Abstract
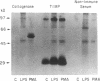
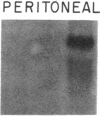
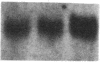
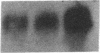
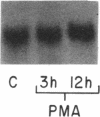
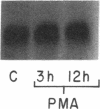
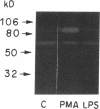
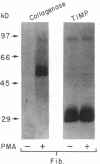
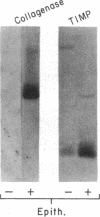
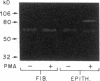
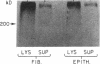
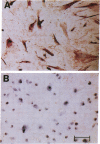
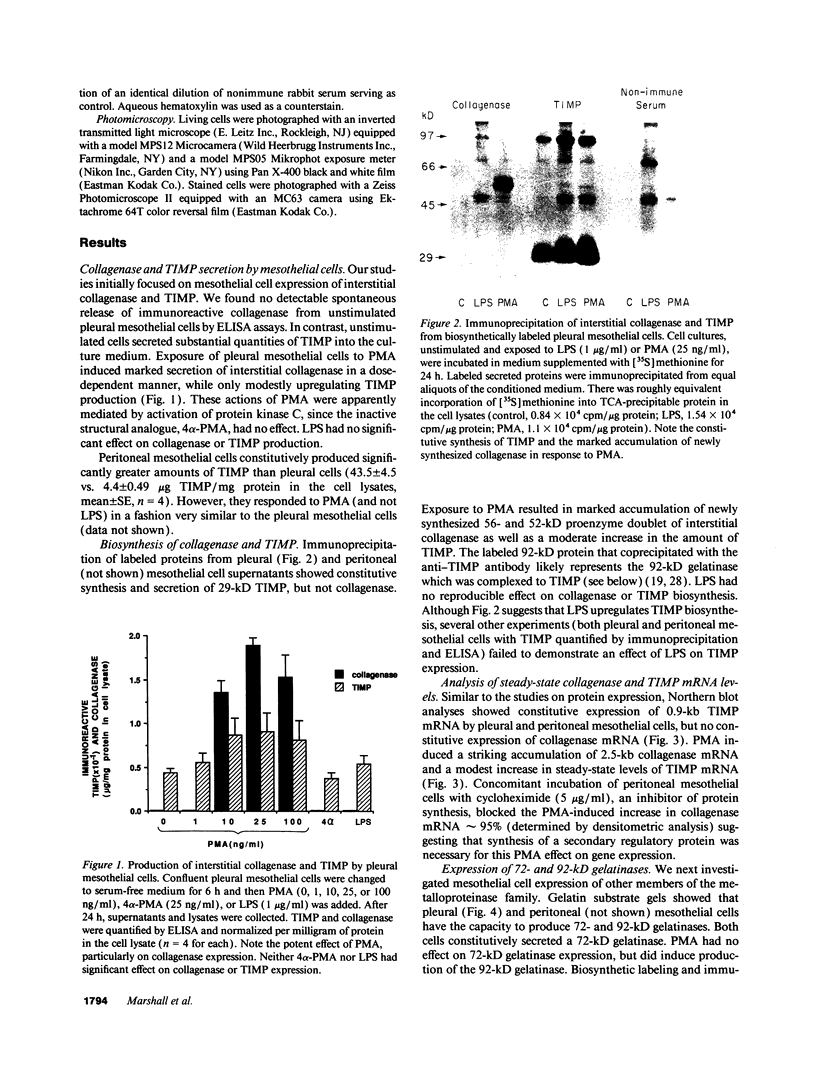
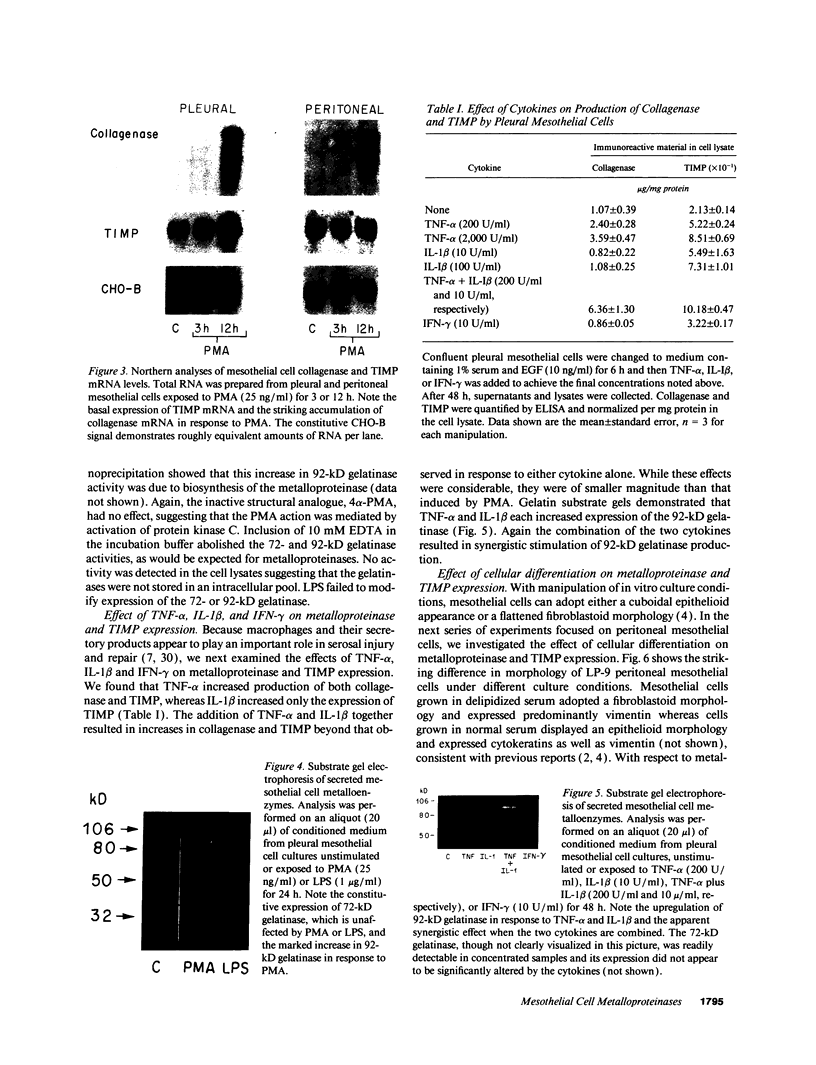
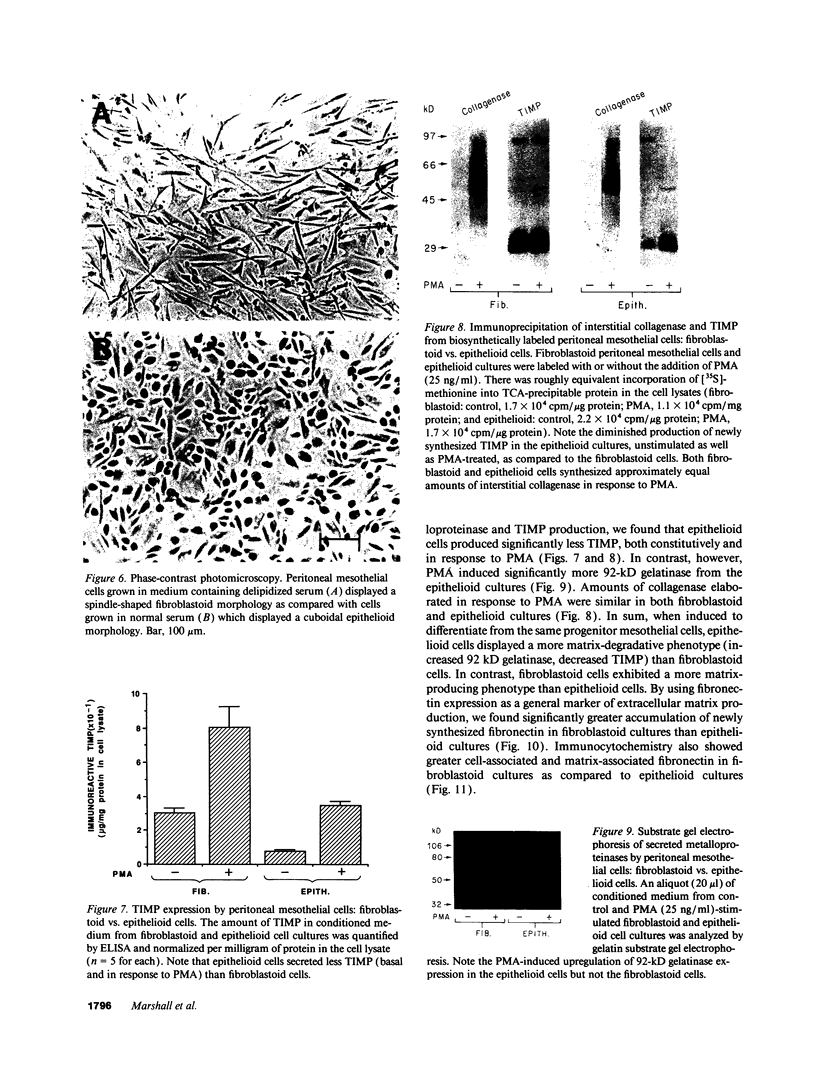
Mesothelial cells play a critical role in the remodeling process that follows serosal injury. Although mesothelial cells are known to synthesize a variety of extracellular matrix components including types I, III, and IV collagens, their potential to participate in matrix degradation has not been explored. We now report that human pleural and peritoneal mesothelial cells express interstitial collagenase, 72- and 92-kD gelatinases (type IV collagenases), and the counterregulatory tissue inhibitor of metalloproteinases (TIMP). Our initial characterization of the mesothelial cell metalloenzymes and TIMP has revealed: (a) they are likely identical to corresponding molecules secreted by other human cells; (b) they are secreted rather than stored in an intracellular pool; (c) a primary site of regulation occurs at a pretranslational level; (d) phorbol myristate acetate, via activation of protein kinase C, upregulates expression of collagenase, 92-kD gelatinase, and TIMP, but has no effect on expression of 72-kD gelatinase; and (e) lipopolysaccharide fails to upregulate the biosynthesis of either metalloproteinases or TIMP. Of particular interest is the observation that the state of cellular differentiation has a striking influence on the expression of metalloenzymes and TIMP, such that epitheloid cells display a more matrix-degradative phenotype (increased 92-kD gelatinase and decreased TIMP) than their