Abstract
Objective
To investigate the novel hypothesis that neurotrophin-3 (NT-3), an established neurotrophic factor that participates in embryonic heart development, promotes blood vessel growth.
Methods and Results
We evaluated the proangiogenic capacity of recombinant NT-3 in vitro and of NT-3 gene transfer in vivo (rat mesenteric angiogenesis assay and mouse normoperfused adductor muscle). Then, we studied whether either transgenic or endogenous NT-3 mediates postischemic neovascularization in a mouse model of limb ischemia. In vitro, NT-3 stimulated endothelial cell survival, proliferation, migration, and network formation on the basement membrane matrix Matrigel. In the mesenteric assay, NT-3 increased the number and size of functional vessels, including vessels covered with mural cells. Consistently, NT-3 overexpression increased muscular capillary and arteriolar densities in either the absence or the presence of ischemia and improved postischemic blood flow recovery in mouse hind limbs. NT-3–induced microvascular responses were accompanied by tropomyosin receptor kinase C (an NT-3 high-affinity receptor) phosphorylation and involved the phosphatidylinositol 3-kinase–Akt kinase–endothelial nitric oxide synthase pathway. Finally, endogenous NT-3 was shown to be essential in native postischemic neovascularization, as demonstrated by using a soluble tropomyosin receptor kinase C receptor domain that neutralizes NT-3.
Conclusion
Our results provide the first insight into the proangiogenic capacity of NT-3 and propose NT-3 as a novel potential agent for the treatment of ischemic disease.
Keywords: angiogenesis, endothelial cells, limb ischemia, neurotrophin-3, PI3K/Akt/eNOS
Angiogenesis is a complex and coordinated process that is deregulated in ischemic diseases, including cardiac and peripheral vascular disorders.1 Understanding the mechanisms involved in blood vessel formation should improve the therapeutic possibilities for the treatment of these pathological features.2,3
Many angiogenic modulators have been discovered, including several that were originally identified as neuronal growth factors. Conversely, vascular endothelial growth factors (VEGFs) have been shown to elicit important neural actions.4
Neurotrophins (NTs) are a family of proteins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), NT-3, and NT-4/5. NTs mediate their actions by binding to 3 tropomyosin receptor kinases (Trks). NT-3 binds with high affinity to TrkC and with lower affinity to TrkA and TrkB.5,6 In addition to their neuronal functions, NTs exert cardiovascular actions under both physiological and pathological conditions5: NGF stimulates reparative neovascularization in ischemic limb muscles7,8 and promotes the survival, proliferation, and migration of endothelial cells (ECs).7-11 BDNF is also critical for EC survival,12 and BDNF gene transfer induces therapeutic angiogenesis in mice with limb ischemia.13 NT-3 participates in cardiac development,14 regulating embryonic cardiomyocyte proliferation.15 Mice with gene knockout for either NT-3 or TrkC present with severe cardiac defects.15,16 The possible vascular functions of NT-3 have never been investigated. To our knowledge, this study provides the first evidence of the proangiogenic potential of NT-3.
Methods
A detailed description of the methods is provided in the online supplemental file (available at http://atvb.ahajournals.org).
Cell Cultures and Cell Biology
Mouse skeletal muscle ECs (SMECs), human umbilical vein ECs (HUVECs; Lonza), and human vascular smooth muscle cells (HVSMCs) were cultured as previously described.17-19 By using jetPEI-HUVEC (iPolyplus-transfection Inc), HUVECs were transfected with the pcDNA3.1 vector containing rat full-length TrkC receptor.20
For experiments, cells were supplemented with human recombinant NT-3, 50 to 100 ng/mL (Promega), or vehicle (PBS). For some experiments, phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002, 50 μmol/L (Calbiochem), or nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME), 6 μmol/L (Sigma), was used. Respective vehicle controls were 0.5% dimethyl sulfoxide and NG-nitro-d-arginine-methyl ester (d-NAME). EC proliferation was measured using a colorimetric 5-bromodeoxyuridine ELISA kit (Roche). The migratory response to NT-3 was evaluated by the scratch assay for SMECs and HUVECs and by direct transwell migration for HVSMC. The SMEC and HUVEC endothelial network forming capacity was studied using a reduced growth factor Matrigel. Serum starvation-induced SMEC apoptosis was assessed by an in situ cell death detection kit (TUNEL; Roche).
Animal Procedures
In vivo experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources and with the prior approval of the UK Home Office and the University of Bristol.
