Summary
Usually, harvesting free flap in the limbs creates an inevitable sequence of aesthetic damage not only in the donor site but also in the area of the graft used to repair the free flap donor site. Aim of the study was to standardize a simple method, defined Autonomous Reparative Unit, that allows closing of the donor site defects with a skin graft from the adjacent cutaneous area, avoiding further aesthetic damage in a third area. We define the “Autonomous Reparative Unit”the rectangular shaped skin area of the flap and the dermoepidermic skin graft designed as an isoscele triangle with the base adjacent to the smaller side of the flap defect. From 2003 to 2008, at the Fondazione IRCCS Istituto Nationale Tumori of Milan, 143 free radial forearm flaps and 42 free osteofasciocutaneus fibula flaps have been performed for head and neck cancer. The autonomous reparative unit has been applicable in 177 cases (92.1%). The autonomous reparative unit method allows a “standard”primary reconstructive unit to be created which can be used in a single or in multiple ways thus avoiding an additional surgical scar and a subsequent additional aesthetic impairment.
Keywords: Radial forearm free flap, Fibula free flap, Donor site morbidity, Donor site closure, Autonomous reparative unit (ARU)
Riassunto
L’utilizzo dei lembi liberi microvascolari crea un inevitabile sequenza di danni estetici prima nella sede donatrice, poi nella sede del prelievo dell’innesto per la riparazione della sede precedente. L’obiettivo è standardizzare una metodica semplice, da noi definita “Autonomous Reparative Unit”(ARU), che permetta la chiusura del difetto dell’area donatrice con innesto dermo-epidermico prelevato da area cutanea adiacente alla sede di prelievo, evitando così l’ulteriore danno estetico in una terza sede. L’ARU è composta dall’area di prelievo di cute del lembo di forma rettangolare più l’area di prelievo dermo-epidermico disegnata come triangolo isoscele a base adiacente al difetto del lembo. Dal 2003 al 2008, presso la Fondazione IRCCS Istituto Nazionale Tumori di Milano, sono stati allestiti 143 lembi radiale di avambraccio e 42 lembi di perone per neoplasie maligne cervico-facciali. L’ARU è stata applicabile in 177 casi (92,1%). La definizione dell’ARU permette di avere una unità ricostruttiva elementare, “standard", di facile applicabilità e ripetibilità, di uso singolo o multiplo per riparazioni più complesse.
Introduction
Revascularized free flaps are currently the “gold standard”in head and neck reconstruction following large oncologic resections. The free radial forearm flap (FRFF) and the free fibula osteofasciocutaneous flap (FFOF) are the most frequently used 1–4.
In both cases, several methods for recovering the exposed donor site have been reported 5.
Direct closure would be the method of choice, but is often not possible due to a too large defect and/or insufficient skin laxity. Other techniques described are: secondary healing, split-thickness skin graft, full-thickness skin graft 6–10, use of alloderm 11, skin expansion 12, local V-Y full-thickness skin transposition 13–15, Z-plasty closure 16, double-opposing rhomboid transposition flap 17, pursestring closure 18, local pedicled ulnar flaps 19, skin-stretching device and negative pressure wound dressing 20 21.
Each of these methods has its own limitations; surgeons must base their choice of one technique, with respect to another, on their clinical experience. Usually the donor site is repaired by a skin graft, which is normally harvested in a cutaneous area distant from the donor site, which causes an additional aesthetic impairment.
Some techniques, to avoid the additional scars, have been described, mainly based on local full-thickness skin graft (FTSG) 8 13–15 22 23. In our experience, it could be the most worthwhile solution to improve aesthetic results in such patients.
In this paper, our personal technique used for harvesting local FTSG is described. A simple solution, suitable for all reconstructions with a FRFF or FOFF, which allows repair of the donor site defect with a FTSG harvested in an adjacent cutaneous area in the same segment of the limb. Attention is focused on a description of the technique as well as an outline of the standard criteria adopted when employing it. This method, which we have called “Autonomous Reparative Unit”(ARU), is applicable for all free flaps needing closure with a skin graft.
