Abstract
Objective
Antiretroviral drugs (ARVs) can prevent HIV mother-to-child transmission (PMTCT), but in utero ARV exposure may be associated with neurologic symptoms due to mitochondrial toxicity (MT). We sought to identify the currently recommended PMTCT regimen that optimally balances risks of pediatric HIV infection and neurologic MT.
Design
Published MTCT and MT data were used in a decision analytic model of MTCT among women in sub-Saharan Africa.
Methods
We investigated the HIV and MT risks associated with no ARV prophylaxis and five recommended regimens ranging from single-dose nevirapine to 3-drug ART. Sensitivity analyses varied all parameters, including infant feeding strategy and the disability of MT relative to HIV.
Results
Provision of no ARVs is the least effective and least toxic strategy, with 18-month HIV risk of 30.4% and MT risk of 0.2% (breastfed infants). With increasing drug number and duration, HIV risk decreases markedly (to 4.9% with 3-drug ART), but MT risk also increases (to 2.2%, also with 3-drug ART). Despite increased toxicity, 3-drug ART minimizes total adverse pediatric outcomes (HIV plus MT), unless the highest published risks are true for both HIV and MT, or the disability from MT exceeds 6.4 times that of HIV infection.
Conclusions
The risk of pediatric MT from effective PMTCT regimens is at least an order of magnitude lower than the risk of HIV infection associated with less effective regimens. Concern regarding MT should not currently limit the use of 3-drug ART for PMTCT where it is available.
Keywords: HIV/AIDS, PMTCT, mitochondrial toxicity, pediatric HIV, antiretroviral therapy, sub-Saharan Africa, decision analysis
Introduction
The use of antiretroviral drugs (ARVs) to prevent mother-to-child transmission of HIV infection (PMTCT) is one of the most successful achievements in HIV prevention. Following the Pediatric AIDS Clinical Trial Group (PACTG) Study 076 in 1994[1], zidovudine (ZDV) monotherapy was widely used for PMTCT, where available [2]. Subsequent trials have investigated antepartum, intrapartum, and postpartum regimens combining ZDV, lamivudine (3TC), nevirapine (NVP), and protease inhibitors (PIs) [3-16]. MTCT rates have been reduced from more than 25% [1, 17] to less than 2% [4, 8, 18] when potent 3-drug antiretroviral therapy (ART) is available and breastfeeding is avoided, and recently reported interventions have reduced transmission to breastfed infants from over 40% (at 24 months of age) [3, 17] to 1-7% (at 6-18 months) [19-26].
Most regimens have demonstrated favorable safety profiles for both mothers and infants during the duration of trial follow-up [3, 8, 10, 16, 27]. In 1999, however, French perinatologists reported 8 cases of severe cognitive and neurological dysfunction among HIV-negative children after in utero ARV exposure [28]. These neurologic symptoms, including hypotonia, seizures, encephalopathy, and neuropathy, were similar to both congenital mitochondrial dysfunction in children and ARV-induced mitochondrial toxicity (MT) in HIV-infected children and adults [28]. Since this first report, other studies of the effects of in utero ARV exposure on mitochondrial function in HIV-negative children have differed in methodologies and conclusions [29-41].
Clinicians and policy makers currently recommend PMTCT regimens in the absence of complete data about both the prevalence and severity of mitochondrial toxicity. Although more intensive ARV regimens substantially reduce MTCT among women with low CD4 counts, the magnitude of MTCT reduction with 3-drug ART compared to shorter-course ARV regimens among women with CD4 >200/μL is less well known [11, 22, 26]. It is also unknown whether exposure to more ARVs for longer durations, or to specific drugs during particular periods of fetal development, causes higher MT risk. Because MT related to nucleoside reverse transcriptase inhibitors (NRTIs) may be rare and remains controversial, the challenge of identifying the optimal ARV regimen to balance efficacy (reduction in MTCT) with toxicity (pediatric neurologic MT) is well-suited for assessment by decision analysis [42]. We used existing data in a decision analytic model to quantify the effects of recommended PMTCT regimens on HIV transmission and neurologic MT at 18 months of age for infants in sub-Saharan Africa and to determine the MT risk that would warrant a change in current PMTCT recommendations.
Methods
We designed a decision analytic model of pregnant, HIV-infected, ART-naïve women in sub-Saharan Africa not meeting 2006 World Health Organization (WHO) criteria for initiation of ART for their own HIV infection (CD4 > 200/μL and no history of AIDS) [43]. Using published MTCT and neurologic MT risks, we examined the pediatric outcomes at 18 months of age associated with no ARV prophylaxis and with five different recommended PMTCT strategies (Table 1) [43, 44].
Table 1.
PMTCT strategies examined in a decision model for sub-Saharan Africa
| Strategy | Prenatal | Intrapartum | Postpartum (to mother) | Neonatal |
|---|---|---|---|---|
| No ARVs | None | None | None | None |
| sdNVP | None | sdNVP | None | sdNVP |
| sdNVP/CBV | None | sdNVP + ZDV/3TC | ZDV/3TC × 7d | sdNVP |
| scZDV/sdNVP | ZDV from 28w | sdNVP | None | ZDV × 7d + sdNVP |
| scZDV/sdNVP/CBV | ZDV from 28w | sdNVP + ZDV/3TC | ZDV/3TC × 7d | ZDV × 7d + sdNVP |
| PI-based 3-drug ART | ZDV/3TC/PI from 8-12w | ZDV/3TC/PI | None | ZDV × 6 w |
Abbreviations: PMTCT: prevention of mother-to-child transmission of HIV; ARVs: antiretroviral drugs; sdNVP: single-dose nevirapine; ZDV: zidovudine; 3TC: lamivudine; CBV: “Combivir tail” (7 days of maternal ZDV/3TC), sc: short-course (from 28 weeks of gestation); PI: protease inhibitor; 28w: 28 weeks of gestational age; 8-12w: between 8 and 12 weeks of gestational age; 7d: 7 days; 6w: 6 weeks.
Modeled PMTCT Strategies
For women in resource-limited settings not meeting criteria for ART themselves, WHO recommends four increasingly intensive PMTCT regimens, depending on resource availability [43]. Table 1 outlines the antenatal, intrapartum, and postnatal components of these regimens. We evaluated all WHO-recommended strategies, as well as no ARVs (for the purpose of comparison) and PI-based 3-drug ART, as recommended in the US [44] (Table 1).