The rat mesenteric assay21,22 was used to characterize the phenotype of NT-3–induced neovascularization. Anesthetized rats underwent laparotomy, and a mesenteric panel was exposed. The panel was imaged, and the surrounding fat pad was infected using Ad.NT-3 or control Ad.eGFP (108 plaque-forming units). Neovascularization of the rat mesenteric connective tissue panel was assessed by intravital microscopy at 0 and 6 days after gene transfer. The percentage area occupied by patent vessels (percentage functional vessel area) was measured. After the rats were euthanized, the mesenteric panel was stained for blood vessel cell markers (isolectin B4 for ECs, nerve glia 2 for pericytes, and α smooth muscle actin for VSMCs) and imaged by confocal microscopy.
Unilateral limb ischemia was induced in anesthetized CD1 mice.7,17 Ad.NT-3 or Ad.Null (108 plaque-forming units) was delivered to the ischemic adductor muscle. Postischemic foot blood flow recovery was assessed by color laser Doppler. Capillary and arteriole densities were evaluated postmortem in paraffin-embedded adductor sections. EC proliferation was measured by staining sections for the proliferation cell nuclear antigen (PCNA) in combination with isolectin B4.
The effect of inhibition of Akt and NO production on Ad.NT-3–induced neovascularization was studied by intramuscular Ad.DNAkt23 (control: Ad.Null, 108 plaque-forming units) and systemic l-NAME (control: d-NAME), respectively.24 To assess the role of endogenous NT-3 on the spontaneous neovascularization response to ischemia, a soluble TrkC receptor domain (TrkCd5), 2 mg/kg body weight intraperitoneally, which neutralizes NT-3, was used.
mRNA and Protein Expression Analyses
Expression levels of TrkC and 18s ribosomal RNA were analyzed in vascular cells and mouse muscles by semiquantitative RT-PCR. Quantitative RT-PCR analyses for mouse NT-3, TrkC, VEGF-A, FGF-2, SDF-1, and 18s rRNA were performed on adductor muscles 3 days after ischemia and gene transfer. Western blot analyses of FGF-2, phosphorylated (serine 1177) and total endothelial NOS (eNOS), phosphorylated (serine 473) and total Akt, total TrkC, and α-β tubulin (loading control) were performed on adductor muscles 3 days after surgery. Phosphorylated TrkC was detected by immunoprecipitation of total TrkC, followed by Western blot analysis for phosphorylated tyrosine.
Statistical Analysis
Data are presented as mean±SEM. Statistical significance was evaluated by using an unpaired t test for comparison between 2 groups. For comparison among more than 2 groups, a 2-way ANOVA was performed, followed by a Bonferroni post hoc test. P<0.05 was considered statistically significant.
Results
Mouse SMECs Express TrkC Receptor
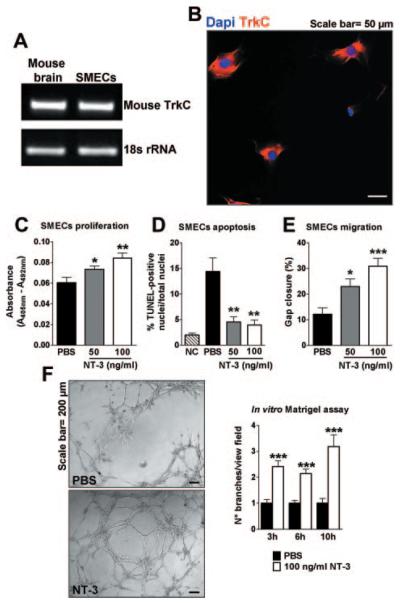
RT-PCR was used to screen TrkC mRNA expression in several EC types, including HUVECs and human micro-vascular ECs (HMVECs) (Supplemental Figure I; available online at http://atvb.ahajournals.org), which are both widely used in angiogenesis studies; and SMECs. Among them, only SMECs expressed TrkC at RNA (Figure 1A and data not shown) and protein (Figure 1B and data not shown) levels, suggesting that these cells may be responsive to NT-3.
Figure 1.
Proangiogenic effects of NT-3 in vitro. A, RT-PCR showing TrkC expression in mouse SMECs. Mouse brain indicates positive control; and 18s ribosomal RNA, loading control. B, Immunocytochemical TrkC staining (red fluorescence) of SMECs. Nuclei are identified by Dapi (blue fluorescence). C, Bar graph shows the effects of recombinant NT-3, 50 and 100 ng/mL, on SMEC proliferation after 24 hours of incubation, as assessed by measurement of 5-bromodeoxyuridine incorporation. D, Bar graph illustrates percentage of TUNEL-positive apoptotic SMECs after serum starvation for 24 hours and NT-3 or PBS. E, Bar graph displays percentage of gap closure measured 24 hours after scratch of SMECs treated with NT-3 or PBS. F, Bar graph showing network formation on Matrigel at different points from SMEC seeding. The number of branches per view field was measured. Microphotographs are representative of tubelike structure generation after 6 hours of incubation in the presence of either PBS or NT-3. Data are given as the mean±SEM (n=3 for each assay). *P<0.05, **P<0.01, and ***P<0.005 vs PBS.