Materials and methods
Description of the technique
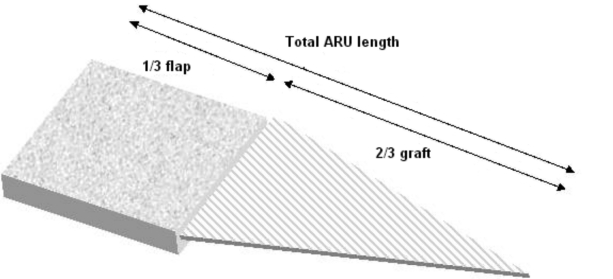
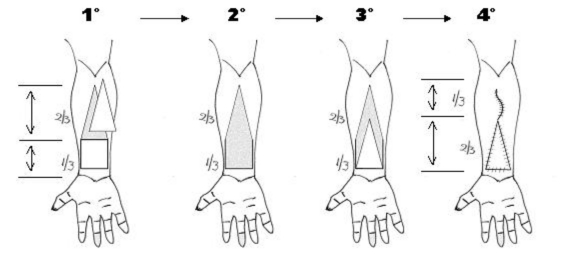
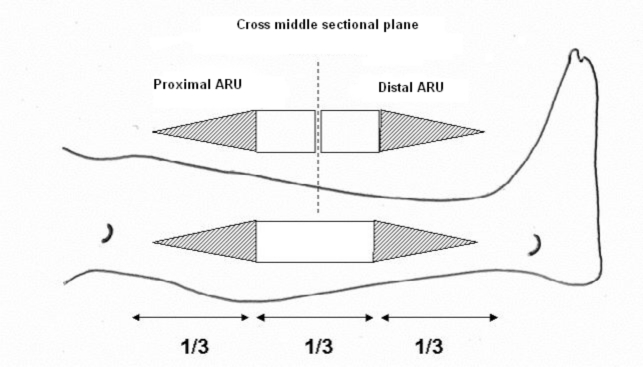
The concept of ARU is based on two components: the rectangular shaped skin flap area and the full-thickness skin graft designed as an isosceles triangle with its base adjacent to the smaller side of the flap defect (Fig. 1).
Fig. 1.
“Autonomous Reparative Unit”(ARU) components.
For the forearm flap it is possible to repair the defect with a single ARU, whereas for the fibula flap two ARUs are necessary.
a) Forearm Site
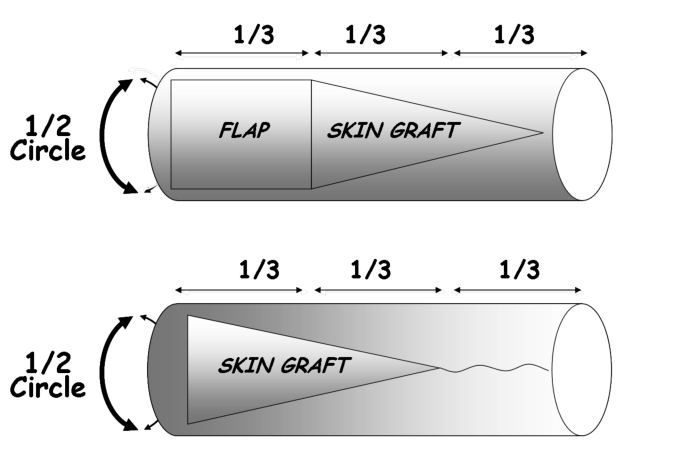
In the forearm, the FRFF is usually harvested, as a rectangle, in the distal third of the segment of the forearm with its larger side in the proximal-distal direction. In this technique, the forearm is to be considered as a cylinder with an axial length and a circumferential width. The maximum width of the ARU must not exceed 50% of the total circumference of the forearm as measured in the distal third (Fig. 2).
Fig. 2.
Top: planning of the forearm free flap by ARU method; Below: final result.
The ARU is based on the fasciocutaneous area of the forearm free flap and the isosceles triangle shaped area of the adjacent full-thickness skin graft. The height of the triangle should be twice the length of the flap (proximal-distal direction) (Fig. 3).