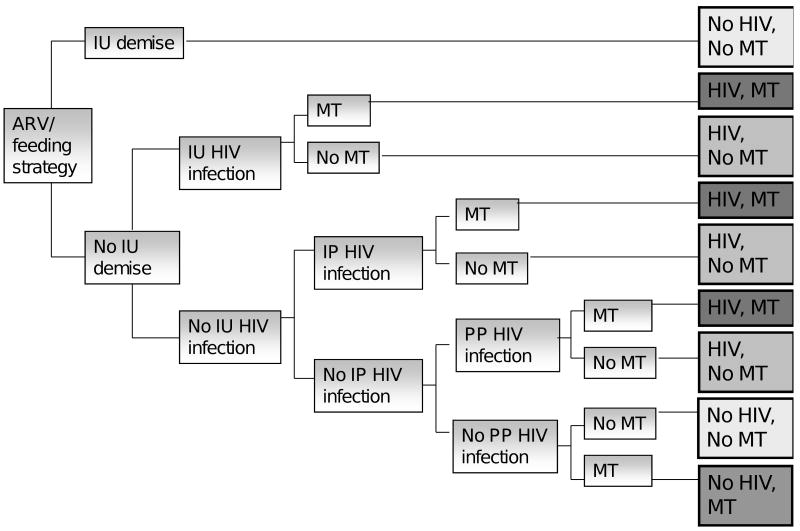
The base case analysis was performed for breastfeeding mothers without access to elective cesarean section. The effects of formula feeding and of the availability and effectiveness of elective cesarean section on reduction in MTCT were evaluated in sensitivity analyses. Model structure is shown in Figure 1. Additional details are provided in the Technical Appendix.
Figure 1.
Women who did not meet WHO criteria for initiation of ART for their own HIV infections were considered eligible for each ARV and feeding strategy. During the period from 10 weeks of gestation through 18 months postpartum, each of the depicted events occurred based on probabilities derived from the published literature: intrauterine (IU) demise or HIV infection, intrapartum or early postpartum (IP) HIV infection, postpartum (PP) HIV infection, or neurologic mitochondrial toxicity (MT), resulting in the outcomes depicted at the end of each path.
Outcomes/Case definitions
Neurologic MT was defined according to the Enquête Périnatale Française (EPF) clinical case definition, excluding any requirement for radiographic, biochemical, or histological findings [32] (Technical Appendix). Intrauterine, intrapartum, and postpartum HIV infections were defined according to the timing of first positive infant virologic test (HIV-1 DNA, RNA, or culture), as typically defined for PMTCT trials [7, 12, 26]. The standard trial definition of intrapartum infection (first positive virologic test between 3 days and 4-8 weeks of age) reflects the limited sensitivity of these assays in the early weeks of life due to delayed viremia [45], but necessitates the inclusion of early postpartum transmission due to breastfeeding in the “intrapartum” category.
Model Parameters
HIV transmission risk
In the base case analysis, risks of HIV transmission for each ARV regimen were derived from publications or presentations meeting the following inclusion criteria: clinical trial of a modeled regimen, conducted in Africa, ≥ 12 months follow-up, and reporting probabilities of HIV infection at each time point among infants who were HIV-negative at the prior time point (or data that permitted these calculations). Clinical trial-based data were used to inform all analyses. With one exception [23] (Technical Appendix), observational reports were excluded, because higher rates of loss to follow-up might bias results. Studies meeting most, but not all, inclusion criteria were included in ranges examined in sensitivity analyses (Technical Appendix Table 1).
For each HIV transmission time point and ARV strategy, the base case transmission risk was derived from the most widely accepted or cited estimate (based on expert opinion), aiming for the midpoint of the range of reported transmission risks (Table 2). PMTCT data were used to calculate the probability of infection during the intrauterine, intrapartum/early postpartum, and late postpartum periods among children who were uninfected at the previous time point.
Table 2.
Selected input parameters for a decision model of PMTCT strategies in sub-Saharan Africa
| Parameter | Base case value for each regimen | |||||
|---|---|---|---|---|---|---|
| (range for sensitivity analysis, breastfed infants)a | ||||||
| No ARVs | sdNVP | sdNVP/CBV | scZDV/sdNVP | scZDV/sdNVP/CBV | 3-drug ART | |
| Probability of intrauterine demise | 0.0049[1] | |||||
| (0 – 1.64%)[7, 8, 11, 14, 15, 17, 88] | ||||||
| Probability of intrauterine HIV infection | 0.07[7, 15, 17, 19, 46, 89] | 0.07[7, 15, 17, 19, 46, 89] | 0.07[7, 15, 17, 19, 46, 89] | 0.038 [26] | 0.038 [26] | 0.0143 [25] |
| (0.031-0.095) [1, 6, 14, 49, 90-93] | (0.031-0.095) [1, 6, 14, 49, 90-93] | (0.031-0.095) [1, 6, 14, 49, 90-93] | (0.01-0.0389) [3, 8, 11] | (0.01-0.0389) [3, 8, 11] | (0.004-0.024) [4, 12, 22-24, 26] | |
| Probability of intrapartum/early postpartum HIV infection | 0.139 [17] | 0.046 [15] | 0.0376 | 0.0259 [11] | 0.0234 | 0.0154 [22] |
| (0.0903-0.1903) [1, 3, 5, 14, 46-48, 90] | (0.0392 - 0.120) [7, 19-22, 49, 93] | (0.0376-0.0564) [6, 95],b | (0.0059 - 0.037) [11, 26] | (0.003 – 0.037) [6],b | (0.009-0.0177) [24-26, 73, 94] | |
| Probability of postpartum HIV infection | 0.0133 [47] | 0.089 [49] | 0.085 | 0.045 [26] | 0.04275 | 0.0208 [22] |
| (0.0718 – 0.1823) [3, 5, 17, 46, 48] | (0.0442-0.0947) [15, 27, 61] [19, 89] | (0.0249 – 0.0947) [95],b | (0.045-0.0737) [26, 61] | (0.03375 – 0.0737) [26, 95],b | (0.009-0.0737) [15, 24-26, 61, 73, 90, 94] | |
| Background probability of pediatric neurologic MT related to maternal HIV | 0.0017 [32] | |||||
| (0.0001-0.0289)[39, 52, 53] | ||||||
| Additional MT above background | 0 b | 0 b | 0 | 0.0188 [39] | 0.0188 [39] | 0.0204 [39] |
| (0 - 0.00006) b | (0 - 0.0290) [39, 40] | (0 - 0.0676) [39, 40] | (0 - 0.0676) [39, 40] | |||
| Reduction in background risk of MT due to reduction in maternal viremia | 0% b | 0% b | 0% b | 0% b | 0% | 0% |
| (0-50%) b | (0-75%) b | |||||
Parallel table provided in technical appendix lists base case values and ranges for sensitivity analysis for formula-fed infants
Parallel table provided in technical appendix provides details of all assumptions used in model
Abbreviations: ARV: antiretroviral drugs; ART: antiretroviral therapy; sdNVP: single-dose nevirapine; MT: mitochondrial toxicity; ZDV: zidovudine; 3TC: lamivudine, CBV: “Combivir” tail (ZDV/3TC)
MTCT risks were assigned to either breastfed or formula-fed strategies according to the predominant feeding practice in each trial. For each breastfed strategy, the median duration of breastfeeding was assumed to be equal to that of the trial population (range for base case analysis, 9 - >20 months) [3, 17, 27, 46-49]. We simulated extended breastfeeding in order to 1) generate 18-month results applicable to African populations in which this practice is common, and 2) conservatively estimate the benefits of peripartum ARVs, the protective effects of which are likely to fade with prolonged breastfeeding [27].
