Summary
The primary aim of this study was to investigate the efficacy of vestibular rehabilitation in a cohort of elderly labyrinthine-defective patients also affected by a moderate cognitive impairment of vascular origin. A secondary aim was to establish whether additional treatment with a cholinergic precursor (choline alphascerate) might enhance the results of the physical therapy in these patients. A retrospective clinical design was employed and data were collected from the vestibular rehabilitation treatment charts of 42 selected elderly patients who attended the tertiary referral centre of the Audiology and Vestibology of the University Hospital of Modena, Italy, in the period 1998-2008. Two groups of patients, well-matched for sex, age, and as close as possible for the vestibular examination upon admittance, were selected; Group A included 20 patients who had undergone vestibular rehabilitation training for one month and Group B included 22 patients who had attended the same physical therapy sessions as the former and had also received daily medication with 1200 mg of choline alphascerate per os. The outcome measures of the two forms of treatments were obtained from comparisons between posturographic and electronystagmographic examinations at baseline and 3 weeks after the end of treatment. Instrumental findings were completed by recording scores of the Dynamic Gait Index, the Dizziness Handicap Inventory and the Hospital Anxiety and Depression Scale before and after treatment. A statistically significant improvement in postural control (p < 0.05) and gait and balance performances (p < 0.005) was recorded in both groups; a relevant and statistically significant reduction of the asymmetry of the vestibular-ocular reflexes was also observed (p < 0.005). The self-rated dizziness handicap and psychological distress were significantly reduced (p < 0.005). Comparisons between the two groups revealed that patients who had also received medication, had achieved significantly better results than the other patients with respect to postural control in response to optokinetic stimulations (p < 0.05) and to Dynamic Gait Index (p < 0.05), thus suggesting, a reinforcement of cholinergic stimulation on vestibular compensation when tested in clinical conditions that require complex perceptual-motor skills and make a significant demand upon cognitive spatial processing resources. Further applications of stimulation of the cholinergic neurotransmission are discussed with particular regard to vestibular compensation in patients with no cognitive impairment or recurrent vertigo attacks of labyrinthine origin.
Keywords: Vertigo, Vestibular compensation, Choline alphascerate, Vascular dementia
Riassunto
Lo scopo primario di questo studio è di verificare l’efficacia della riabilitazione vestibolare in un gruppo di pazienti labirintopatici affetti da deficit cognitivo d’origine cerebro-vascolare. Un secondo obiettivo è di valutare se un concomitante trattamento farmacologico basato sulla stimolazione neurocolinergica (colina alfascerato) sia in grado di aumentare l’efficacia del protocollo riabilitativo. Si tratta di uno studio retrospettivo i cui dati sono stati raccolti dalle cartelle cliniche di 42 pazienti anziani selezionati tra coloro che sono stati sottoposti a riabilitazione vestibolare presso il Laboratorio di Audiologia e Vestibologia dell’Azienda Ospedaliero-Universitaria Policlinico di Modena nel periodo 1998-2008. Sono stati così composti due gruppi di pazienti sovrapponibili gli uni agli altri per sesso, età e, per quanto possibile, per le risultanze dell’esame vestibolare al momento dell’ammissione; nel Gruppo A sono stati inclusi 20 pazienti sottoposti ad un ciclo di riabilitazione vestibolare per un periodo di un mese e nel Gruppo B sono stati inclusi 22 pazienti che avevano inoltre assunto alfascerato di colina alla dose di 1200 mg/die per os per 1 mese. I risultati delle due modalità di trattamento sono stati confrontati analizzando i dati posturografici ed elettronistagmografici all’inizio e tre settimane dopo la fine della terapia. L’analisi dei dati strumentali è stata completata dai punteggi relativi al Dynamic Gait Index, dal Dizziness Handicap Inventory e dall’Hospital Anxiety and Depression Scale somministrati ai pazienti prima e dopo terapia. È stato osservato un netto miglioramento sia del controllo posturale statico che dinamico in entrambi i gruppi (p < 0,05), e un miglioramento ancora più significativo dei riflessi vestibolo-oculo-motori (p < 0,005); parallelamente si è rilevata una riduzione significativa della percezione dell’ handicap correlato alla vertigine e del distress psicologico (p < 0,05). Dallo studio comparativo tra i due gruppi si è potuto osservare che i pazienti sottoposti sia a terapia fisica che farmacologica avevano ottenuto miglioramenti superiori agli altri nei test posturografici eseguiti con stimolazione otticocinetica e del Dynamic Gait Index (p < 0,05); ciò documenta un’azione di rinforzo della stimolazione neurocolinergica sul compenso vestibolare laddove questo venga testato in condizioni di esame che richiedono il possesso di maggior abilità percettivo-motorie e maggiori risorse a livello cognitivo per l’orientamento spaziale. Ulteriori studi sono necessari per verificare l’eventuale efficacia della stimolazione neurocolinergica nei pazienti labirintopatici senza impairment cognitivo.