NT-3 Exerts In Vitro Proangiogenic Effects
To evaluate the possible mechanisms of NT-3 angiogenic activities on SMECs, we examined the effects of this NT on the major steps of angiogenesis, specifically EC proliferation, survival, migration, and endothelial network formation. As shown in Figure 1C through F, NT-3 strongly enhanced SMEC proliferation, survival, migration, and network formation. Similar results were obtained when using NT-3 to stimulate TrkC-transduced HUVECs (Supplemental Figure II).
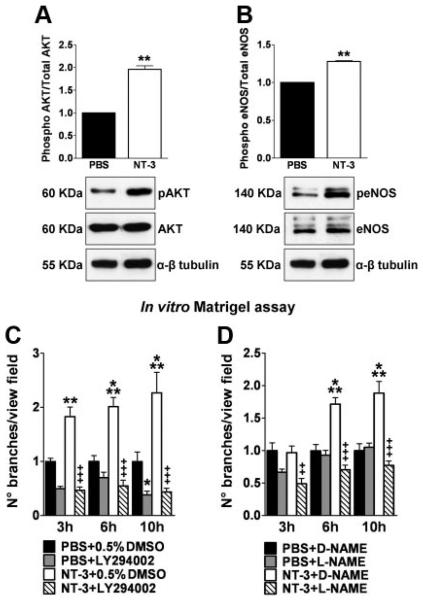
NT-3–Induced Angiogenesis Is Mediated by the PI3K-Akt-eNOS Signaling Pathway
The PI3K-Akt-eNOS signaling pathway is well-known to promote EC survival and angiogenesis, including in response to NGF.7,9 Moreover, NT-3, via TrkC, was shown to activate this pathway in neural cells.25 Western blot analyses showed that NT-3 induced Akt and eNOS phosphorylation (Figure 2A and B). Moreover, PI3K inhibition by LY294002 and NOS inhibition by l-NAME abrogated NT-3–induced network formation on Matrigel (Figure 2C and D). These cumulative data provide evidence that the PI3K-Akt-eNOS pathway mediates the proangiogenic responses of ECs to NT-3 stimulation.
Figure 2.
Akt-eNOS activation is critical for NT-3–induced angiogenesis in vitro. SMECs were incubated in their basal medium, added with either NT-3, 100 ng/mL, or PBS for 30 minutes. Immunoblot analysis and averaged densitometric data show the effects of NT-3 stimulation on Akt (A) and eNOS (B) phosphorylation. α-β tubulin indicates the loading control. Data are given as the mean±SEM (n=3). **P<0.01 vs PBS. Bar graphs display the effect of PI3K-Akt inhibition by LY294002 (C) and NO production by L-NAME (D) on NT-3–induced endothelial network formation in vitro. At different points from cell seeding, the number of branches per view field was measured. Data are given as mean±SEM (n=3). *P<0.05, **P<0.01, and ***P<0.005 vs PBS plus 0.5% dimethyl sulfoxide (DMSO) or D-NAME. ++P<0.01 and +++P<0.005 vs NT-3 plus 0.5% dimethyl sulfoxide (DMSO) or D-NAME.
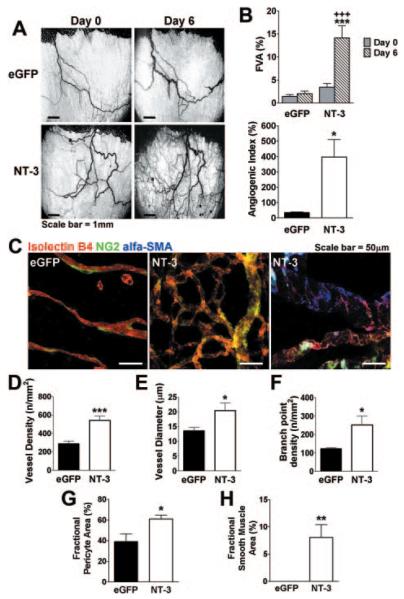
NT-3 Promotes New Vessel Formation in the Rat Mesenteric Angiogenesis Assay
Next, we investigated whether NT-3 overexpression induces neovascularization in vivo. As shown in Figure 3A, in the rat mesenteric assay, Ad.NT-3 significantly increased the area occupied by functional blood vessels (percentage functional vessel area) 6 versus 0 days after gene transfer, whereas Ad.eGFP did not change the percentage functional vessel area. Similarly, the overtime increase in vessel area (percentage angiogenic index) was higher in Ad.NT-3–injected mesentery (Figure 3B). To investigate the phenotype of Ad.NT-3–induced neovascularization, confocal microscopy was performed to determine endothelial and periendothelial vessel composition (Figure 3C). Moreover, vessel density, branch point density, and diameter were measured. Morphometric analyses indicated that NT-3 overexpression enhanced vessel density, vessel diameter, and branch point density (Figure 3D–F). Most important, Ad.NT-3 increased the fractional coverage of newly formed vessels with pericytes and VSMCs (Figure 3G and H), indicating that it was able to generate new arterioles in this system.
