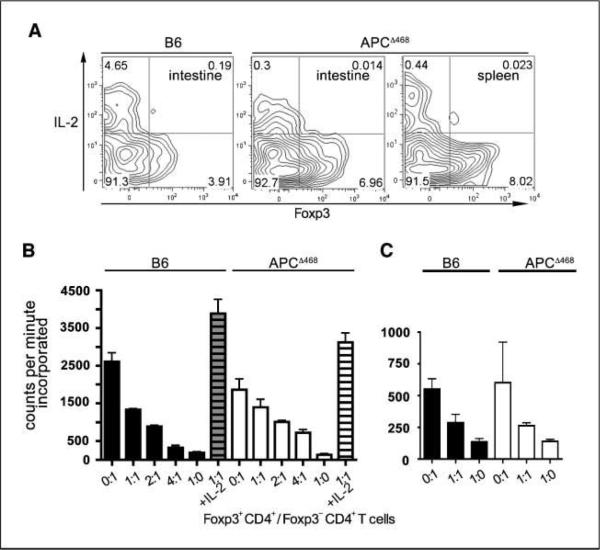
These observations established that frequencies and absolute numbers of CD4+CD25+Foxp3+ T cells were elevated in the polyp-ridden mice, raising the intriguing question of why, in spite of their increased numbers, cancer-associated mastocytosis was unhindered. We therefore proceeded to check for Treg functions. To distinguish activated CD4 T cells from Treg cells, we crossed APCΔ468 mice with Foxp3-GFP reporter mice (20). T cells were isolated from the spleen and intestine of aged APCΔ468Foxp3-GFP mice, stimulated with anti-CD3 and anti-CD28 for 3 days, and then stained for CD4, CD25, and intracellular IL-2. Treg cells do not produce IL-2 but constitutively express the IL-2α receptor (CD25) and effectively compete with helper CD4 T cells for IL-2 and hence for proliferation. As expected, CD4+Foxp3+ cells derived from control wt C57BL/6 mice failed to produce IL-2, whereas intracellular IL-2 was readily detected in Foxp3− cells (Fig. 3A). CD4+Foxp3+ cells derived from the intestine or spleen of APCΔ468 mice also did not produce IL-2. Additionally, we performed a widely used standard in vitro assay that measures the ability of Treg cells to inhibit proliferation of CD4 helper T cells in the absence of exogenous IL-2. Treg cells from both spleen and intestine of polyp-ridden APCΔ468-Foxp3-GFP mice readily suppressed proliferation of anti-CD3–stimulated and anti-CD28–stimulated CD4 T cell in coculture experiments (Fig. 3B and C). Together, these results indicate that Foxp3-GFP cells from polyp-bearing APCΔ468 mice share morphologic characteristics with and behave as Treg cells.
Figure 3.
CD4+CD25+Foxp3+ cells from polyp-bearing APCΔ468 mice do not produce IL-2 and suppress proliferation of helper CD4 T cells. A, FACS contour plots of total T cells derived from 4-mo-old C57BL/6 or polyp-bearing APCΔ468 mice, showing CD4+CD25+Foxp3+ cells stained for intracellular IL-2. B and C, inhibition of proliferation of naive T cells derived from the spleen (B) or small intestine (C). CD4+Foxp3− cells (2 × 104) were cultured alone (0:1) or with increasing numbers of CD4+Foxp3+ T cells as indicated, together with 2 × 105 irradiated spleen cells as feeder cells. In control plates, IL-2 was added to overcome Treg suppression. The results shown are representative of five independent experiments.