Fig. 3.
Stages of preparation and repair of the forearm donor site according to ARU method (1st: Full-thickness skin graft harvesting; 2nd Fasciocutaneous free flap harvesting; 3rd: Triangular skin graft positioning; 4th: Direct suture of the proximal third of the defect).
This means that the total length of the forearm is divided into three parts: the distal third corresponding to the design of the fasciocutaneous flap and the proximal two thirds corresponding to the skin graft.
In the first phase of this procedure, the triangular area of the full-thickness skin graft is harvested; then the radial forearm free flap is harvested with a conventional technique. The residual subcutaneous tissue of the proximal two thirds of the triangular area should be removed.
The donor site is closed by direct suture of the proximal third and by grafting the two distal thirds (Fig. 3).
b) Leg Site
Major reconstructive problems, in this site, are represented by the lesser elasticity of the cutaneous tissue and the central localization of the skin component of the flap. This makes the reparative design more complex.
Correct performance of the ARU technique requires that also the leg be ideally considered as a cylinder with an axial length and a circumferential width (Fig. 4).
Fig. 4.
Planning of the fibula free flap by Double-Rau method.
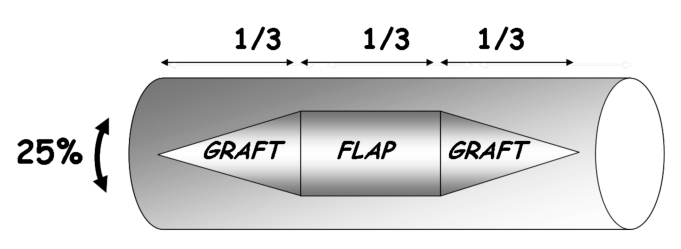
In order to better establish the theoretical design, it is necessary to hypotheitcally divide the leg into two hemi-cylinders of equal length: one cephalic and one caudal. For successful repair, two ARUs (proximal and distal) are necessary: each one must be the mirror image to a cross-sectional plane passing in the middle of the leg (Fig. 5).
Fig. 5.
Repair of donor site of leg divides this segment into two ARUs.
The initial design delimits a central rectangular area located over the central third of the leg and two triangular adjacent areas (proximal and distal third). This entity represents an approximately elliptic-shaped defect (Fig. 5).
The flap design is based on two ARUs (cephalic and caudal), each composed of 50% of the cutaneous rectangular area and an adjacent triangular skin graft area (Fig. 5).
Once the flap has been properly designed, the two triangular areas of full-thickness skin graft can be harvested, leaving a rectangular skin area in the central third.
Subsequently, the conventional osteofasciocutaneous free flap can be harvested.
The osteofasciocutaneous flap is eventually performed with a cutaneous area in which the central third is composed of total skin and the two lateral thirds are composed only of subcutaneous and dermal tissue. The two lateral thirds can either be sacrificed in order to obtain the initial design of the flap or spared, if necessary.
The donor site defect is closed by direct suture on the proximal and distal extremities; the central area is covered by two triangular skin grafts (Fig. 6).
Fig. 6.
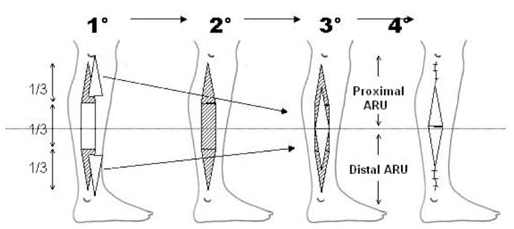
Stages of preparation and repair of donor site of leg according to ARU method (1st: harvesting of dermoepidermic skin grafts; 2nd: fasciocutaneous flap harvesting; 3rd: positioning of grafts over central third; 4th: direct suture of proximal and distal portions of defect).
Description of the series
Since 2003, 185 patients with oral and oro-pharyngeal cancers were submitted for the ARU technique and underwent a large surgical resection at the Cranio-Maxillo-Facial Surgery Unit of the National Cancer Institute (IRCCS Fondazione Istituto Nazionale Tumori) in Milan, Italy.
