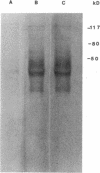
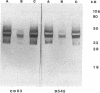
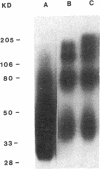
Abstract
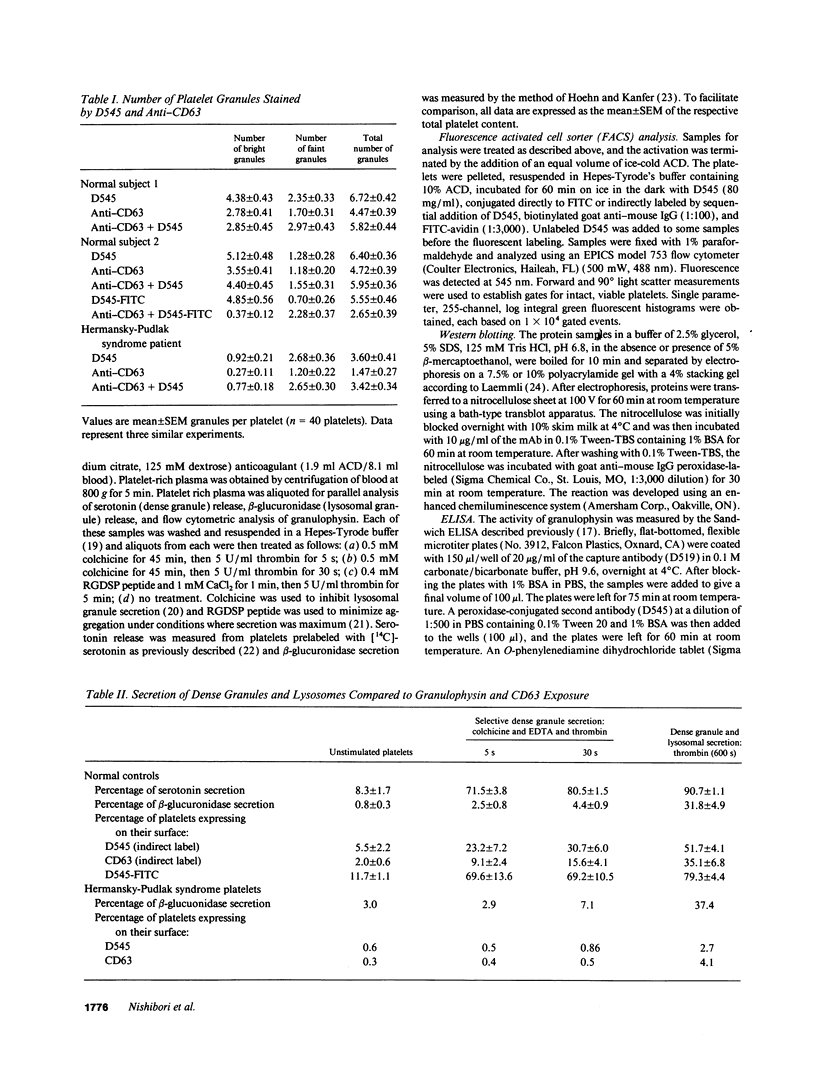
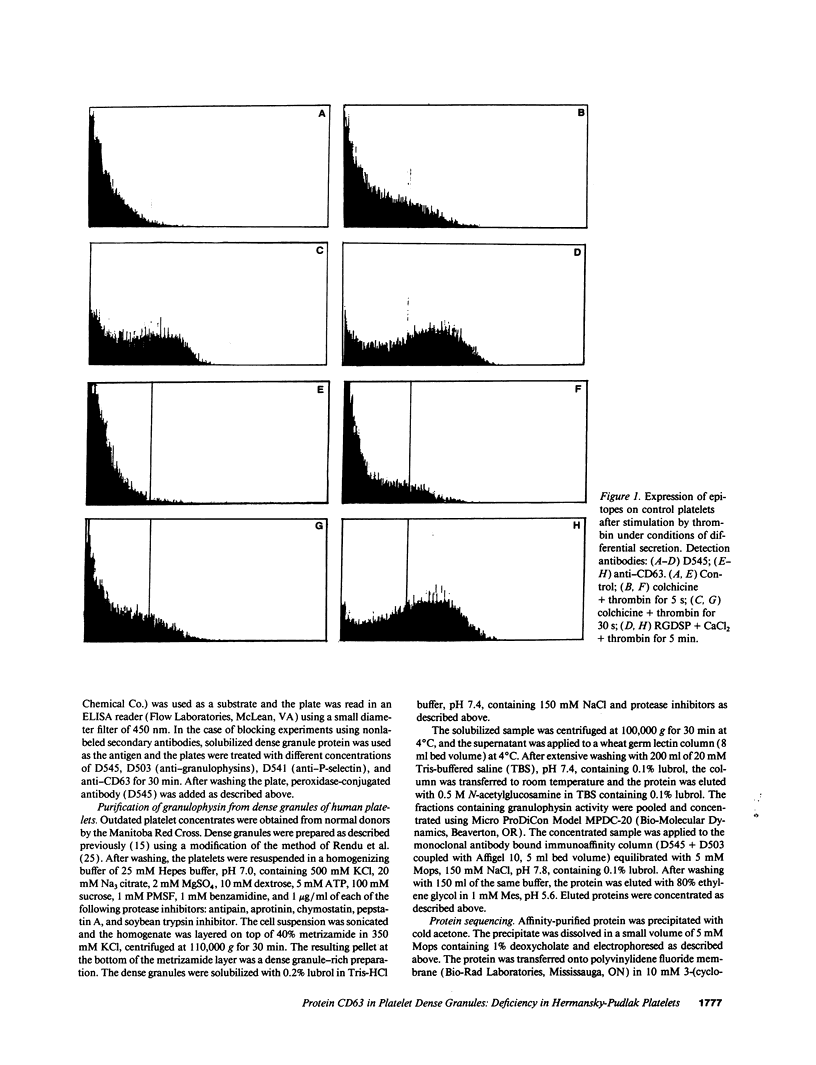
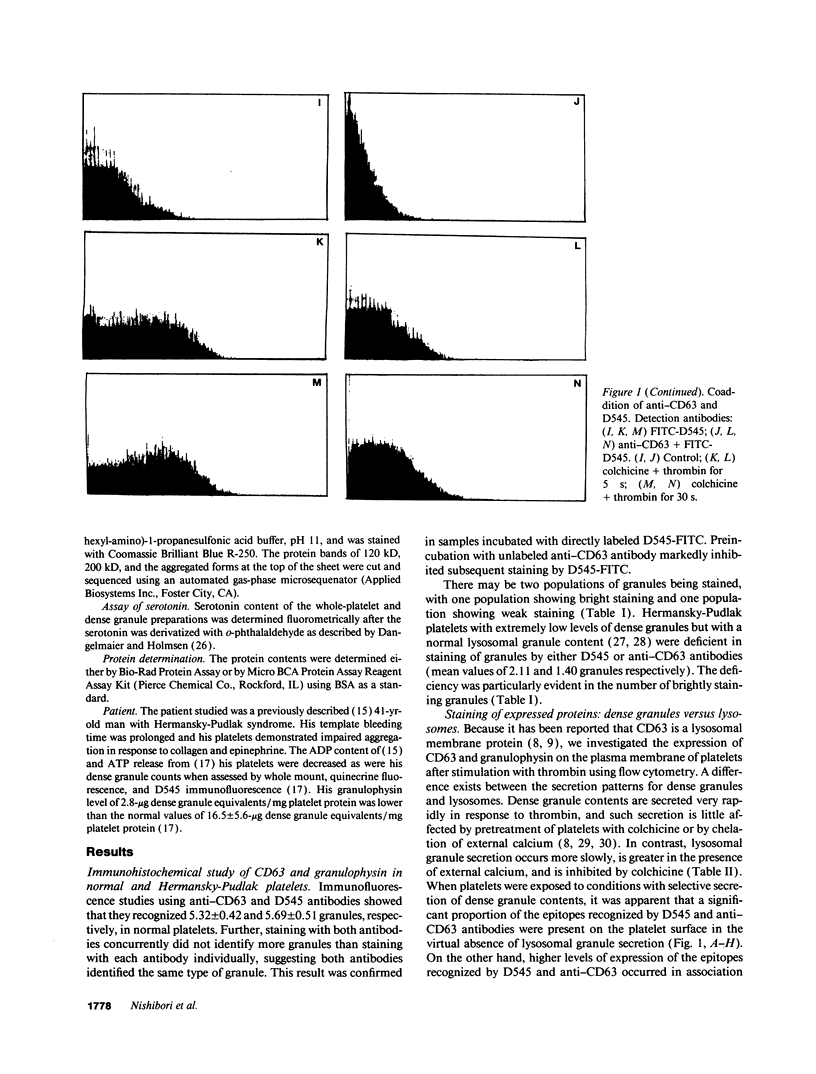
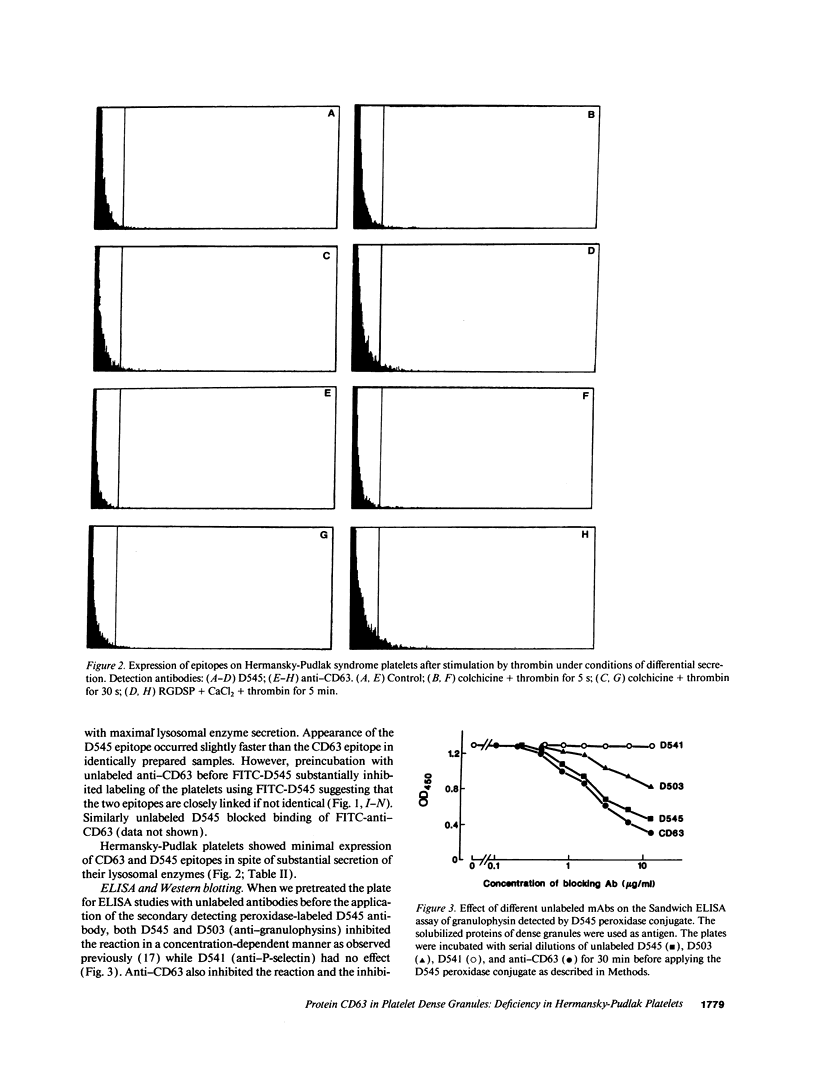
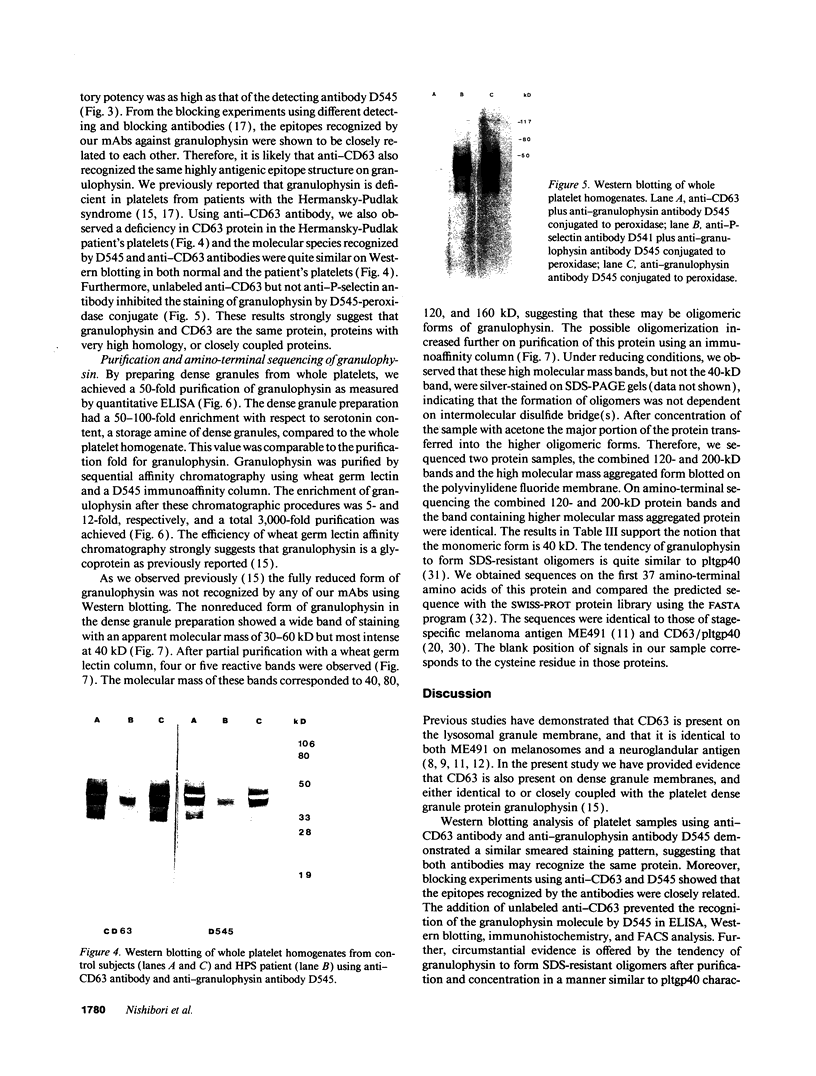
The levels and expression of the proteins CD63 and granulophysin in platelets from control and from a Hermansky-Pudlak syndrome subject (a condition characterized by dense granule and lysosomal deficiencies and the accumulation of ceroid-like material in reticuloendothelial cells) were examined. Immunofluorescence studies indicated that anti-CD63 and anti-granulophysin antibodies recognized similar numbers of granules; coapplication of antibodies did not identify more granules than the individual antibodies. Significantly fewer granules were recognized in Hermansky-Pudlak syndrome platelets than in control using either antibody. Immunoblotting studies demonstrated that anti-CD63 and anti-granulophysin antibodies apparently recognize the same protein, which was deficient in Hermansky-Pudlak platelets. Analysis by fluorescence-activated cell sorter (FACS) showed biphasic expression of CD63 and granulophysin after thrombin stimulation of control but not Hermansky-Pudlak platelets. Anti-CD63 effectively blocked detection of the protein by anti-granulophysin using immunofluorescence, ELISA, immunoblotting, and FACS analysis. Amino-terminal sequencing over the first 37 amino acids revealed that granulophysin was homologous to CD63, melanoma antigen ME491, and pltgp40. These results suggest that granulophysin and CD63 are possibly identical proteins. This is the first report of a protein present in platelet dense granules, lysosomes, and melanocytes, but deficient in a patient with Hermansky-Pudlak syndrome.