CD4+CD25+Foxp3+ cells in polyp-ridden mice are functionally altered
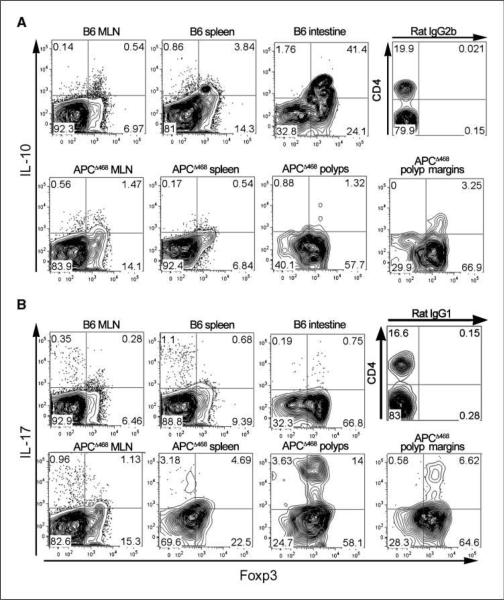
We had previously reported that adenomatous polyps are infiltrated with proinflammatory cells, including mast cells (10). The observation that mast cell frequency is controlled by nTreg cells is in line with the role of nTreg cells in regulation of inflammation (21) and control of inflammation-induced cancer (18). There are reports of defective Treg cells in arthritis, a chronic autoimmune inflammatory disease (22, 23). In our current animal model, accumulation of endogenous CD4+CD25+Foxp3+ cells in polyps in progressive disease was consistent with a functional failure. IL-10 is critical for suppression of inflammation (24) and cancer by Treg cells (18). To test possibility of defects in Treg function related to IL-10, we stimulated CD4+CD25+Foxp3-GFP+ cells from the intestine of APCΔ468Foxp3-GFP mice with anti-CD3 and anti-CD28 for 3 days and stained for IL-10. Approximately 4% of Treg cells from spleen and >40% from intestine of healthy control C57BL/6 mice produced IL-10 (Fig. 4A). In contrast, Treg cells from the spleen of APCΔ468Foxp3-GFP mice were devoid of IL-10 (Fig. 4A). IL-10-expressing Treg cells were reduced by at least an order of magnitude in the healthy intestine tissue marginal to the polyps in comparison with intestine of healthy mice (Fig. 4). Adenomatous polyps contained even fewer IL-10-expressing Treg cells (Fig. 4A). We concluded that the endogenous Treg cells in polyp-ridden mice were defective in secretion of IL-10. Based on the crucial role of IL-10 in control of inflammation and the accumulation of Treg cells in adenomatous polyps, we predicted that the polyp-infiltrating Treg cells either were unable to control inflammation or were proinflammatory and thus functionally distinct from Treg cells from healthy mice. To further characterize polyp-infiltrating Treg cells, we tested the cells for the synthesis of TH1 (IFN-γ) or TH2 (IL-4) cytokines but failed to detect either (data not shown). However, up to a quarter of CD4+CD25+Foxp3+ cells derived from APCΔ468 mice expressed IL-17 after 3 days of ex vivo stimulation (Fig. 4B). Isotype control antibody for the cytokines did not stain (Fig. 4B). These observations were consistent with polyposis being a TH17-driven disease.
Figure 4.
CD4+Foxp3+ cells derived from polyp-ridden APCΔ468 mice are deficient in IL-10 but produce IL-17. MNCs isolated from MLN, spleen, intestine, microdissected adenomatous polyps, or healthy tissue from the margin of the polyps were cultured with anti-CD3/CD28 beads for 3 d and then stained for cell surface expression of CD4, CD25, intracellular Foxp3, and IL-10 (A) or IL-17 (B) and analyzed by FACS. All contour plots except for isotype control have been pregated for CD4. Representative contour plots from one of three independent assays are shown, depicting expression of IL-10 or IL-17 and Foxp3. Isotype controls are shown for IL-10 (IgG2b) and IL-17(IgG1).
TH17 cytokines are suppressed by adoptive transfer of healthy Treg
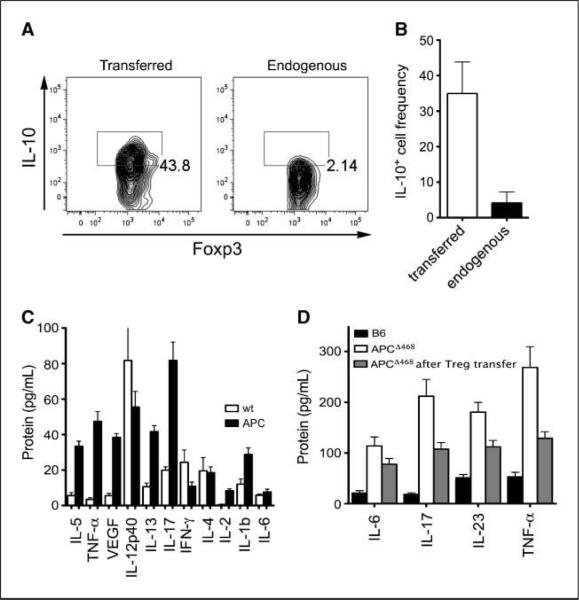
Treg cells adoptively transferred from healthy mice homed to the intestine and expressed IL-10 (Fig. 5A), in contrast to the endogenous Treg cells in the same mice (Fig. 5A and B). As reported before (19), the adoptive transfer of Treg cells resulted in suppression of polyposis, and we have already mentioned that it causes significant reductions in the frequencies of gut-infiltrating mast cells and progenitors (see Fig. 1). To further investigate the effect of Treg transfer on inflammation, we carried out multiplex ELISA on serum of diseased or age-matched healthy control mice for key cytokines associated with TH1, TH2, or TH17 responses. TH1 cytokines (IL-12 and IFN-γ) were lowered with the exception of TNF-α that was elevated in the sera of APCΔ468 mice compared with C57BL/6 (Fig. 5C). TH2 cytokines showed a disparate pattern, with IL-13 being elevated and IL-4 reduced (Fig. 5C). IL-17 was markedly elevated (Fig. 5C). We focused on TH17 cytokines and TNF-α, monitoring with standard ELISA mice that had received CD4+CD25+ nTreg cells from healthy donors. Treg transfer resulted in a clear drop in the level of serum IL-6, IL-17, IL-23, and TNF-α as early as 3 weeks after adoptive transfer, as detected by standard ELISA assays (Fig. 5D). Together, these observations showed that adoptively transferred Treg cells home to the intestine, secrete IL-10 and control inflammation, and are distinct from the endogenous Treg cells that did not produce IL-10. This raised the question of the function of endogenous Treg cells about inflammation.