Average age of patients was 59.3 years, range (16-82 years), M/F ratio 1.4. All operations were performed by the same surgical team.
In 143/185 cases, reconstruction was performed with a FRFF. In 2 cases with small defects, these were closed by local flap. In other cases, 132/141 (93.6%) met the pre-operative criteria of the ARU method; in 9/141 (6.4%), the ARU method was not performed because the defects were more than 1/3 of the forearm needing a groin full-thickness skin graft.
In 42/185 patients, who needed mandibular reconstruction, a FOFF was used; in 6 cases, direct closure was performed, in 31/36 (86.2%) patients, double ARU was performed, only 5/36 (13.8%) cases did not meet the pre-operative criteria of the ARU method because the defects were more than 1/3 of the leg thus needing a groin full-thickness skin graft.
For the final analysis of data, the following variables were considered:
feasibility of the ARU method;
vitality of the graft evaluated in the post-operative time expressed as total loss, partial loss and absent/minimal (< 5%) loss;
scar evolution of the graft (30, 60, 180 days) assessed by clinical examination.
Results
The median follow-up was 18 months (range 4 months-6 years).
Forearm Site analysis
In all the 132 cases submitted to the ARU method, the forearm defect was successfully closed with an adjacent skin graft without tension. All the pre-operative criteria were applied and proved feasible in all cases. Special care was taken for the young patients and obese patients, for whom all the ARU criteria had to be strictly observed due to the reduced elasticity of the skin and the muscular mass.
As far as concerns the final evaluation of the vitality of the graft, 117/132 patients (88.6%) healed without or with only minimal (5%) loss, 15/132 (11.4%) showed partial loss. No total loss was recorded.
Exposure of the tendons occurred in two cases of partial skin graft loss; due to technical errors, as the paratenon was not respected during the flap harvesting, and thus not in direct correlation with the ARU method. Complete healing was achieved with O2 hyperbaric therapy.
As far as the scar evolution is concerned, in 108/132 cases (82%) a normal scar was reported, 24/132 cases (18%) had hypertrophic scars, whereas no keloid scar was observed. In those cases of hypertrophic scars and in the absence of exudations, elastocompressive supports and laser therapy were used. One patient, after 6 months showed severe and persistent dysaesthesia.
Leg Site analysis
In all 31 cases of fibula free flap submitted to the ARU method, the donor site was repaired with the double ARU technique and reconstruction was obtained without tension. Due to the reduced elasticity of the leg skin, it was necessary to strictly comply with the ARU application criteria.
From the final evaluation of the vitality of the graft, 23/31 patients (74.1%) healed without or only minimal (< 5%) loss, 7/31 (22.5%) partial loss while in one case total loss was recorded.
Discussion
The main aim of surgery, in head and neck oncology, is radical tumour resection, but the optimal restoration of the physiological functions remains mandatory.
Last but not least, all efforts must be made in order to limit the aesthetic impairments caused by cancer ablation, which may affect the patient’s quality of life 24.
The use of microvascular free flaps has become a widely accepted therapeutic choice in extensive head and neck reconstruction, due to the advantages it offers over conventional procedures. This extensive use of the free flap has shifted some of the focus, in head and neck reconstruction, towards limiting donor site morbidity 25–34.
Numerous techniques have been described for the repair of the donor site, most Authors have used skin grafts from distant sites (abdomen, thigh, groin, arm) 5–21, this obviously results in aesthetic and functional damage, both in the donor site and in the site in which the skin graft, for the wound closure, is harvested.
Local fasciocutaneous transposition flaps have been reported to yield good-quality skin and to heal rapidly without functional deficit 35. However, they usually require extensive dissection of the forearm, with subsequent sensory loss and oedema 36.
A bilobed flap, based on the fasciocutaneous perforator of the ulnar artery, does not require an additional donor site but is suitable for the closure of only small or medium-size donor defects. A long post-operative scar is the major disadvantage and it has a high sensory nerve damage rate. Furthermore, it requires a reatively highly skilled surgical performance 5.