Introduction
Vertigo and dizziness, as a consequence of damage to the vestibular end organ, are known to improve spontaneously due to central adaptive mechanisms. These mechanisms recalibrate the input from the undamaged vestibular system and from visual and somatosensory systems, and substitute for the input from the damaged labyrinth 1. However, the recalibration of the vestibulo-ocular and the vestibulo-spinal reflexes is not always complete as it is generally accepted and several patients with labyrinthine hypofunction complain of a residual symptomatology, namely oscillopsia and unsteadiness, and become chronically disabled.
At present, there is no explanation for patients’ incomplete recovery even if the tendency to chronic vertigo and dizziness is more frequently observed in patients with restricted daily activities 2 that prevent them from being exposed to body motion-induced vertigo, which is considered the most appropriate stimulus for vestibular compensation 3 4. Another cause that could potentially interfere with functional recovery is co-morbidity with vascular diseases that affect specific areas and neural pathways of the central nervous system that are thought to be directly involved in vestibular compensation 5. It should also be pointed out that multisensory deficits, drugs, or systemic diseases may cause postural and gait disturbances per se, and also account for persistent dizziness together with vestibular disorders 6. Moreover, it has been demonstrated that patients with peripheral vestibular lesions 7 8 require more attention, for postural control, than healthy adults and it has also been shown that vestibulo-ocular-reflexes are partially dependent on cognitive resources and are not completely automatic 9 thus suggesting the hypothesis that cognitive impairment might interfere with vestibular compensation. Overall, these results suggest that the progression of compensation, from vestibular damage, in the elderly, could be critical for more than one reason.
It is not surprising, therefore, that even if the efficacy of vestibular rehabilitation treatment (VRT) in improving both symptoms and physical performances of subjects with unilateral vestibular loss is proved both in adults and the elderly 10 11, this last age group is not expected to reach such good results as those in younger age groups 12. It has been suggested that elderly patients show less improvement in their balance performances than younger adults especially when affected by a multifactorial reduction in central nervous system plasticity and cognitive resources that may interfere with recovery mediated by adaptation training of the vestibular and spinal systems 13.
Aim of this retrospective study was: 1. to test the hypothesis that results of VRT in elderly patients with peripheral vestibular lesions and impaired cognitive resources, due to reduced cerebral vascular perfusion, could lead to a significant improvement in physical balance, and, 2. to establish whether this achievement could be further enhanced by neurocholinergic stimulation, known to be effective in the treatment of cognitive impairment of different origin, namely vascular 14 15, and is reported to directly improve vestibular compensation 16 17.
Material and Methods
Data were collected from the clinical charts of 42 elderly patients (22 male, 20 female, mean age = 69.9 yrs, SD = 7.3, range 57-81 yrs) selected from all dizzy patients, referred by other physicians, to the tertiary centre of Audiology and Vestibology of the University Hospital of Modena, Italy, where the physical therapy exercise programme was completed in the period 1998-2008.