fibroblastoid counterparts. We speculate that mesothelial cells directly participate in the extracellular matrix turnover that follows serosal injury via elaboration of metalloproteinases and TIMP. Additionally, the reactive cuboidal mesothelium which is characteristic of the early response to serosal injury may manifest a matrix-degenerative phenotype favoring normal repair rather than fibrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Cooper T. W., Bauer E. A., Eisen A. Z. Enzyme-linked immunosorbent assay for human skin collagenase. Coll Relat Res. 1983 May;3(3):205–215. doi: 10.1016/s0174-173x(83)80004-1. [DOI] [PubMed] [Google Scholar]
- Cury J. D., Campbell E. J., Lazarus C. J., Albin R. J., Welgus H. G. Selective up-regulation of human alveolar macrophage collagenase production by lipopolysaccharide and comparison to collagenase production by fibroblasts. J Immunol. 1988 Dec 15;141(12):4306–4312. [PubMed] [Google Scholar]
- Demetri G. D., Zenzie B. W., Rheinwald J. G., Griffin J. D. Expression of colony-stimulating factor genes by normal human mesothelial cells and human malignant mesothelioma cells lines in vitro. Blood. 1989 Aug 15;74(3):940–946. [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J Immunol. 1990 Jul 1;145(1):161–166. [PubMed] [Google Scholar]
- Elias J. A., Reynolds M. M., Kotloff R. M., Kern J. A. Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6171–6175. doi: 10.1073/pnas.86.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonard H., Grimaud J. A. Matrix metalloproteinases. A review. Cell Mol Biol. 1990;36(2):131–153. [PubMed] [Google Scholar]
- Fotev Z., Whitaker D., Papadimitriou J. M. Role of macrophages in mesothelial healing. J Pathol. 1987 Mar;151(3):209–219. doi: 10.1002/path.1711510309. [DOI] [PubMed] [Google Scholar]