Split skin graft, usually from the thigh, has been reported to cause very poor cosmetic appearance of the scar; and contraction of the split skin graft is significantly high, particularly on a poorly vascularised recipient bed 1.
Full-thickness grafts, often preferred on account of the better aesthetic result and better quality tissue, are commonly taken from the groin, requiring a separate operation field, additional operation time, more dressings, delayed healing, discomfort, itching, and conspicuous scarring 19. The scar may be mistaken for an abdominal operation, and Sleeman et al. 9 recommended using the left side presumably to avoid confusion with an appendicectomy scar.
The inner arm is another convenient donor site but requires additional time for removal of the tourniquet as well as further preparation and draping of the arm 4.
All these techniques can lead to a good result but still need a third scar, unlike the present ARU method.
Some Authors, concerning the use of FTSG from the ipsilateral proximal forearm, have reported good results in terms of aesthetics and function 15 37.
Gonzàles-Garcìa, et al. 22, reported that the combined local triangular FTSG closure of the FRFF donor site is a reliable technique, without the need of a distant donor site, which may preclude donor-site problems. However, although the results are good as far as concerns associated morbidity, the closure of the donor site, in a combined local FTSG triangular fashion, is limited by the size of the defect. This disadvantage can be eliminated with the use of the our ARU method, which proved reliable for large donor site defects 22.
The advantage of our ARU method is the possibility to establish well-defined criteria, easily applicable and repeatable, useful to the surgeons who wants to apply local FTSG as a technique for donor site repair.
The donor site of the skin graft can be associated with pain, infection and hypertrophic scar formation 38. Many donor site complications with the FRFF and FFOF procedures have been described 23 39–42.
Richardson et al. reported that healing of the donor site of the radial fasciocutaneous flap was compromised by loss of the skin graft (16%), exposure of a tendon (13%), and delayed healing (22%), with the additional problem of delayed healing at the split-skin graft donor site (24%) 19.
Suprafascial dissection of the FRFF limited donor-site complications even further 43–45.
In our series, harvested in a conventional fashion, without suprafascial dissection, considerably better results were achieved: 89% successful healing, 11% partial loss of the skin graft, 1.4% exposure of a tendon. No additional problems of delayed healing occurred at the split-skin graft donor site, simply because it doesn’t exist.
The only disadvantage of our method is the wider area grafted in the forearm; but our high rate of successful healing is probably due to the fact that the skin graft, although wider, is placed without tension.
The importance of our study is the introduction of the ARU technique which offers the possibility to restore the free flap bedside by using an adjacent skin area, without further morbidity and additional scar. Furthermore, the procedure is easily and rapidly performed and needs no special skill.
The ARU method, we developed, has been applied, so far, to the most common types of limb flaps, but appropriate modifications to the above criteria might result in this procedure becoming suitable for all cutaneous regions.
The ARU method permits the creation of a “standard”primary reconstructive unit, which is both manageable and repeatable. It can either be used as a single or as multiple units, depending on the type of reconstruction.
The major advantage of this technique is that it offers the possibility to reconstruct donor defects with adjacent tissue in a one-stage operation minimizing donor-site morbidity and improving overall aesthetic appearance since there is no longer a third scar in another site.
Fig. 7.
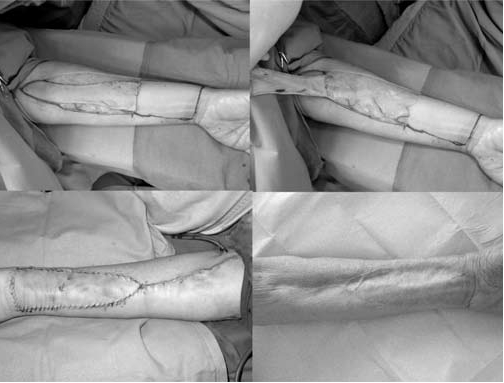
Intra-operative images of RFFF donor site repaired with ARU method.
Fig. 8.