Full text
PDF
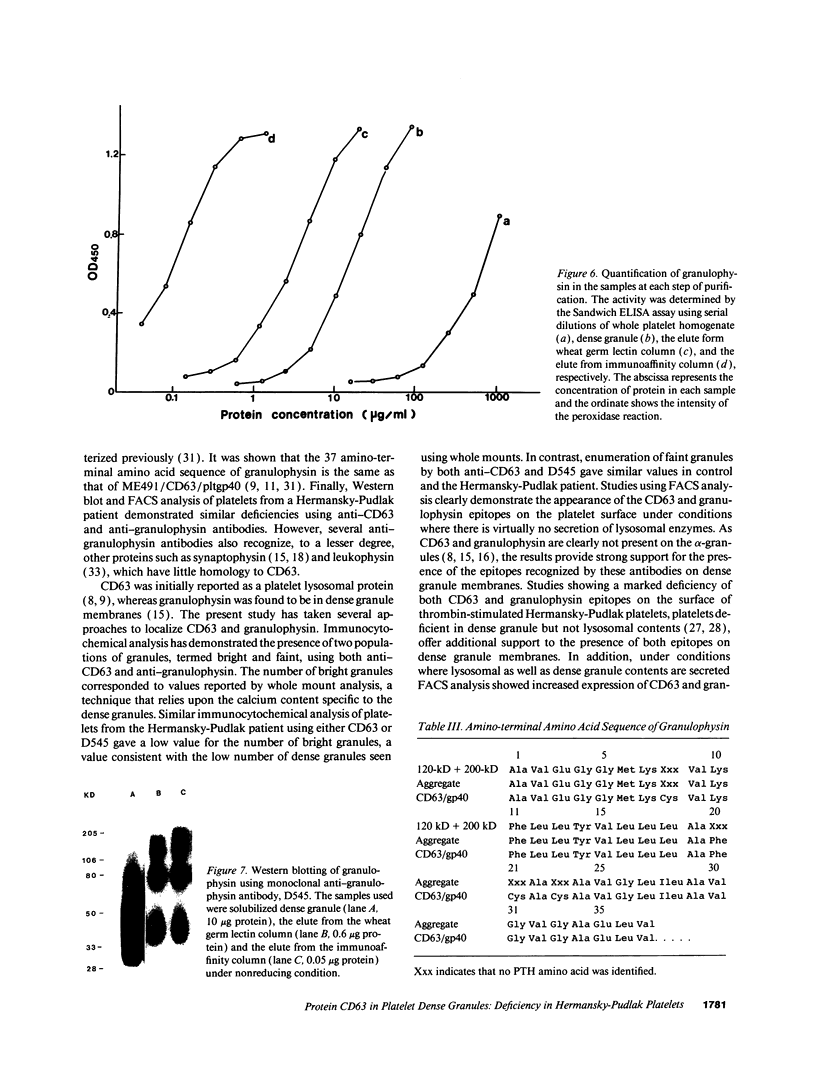

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelhaleem M. M., Hatskelzon L., Dalal B. I., Gerrard J. M., Greenberg A. H. Leukophysin: a 28-kDa granule membrane protein of leukocytes. J Immunol. 1991 Nov 1;147(9):3053–3059. [PubMed] [Google Scholar]
- Atkinson B., Ernst C. S., Ghrist B. F., Herlyn M., Blaszczyk M., Ross A. H., Herlyn D., Steplewski Z., Koprowski H. Identification of melanoma-associated antigens using fixed tissue screening of antibodies. Cancer Res. 1984 Jun;44(6):2577–2581. [PubMed] [Google Scholar]
- Azorsa D. O., Hyman J. A., Hildreth J. E. CD63/Pltgp40: a platelet activation antigen identical to the stage-specific, melanoma-associated antigen ME491. Blood. 1991 Jul 15;78(2):280–284. [PubMed] [Google Scholar]
- Brandt E. J., Elliott R. W., Swank R. T. Defective lysosomal enzyme secretion in kidneys of Chediak-Higashi (beige) mice. J Cell Biol. 1975 Dec;67(3):774–788. doi: 10.1083/jcb.67.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Demetrick D. J., Herlyn D., Tretiak M., Creasey D., Clevers H., Donoso L. A., Vennegoor C. J., Dixon W. T., Jerry L. M. ME491 melanoma-associated glycoprotein family: antigenic identity of ME491, NKI/C-3, neuroglandular antigen (NGA), and CD63 proteins. J Natl Cancer Inst. 1992 Mar 18;84(6):422–429. doi: 10.1093/jnci/84.6.422. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Beattie L. L., Park J., Israels S. J., McNicol A., Lint D., Cragoe E. J., Jr A role for protein kinase C in the membrane fusion necessary for platelet granule secretion. Blood. 1989 Nov 15;74(7):2405–2413. [PubMed] [Google Scholar]
- Gerrard J. M., Lint D., Sims P. J., Wiedmer T., Fugate R. D., McMillan E., Robertson C., Israels S. J. Identification of a platelet dense granule membrane protein that is deficient in a patient with the Hermansky-Pudlak syndrome. Blood. 1991 Jan 1;77(1):101–112. [PubMed] [Google Scholar]
- Gerrard J. M. Platelet aggregation: cellular regulation and physiologic role. Hosp Pract (Off Ed) 1988 Jan 15;23(1):89-98, 103-4, 107-8. doi: 10.1080/21548331.1988.11703404. [DOI] [PubMed] [Google Scholar]