- Gabrielson E. W., Gerwin B. I., Harris C. C., Roberts A. B., Sporn M. B., Lechner J. F. Stimulation of DNA synthesis in cultured primary human mesothelial cells by specific growth factors. FASEB J. 1988 Aug;2(11):2717–2721. doi: 10.1096/fasebj.2.11.3260881. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Lima F. B., Birnbaum M. J. Expression of a glucose transporter gene cloned from brain in cellular models of insulin resistance: dexamethasone decreases transporter mRNA in primary cultured adipocytes. Mol Endocrinol. 1989 Jul;3(7):1132–1141. doi: 10.1210/mend-3-7-1132. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Goodman R. B., Wood R. G., Martin T. R., Hanson-Painton O., Kinasewitz G. T. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992 Jan 15;148(2):457–465. [PubMed] [Google Scholar]
- Harvey W., Amlot P. L. Collagen production by human mesothelial cells in vitro. J Pathol. 1983 Mar;139(3):337–347. doi: 10.1002/path.1711390309. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Hibbs M. S., Kang A. H., Mainardi C. L. Secreted forms of human neutrophil collagenase. J Biol Chem. 1986 Apr 25;261(12):5645–5650. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Tuuttila A., Chow L. T., Lohi J., Keski-Oja J., Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991 Sep 5;266(25):16485–16490. [PubMed] [Google Scholar]
- Idell S., Zwieb C., Kumar A., Koenig K. B., Johnson A. R. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol. 1992 Oct;7(4):414–426. doi: 10.1165/ajrcmb/7.4.414. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato T., Ojima Y., Chen L. C., Nagase H., Mori Y. Calmodulin differentially modulates the interleukin 1-induced biosynthesis of tissue inhibitor of metalloproteinases and matrix metalloproteinases in human uterine cervical fibroblasts. J Biol Chem. 1991 Jul 25;266(21):13598–13601. [PubMed] [Google Scholar]
- Kim K. H., Stellmach V., Javors J., Fuchs E. Regulation of human mesothelial cell differentiation: opposing roles of retinoids and epidermal growth factor in the expression of intermediate filament proteins. J Cell Biol. 1987 Dec;105(6 Pt 2):3039–3051. doi: 10.1083/jcb.105.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca P. J., Rheinwald J. G. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 1984 Jul;44(7):2991–2999. [PubMed] [Google Scholar]