Patients’ charts were taken into consideration and included in the analysis according to the following criteria:
presence of a well-documented unilateral and persistent vestibular hypofunction (as a consequence of various labyrinthine disorders, such as vestibular neuritis, labyrinthitis, labyrinthine concussion and infarction, Ménière’s disease);
complaints of persistent vertigo/dizziness lasting at least 6 months (mean duration = 25.4 SD = 9.2) before admittance;
Mini-Mental State Examination (MMSE) 18 suggesting the presence of a mild cognitive impairment i.e.,with a score ≤ 24 and >18, according to age and educational level using the score-adjustment coefficients proposed by Magni et al. 19;
Cerebral Magnetic Resonance Imaging (MRI) positive for cerebral vascular disease (ischaemic white matter lesions, lacunar infarction of cortical and sub-cortical areas) and 99mTc single-photon emission tomography (SPET) positive for reduced regional cortical perfusion 20.
Patients were divided into 2 subgroups (A and B), well-matched for age, sex, cognitive decline, and as close as possible also results of vestibular tests, in order to obtain comparisons between two homogeneous samples.
Patients from both Groups A and B had given informed consent to the proposed treatment and had been submitted to the VRT programme for one month, attending 8 sessions, but only Group B patients had also received medication with choline alphascerate (L-α- glyceryl phosphorylcholine – GFC) per os at a dose of 1200 mg/day for one month. GFC, a semi-synthetic derivative of phosphatidylcholine, was initially hypothesised to improve the cognitive deficit in experimental models of aging brain 21 22 and, later, its efficacy was confirmed in patients affected by a cognitive decline of vascular origin 23. In these subjects, GFC is effective in reducing clinical symptoms such as memory and attention impairment, affective disorders, as well as somatic symptoms, such as fatigue and dizziness and, therefore, it has been widely used for this purpose in elderly patients 24.
Instrumental investigation
According to our standard diagnostic protocol, all patients, upon admittance, are submitted to an electro-oculo-graphic battery that includes tests for exploring oculomotor (saccades and smooth-pursuit) and optokinetic functions, spontaneous and gaze nystagmus, three cycles of sinusoidal rotation testing with a maximum speed of 60°/sec for vestibulo-oculomotor reflex (VOR) and bi-thermal irrigation (30°C and 44°C) of both ear canals for which the vestibular paresis formula of Jongkees et al. 25 is used. Vestibular paresis is defined as > 25% asymmetry between the right-side and left-side responses at caloric test 26.
The asymmetry of the vestibulo-ocular reflexes, in the pendular rotation chair testing, is used as a measure of vestibular compensation. The asymmetry index is established by computing the mean asymmetry of the maximal angular velocity of the slow phase of pendular nystagmus across the three-cycle rotational test expressed in degree according to the formula: (AVSP, normal labyrinth – AVSP, defective labyrinth)/(AVSP, normal labyrinth + AVSP, defective labyrinth)*100 as originally suggested by Norrè et al. 26.
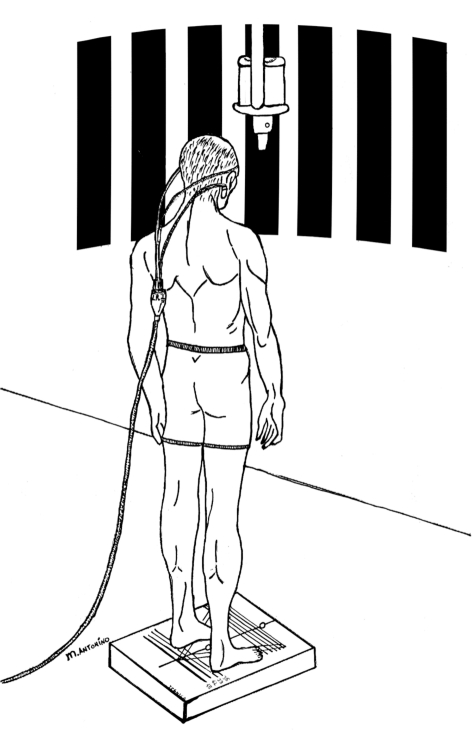
Posturography is performed by means of a stable force-plate sensitive to vertical forces. Patients were requested to maintain a relaxed, motionless upright stance, stand bare foot with feet at an angle of 30°, with a natural head-neck posture, under 4 different conditions: 1) gazing at a steady, vertical light bar at a distance of 150 cm (EO), 2) with eyes closed in total darkness (EC), 3-4) facing the wall in front on which a special projector offered a full-field linear optokinetic stimulation (OKN) represented by vertical alternating dark and light stripes 30 cm in diameter, delivered at a speed of 30°/s, in the horizontal plane (Fig. 1). These two trails are routinely employed, in our laboratory, to test postural reaction to visual-vestibular conflicting environmental conditions which are known to be critical for patients with an uncompensated vestibular lesion 28 29. The direction of the OKS is first delivered towards the normal labyrinth (OKN - NL) and then to the defective one (OKN - DL). Patients are instructed to look carefully at the moving stripes and to mentally count them; they are also informed that they will be asked for the result after each test. This mental task was originally introduced in order to avoid a decrease in alertness which could negatively affect the optokinetic reflex 30. The sway path is computed by continuously detecting the body’s centre of pressure and calculating an elliptic area which corresponds to 90% of the positions of the centre of pressure over time and expressed in millimetres. This procedure was designed to eliminate 10% of the more extreme positions which could be due to involuntary perturbations of quiet stance.