Figure 3.
Vascular effects of NT-3 overexpression in the rat mesenteric assay. A, Intravital microscopy images displaying vasculature of a rat mesenteric panel on days 0 and 6 from NT-3 or eGFP gene transfer. B, Top: percentage functional vessel area on 0 and 6 days from gene transfer. Bottom: overtime increase in vessel area (angiogenic index, calculated as percentage increase of vessel area). C, Confocal-stack images of whole-mount mesenteric panels harvested on day 6 from gene transfer. Mesenteric panels were stained for cell markers (isolectin B4 for ECs, nerve glia 2 for pericytes, and α smooth muscle actin for VSMCs). Ad.NT-3 increases vessel density (D), vessel diameter (E), branch point density (F), and perivascular cell coverage (G and H). Data are given as the mean ± SEM (n=5 rats per group). *P<0.05, **P<0.01, and ***P<0.005 vs Ad.eGFP. +++P<0.005 vs Ad.NT-3 on day 0.
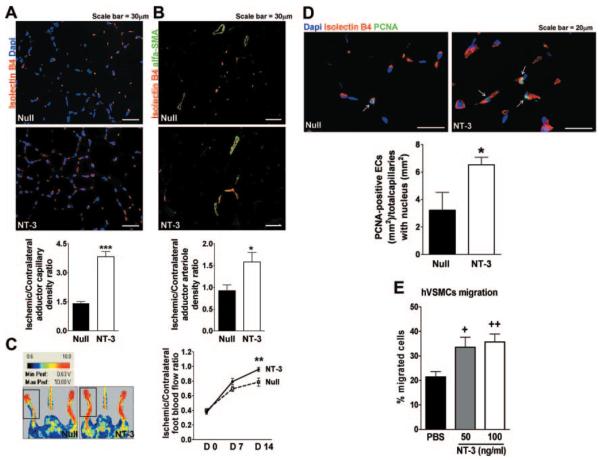
NT-3 Enhances Reparative Neovascularization in a Mouse Model of Limb Ischemia
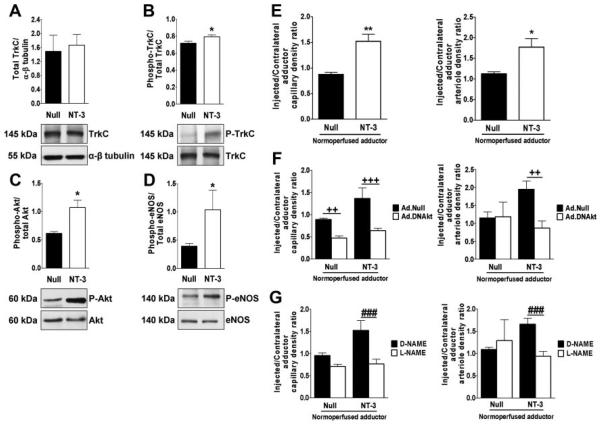
The observation that NT-3 overexpression promotes angiogenesis in vivo prompted us to investigate the therapeutic potential of Ad.NT-3 for ischemic disease. TrkC is expressed by capillary ECs of mouse limb muscles (Supplemental Figure III), thus suggesting a possible role for NT-3 in this vascular district. Therefore, we used a mouse model of limb ischemia to test the therapeutic potential of NT-3–induced neovascularization. Mice underwent femoral artery ligation, followed by Ad.NT-3 or Ad.Null delivery to the ischemic adductor muscle. Ad.NT-3 resulted in transgenic (rat) NT-3 expression in the ischemic muscle for the entire duration (14 days) of the experiment (Supplemental Figure IV). As shown in Figure 4A and B, Ad.NT-3 increased the densities of capillaries and small (diameter, 10 to 20 μm) arterioles in ischemic adductors. These microvascular responses to Ad.NT-3 were associated with improved blood flow recovery 14 days after surgery (Figure 4C).
Figure 4.