Neurologic MT risk
Because an independent risk of fetal mitochondrial dysfunction has been postulated to result from maternal HIV viremia [39, 50], the base case analysis derived the risk of mitochondrial dysfunction among HIV-exposed but ARV-unexposed children from the upper confidence limit reported in the EPF cohort: 0 of 1748 ARV-unexposed children, 95% CI (0, 0.17%) [32, 51]. Sensitivity analyses evaluated MT risks ranging from the general population risk (0.01%) [52, 53] to 2.9%, as reported among ARV-exposed children in PACTG 219/219C (2.9%, 95% CI (0.6%-8.4%)) [39] (Table 2).
The risks of MT among HIV- and ARV-exposed children were derived from two studies of living, uninfected children incorporating routine neuropsychiatric assessment [32, 39]. The base case analysis made use of the MT risks associated with exposure to ZDV (1.88%) and ZDV/3TC (2.04%) at any time during pregnancy in the PACTG 219/219C report [39] (Table 2). Sensitivity analyses incorporated the range of risks reported in these two studies: the overall MT risk was 0.26% [32]- 1.8% [39]; when stratified by time of earliest exposure to specific NRTIs, MT risks in the PACTG 219/219C cohort ranged from 0.4% (first exposure to 3TC in 2nd trimester) to 6.9% (first exposure to 3TC in 3rd trimester) [39].
Assumptions
When data to inform HIV transmission and neurologic MT risks were incomplete for any PMTCT strategy, we assumed differences in transmission or toxicity risks compared to the most similar strategy, based on individual components of each regimen (Table 2; Technical Appendix). In the base case analysis, because MTCT risks among women with CD4 > 200/μL were rarely available [22], we relied on transmission risks from all women participating in the included PMTCT trials (participants reported with CD4 < 200/μL: range 5-24%, mean 12.9%). We then used the lowest published transmission risks to create a “best case” scenario for HIV risk which may better reflect MTCT from women with less advanced disease. Adherence to ARV and feeding strategies was assumed to be equal to that in the trials (Table 2).
Sensitivity analyses
Sensitivity analyses were performed on all model parameters and assumptions, feeding strategy, the availability of elective cesarean section, and all uncertain input parameters (Table 2; Technical Appendix). In order to determine the prevalence of MT which would change current practice, we varied the risks of neurologic MT associated with each ARV strategy, with individual components of each strategy, and with maternal HIV viremia over published and clinically plausible ranges (Table 2). The highest and lowest published risks of HIV transmission and MT were used to create “best case” and “worst case” scenarios for each outcome. The disability of MT relative to pediatric HIV infection was also evaluated in sensitivity analyses.
Results
Base case analysis
Among breastfeeding women, provision of no ARVs is the least effective and least toxic PMTCT strategy (HIV transmission risk of 30.4% and MT risk of 0.2%, at 18 months of age). With increasing number and duration of ARVs, the 18-month HIV transmission risk declines markedly (to 4.9% with 3-drug ART). When antepartum NRTIs are used, MT risks rise from 0.2% (no ARVs; sdNVP) to 2.0% (both scZDV regimens) and 2.2% (3-drug ART). Despite this increased toxicity, 3-drug ART minimizes total adverse events, defined as the sum of HIV infections and MT cases (Table 3, Section I).
Table 3.
Model results: prevalence of pediatric HIV infection and neurologic mitochondrial toxicity at 18 months of age associated with each ARV strategy, under base case and “worst-case” scenarios*
| I. Primary results (base case estimates) for HIV and neurologic MT risk | ||||||
|---|---|---|---|---|---|---|
| Breastfed Infants (%) | Formula-fed infants (%) | |||||
| HIV | MT | Totalb | HIV | MT | Totalb | |
| No ARVs | 30.4 | 0.2 | 30.6 | 17.4 | 0.2 | 17.5 |
| sdNVP | 19.1 | 0.2 | 19.2 | 10.4 | 0.2 | 10.5 |
| sdNVP/CBV | 18.0 | 0.2 | 18.1 | 9.8 | 0.2 | 10.0 |
| scZDV/sdNVP | 10.5 | 2.0 | 12.5 | 4.3 | 2.0 | 6.4 |
| scZDV/sdNVP/CBV | 10.0 | 2.0 | 12.1 | 4.3 | 2.0 | 6.4 |
| 3-drug ART | 4.9 | 2.2 | 7.1 | 2.3 | 2.2 | 4.5 |
| II. “Worst-case” results (highest published estimates) for both HIV and neurologic MT riska | ||||||
| Breastfed Infants (%) | Formula-fed infants (%) | |||||
| HIV | MT | Totalb | HIV | MT | Totalb | |
| No ARVs | 39.9 | 2.9 | 42.8 | 29.9 | 2.9 | 32.8 |
| sdNVP | 27.8 | 2.9 | 30.7 | 14.5 | 2.9 | 17.4 |
| sdNVP/CBV | 23.7 | 2.9 | 26.6 | 12.9 | 2.9 | 15.8 |
| scZDV/sdNVP | 14.2 | 5.8 | 20.0 | 5.9 | 5.8 | 11.7 |
| scZDV/sdNVP/CBV | 13.5 | 5.8 | 19.3 | 5.8 | 5.8 | 11.6 |
| 3-drug ART | 11.1 | 9.6 | 20.7 | 4.0 | 9.6 | 13.6 |
| III. “Worst-case results” (highest published estimates) for neurologic MT risk; base case estimates for HIVa | ||||||
| Breastfed Infants (%) | Formula-fed infants (%) | |||||
| HIV | MT | Totalb | HIV | MT | Totalb | |
| No ARVs | 30.4 | 2.9 | 33.3 | 17.4 | 2.9 | 20.3 |