Fig. 1.
Experimental setting for detecting postural response to horizontal optokinetic stimulation in labyrinthine-defective patients.
Clinical Examination and Questionnaires
Dynamic Gait Index (DGI) 31 is the usual scale employed to measure mobility function and dynamic balance. The 8 tasks of this scale include: walking, walking while changing speed and direction, walking while turning the head, walking over and around obstacles and stair climbing. Scoring of the DGI is based on a 4-point scale from 0 to 3, with 0 indicating severe impairment and 3 indicating normal ability.
The Dizziness Handicap Inventory (DHI) 32 is the questionnaire routinely used to measure self-perceived dizziness disability. It consists of 25 items, sub-grouped into 3 domains, that address physical (7 items), emotional (9 items) and functional (9 items) perceived disability. Possible answers are "yes" (scored as 4), "sometimes" (scored as 2) and "no" (scored as 0). The Total DHI score ranges from 100, indicating maximal perceived disability to 0, indicating no perception of disability.
Patients are also given a second list of 14 items, the Hospital Anxiety and Depression Scale (HADS) 33, which is a self-rating questionnaire used to measure anxiety and depression on two separate scales and it is routinely employed as a screening tool to assess the level of psychological distress that could modify the perception of the dizziness handicap in otoneurological patients 34. There are 4 possible answers for each item (score: 0-3) expressing an increasing level of severity. Each participant provides informed consent before starting the rehabilitation programme.
VRT protocol
Each patient underwent a 2-hour session twice a week for one month including optokinetic stimulation 35 and saccadic eye tracking 36 designed to improve postural control during quiet stance, standing on an unstable platform with a LC screen in front of them providing a visual feedback of their own body oscillation designed to improve dynamic balance control by impairing the exploitation of the kinematic ankle proprioceptive and plantar pressure somatosensation 37. Gaze stabilization exercises, i.e., head turning in the horizontal plane while staring at a target held at eye level and at a distance of 1 metre from their eye, at an increasing speed until oscillopsia and/or dizziness occurs, and repeated, 10 times consecutively, also on the vertical plane, completed the session.
A final re-examination is performed 2 weeks after VRT including the rotatory chair test to evaluate VOR asymmetry, posturography, DGI, DHI and HADS.
Statistical analysis
A preliminary χ2 test was used to define sex distribution (categorical variable) and an independent T-test was used to test age and MMSE in the two groups (continuous variables). Thereafter, in order to determine the assumption that VHT is really effective in reducing symptoms and increasing physical performances, the paired T-test test was used to compare posturographic findings, VOR asymmetry, DGI, DHI, HADS scores before and after vestibular rehabilitation treatment in the two samples. Finally, an independent T-test was used to compare the results of VHT alone and in association with GFC in the two groups. The level of statistical significance was set at p < 0.05 in all procedures. The statistical Package SPSS version 14 was used.