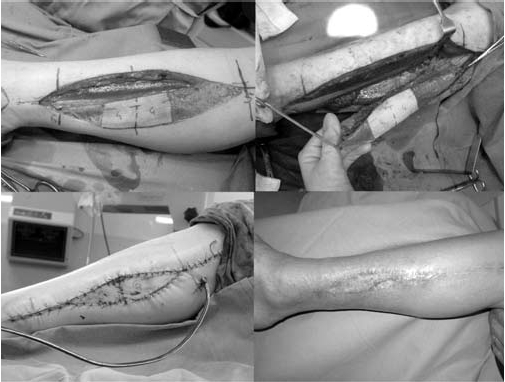
Case of fibula free flap donor site repaired with Double-ARU method.
Table I. Feasibility criteria of the ARU in the forearm free flap.
| 1) | The maximum width of the ARU must not exceed 50% of the total circumference of the forearm in the distal third |
| 2) | The length of the flap must be equal to one third of the total length of the ARU |
| 3) | The skin graft area must be an isoscele triangle with its base adjacent to the proximal side of the flap and an equal width |
| 4) | The length of the triangle must be twice the axial length of the flap, corresponding to the 2/3 proximal of the ARU |
| 5) | The apex of the ARU must not exceed the fold of the elbow in order to avoid retracting scars limiting the arm functionality |
Table II. Feasibility criteria of ARU in fibula free flap.
| 1) | The maximum width of the ARU must not exceed 25% of the total of the leg circumference |
| 2) | The length of the harvested cutaneous rectangular area must not exceed one third of the total length of the surgical defect |
| 3) | The area of the harvested skin graft must be an isoscele triangle with the base adjacent to the proximal and distal sides of the rectangular area, and with equal width |
| 4) | The length of every triangle must be equal to the length of the rectangular area |
References
- 1.González-García R, Naval-Gías L, Rodríguez-Campo FJ, et al. Vascularized free fibular flap for the reconstruction of mandibular defects: clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria E, Granados M, Barrera-Franco JL. Radial forearm free tissue transfer for head and neck reconstruction: versatility and reliability of a single donor site. Microsurgery 2000;20:195-201. [DOI] [PubMed] [Google Scholar]
- 3.González-García R, Naval-Gías L, Rodríguez-Campo FJ, et al. Reconstruction of oromandibular defects by vascularized free flaps: the radial forearm free flap and fibular free flap as major donor sites. J Oral Maxillofac Surg 2009;67:1473-7. [DOI] [PubMed] [Google Scholar]
- 4.Médard de Chardon V, Balaguer T, Chignon-Sicard B, et al. The radial forearm free flap: a review of microsurgical options. J Plast Reconstr Aesthet Surg 2009;62:5-10. [DOI] [PubMed] [Google Scholar]
- 5.Chau J, Harris J, Nesbitt P, et al. Radial forearm donor site: comparison of the functional and cosmetic outcomes of different reconstructive methods. J Otolaryngol Head Neck Surg 2009;38:294-301. [PubMed] [Google Scholar]
- 6.Avery CM, Iqbal M, Orr R, et al. Repair of radial free flap donor site by full-thickness skin graft from inner arm. Br J Oral Maxillofac Surg 2005;43, 161-5. [DOI] [PubMed] [Google Scholar]
- 7.Gaukroger MC, Langdon JD, Whear NM, et al. Repair of the radial forearm flap donor site with a full-thickness graft. Int J Oral Maxillofac Surg 1994;23:205-8. [DOI] [PubMed] [Google Scholar]
- 8.Van der Lei B, Spronk CA, de Visscher JG. Closure of radial forearm free flap donor site with local full-thickness skin graft. Br J Oral Maxillofac Surg 1999;37:119-22. [DOI] [PubMed] [Google Scholar]
- 9.Sleeman D, Carton AT, Stassen LF. Closure of radial forearm free flap defect using full-thickness skin from the anterior abdominal wall. Br J Oral Maxillofac Surg 1994;32:54-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim TB, Moe KS, Eisele DW, et al. Full-thickness skin graft from the groin for coverage of the radial forearm free flap donor site. Am J Otolaryngol 2007;28:325-9. [DOI] [PubMed] [Google Scholar]