Figure 5.
Elevated levels of proinflammatory cytokines in sera of APCΔ468 mice are corrected after adoptive transfer of nTreg cells from healthy mice. A, adoptively transferred Treg cells were explanted from the intestine of polyp-ridden mice 3 wkafter transfer and analyzed by FACS for expression of IL-10; a representative contour plot is shown. B, summary of three independent experiments; transferred and endogenous Treg cells were distinguished by expression of Ly5.1 versus Ly5.2 and staining for intracellular Foxp3. C, sera from 4-mo-old APCΔ468 and age-matched wt mice were analyzed with multiplex ELISA for 10 cytokines. The results of three independent experiments conducted in triplicate were used to calculate the positive or negative changes using the wt values as baseline. D, standard ELISA used to measure the level of TH17-associated cytokines 3 wk after adoptive transfer of 1×106 nTreg cells from healthy donors to polyp-ridden APCΔ468 mice of 2.5 to 3 mo of age. Sera from untreated age-matched APCΔ468 mice were used as control. White columns, APCΔ468; black columns, C57BL/6; gray columns, Treg-treated APCΔ468.
CD4+Foxp3+ cells from tumor-bearing mice are proinflammatory
The CD4 T-cell proliferation assay that is classically used to test Treg activity is primarily based on competition for IL-2 and is not sufficiently relevant to inflammation. To test the effect of Treg cells on inflammation, we devised a new in vitro assay that relied on the ability of Treg cells to suppress the differentiation and expansion of mast cells ex vivo. The assay was based on the MCp assay reported by us earlier (10) but included IL-2 and exogenous T cells.
To avoid complications due to endogenous Treg cells, we crossed the APCΔ468 mice to the Rag2−/− background and used these as donors of MNCs for the MCp assay. The MNCs were then mixed with CD4+Foxp3+ Treg cells sorted from the spleens of healthy, polyp-ridden, or IL-10−/− mice. MNCs with or without T cells were then plated in limiting dilution into 96-well dishes with medium containing IL-2, SCF, and IL-3. MCp-derived mast cell colonies were scored after 11 to 15 days (Fig. 6A). Addition of CD4+Foxp3-GFP+ cells from healthy Foxp3-GFP mice to the cultures significantly reduced the frequency of mast cell colonies (APCΔ468×Rag−/−, 1,600 ±100 MCps/106 MNCs; APCΔ468×Rag−/− in the presence of 5 × 104 nTreg cells, 1,100 ± 120 MCps/106 MNCs; P = 0.0014). However, addition of CD4+Foxp3-GFP+ cells derived from polyp-ridden mice almost doubled the number of progenitor-derived mast cell colonies (2,700 ± 170 MCps/106 MNCs; P < 0.0001; Fig. 6A). Because these Treg cells could not produce IL-10, we tested the role of IL-10. Treg cells from healthy mice failed to control MCp differentiation and expansion when they were depleted of IL-10 either through adding anti-IL-10 (2,000 ± 170 MCps/106 MNCs; P = 0.058) or by using IL-10−/− mice as source of Treg cells (1,800 ± 200 MCps/106 MNCs; P = 0.2468; Fig. 6A). These observations suggested that Treg cells may be controlling the natural frequency of mucosal MCps. To test this, we depleted MNCs isolated from the intestine of healthy mice (B6, 220 ± 28 MCps/106 MNCs) from CD4+ (740 ± 160 MCps/106 MNCs; P = 0.0427) or CD25+ T cells (950 ± 130 MCps/106 MNCs; P = 0.0002). In both cases, we observed marked increase in the mast cell colonies (Fig. 6B). Addition of anti-IL-10 produced a similar positive effect, arguing that the suppression by Treg cells required IL-10 (760 ± 170 MCps/106 MNCs; P = 0.0078, all analyzed with unpaired t test with Welch's correction). Based on these observations, we conclude that mast progenitor frequencies in the gut are controlled by Treg cells in a process that requires IL-10. In dysplasia, Treg cells are rendered incapable of producing IL-10 and convert to a proinflammatory phenotype while maintaining their expression of Foxp3 and ability to compete for IL-2. The altered functional status of Treg cells in tumor-bearing mice favors tumor progression.