Results
Preliminary statistics
Mean ages of Group A (VHT alone) and Group B (VHT + GFC) were 69.8 (SD = 7.2) and 70.1 (SD = 7.5), respectively, and were not statistically different (t = -.106, p = 916). Groups A and B comprised, respectively, 9 males and 11 females and 6 males and 16 females and the Pearson chi-square value resulted 1.434 (Asymp. Sig, two-tailed = 0.231) indicating no statistically significant difference in the distribution of patients with respect to sex. MMSE scores were 22.1 (SD = 1.3) and 21.4 (SD = 1.5) for Groups A and B, respectively, with no statistically significant difference (t = 1.5, p = 0.129).
Outcome measurements in the two groups
Overall, static balance control improved after VRT in Group A as documented by a significant reduction of body sway in the open eye condition (p < 0.05) and during both OKS towards the normal labyrinth (p = 0.005) and the defective one (p < 0.005). No difference was found for body sway in the EC condition (p > 0.05). Group B patients also exhibited a significant reduction of body sway in EO, OKS-NL and OKS-DL and no improvement in EO condition (Table I).
Table I. Mean values and standard deviations of posturographic parameters, VOR asymmetry, DGI, DHI and its subscale scores, HADS and its subscale scores before and after treatment in the two groups (A and B).
| Before treatment | After treatment | Paired t-test | |||||
| Mean | SD | Mean | SD | t | p | ||
| EO | A | 459.4 | 105.7 | 397.0 | 68.6 | 2.4 | 0.027* |
| B | 361.8 | 142.2 | 240.6 | 105.2 | 6.7 | < 0.005** | |
| EC | A | 885.4 | 415.5 | 807.0 | 408.0 | 1.2 | 0.237 |
| B | 788.9 | 336.9 | 695.2 | 301.5 | 2.0 | 0.54 | |
| OKS-NL | A | 405.3 | 124.8 | 374.3 | 116.6 | 3.1 | 0.005** |
| B | 436.4 | 146.7 | 325.9 | 93.9 | 3.6 | 0.002** | |
| OKS-DL | A | 780.3 | 327.4 | 618.9 | 230.3 | 4.2 | 0.001** |
| B | 677.1 | 203.5 | 359.9 | 75.2 | 6.6 | < 0.005** | |
| DP | A | 15.1 | 7.3 | 5.3 | 4.7 | 8.1 | < 0.005** |
| B | 16.4 | 6.0 | 3.4 | 4.7 | 11.4 | < 0.005** | |
| DGI | A | 14.6 | 3.4 | 16.9 | 3.3 | -3.0 | 0.007* |
| B | 15.4 | 4.2 | 20.5 | 3.1 | -6.6 | < 0.005** | |
| DHI | A | 48.6 | 15.6 | 40.4 | 14.2 | 8.7 | < 0.005** |
| B | 53.6 | 19.5 | 37.4 | 8.9 | 5.9 | < 0.005** | |
| DHI (phy) | A | 18.1 | 8.5 | 15.8 | 8.2 | 4.2 | < 0.005** |
| B | 19.9 | 8.7 | 11.2 | 3.2 | 5.5 | < 0.005** | |
| DHI (func) | A | 13.7 | 8.3 | 11.6 | 7.1 | 5.5 | < 0.005** |
| B | 15.2 | 8.3 | 11.2 | 6.0 | 5.3 | < 0.005** | |
| DHI (emot) | A | 16.8 | 4.6 | 13.0 | 3.7 | 5.9 | < 0.005** |
| B | 18.4 | 6.1 | 15.0 | 2.8 | 3.0 | 0.006* | |
| HADS | A | 22.3 | 3.7 | 17.5 | 3.3 | 5.3 | < 0.005** |
| B | 21.3 | 5.0 | 15.6 | 2.8 | 7.2 | < 0.005** | |
| HADS (anx) | A | 10.3 | 2.9 | 7.8 | 2.6 | 2.7 | 0.14* |
| B | 10.9 | 3.0 | 8.4 | 2.3 | 5.0 | < 0.005** | |
| HADS (dep) | A | 12.0 | 2.5 | 9.6 | 1.8 | 4.0 | 0.001** |
| B | 10.4 | 2.7 | 7.2 | 1.3 | 6.7 | < 0.005** | |
* p < 0.05 and ** p < 0.005.