- 11.Sinha UK, Shih C, Chang K, et al. Use of alloderm for coverage of radial forearm free flap donor site. Laryngoscope 2002;112:230-4. [DOI] [PubMed] [Google Scholar]
- 12.Hallock GG. Refinement of the radial forearm flap donor site using skin expansion. Plast Reconstr Surg 1988;81:21-5. [DOI] [PubMed] [Google Scholar]
- 13.Karimi A, Mahy P, Reychler H. Closure of radial forearm free flap donor site defect with a local meshed full-thickness skin graft: A retrospective study of an original technique. J Craniomaxillofac Surg 2007;35:369-73. [DOI] [PubMed] [Google Scholar]
- 14.Liang MD, Swartz WM, Jones NF. Local full-thickness skin-graft coverage for the radial forearm flap donor site. Plast Reconstr Surg 1994;93:621-5. [PubMed] [Google Scholar]
- 15.Shiba K, Iida Y, Numata T. Ipsilateral full-thickness forearm skin graft for covering the radial forearm flap donor site. Laryngoscope 2003;113:1043-6. [DOI] [PubMed] [Google Scholar]
- 16.Hui KC, Zhang F, Lineaweaver WC. Z-plasty closure of the donor defect of the radial forearm free flap. J Reconstr Microsurg 1999;15:19-21. [DOI] [PubMed] [Google Scholar]
- 17.Akyurek M, Safak T. Direct closure of radial forearm free flap donor sites by double-opposing rhomboid transposition flaps. J Reconstr Microsurg 2002;18:33-6. [DOI] [PubMed] [Google Scholar]
- 18.Winslow CP, Hansen J, Mackenzie D, et al. Pursestring closure of radial forearm fasciocutaneous donor sites. Laryngoscope 2000;110:1815-8. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CH, Kuo YR, Yao SF, et al. Primary closure of radial forearm flap donor defects with a bilobed flap based on the fasciocutaneous perforator of the ulnar artery. Plast Reconstr Surg 2004;113:1355-60. [DOI] [PubMed] [Google Scholar]
- 20.Avery C, Pereira J, Moody A, et al. Negative pressure wound dressing of the radial forearm donor site. Int J Oral Maxillofac Surg 2000;29:198-200. [PubMed] [Google Scholar]
- 21.Andrews BT, Smith RB, Chang KE, et al. Management of the radial forearm free flap donor site with the vacuum-assisted closure (VAC) system. Laryngoscope 2006;116:1918-22. [DOI] [PubMed] [Google Scholar]
- 22.González-García R, Ruiz-Laza L, Manzano D, et al. Combined local triangular full-thickness skin graft for the closure of the radial forearm free flap donor site: a new technique. J Oral Maxillofac Surg 2009;67:1562-7. [DOI] [PubMed] [Google Scholar]
- 23.Zuidam JM, Coert JH, Hofer SOP. Closure of the donor site of the free radial forearm flap. A comparison of full-thickness graft and split-thickness skin graft. Ann Plast Surg 2005;55:612-6. [DOI] [PubMed] [Google Scholar]
- 24.Bozec A, Poissonnet G, Chamorey E, et al. Quality of life after oral and oropharyngeal reconstruction with a radial forearm free flap: prospective study. J Otolaryngol Head Neck Surg 2009;38:401-8. [PubMed] [Google Scholar]
- 25.Chau J, Harris J, Nesbitt P, et al. Radial forearm donor site: comparison of the functional and cosmetic outcomes of different reconstructive methods. J Otolaryngol Head Neck Surg 2009;38:294-301. [PubMed] [Google Scholar]
- 26.Richardson D, Fisher SE, Vaughan FD, et al. Radial forearm flap donor-site complications morbidity: a prospective study. Plast Reconstr Surg 1997;99:109-15. [DOI] [PubMed] [Google Scholar]
- 27.Bardsley AF, Soutar DS, Elliot D, et al. Reducing morbidity in the radial forearm flap donor site. Plast Reconstr Surg 1990;86:287-92. [PubMed] [Google Scholar]