Figure 6.
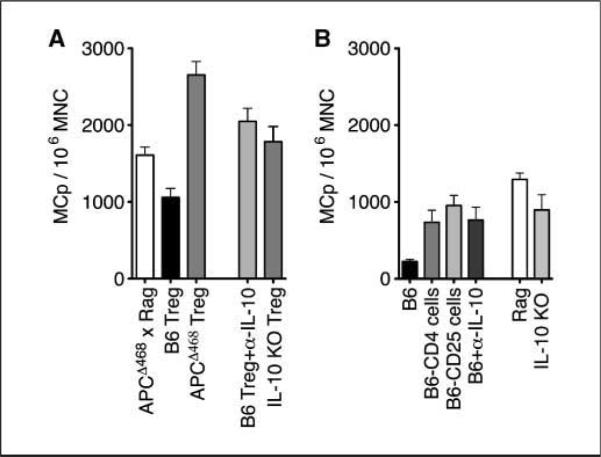
Healthy Treg cells suppress, whereas diseased Treg cells promote, MCp differentiation and expansion. A, frequency of MCps among total MNCs isolated from the intestine of APCΔ468Rag−/− mice (white column) and cocultured with CD4+CD25+Foxp3+ cells at 1:1 ratio from wt B6, APCΔ468, or IL-10−/− mice. B, frequencies of MCps isolated from the intestine of wt B6 mice (black column), depleted of CD4+ (B6-CD4) or CD25+ (B6-CD25) cells, or cultured in the presence of anti–IL-10 or from the intestine of Rag−/− or IL-10−/− mice.
Discussion
Polyposis is reversed through the adoptive transfer of Treg cells from healthy mice to polyp-ridden mice. We show that this is in part due to Treg suppression of focal mastocytosis, a critical tumor-promoting inflammatory response (10). Because Treg cells are known to expand in cancer, it is perplexing why the transfer of a limited number of Treg cells to mice harboring preneoplastic lesions should have such dramatic protective results. We show for the first time that the diseased recipients harbor functionally altered Treg cells. These express Foxp3 and suppress CD4 T-cell expansion but do not produce IL-10 and in the polyp microenvironment produce IL-17. CD4+Foxp3+ T cells derived from polyp-ridden mice promote rather than suppress mast cell maturation and expansion and are therefore “protumor” T cells that support cancer inflammation and tumor growth. While this manuscript was in preparation, a report documented the suppression of mast cell degranulation and allergic responses by Treg cells (17). This is consistent with earlier observations suggesting that Treg cells in arthritic patients fail to suppress inflammation and that a major benefit of anti-TNF treatment is the recovery of Treg functions (23).
The present report is the first to show that Treg cells are altered in the preneoplastic stage and that this alteration specifically affects mast cell expansion. Treg cells are important in immune homeostasis in the intestine. Loss of production of IL-10 is a major functional defect that could explain the inability of the Treg cells to suppress focal mastocytosis and systemic inflammation in the polyp-ridden mice. IL-10 is well accepted as a major regulator of mucosal immune responses in mice (24). Interestingly, IL-10 can also be protective in carcinogenesis, causing profound inhibition of tumor establishment, growth, and metastasis (25). Much less is known about the role of IL-10 in control of TH17-driven inflammation. Our observations are consistent with IL-10 mediating the Treg suppression of MCp differentiation/expansion and maintenance of gut homeostasis.