- Hardisty R. M., Mills D. C., Ketsa-Ard K. The platelet defect associated with albumism. Br J Haematol. 1972 Dec;23(6):679–692. doi: 10.1111/j.1365-2141.1972.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Hoehn S. K., Kanfer J. N. L-Ascorbic acid and lysosomal acid hydrolase activities of guinea pig liver and brain. Can J Biochem. 1978 May;56(5):352–356. doi: 10.1139/o78-056. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- Holmsen H., Robkin L., Day H. J. Effects of antimycin A and 2-deoxyglucose on secretion in human platelets. Differential inhibition of the secretion of acid hydrolases and adenine nucleotides. Biochem J. 1979 Aug 15;182(2):413–419. doi: 10.1042/bj1820413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta H., Ross A. H., Huebner K., Isobe M., Wendeborn S., Chao M. V., Ricciardi R. P., Tsujimoto Y., Croce C. M., Koprowski H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988 Jun 1;48(11):2955–2962. [PubMed] [Google Scholar]
- Israels S. J., Gerrard J. M., Jacques Y. V., McNicol A., Cham B., Nishibori M., Bainton D. F. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140). Blood. 1992 Jul 1;80(1):143–152. [PubMed] [Google Scholar]
- Kenney D. M., Chao F. C. Microtubule inhibitors alter the secretion of beta-glucuronidase by human blood platelets: involvement of microtubules in release reaction II. J Cell Physiol. 1978 Jul;96(1):43–52. doi: 10.1002/jcp.1040960106. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metzelaar M. J., Wijngaard P. L., Peters P. J., Sixma J. J., Nieuwenhuis H. K., Clevers H. C. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991 Feb 15;266(5):3239–3245. [PubMed] [Google Scholar]
- Murayama T., Kajiyama Y., Nomura Y. Histamine-stimulated and GTP-binding proteins-mediated phospholipase A2 activation in rabbit platelets. J Biol Chem. 1990 Mar 15;265(8):4290–4295. [PubMed] [Google Scholar]
- Nieuwenhuis H. K., van Oosterhout J. J., Rozemuller E., van Iwaarden F., Sixma J. J. Studies with a monoclonal antibody against activated platelets: evidence that a secreted 53,000-molecular weight lysosome-like granule protein is exposed on the surface of activated platelets in the circulation. Blood. 1987 Sep;70(3):838–845. [PubMed] [Google Scholar]
- Novak E. K., Hui S. W., Swank R. T. Platelet storage pool deficiency in mouse pigment mutations associated with seven distinct genetic loci. Blood. 1984 Mar;63(3):536–544. [PubMed] [Google Scholar]