- MacNaul K. L., Chartrain N., Lark M., Tocci M. J., Hutchinson N. I. Discoordinate expression of stromelysin, collagenase, and tissue inhibitor of metalloproteinases-1 in rheumatoid human synovial fibroblasts. Synergistic effects of interleukin-1 and tumor necrosis factor-alpha on stromelysin expression. J Biol Chem. 1990 Oct 5;265(28):17238–17245. [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Phan S. H., Gharaee-Kermani M., McGarry B., Kunkel S. L., Wolber F. W. Regulation of rat pulmonary artery endothelial cell transforming growth factor-beta production by IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992 Jul 1;149(1):103–106. [PubMed] [Google Scholar]
- Rennard S. I., Jaurand M. C., Bignon J., Kawanami O., Ferrans V. J., Davidson J., Crystal R. G. Role of pleural mesothelial cells in the production of the submesothelial connective tissue matrix of lung. Am Rev Respir Dis. 1984 Aug;130(2):267–274. doi: 10.1164/arrd.1984.130.2.267. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Strange C., Tomlinson J. R., Wilson C., Harley R., Miller K. S., Sahn S. A. The histology of experimental pleural injury with tetracycline, empyema, and carrageenan. Exp Mol Pathol. 1989 Dec;51(3):205–219. doi: 10.1016/0014-4800(89)90020-8. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stylianou E., Jenner L. A., Davies M., Coles G. A., Williams J. D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990 Jun;37(6):1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P. Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem. 1983 Oct 25;258(20):12259–12264. [PubMed] [Google Scholar]
- Whitaker D., Papadimitriou J. Mesothelial healing: morphological and kinetic investigations. J Pathol. 1985 Feb;145(2):159–175. doi: 10.1002/path.1711450204. [DOI] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Kooistra T., Scheffer M. A., Hajo van Bockel J., van Muijen G. N. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood. 1990 Apr 1;75(7):1490–1497. [PubMed] [Google Scholar]