Our report is also the first to propose a pathologic function for the earlier reported but thus far poorly studied “Foxp3/IL-17 double-positive cells” (9) in cancer promotion. Adenomatous polyps are immediate precursors to intestinal and colonic carcinomas, and therefore, we propose that the shift in function of CD4+CD25+ T cells is likely to be an early event in colon cancer. At this point, we cannot rule out the possibility that the CD4+Foxp3+IL-17+ cells are generated elsewhere and targeted to the polyps. However, we failed to identify such cells in the thymus, spleen, or lymphatics, including the MLN, leaving the possibility open that these are bona fide Treg cells that are converted to produce IL-17 in the preneoplastic stromal microenvironment. Whether this is a true conversion of nTreg cells or of naive CD4 T cells remains to be clarified.
Recent studies have shown the plasticity and ready conversion of Foxp3+ cells to a CD4+Foxp3+IL-17+ phenotype (26, 27). The elevated levels of IL-6, IL-1β, and IL-23 in sera of polyp-ridden mice are consistent with the notion of Treg conversion to TH17 lineage; however, the persistent expression of Foxp3 raises new questions. Treg differentiation from naive CD4 T cells needs TGF-β but is inhibited by IL-6 (28). In contrast, differentiation of naive CD4 T cells into TH17 cells requires local production of TGF-β and IL-6, with IL-23 being necessary for prolonged stabilization (28, 29). The concomitant expression of Foxp3 and production of IL-17 is intriguing. Foxp3, a key transcription factor expressed by Treg cells, antagonized the function of ROR-γt, which is essential for the TH17 phenotype (4). Nevertheless, the concomitant expression of IL-17 and Foxp3 by CD4 T cells has been reported before (9, 30). Several recent studies have shown that Treg and TH17 cells are simultaneously enriched in tumors. Needle aspirates from localized adenocarcinoma and peripheral blood from patients undergoing prostatectomy show skewing of T cells toward both a Treg and TH17 phenotype (31). Cells double positive for IL-17 and Foxp3 have been observed in mismatch repair–proficient colorectal cancer (32). Our observations are consistent with the findings and extend these by showing that the CD4+Foxp3+IL-17+ T cells can have a potent proinflammatory and therefore tumor-promoting role.
We have noticed high levels of IL-17 and IL-23 in the sera of polyp-ridden mice and that polyp-infiltrating CD4+Foxp3+ cells express IL-17. Based on these findings and the ability of these Treg cells to promote mast cell expansion, we propose the term protumor T cells to describe the CD4+Foxp3+IL-17+ T cells. A plethora of recent data has emerged on the prevalence of IL-17–producing T cells in cancer. Tumor-secreted lactic acid is reported to promote TH17 differentiation (33). IL-17–producing CD8 T cell promotes growth of transplanted tumors in mice by suppressing apoptosis (34). IL-17–producing T cells promote inflammation and activate the p38, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase pathways in xenogenic transplanted human gastric cancer cells (35). IL-17 is up-regulated in prostate cancer (36), and IL-17–positive cells are enriched in the bone marrow of myeloma patients (37). Up-regulation of IL-17 in progressive cancer is consistent with a pathogenic role in tumor promotion. However, we cannot rule out the possibility that expression of IL-17 by tumor-specific cytotoxic cells may be in other circumstances protective.
An intimate link between IL-17 and focal mastocytosis in cancer is emerging. IL-17 is associated with the increased production of SCF, a critical mediator of mast cell expansion and maturation (38). Mast cells in turn promote IL-17–dependent recruitment of secondary inflammatory cells (39). In an earlier study with APCΔ468 mice (10), we provided evidence for mast cells being causatively involved in the progression of preneoplastic adenomatous polyps (10). Animal modeling and studies with human tumor samples suggest that the tumor-promoting role of mast cells is not unique to polyposis. Angiogenesis has been a focus of attention as a potential mechanism of action of mast cells in promoting skin cancer (40) and pancreatic islet cell dysplasia (41) in mice genetically predisposed to these diseases. Similar observations were reported with transplantable tumor cell lines, where secretion of SCF tumor by tumor cells seemed to be responsible for homing of mast cells (42). Mast cells are common in several human cancers, including Merkel cell carcinoma (43), breast (44), lung (45), and Hodgkin's lymphoma (46). Mast cell infiltration correlates well with tumor angiogenesis and metastases in gastric cancer (47), colorectal cancer (48), pulmonary adenocarcinoma (45), renal cell cancer (49), and prostate cancer (50). Altogether, these observations justify the choice of mast cells as candidate proinflammatory cells etio-logically linked with tumor progression.