Ad.NT-3 enhances postischemic neovascularization. Ischemic adductor sections are stained for identification of capillaries (A) and arterioles (B). Capillary and arteriole densities are expressed as ratio of ischemic to contralateral adductor (n=5 mice per group). C, Left: representative color laser Doppler images taken 14 days after ischemia are shown together with the color code (blood flow increases from black to red). Right: course of postischemic recovery of foot blood flow (n=12 mice per group). D, Bar graph and representative immunofluorescence staining display the number of proliferating (PCNA-positive, green fluorescence) capillary ECs (isolectin B4, red fluorescence) in Ad.NT-3- or Ad.Null-treated ischemic adductors. Nuclei are stained by Dapi (blue fluorescence) (n=5 mice per group). E, Bar graph presents the effect of NT-3, 50 and 100 ng/mL, or PBS on human VSMC migration (n=3). Data are given as mean±SEM. *P<0.05, **P<0.01, and ***P<0.005 vs Ad.Null. +P<0.05 and ++P<0.01 vs PBS.
To evaluate whether in vivo EC proliferation was instrumental to the angiogenic process induced by Ad.NT-3, we performed PCNA staining in ischemic muscles 3 days after surgery. As shown in Figure 4D, ischemic muscles injected with Ad.NT-3 presented a 2-fold increase in PCNA-positive capillary ECs compared with controls. This finding is in line with the reported proliferative effect of NT-3 in cultured SMECs and TrkC-transduced HUVECs.
To investigate how NT-3 could promote VSMC recruitment, thus leading to arteriole formation, we first examined TrkC presence by HVSMCs. After finding the receptor expressed by these cells (Supplemental Figure IA), we studied whether HVSMCs migrate toward NT-3. As displayed in Figure 4E, NT-3 promotes HVSMC migration. We also evaluated whether NT-3 may induce arteriole formation by indirect mechanisms involving growth factors and cytokines, known to mediate capillary formation and/or maturation, such as VEGF-A, FGF-2, and SDF-1. However, we found that at 3 days after ischemia, the expression of none of these is modified by NT-3 overexpression (Supplemental Figures V and VI).
TrkC, Akt, and eNOS Are Involved in Ad.NT-3–Induced Neovascularization
To determine whether NT-3 could act by binding to its TrkC receptor and subsequently lead to the activation of the Akt-eNOS pathway, we analyzed the phosphorylation levels of TrkC (tyrosine 490), Akt (serine 473), and eNOS (serine 1177) in ischemic adductors harvested 3 days after surgery and gene transfer. The results of Western blot analyses showed increased phosphorylated TrkC, phosphorylated Akt, and phosphorylated eNOS contents in Ad.NT-3–treated muscles (Figure 5A–D). These results confirm the effect of NT-3 on Akt and eNOS phosphorylation observed in SMECs. We already proved that the PI3K-Akt-eNOS pathway mediates the in vitro angiogenesis responses of SMECs treated with NT-3. To investigate whether Akt and eNOS are also important for NT-3–induced neovascularization in limb muscles, we used normoperfused mice to avoid the possible confounding effects of ischemia. As shown in Figure 5E, Ad.NT-3 increased capillary and arteriole densities in normoperfused adductors. Both of these responses to NT-3 were abrogated by either Ad.DNAkt or L-NAME (Figure 5F and G).
Figure 5.
Akt and eNOS mediate NT-3–induced neovascularization. Western blot bands and averaged densitometric measurements showing the effect of NT-3 or Null gene transfer on total and phosphorylated TrkC (A and B), Akt (C), and eNOS (D) in muscles 3 days after ischemia (n=5 mice per group). E, Ad.NT-3 stimulates growth of capillaries and small arterioles in normoperfused muscles. Dominant-negative Akt (Ad.DNAkt) (F) and L-NAME (G) abrogate Ad.NT-3–induced neovascularization (n=6 mice per group). Data are given as mean±SEM. *P<0.05 and **P<0.01 vs Ad.Null. ++P<0.01 and +++P<0.005 vs controls given Ad.Null instead of Ad.DNAkt. ###P<0.005 vs D-NAME.
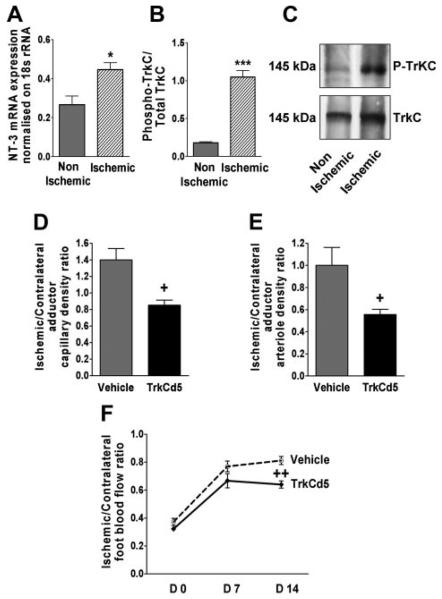
Ischemia Upregulates the Expression of Endogenous NT-3 and Enhances TrkC Phosphorylation in Skeletal Muscles
As displayed in Figure 6A, NT-3 mRNA expression was increased in muscles 3 days after ischemia. In contrast, ischemia did not modulate TrkC mRNA or protein levels (Supplemental Figure VII). Nevertheless, the content of phoshporylated TrkC was enhanced in ischemic muscles, probably as a consequence of increased expression of the endogenous NT-3 ligand (Figure 6B and C).