| sdNVP | 19.1 | 2.9 | 22.0 | 10.4 | 2.9 | 13.2 |
| sdNVP/CBV | 18.0 | 2.9 | 20.9 | 9.8 | 2.9 | 12.7 |
| scZDV/sdNVP | 10.5 | 5.8 | 16.2 | 4.3 | 5.8 | 10.1 |
| scZDV/sdNVP/CBV | 10.0 | 5.8 | 15.8 | 4.3 | 5.8 | 10.1 |
| 3-drug ART | 4.9 | 9.6 | 14.5 | 2.3 | 9.6 | 11.9 |
| IV. “Best-case results” (lowest published estimates) for HIV risk; base case estimates for neurologic MT | ||||||
| Breastfed Infants (%) | Formula-fed infants (%) | |||||
| HIV | MT | Totalb | HIV | MT | Totalb | |
| No ARVs | 18.1 | 0.2 | 18.3 | 9.7 | 0.2 | 9.8 |
| sdNVP | 11.0 | 0.2 | 11.1 | 6.0 | 0.2 | 6.1 |
| sdNVP/CBV | 9.8 | 0.2 | 10.0 | 4.9 | 0.2 | 5.1 |
| scZDV/sdNVP | 6.0 | 2.0 | 8.0 | 1.6 | 2.0 | 3.6 |
| scZDV/sdNVP/CBV | 4.6 | 2.0 | 6.7 | 1.4 | 2.0 | 3.5 |
| 3-drug ART | 1.3 | 2.2 | 3.5 | 0.9 | 2.2 | 3.1 |
| V. “Best-case results” (lowest published estimates) for HIV risk and “worst-case results” (highest published estimates) for neurologic MT risk | ||||||
| Breastfed Infants (%) | Formula-fed infants (%) | |||||
| HIV | MT | Totalb | HIV | MT | Totalb | |
| No ARVs | 18.1 | 2.9 | 21.0 | 9.7 | 2.9 | 12.6 |
| sdNVP | 11.0 | 2.9 | 13.9 | 6.0 | 2.9 | 8.9 |
| sdNVP/CBV | 9.8 | 2.9 | 12.7 | 4.9 | 2.9 | 7.8 |
| scZDV/sdNVP | 6.0 | 5.8 | 11.8 | 1.6 | 5.8 | 7.4 |
| scZDV/sdNVP/CBV | 4.6 | 5.8 | 10.4 | 1.4 | 5.8 | 7.2 |
| 3-drug ART | 1.3 | 9.6 | 10.9 | 0.9 | 9.6 | 10.5 |
Strategies that minimize overall adverse events (HIV and MT) are shown in boldface type.
Results of analysis using highest published rates for HIV only are provided in technical appendix.
Totals may not appear to equal exact sums, due to rounding of HIV and MT risks.
Abbreviations: ARV: antiretroviral drugs; ART: antiretroviral therapy; sdNVP: single-dose nevirapine; scZDV: short-course zidovudine; 3TC: lamivudine, CBV: “Combivir” tail (ZDV/3TC)
Using population-level estimates, treating 10,000 breastfeeding mothers with the scZDV/sdNVP/CBV regimen (the next most effective and next least toxic regimen) compared to 3-drug ART would prevent 15 cases of MT, but would allow 507 additional HIV infections. To substantially reduce MT compared to 3-drug ART (0.2% vs. 2.2%), one would choose the sdNVP/CBV regimen; this choice would prevent 202 cases of MT in the same population, but 1303 additional HIV infections would occur.
Sensitivity analyses
Formula feeding
When formula-fed infants are evaluated using other base case parameters, 3-drug ART remains the strategy that minimizes total adverse events (Table 3, Section I).
Worst- and best-case scenarios
We used the highest published risks of HIV transmission and MT associated with each PMTCT regimen to create a “worst-case” (highest toxicity and lowest efficacy) scenario. In contrast to the base case, if the “worst-case” risks are simultaneously true for both MT and HIV, then both scZDV regimens minimize total adverse outcomes compared to 3-drug ART in breast- and formula-fed infants (Table 3, Section II). When the “worst-case” estimates are used only for MT risk (Table 3, Section III), the order of strategies is identical to that in the base case for breastfed infants, but both scZDV regimens are superior to 3-drug ART in formula-fed infants. When the “worst-case” estimates are used only for HIV risk, the order of the strategies is unchanged from the base case, regardless of feeding strategy (Technical Appendix).
When “best case” HIV risks, which may better reflect MTCT risks from mothers with CD4 > 200/μL, are combined with base case MT risks, the order of strategies is also unchanged from the base case (Table 3, Section IV). However, when “best case” HIV risks and “worst case” MT risks are simultaneously examined (Table 3, Section V), the less intensive regimens (scZDV/sdNVP/CBV in breastfed infants, and all ARV regimens in formula-fed infants) minimize total adverse outcomes, compared to 3-drug ART (Technical Appendix).
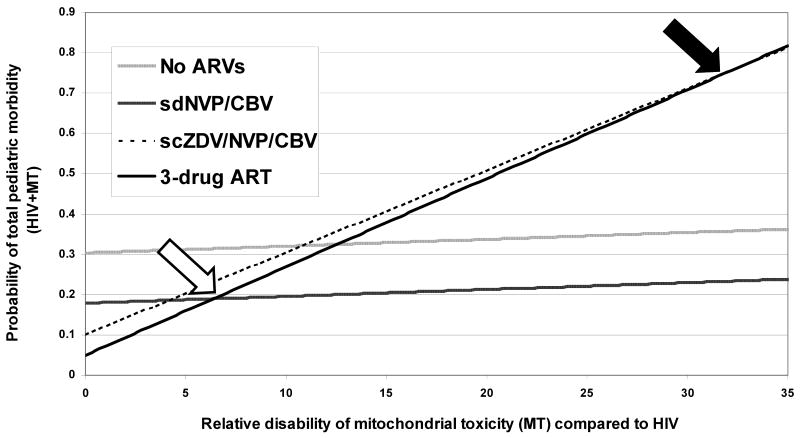
Relative disability of MT and HIV
We varied the degree of disability associated with neurologic MT as a function of the disability associated with pediatric HIV (Figure 2) and compared the total adverse pediatric outcomes (HIV and MT) associated with each strategy. Using the base case input parameters, we first compared 3-drug ART to the most effective regimen that excludes an NRTI, confering a substantial decrease in toxicity (sdNVP/CBV). In breastfed infants, the morbidity of MT would have to exceed 6.4 times the morbidity of pediatric HIV infection to recommend the sdNVP/CBV regimen over 3-drug ART (open arrow). We then compared 3-drug ART to the next most effective and next least toxic regimen (scZDV/sdNVP/CBV). Here, the threshold is higher, because the number of excess cases of HIV that occur when the scZDV/sdNVP/CBV regimen is substituted for 3-drug ART greatly exceeds the number of MT cases prevented: the morbidity of MT would need to exceed 32.1 times that of HIV in breastfed infants in order to recommend the scZDV/sdNVP/CBV regimen over 3-drug ART (closed arrow).
Figure 2.