Based on our findings, we propose that early in cancer Treg cells are functionally diverted into protumor T cells and play an important role in shaping the microenvironment of developing tumors. A major contribution of such tumor-promoting T cells to cancer is the escalation of cancer-associated inflammation. At the same time, the cells intercept tumor-specific T cells, providing a dual advantage to the developing tumor. These observations provide the rationale for targeting Treg cells and their interaction with mast cells for therapeutic intervention in dysplasia and cancer.
Acknowledgments
Grant support: RO1-CA104547. K. Khazaie is the recipient of American Cancer Society Research Scholar grant 113422 RSG and Zell Family Award of Robert H. Lurie Cancer Center.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Khazaie K, von Boehmer H. The impact of CD4(+) CD25(+) Treg on tumor specific CD8(+) T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–36. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–40. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 7.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 8.Ichiyama K, Yoshida H, Wakabayashi Y, et al. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J Biol Chem. 2008;283:17003–8. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 9.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 10.Gounaris E, Erdman SE, Restaino C, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–82. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent-Schneider H, Thery C, Mazzeo D, Tenza D, Raposo G, Bonnerot C. Secretory granules of mast cells accumulate mature and immature MHC class II molecules. J Cell Sci. 2001;114:323–34. doi: 10.1242/jcs.114.2.323. [DOI] [PubMed] [Google Scholar]
- 12.McLachlan JB, Hart JP, Pizzo SV, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 13.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–12. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 14.Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–48. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 15.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–62. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 16.Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 17.Gri G, Piconese S, Frossi B, et al. CD4(+)CD25(+) regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–81. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdman SE, Rao VP, Poutahidis T, et al. CD4(+)CD25 (+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–50. [PubMed] [Google Scholar]
- 19.Erdman SE, Sohn JJ, Rao VP, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 20.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 22.Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med. 2007;204:33–9. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kundu N, Fulton AM. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, enhances NK lysis. Cell Immunol. 1997;180:55–61. doi: 10.1006/cimm.1997.1176. [DOI] [PubMed] [Google Scholar]
- 26.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 27.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 29.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-β induces development of the T (H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 30.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci US A. 2009;106:4793–8. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Gouvello S, Bastuji-Garin S, Aloulou N, et al. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–9. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 33.Shime H, Yabu M, Akazawa T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–83. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 34.Nam JS, Terabe M, Kang MJ, et al. Transforming growth factor β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008;68:3915–23. doi: 10.1158/0008-5472.CAN-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Toh ML, Zrioual S, Miossec P. IL-17A versus IL-17F induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in AGS gastric adenocarcinoma cells. Cytokine. 2007;38:157–64. doi: 10.1016/j.cyto.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Fujita K, Ewing CM, Sokoll LJ, et al. Cytokine profiling of prostatic fluid from cancerous prostate glands identifies cytokines associated with extent of tumor and inflammation. Prostate. 2008;68:872–82. doi: 10.1002/pros.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhodapkar KM, Barbuto S, Matthews P, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–85. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzenberger P, Huang W, Ye P, et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–9. doi: 10.4049/jimmunol.164.9.4783. [DOI] [PubMed] [Google Scholar]
- 39.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–8. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–8. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 42.Huang B, Lei Z, Zhang GM, et al. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–79. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beer TW, Ng LB, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:27–30. doi: 10.1097/DAD.0b013e31815c932a. [DOI] [PubMed] [Google Scholar]
- 44.Amini RM, Aaltonen K, Nevanlinna H, et al. Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer. 2007;7:165. doi: 10.1186/1471-2407-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–92. [PubMed] [Google Scholar]
- 46.Molin D, Edstrom A, Glimelius I, et al. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol. 2002;119:122–4. doi: 10.1046/j.1365-2141.2002.03768.x. [DOI] [PubMed] [Google Scholar]
- 47.Yano H, Kinuta M, Tateishi H, et al. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2:26–32. doi: 10.1007/s101200050017. [DOI] [PubMed] [Google Scholar]
- 48.Lachter J, Stein M, Lichtig C, Eidelman S, Munichor M. Mast cells in colorectal neoplasias and premalignant disorders. Dis Colon Rectum. 1995;38:290–3. doi: 10.1007/BF02055605. [DOI] [PubMed] [Google Scholar]
- 49.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur Urol. 2006;50:530–4. doi: 10.1016/j.eururo.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 50.Nonomura N, Takayama H, Nishimura K, et al. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007;97:952–6. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]