- Novak E. K., Hui S. W., Swank R. T. The mouse pale ear pigment mutant as a possible animal model for human platelet storage pool deficiency. Blood. 1981 Jan;57(1):38–43. [PubMed] [Google Scholar]
- Novak E. K., Swank R. T. Lysosomal dysfunctions associated with mutations at mouse pigment genes. Genetics. 1979 May;92(1):189–204. doi: 10.1093/genetics/92.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddington M., Novak E. K., Hurley E., Medda C., McGarry M. P., Swank R. T. Immature dense granules in platelets from mice with platelet storage pool disease. Blood. 1987 May;69(5):1300–1306. [PubMed] [Google Scholar]
- Rendu F., Lebret M., Nurden A. T., Caen J. P. Initial characterization of human platelet mepacrine-labelled granules isolated using a short metrizamide gradient. Br J Haematol. 1982 Oct;52(2):241–251. doi: 10.1111/j.1365-2141.1982.tb03886.x. [DOI] [PubMed] [Google Scholar]
- Rendu F., Maclouf J., Launay J. M., Boinot C., Levy-Toledano S., Tanzer J., Caen J. Hermansky-Pudlak platelets: further studies on release reaction and protein phosphorylations. Am J Hematol. 1987 Jun;25(2):165–174. doi: 10.1002/ajh.2830250206. [DOI] [PubMed] [Google Scholar]
- Shalev A., Gerrard J. M., Robertson C., Greenberg A. H., Linial M. Sharing of antigenic epitopes between synaptophysin and granulophysin. J Cell Biochem. 1992 May;49(1):59–65. doi: 10.1002/jcb.240490111. [DOI] [PubMed] [Google Scholar]
- Shalev A., Michaud G., Israels S. J., McNicol A., Singhroy S., McMillan E. M., White J. G., Witkop C. J., Nichols W. L., Greenberg A. H. Quantification of a novel dense granule protein (granulophysin) in platelets of patients with dense granule storage pool deficiency. Blood. 1992 Sep 1;80(5):1231–1237. [PubMed] [Google Scholar]
- Swank R. T., Reddington M., Howlett O., Novak E. K. Platelet storage pool deficiency associated with inherited abnormalities of the inner ear in the mouse pigment mutants muted and mocha. Blood. 1991 Oct 15;78(8):2036–2044. [PubMed] [Google Scholar]
- Weiss H. J., Chervenick P. A., Zalusky R., Factor A. A familialdefect in platelet function associated with imapired release of adenosine diphosphate. N Engl J Med. 1969 Dec 4;281(23):1264–1270. doi: 10.1056/NEJM196912042812303. [DOI] [PubMed] [Google Scholar]
- Witkop C. J., Jr Albinism. Adv Hum Genet. 1971;2:61–142. [PubMed] [Google Scholar]