Effect of varying the relative disability of pediatric neurologic MT compared to pediatric HIV infection on total adverse pediatric outcomes (HIV plus MT): base case analysis. On the horizontal axis is the relative disability of MT compared to HIV infection (MT ranging from 0 – 35 times more disabling than HIV). On the vertical axis is the probability of total pediatric morbidity (HIV plus MT). Thresholds are highlighted at which the sdNVP/CBV regimen (open arrow) and the scZDV/sdNVP/CBV regimen (closed arrow) minimize total morbidity compared to 3-drug ART.
Discussion
The efficacy of ARVs in the prevention of MTCT of HIV is widely accepted[54]. Two studies report an association between in utero ARV exposure and infant neurologic dysfunction possibly related to MT [32, 39], but the true prevalence and severity of this postulated MT remain controversial [55, 56]. Motivated by the possibility that the association between ARV exposure and MT is causal [39], we conducted an exploratory analysis in order to determine the prevalence and severity of MT at which current ARV recommendations for PMTCT would merit change [43, 44].
This decision analytic model assesses the 18-month risks for pediatric HIV transmission and neurologic MT, following the administration of six different PMTCT regimens to pregnant, HIV-infected women in sub-Saharan Africa. Model results reflect the published efficacy of ARVs for PMTCT; base case estimates of HIV transmission at 18 months in breastfed infants range from 4.9% with 3-drug ART to 30.4% with no ARVs. These results calibrate with results from observational studies [57, 58] and with syntheses of PMTCT trials in sub-Saharan Africa [3, 18, 59, 60], including 40-50% (relative) [59, 61] and 14-15% (absolute) [59, 62, 63] increases in MTCT risk due to breastfeeding. Because MT has primarily been attributed to in utero NRTI exposure, the three least effective PMTCT regimens, all of which exclude antepartum NRTIs, demonstrate low toxicity risks. Overall, 3-drug ART initiated in the first trimester results in many fewer pediatric HIV infections, slightly more cases of pediatric neurologic MT, and substantially fewer total adverse pediatric outcomes (HIV infections and MT cases) than the less toxic but less effective regimens.
Published studies of ARV-associated MT have differed in methodology and MT case definition, which may explain inconsistent findings [2, 5, 29-38, 40, 64-69]. In addition, most studies have not controlled for maternal substance abuse and socioeconomic factors [68], high maternal viral load [50], and maternal disease stage [39], all of which have been hypothesized to cause infant mitochondrial dysfunction and adverse neurologic outcomes [39, 50, 68]. We therefore chose data from the only two studies using routine neuropsychiatric evaluations of living, HIV-uninfected children [32, 39]. Brogly et al. demonstrated a significantly higher risk of MT when NRTIs (ZDV, 3TC, or both) were initiated in the third trimester than in the first trimester [39]. They postulate a period of neurodevelopment late in gestation in which the fetal brain is uniquely sensitive to NRTI-induced MT [70-72]. The authors were unable to control for high maternal RNA at delivery (likely a result of late ARV initiation) and maternal drug use (a potential cause of late ARV initiation, although not associated with MT in this study). Because these factors may have led to the overestimation of MT risk from 3rd-trimester NRTI initiation, our base case analysis conservatively relied on the MT risk associated with any ZDV, 3TC, or ZDV/3TC exposure, regardless of timing (1.88% - 2.04%).
Our results demonstrate that at MT prevalences lower than the base case risks (as in the EPF, range 0.26-0.87%) [32], 3-drug ART would still minimize total adverse outcomes. More importantly, these results remain true at MT prevalences higher than the base case scenario. Our “worst-case” MT scenario used data from subgroups with 3rd-trimester initiation of ZDV, 3TC, or ZDV/3TC in PACTG 219/219C; results suggest that even if these “worst-case” MT estimates were correct, a change in recommended ARVs for PMTCT would be warranted only if the very highest or lowest published HIV risks associated with each regimen were also true. HIV transmission risks at 18 months in the “best-case” and “worst-case” scenarios are well outside commonly reported ranges [1, 8, 18, 23, 26, 59, 73].
Currently reported prevalences of MT are therefore unlikely to change PMTCT recommendations. However, little is known about the morbidity and mortality of ARV-associated neurologic MT [32, 39, 40, 55], and prognostic information must be extrapolated from reports of congenital mitochondrial dysfunction [52, 53, 74, 75]. As new data specific to ARV-associated MT emerge [4, 32, 39, 40, 55, 76], a primary factor in the choice of PMTCT regimen will be the relative disability of MT compared to that of pediatric HIV infection. Pediatric HIV disease substantially reduces life expectancy in sub-Saharan Africa, even when therapy is available [77-80], and may itself be associated with significant neurodevelopmental delay [81]. If pediatric HIV infection consistently causes greater morbidity and mortality than MT, then the balance of risk and benefit will always favor more effective regimens for PMTCT. If, however, MT is markedly more disabling than pediatric HIV (for example, if effective therapies for pediatric HIV become widely available), then policy makers may choose PMTCT strategies that permit more cases of HIV infection in order to avoid MT in uninfected children. The combined outcome of HIV infections plus MT cases allowed us to estimate that, at base-case MT risks, neurologic MT will need to be at least 6.4 times more disabling than HIV infection in order to prompt a change in current PMTCT recommendations.
Of note, this model did not examine maternal outcomes. Emerging data suggest a benefit to ART initiation at CD4 > 200/μL, as reflected in recent changes to US treatment guidelines [82]. Therefore, women included in our model are likely to benefit from 3-drug ART during pregnancy, and the effects on maternal health of withdrawing ART after use for PMTCT remain unknown [44, 83, 84]. Maternal drug-resistant HIV resulting from single- or dual-drug PMTCT regimens may also result in reduced efficacy of ART when it is eventually initiated [85]. These maternal effects may tip the balance of risk and benefit in favor of 3-drug ART when both maternal and pediatric outcomes are considered. Additionally, the model did not incorporate the costs of each PMTCT regimen or of clinical care for HIV- or MT-affected children after birth. In settings with severely constrained health care resources, concerns for costs may outweigh concerns for toxicity in the selection of ARV regimens for PMTCT.
This analysis required several simplifying assumptions. First, data are limited on late postpartum transmission rates by actual infant feeding practices [7, 17, 58]. Second, the model did not account for the neurodevelopmental effects of maternal age, preterm delivery, or stage of maternal HIV disease, which may affect pediatric neurologic outcomes [86]. Finally, women with CD4 < 200/μL merit 3-drug ART for their own HIV infections as well as for PMTCT[43], and therefore were intentionally excluded from the model. Because MTCT data were not limited to women with high CD4 counts, the base case analysis likely overestimates transmission risks for women not requiring ART themselves [19, 87].