Figure 6.
Endogenous NT-3 participates in postischemic angiogenesis and blood flow recovery. Ischemia upregulates endogenous NT-3 expression (A) and increases phosphorylated TrkC (B and C) in skeletal muscle. TrkCd5 induced neutralization of endogenous NT-3 and impaired the native neovascularization response to ischemia (D and E) (n=6 mice per group). (F) Foot blood flow recovery after ischemia and treatment with TrkCd5 or vehicle (n=7 mice per group). Data are given as mean±SEM. *P<0.05 and ***P<0.005 vs nonischemic. +P<0.05 and ++P<0.01 vs vehicle.
Neutralization of Endogenous NT-3 Impairs Native Postischemic Reparative Angiogenesis in Mice
To evaluate the role of the endogenous NT-3 in the native neovascularization response to limb ischemia, we used a soluble TrkC mutant (TrkCd5), which prevents NT-3 receptor binding. The inhibitory capacity of TrkCd5 on Ad.NT-3–induced angiogenesis was preliminarily validated (Supplemental Figure VIII). Then, TrkCd5 or its vehicle was used to treat mice after the induction of limb ischemia. TrkCd5 impaired the ischemia-induced reparative angiogenesis response at both capillary (Figure 6D) and arteriole (Figure 6E) levels. In line with the reduced microvascular growth, blood perfusion recovery was deficient in TrkCd5-treated mice (Figure 6F).
Discussion
Vascular regeneration by therapeutic neovascularization is an ambitious strategy for improving the prognosis of patients with cardiovascular ischemic disease. Revascularization of adult tissues is a complex process and is modulated by the collaboration of multiple factors, some of which may still be unrecognized. In this study, we document, for the first time to our knowledge, the proangiogenic potential of NT-3. Although well-known as regulators of neuronal survival, differentiation, and regeneration, NTs have only recently emerged as important mediators of vessel survival and stabilization in late embryogenesis12 and postnatal angiogenesis after vascular injury.7,13 NT-3 was previously implicated in cardiac development14-16 and cardiomyocyte hypertrophy26; however, NT-3 functions have not been investigated in the context of vascular biology. To our knowledge, this study is the first to describe the role of NT-3 in blood vessel formation. Herein, using cultured mouse SMECs, we provided evidence of the ability of NT-3 to promote EC proliferation, survival, migration, and network formation on Matrigel. In vitro proangiogenic responses to NT-3 were also observed using TrkC-transduced HUVECs, thus strengthening the concept of a direct proangiogenic effect of NT-3 in ECs. This first set of evidence prompted us to perform further investigations in vivo. To characterize the cellular mechanisms of Ad.NT-3–induced angiogenesis, we used the mesenteric angiogenesis assay, which allows the detailed phenotypic description of neovessels.21,22 In this model, NT-3 overexpression increased the functional vessels available for flowing blood. The newly formed vessels showed an enhanced branch point density and diameter compared with the control group. Moreover, they displayed increased coverage by mural cells. We also observed proangiogenic responses to NT-3 in mouse normoperfused adductor muscles, which we documented to express TrkC at the capillary EC level. Our next step was to evaluate the potential of NT-3 supplementation after vascular injury. Thus, we used a mouse model of hind limb ischemia. We found that intramuscular NT-3 gene delivery induced reparative capillarization and increased the number of small arterioles in ischemic adductors. Enhanced reparative neovascularization was associated with improved postischemic blood flow recovery.
To gain further insight into the action of NT-3 in reparative angiogenesis, we first investigated whether NT-3 exerts its angiogenic effect by promoting EC proliferation. Consistently with our in vitro results, we demonstrated that NT-3 overexpression induces capillary EC proliferation during the mounting phase of neovascularization in ischemic muscles (3 days after ischemia). Then, we addressed our attention to the arteriole formation process, a key step in the vessel maturation mechanism, and showed that NT-3 directly acts on VSMCs to promote their migration, without modulating the expression of growth factors and cytokines (eg, FGF-2 and SDF-1) involved in mural cell recruitment.