- 28.Avery C. Prospective study of the septocutaneous radial free flap and suprafascial donor site. Br J Oral Maxillofacial Surg 2007;45:611-6. [DOI] [PubMed] [Google Scholar]
- 29.Lutz BS, Wei FC, Chang SC, et al. Donor site morbidity after suprafascial elevation of the radial forearm flap: A prospective study in 95 consecutive cases. Plast Reconstr Surg 1999;103:132-7. [DOI] [PubMed] [Google Scholar]
- 30.De Witt CA, De Bree R, Verdonck-de Leeuw IM, et al. Donor site morbidity of the fasciocutaneous radial forearm flap: what does the patient really bother? Eur Arch Otorhinolaryngol 2007;264:929-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadopulos NA, Schaff J, Bucher H, et al. Donor site morbidity after harvest of free osteofasciocutaneous fibular flaps with an extended skin island. Ann Plast Surg 2002;49:138-44. [DOI] [PubMed] [Google Scholar]
- 32.Shpitzer T, Neligan P, Boyd B, et al. Leg morbidity and function following fibular free flap harvest. Ann Plast Surg 1997;38:460-4. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo DA, Rekow A. A review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg 1995;96:585-602. [PubMed] [Google Scholar]
- 34.Hidalgo DA, Disa JJ, Cordeiro PG, et al. A review of 716 consecutive free flaps for oncologic surgical defects: refinement in donor-site selection and technique. Plast Reconstr Surg 1998;102:722-34. [PubMed] [Google Scholar]
- 35.Sidebottom AJ, Stevens L, Moore M, et al. Repair of the radial forearm free flap donor site with full or partial thickness skin graft: a prospective randomized controlled trial. Int J Oral Maxillofac Surg 2000;29:194-7. [PubMed] [Google Scholar]
- 36.Hui KC, Zhang F, Lineaweaver WC. Z-plasty closure of the donor defect of the radial forearm free flap. J Reconstr Microsurg 1999;15:19-21. [DOI] [PubMed] [Google Scholar]
- 37.Elliot D, Bardsley AF, Batchelor AG, et al. Direct closure of the radial forearm flap donor defect. Br J Plast Surg 1988;41:358-60. [DOI] [PubMed] [Google Scholar]
- 38.Emerick KS, Deschler DG. Incidence of donor site skin graft loss requiring surgical intervention with the radial forearm free flap. Head Neck 2007;29:573-6. [DOI] [PubMed] [Google Scholar]
- 39.Ho T, Couch M, Carson K, et al. Radial forearm free flap donor site outcomes comparison by closure methods. Otolaryngol Head Neck Surg 2006;134:309-15. [DOI] [PubMed] [Google Scholar]
- 40.Juretic M, Car M, Zambelli M. The radial forearm free flap: our experience in solving donor site problems. J Cranio Maxillo Fac Surg 1992;20:184-6. [DOI] [PubMed] [Google Scholar]
- 41.Brown MT, Couch ME, Huchton DM. Assessment of donor-site functional morbidity from radial forearm fasciocutaneous free flap harvest. Arch Otolaryngol Head Neck Surg 1999;125:1371-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim PD, Fleck T, Heffelfinger R, et al. Avoiding secondary skin graft donor site morbidity in the fibula free flap harvest. Arch Otolaryngol Head Neck Surg 2008;134:1324-7. [DOI] [PubMed] [Google Scholar]
- 43.Avery CME, Pereira J, Brown AE. Suprafascial dissection of the radial forearm flap and donor site morbidity. Int J Oral Maxillofac Surg 2001;30:37-41. [DOI] [PubMed] [Google Scholar]
- 44.Kamal D, Fassio E, Goudot P, et al. Suprafascial dissection of the radial forearm flap. Ann Chir Plast Esthet 2009; in press. [DOI] [PubMed] [Google Scholar]
- 45.Chang SC, Miller G, Halbert CF, et al. Limiting donor site morbidity by suprafascial dissection of the radial forearm flap. Microsurgery 1996;17:136-40. [DOI] [PubMed] [Google Scholar]