The “best-case” scenario, in which the lowest published HIV transmission risks were attributed to each regimen, may more closely approximate true MTCT risks from women with less advanced disease. The results of the “best case” HIV scenario were unchanged from the base case, except when the highest published MT risks for 3-drug ART were simultaneously considered. For breastfed infants in this “best-case HIV/worst-case MT” scenario, scZDV/sdNVP/CBV was superior to 3-drug ART, due primarily to the high MT risk assigned to 3rd-trimester 3TC exposure. For formula-fed infants, all ARV regimens were superior to 3-drug ART, due to very low HIV risks assigned to less intensive regimens. The small differences in total adverse outcomes between strategies suggest that further studies are required to investigate 1) whether scZDV regimens are effective among women with CD4 > 200/μL, and 2) whether MT risks with 3-drug ART approach those observed in select subgroups of PACTG 219/219C [39]. If such data emerge and are simultaneously true, short-course regimens may be appropriate alternatives to 3-drug ART in women with high CD4 counts, especially when formula-feeding is feasible.
In resource-limited settings, concerns for toxicity, as well as for cost, may influence the selection of less effective ARV regimens for PMTCT than are recommended in developed nations [43]. Currently available data suggest that total pediatric adverse outcomes (HIV infections and cases of neurologic mitochondrial toxicity) are minimized by the use of PI-based 3-drug ART for PMTCT. Less effective ARV regimens would only be substantially superior to 3-drug ART if the very highest or lowest published risks of HIV, as well as the highest published risks of MT, associated with each strategy were simultaneously true, or if ARV-related mitochondrial toxicity were markedly more disabling than pediatric HIV infection. Access to diagnosis, prenatal care, and ARVs for HIV-infected women in resource-limited settings remain crucial to reducing the more than 500,000 perinatal infections that occur worldwide each year, and every effort should be made to provide 3-drug ART to women who require therapy for their own health [54]. For women with less advanced HIV disease, nucleoside-sparing PMTCT regimens, or regimens that avoid combination nucleosides, may warrant further investigation. In the meantime, currently reported risks of mitochondrial toxicity should not lead providers or patients to avoid the use of 3-drug ART during pregnancy for PMTCT, and efforts should be expanded to increase the availability of 3-drug ART for PMTCT in resource-limited settings.
Acknowledgments
The authors would like to acknowledge Jennifer Chu, BS, and Brandon Morris, BA, for assistance with manuscript preparation. Funding for this work was provided by the National Institute of Allergy and Infectious Disease (T32 AI07433 (Ciaranello; PI: Freedberg); R01 AI058736 and R37 AI42006-10A1 (Freedberg, Weinstein, Walensky); and U01 AI 069456-01 (Lockman)); the National Institute of Child Health and Human Development (Cooperative Agreement U01 HD052102-03 (Seage); R01 HD044391 (Lockman)); and the Doris Duke Charitable Foundation (Clinical Scientist Development Award (Walensky)). The authors report the following potential conflicts of interest: Andrea L. Ciaranello, no conflict; George R. Seage III, no conflict; Kenneth A. Freedberg, no conflict; Milton C. Weinstein, no conflict; Shahin Lockman, no conflict; Rochelle P. Walensky, no conflict.
Footnotes
This work was presented in poster form at the May, 2007 Harvard University Center for AIDS Research Pediatric HIV Symposium (Boston, Massachusetts), and at the October, 2007 Annual Meeting of the Society for Medical Decision Making (Pittsburgh, Pennsylvania).
Author contributions include formulation of the research question (Ciaranello, Freedberg, Lockman, Seage, Walenksy), design of the simulation model and analytic plan (Ciaranello, Freedberg, Weinstein, Walenksy), selection of the input data for simulation model (Ciaranello, Lockman, Walensky), and preparation (Ciaranello) and critical editing (Freedberg, Lockman, Seage, Weinstein, Walensky) of the manuscript.
References
- 1.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Do minguez K, Bertolli J, Fowler M, Peters V, Ortiz I, Melville S, et al. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children < or = 5 years of age, Pediatric Spectrum of HIV Disease project (PSD), USA. Ann N Y Acad Sci. 2000;918:236–246. doi: 10.1111/j.1749-6632.2000.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 3.Leroy V, Karon JM, Alioum A, Ekpini ER, Meda N, Greenberg AE, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–641. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 4.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind placebo-controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre JA, M N, Gray GE, Hopley M, Kimura T, Robinson P, Mayers D. Abstract no TuFo0204: Addition of short course Combivir (CBV) to single dose Viramune (sdNVP) for the prevention of mother to child transmission (pMTCT) of HIV-1 can significantly decrease the subsequent development of maternal and paediatric NNRTI-resistant virus. International AIDS Society. 2005 Rio de Janeiro. [Google Scholar]
- 7.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 8.Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 9.Lallemant M, Jourdain G, Le Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med. 2000;343:982–991. doi: 10.1056/NEJM200010053431401. [DOI] [PubMed] [Google Scholar]
- 10.Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, Berrebi A, Benifla JL, Burgard M, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285:2083–2093. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]
- 11.Dabis F, Bequet L, Ekouevi DK, Viho I, Rouet F, Horo A, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–318. [PMC free article] [PubMed] [Google Scholar]
- 12.Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288:189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Thistle P, Gottesman M, Pilon R, Glazier RH, Arbess G, Phillips E, et al. A randomized control trial of an Ultra-Short zidovudine regimen in the prevention of perinatal HIV transmission in rural Zimbabwe. Cent Afr J Med. 2004;50:79–84. [PubMed] [Google Scholar]
- 14.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 15.Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 16.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292:202–209. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]
- 17.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 18.Leroy V, Sakarovitch C, Cortina-Borja M, McIntyre J, Coovadia H, Dabis F, et al. Is there a difference in the efficacy of peripartum antiretroviral regimens in reducing mother-to-child transmission of HIV in Africa? AIDS. 2005;19:1865–1875. doi: 10.1097/01.aids.0000188423.02786.55. [DOI] [PubMed] [Google Scholar]