Various molecular mechanisms may be implicated in the vascular effects of NT-3. Classically, proangiogenic factors activate cognate receptors expressed on the EC plasma membrane, which in turn stimulate signal transduction kinases. It has been previously documented that NGF, after TrkA binding, activates the PI3K/Akt and Ras/mitogen-activated protein kinase pathways in ECs.9,27 We did not find any change in Ras/mitogen-activated protein kinase signaling (data not shown). Conversely, we provided evidence that activation of the PI3K-Akt-eNOS pathway is critical for NT-3–induced angiogenesis both in vitro and in vivo. NT-3 binds to TrkC with high affinity. Also, we found that endogenous and transgenic NT-3 stimulated TrkC phosphorylation/activation in mouse ischemic muscles. However, because NT-3 also binds to both TrkA and TrkB with lower affinity, we cannot exclude that these receptors played a role in NT-3–induced angiogenesis. It was previously reported that VEGF contributes to the proangiogenic response to NGF in mouse ischemic limb muscles.7 However, NT-3 gene transfer did not increase VEGF-A mRNA or protein levels. This suggests that VEGF-A may not be involved with NT-3–induced neovascularization.
Finally, we assessed the importance of endogenous NT-3 in vascular repair. Consistent with previous studies28-30 demonstrating that NTs promote nerve regeneration and neuronal protection in different models of injury, we showed, for the first time to our knowledge, that the levels of both endogenous NT-3 and phosphorylated TrkC increased in response to ischemia. This finding is compatible with ischemia-induced activation of a prosurvival and regenerative mechanism. This result prompted us to evaluate whether endogenous NT-3 plays a role in the native postischemic vascular regeneration process. To neutralize endogenous NT-3 in ischemic mice, we used TrkCd5. This compound was modeled after TrkA and TrkB soluble mutant forms (TrkAd5 and TrkBd5, respectively). Previously, TrkAd5 and TrkBd5 were used to block NGF binding to TrkA and BDNF binding to TrkB, respectively.31-33 TrkCd5 treatment reduced both capillary and arteriole densities in ischemic muscles and impaired blood flow recovery. These data suggest that endogenous NT-3 is a key player in the native healing response to ischemia.
In summary, we showed that NT-3 promotes angiogenesis. An ischemia-induced increase in endogenous NT-3 expression is fundamental for reparative angiogenesis. NT-3 overexpression has a profound impact on neovascularization and consequently accelerates the rate of perfusion recovery. Our results, together with the previous studies on NGF and BDNF, provide solid evidence that NTs are able to promote reparative angiogenesis and, thus, enhance blood supply to ischemic limbs.
Taken together, these findings, in addition to being relevant for the treatment of ischemic vasculopathies, give important new insights into common mechanisms driving the behavior of vascular and neuronal cells and suggest further exciting discoveries in the years to come.
Supplementary Material
Acknowledgments
We thank Luciola Barcelos, PhD, Nicolle Krankel, PhD, Rajesh Katare, MD, PhD, Ayman Al Haj Zen, PhD, Paola Campagnolo, PhD, Mauro Siragusa, PhD, and Paul Savage for their valuable advice and technical support; Yanhua Hu, PhD for providing the HVSMCs; and Lino Tessarollo, PhD for providing the pcDNA3.1 vector containing rat full-length TrkC receptor.
Sources of Funding
The study was supported by the British Heart Foundation (grant RJ4430 to Dr Emanueli). Dr Cristofaro was a recipient of a doctoral student bursary from the University of Bristol.
Footnotes
Disclosures
None.
References
- 1.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 3.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 5.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev. 2009;89:279–308. doi: 10.1152/physrev.00007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofaro B, Emanueli C. Possible novel targets for therapeutic angiogenesis. Curr Opin Pharmacol. 2009;9:102–108. doi: 10.1016/j.coph.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106:2257–2262. doi: 10.1161/01.cir.0000033971.56802.c5. [DOI] [PubMed] [Google Scholar]
- 8.Turrini P, Gaetano C, Antonelli A, Capogrossi MC, Aloe L. Nerve growth factor induces angiogenic activity in a mouse model of hindlimb ischemia. Neurosci Lett. 2002;32:109–112. doi: 10.1016/s0304-3940(02)00090-3. [DOI] [PubMed] [Google Scholar]
- 9.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 10.Graiani G, Emanueli C, Desortes E, Van Linthout S, Pinna A, Figueroa CD, Manni L, Madeddu P. Nerve growth factor promotes reparative angiogenesis and inhibits endothelial apoptosis in cutaneous wounds of type 1 diabetic mice. Diabetologia. 2004;47:1047–1054. doi: 10.1007/s00125-004-1414-7. [DOI] [PubMed] [Google Scholar]
- 11.Salis MB, Graiani G, Desortes E, Caldwell RB, Madeddu P, Emanueli C. Nerve growth factor supplementation reverses the impairment, induced by type 1 diabetes, of hindlimb post-ischaemic recovery in mice. Diabetologia. 2004;47:1055–1063. doi: 10.1007/s00125-004-1424-5. [DOI] [PubMed] [Google Scholar]