- 19.Kumwenda NI, Hoover DR, Mofenson LM, Thigpen MC, Kafulafula G, Li Q, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 20.Sastry J, The Six Week Extended Dose Nevirapine (SWEN) Study Team Abstract 43: Extended-dose nevirapine to 6 Weeks of age for infants in Ethiopia, India, and Uganda: A randomized trial for prevention of HIV transmission through breastfeeding. Conference on Retroviruses and Opportunistic Infections; Boston. 2008. [Google Scholar]
- 21.Bedri A, Gudetta B, Isehak A, Kumbi S, Lulseged S, Mengistu Y, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 22.Thomas T, M R, Ndivo R, Zeh C, Borkowf C, Thigpen M, De Cock K, Amornkul P, Greenberg A, Fowler M, Kisumu Breastfeeding Study Team Abstract 45aLB: Prevention of Mother-to-Child Transmission of HIV-1 among Breastfeeding Mothers Using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003-2007. Conference on Retroviruses and Opportunistic Infections; Boston. 2008. [Google Scholar]
- 23.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21 4:S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 24.Kilewo C, K K, Ngarina M, Massawe A, Lyamuya E, Lipyoga R, Msemo G, Swai A, Mhalu F, Biberfeld G. International AIDS Society. Sydney, Australia: 2007. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers prophylactically with triple antiretroviral therapy in Dar es Salaam, Tanzania - the MITRA PLUS study. [DOI] [PubMed] [Google Scholar]
- 25.Arendt V, N P, Vyankandondera J, Ndayisaba G, Muganda j, Courteille O, Rutanga C, Havuga E, Dhont N, Mujawamassiga A, Omes C, Peltier A. International AIDS Society. Sydney, Australia: 2007. AMATA study: effectiveness of antiretroviral therapy in breastfeeding mothers to prevent post-natal vertical transmission in Rwanda. [Google Scholar]
- 26.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 27.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 28.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 29.Aldrovandi G, M J, Chu C, Ha B, Handelsman E, Shearer W, Foca M, Rich K, McIntosh K for the WITS Study Group. Paper #695: Mitochondrial DNA Content of Peripheral Blood Mononuclear Cells in Uninfected Infants Born to HIV-infected Women with or without ART Exposure in the Women and Infants Transmission Study. Conference on Retroviruses and Opportunistic Infections; Denver. 2006. [Google Scholar]
- 30.Vigano A, B R, Schneider L, Cafarelli L, Tornaghi R, Pinti M, Prada N, Cossarizza A. Paper #942: Lack of Hyperlactatemia and Impaired Mitochondrial DNA Content in CD4+ Cells of HIV-uninfected Infants Exposed to Perinatal Antiretroviral Therapy. Conference on Retroviruses and Opportunistic Infections; San Francisco. 2004. [Google Scholar]
- 31.Maagaard A, Holberg-Petersen M, Kollberg G, Oldfors A, Sandvik L, Bruun JN. Mitochondrial (mt)DNA changes in tissue may not be reflected by depletion of mtDNA in peripheral blood mononuclear cells in HIV-infected patients. Antivir Ther. 2006;11:601–608. [PubMed] [Google Scholar]
- 32.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Culnane M, Fowler M, Lee SS, McSherry G, Brady M, O'Donnell K, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 34.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, Blanche S. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 35.O'Meara M, G M, Hayes E, Butler K. Abstract #853: Simplification of Neonatal Component of Regimens to Prevent Perinatal HIV Transmission. Conference on Retroviruses and Opportunistic Infections; Boston. 2003. [Google Scholar]
- 36.Noguera A, Fortuny C, Munoz-Almagro C, Sanchez E, Vilaseca MA, Artuch R, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 37.Giaquinto C, De Romeo A, Giacomet V, Rampon O, Ruga E, Burlina A, et al. Lactic acid levels in children perinatally treated with antiretroviral agents to prevent HIV transmission. AIDS. 2001;15:1074–1075. doi: 10.1097/00002030-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 38.Ekouevi DK, Toure R, Becquet R, Viho I, Sakarovitch C, Rouet F, et al. Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV: Agence Nationale de Recherches Sur le SIDA et les Hepatites Virales 1209 study, Abidjan, Ivory Coast. Pediatrics. 2006;118:e1071–1077. doi: 10.1542/peds.2006-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brogly S, Y N, Mofenson L, Oleskee J, Van Dyke R, Craing M, Abzugh M, Brady M, Patrick JP, Hughes M, Seage G., III In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 40.Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Benhammou V, Tardieu M, Warszawski J, Rustin P, Blanche S. Clinical mitochondrial dysfunction in uninfected children born to HIV-infected mothers following perinatal exposure to nucleoside analogues. Environ Mol Mutagen. 2007;48:173–178. doi: 10.1002/em.20279. [DOI] [PubMed] [Google Scholar]
- 42.Hunink M, G P, Siegel J, et al. Decision making in health and medicine: integrating evidence and values. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 43.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants in resource-limited settings: towards universal access - recommendations for a public health approach. [October 15, 2006. July 15. 2008];2006 http://www.who.int/hiv/pub/guidelines/en/
- 44.US Public Health Service Task Force. Perinatal HIV Guidelines Working Group. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV-1 Transmission in the United States. 2006 http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf. June 27. 2008.
- 45.Feeney ME, Tang Y, Pfafferott K, Roosevelt KA, Draenert R, Trocha A, et al. HIV-1 viral escape in infancy followed by emergence of a variant-specific CTL response. J Immunol. 2005;174:7524–7530. doi: 10.4049/jimmunol.174.12.7524. [DOI] [PubMed] [Google Scholar]
- 46.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 47.Fawzi W, Msamanga G, Spiegelman D, Renjifo B, Bang H, Kapiga S, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–338. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 48.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 49.Taha TE, Hoover DR, Kumwenda NI, Fiscus SA, Kafulafula G, Nkhoma C, et al. Late postnatal transmission of HIV-1 and associated factors. J Infect Dis. 2007;196:10–14. doi: 10.1086/518511. [DOI] [PubMed] [Google Scholar]
- 50.Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 51.Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. Bmj. 1995;311:619–620. doi: 10.1136/bmj.311.7005.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uusimaa J, Remes AM, Rantala H, Vainionpaa L, Herva R, Vuopala K, et al. Childhood encephalopathies and myopathies: a prospective study in a defined population to assess the frequency of mitochondrial disorders. Pediatrics. 2000;105:598–603. doi: 10.1542/peds.105.3.598. [DOI] [PubMed] [Google Scholar]
- 53.Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann Neurol. 2001;49:377–383. [PubMed] [Google Scholar]
- 54.World Health Organization. Mother-to-child transmission of HIV. 2007 http://www.who.int/hiv/mtct/en/index.html. May 23, 2007. 2007.