- 12.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 13.Kermani P, Rafii D, Jin D, Whitlock P, Schaffer W, Chiang A, Vincent L, Friedrich M, Shido K, Hackett NR, Crystal RG, Rafii S, Hempstead BL. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–663. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donovan MJ, Hahn R, Tessarollo L, Hempstead BL. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat Genet. 1996;14:210–213. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- 15.Lin M, Das I, Schwartz G, Tsoulfas P, Mikawa T, Hempstead B. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev Biol. 2000;226:180–191. doi: 10.1006/dbio.2000.9850. [DOI] [PubMed] [Google Scholar]
- 16.Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci U S A. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporali A, Pani E, Horrevoets AJG, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. 2008;103:e15–e26. doi: 10.1161/CIRCRESAHA.108.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ieronimakis N, Balasundaram G, Reyes M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS ONE. 2008;3:1–16. doi: 10.1371/journal.pone.0001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, Hu Y, Kleindienst R, Wick G. Nitric oxide induces heat-shock protein 70 expression in vascular smooth muscle cells via activation of heat shock factor 1. J Clin Invest. 1997;100:1089–1097. doi: 10.1172/JCI119619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban PF, Yoon H-Y, Becker J, Dorsey SG, Caprari P, Palko ME, Coppola V, Saragovi HU, Randazzo PA, Tessarollo L. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J Cell Biol. 2006;173:291–299. doi: 10.1083/jcb.200512013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone OA, Richer C, Emanueli C, van Weel V, Quax PHA, Katare R, Kraenkel N, Campagnolo P, Barcelos LS, Siragusa M, Sala-Newby GB, Baldessari D, Mione M, Vincent MP, Benest AV, Al Haj Zen A, Gonzalez J, Bates DO, Alhenc-Gelas F, Madeddu P. Critical role of tissue kallikrein in vessel formation and maturation: implications for therapeutic revascularization. Arterioscler Thromb Vasc Biol. 2009;29:657–664. doi: 10.1161/ATVBAHA.108.182139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W-Y, Whittles CE, Harper SJ, Bates DO. An adenovirus-mediated gene-transfer model of angiogenesis in rat mesentery. Microcirculation. 2004;11:361–375. doi: 10.1080/10739680490437568. [DOI] [PubMed] [Google Scholar]
- 23.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MVG, Napoli C, Sadoshima J, Croce CM, Ross J. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emanueli C, Salis MB, Van Linthout S, Meloni M, Desortes E, Silvestre J-S, Clergue M, Figueroa CD, Gadau S, Condorelli G, Madeddu P. Akt/protein kinase B and endothelial nitric oxide synthase mediate muscular neovascularization induced by tissue kallikrein gene transfer. Circulation. 2004;110:1638–1644. doi: 10.1161/01.CIR.0000142051.36244.83. [DOI] [PubMed] [Google Scholar]
- 25.Ness JK, Mitchell NE, Wood TL. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: differential regulation and activation of receptors and the downstream effector Akt. Dev Neurosci. 2002;24:437–445. doi: 10.1159/000069050. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi-Manabe H, Ieda M, Kimura K, Manabe T, Miyatake S, Kanazawa H, Kawakami T, Ogawa S, Suematsu M, Fukuda K. A novel cardiac hypertrophic factor, neurotrophin-3, is paradoxically downregulated in cardiac hypertrophy. Life Sci. 2007;81:385–392. doi: 10.1016/j.lfs.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Rahbek UL, Dissing S, Thomassen C, Hansen AJ, Tritsaris K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflügers Archiv. 2005;450:355–361. doi: 10.1007/s00424-005-1436-0. [DOI] [PubMed] [Google Scholar]
- 28.Lin C-H, Cheng F-C, Lu Y-Z, Chu L-F, Wang C-H, Hsueh C-M. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Exp Neurol. 2006;201:225–233. doi: 10.1016/j.expneurol.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: evidence from engraftment of neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006;199:179–190. doi: 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z-H, Wang R-Z, Li G-L, Wei J-J, Li Z-J, Feng M, Kang J, Du W-C, Ma W-B, Li Y-N, Yang Y, Kong Y-G. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci Lett. 2008;444:227–230. doi: 10.1016/j.neulet.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 31.Banfield MJ, Naylor RL, Robertson AGS, Allen SJ, Dawbarn D, Brady RL. Specificity in Trk receptor:neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure. 2001;9:1191–1199. doi: 10.1016/s0969-2126(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 32.Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, Colmers WF, Dawbarn D, Ballanyi K, Smith PA. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–1032. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson JJ, Fahey MS, van den Worm E, Engels F, Nijkamp FP, Stroemer P, McMahon S, Allen SJ, Dawbarn D. TrkAd5: a novel therapeutic agent for treatment of inflammatory pain and asthma. J Pharmacol Exp Ther. 2006;316:1122–1129. doi: 10.1124/jpet.105.095844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.