- 55.Spector SA, Saitoh A. Mitochondrial dysfunction: prevention of HIV-1 mother-to-infant transmission outweighs fear. AIDS. 2006;20:1777–1778. doi: 10.1097/01.aids.0000242825.97495.a7. [DOI] [PubMed] [Google Scholar]
- 56.Blanche S, Tardieu M, Benhammou V, Warszawski J, Rustin P. Mitochondrial dysfunction following perinatal exposure to nucleoside analogues. AIDS. 2006;20:1685–1690. doi: 10.1097/01.aids.0000242814.42344.77. [DOI] [PubMed] [Google Scholar]
- 57.Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 59.De Cock KM, Fowler MG, Mercier E, De Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 60.Chigwedere P, Seage GR, Lee TH, Essex M. Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: a meta-analysis of published clinical trials. AIDS Res Hum Retroviruses. 2008;24:827–837. doi: 10.1089/aid.2007.0291. [DOI] [PubMed] [Google Scholar]
- 61.Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 62.Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis. 2006;6:726–732. doi: 10.1016/S1473-3099(06)70629-6. [DOI] [PubMed] [Google Scholar]
- 63.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 64.Bulterys M, Nesheim S, Abrams EJ, Palumbo P, Farley J, Lampe M, Fowler MG. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women. Retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918:212–221. doi: 10.1111/j.1749-6632.2000.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 65.Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25:261–268. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
- 66.Tovo PA, Chiapello N, Gabiano C, Zeviani M, Spada M. Zidovudine administration during pregnancy and mitochondrial disease in the offspring. Antivir Ther. 2005;10:697–699. [PubMed] [Google Scholar]
- 67.Chotpitayasunondh T, Vanprapar N, Simonds RJ, Chokephaibulkit K, Waranawat N, Mock P, et al. Safety of late in utero exposure to zidovudine in infants born to human immunodeficiency virus-infected mothers: Bangkok. Bangkok Collaborative Perinatal HIV Transmission Study Group. Pediatrics. 2001;107:E5. doi: 10.1542/peds.107.1.e5. [DOI] [PubMed] [Google Scholar]
- 68.Alimenti A, Forbes JC, Oberlander TF, Money DM, Grunau RE, Papsdorf MP, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118:e1139–1145. doi: 10.1542/peds.2006-0525. [DOI] [PubMed] [Google Scholar]
- 69.Paul ME, Chantry CJ, Read JS, Frederick MM, Lu M, Pitt J, et al. Morbidity and mortality during the first two years of life among uninfected children born to human immunodeficiency virus type 1-infected women: the women and infants transmission study. Pediatr Infect Dis J. 2005;24:46–56. doi: 10.1097/01.inf.0000148879.83854.7e. [DOI] [PubMed] [Google Scholar]
- 70.Divi RL, Haverkos KJ, Humsi JA, Shockley ME, Thamire C, Nagashima K, et al. Morphological and molecular course of mitochondrial pathology in cultured human cells exposed long-term to Zidovudine. Environ Mol Mutagen. 2007;48:179–189. doi: 10.1002/em.20245. [DOI] [PubMed] [Google Scholar]
- 71.Divi RL, Leonard SL, Kuo MM, Nagashima K, Thamire C, St Claire MC, et al. Transplacentally exposed human and monkey newborn infants show similar evidence of nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity. Environ Mol Mutagen. 2007;48:201–209. doi: 10.1002/em.20201. [DOI] [PubMed] [Google Scholar]
- 72.Venerosi A, Valanzano A, Alleva E, Calamandrei G. Prenatal exposure to anti-HIV drugs: neurobehavioral effects of zidovudine (AZT) + lamivudine (3TC) treatment in mice. Teratology. 2001;63:26–37. doi: 10.1002/1096-9926(200101)63:1<26::AID-TERA1005>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 73.Jamisse L, B J, Farquhar C, Osman N, Djedje M, Hitti J. Abstract 756: Perinatal HIV transmission with HAART during late pregnancy and post-partum. Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 74.Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 75.Haas R, Dietrich R. Neuroimaging of mitochondrial disorders. Mitochondrion. 2004;4:471–490. doi: 10.1016/j.mito.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 76.National Institute of Child Health and Human Development. Pediatric HIV/AIDS Cohort Study Home Page. 2007 https://phacs.nichdclinicalstudies.org/overview.asp.
- 77.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 78.Fassinou P, Elenga N, Rouet F, Laguide R, Kouakoussui KA, Timite M, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d'Ivoire. AIDS. 2004;18:1905–1913. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 79.Violari A, C M, Gibb D, Babiker A, Steyn J, Jean-Phillip P, McIntyre J. International AIDS Society. Sydney, Australia: 2007. Antiretroviral therapy initiated before 12 weeks of age reduces early mortality in young HIV-infected infants: evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study. [Google Scholar]
- 80.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 81.Foster CJ, Biggs RL, Melvin D, Walters MD, Tudor-Williams G, Lyall EG. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. 2006;48:677–682. doi: 10.1017/S0012162206001423. [DOI] [PubMed] [Google Scholar]
- 82.Hammer SM, E JJ, Jr, R P, S RT, T MA, W S, C P, F MA, G JM. Antiretroviral Treatment of Adult HIV Infection: 2008 Recommendations of the International AIDS Society–USA Panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 83.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 84.Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, Neaton JD, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis. 2008;197:1145–1155. doi: 10.1086/529523. [DOI] [PubMed] [Google Scholar]
- 85.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 86.Llorente A, Brouwers P, Charurat M, Magder L, Malee K, Mellins C, et al. Early neurodevelopmental markers predictive of mortality in infants infected with HIV-1. Dev Med Child Neurol. 2003;45:76–84. [PubMed] [Google Scholar]
- 87.Rouet F, Sakarovitch C, Msellati P, Elenga N, Montcho C, Viho I, et al. Pediatric viral human immunodeficiency virus type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics. 2003;112:e289. doi: 10.1542/peds.112.4.e289. [DOI] [PubMed] [Google Scholar]
- 88.Massad LS, Springer G, Jacobson L, Watts H, Anastos K, Korn A, et al. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS. 2004;18:281–286. doi: 10.1097/00002030-200401230-00018. [DOI] [PubMed] [Google Scholar]
- 89.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Magder LS, Mofenson L, Paul ME, Zorrilla CD, Blattner WA, Tuomala RE, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38:87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 91.Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 92.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354:471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 93.Thistle P, Spitzer RF, Glazier RH, Pilon R, Arbess G, Simor A, et al. A randomized, double-blind, placebo-controlled trial of combined nevirapine and zidovudine compared with nevirapine alone in the prevention of perinatal transmission of HIV in Zimbabwe. Clin Infect Dis. 2007;44:111–119. doi: 10.1086/508869. [DOI] [PubMed] [Google Scholar]
- 94.Palombi L, G P, Liotta G, Magnano san Lio M, Guidotti G, Assane A, Narciso P, Ceffa S, Nielsen-Saines K, Marazzi M. Paper # 747: Safety and efficacy of maternal HAART in the prevention of early and late postnatal HIV-1 transmission in Mozambique. Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 95.Chi BH, Chintu N, Cantrell RA, Kankasa C, Kruse G, Mbewe F, et al. Addition of single-dose tenofovir and emtricitabine to intrapartum nevirapine to reduce perinatal HIV transmission. J Acquir Immune Defic Syndr. 2008;48:220–223. doi: 10.1097/QAI.0b013e3181743969. [DOI] [PubMed] [Google Scholar]