VOR asymmetry, as tested by the pendular rotational test, was significantly reduced after treatment in both Groups A and B (p < 0.005) (Table II) as a further confirmation of the efficacy of gaze stabilisation exercises.
Table II. Independent T-test for mean values of differences in posturographic parameters (body sway area) in the 4 trials, VOR asymmetry, DGI, DHI and its subscale scores, HADS and its subscale scores before and after treatment in the 2 groups (A and B).
| Before therapy | After therapy | Δ | Independent T-test (for Δ) | ||||||
| Mean | SD | Mean | SD | Mean | SD | T | p | ||
| EO | A | 459.4 | 105.7 | 397.0 | 68.6 | 62.3 | 116.6 | -1.9 | 0.068 |
| B | 361.8 | 142.2 | 240.6 | 105.2 | 121.1 | 85.0 | |||
| EC | A | 885.4 | 415.5 | 807.0 | 408.0 | 78.4 | 286.9 | -0.197 | 0.845 |
| B | 788.9 | 336.9 | > 695.2 | 301.5 | 93.7 | 216.5 | |||
| OKS-NL | A | 405.3 | 124.8 | 374.3 | 116.6 | 31.0 | 43.3 | -2.3 | 0.023* |
| B | 436.4 | 146.7 | 325.9 | 93.9 | 110.5 | 144.2 | |||
| OKS-DL | A | 780.3 | 327.4 | 618.9 | 230.3 | 161.4 | 173.2 | -2.5 | 0.017* |
| B | 677.1 | 203.5 | 359.9 | 75.2 | 317.2 | 225.7 | |||
| DP | A | 15.1 | 7.3 | 5.3 | 4.7 | 9.8 | 5.4 | -1.8 | 0.70 |
| B | 16.4 | 6.0 | 3.4 | 4.7 | 12.9 | 5.3 | |||
| DGI | A | 14.6 | 3.4 | 16.9 | 3.3 | -2.3 | 3.4 | 2.4 | 0.015* |
| B | 15.4 | 4.2 | 20.5 | 3.1 | -5.0 | 3.6 | |||
| DHI | A | 48.6 | 15.6 | 43.0 | 13.5 | 8.2 | 4.2 | -3.5 | 0.012* |
| B | 53.6 | 19.5 | 37.4 | 8.9 | 16.2 | 12.9 | |||
| DHI (phy) | A | 18.1 | 8.5 | 15.8 | 8.2 | 2.3 | 2.4 | -3.7 | 0.001** |
| B | 19.9 | 8.7 | 11.2 | 3.2 | 8.7 | 7.4 | |||
| DHI (func) | A | 13.7 | 8.3 | 11.6 | 7.1 | 2.1 | 1.8 | -2.2 | 0.032* |
| B | 15.2 | 8.3 | 11.2 | 6.0 | 4.0 | 3.4 | |||
| DHI (emot) | A | 16.8 | 4.6 | 13.0 | 3.8 | 3.8 | 2.3 | 0.332 | 0.741 |
| B | 18.4 | 6.1 | 15.0 | 3.4 | 3.4 | 5.2 | |||
| HADS | A | 22.3 | 3.7 | 17.5 | 3.3 | 4.8 | 4.1 | -0.691 | 0.494 |
| B | 21.3 | 5.0 | 15.6 | 2.8 | 0.0 | 4.0 | |||
| HADS (anx) | A | 10.3 | 2.9 | 7.8 | 2.6 | 2.1 | 4.4 | 1.4 | 0.928 |
| B | 10.9 | 3.0 | 8.4 | 2.7 | 0.5 | 3.2 | |||
| HADS (dep) | A | 12.0 | 2.5 | 9.6 | 1.8 | 2.3 | 2.6 | -1.2 | 0.231 |
| B | 10.4 | 2.7 | 7.2 | 1.3 | 3.3 | 2.3 | |||
* p < 0.05 and ** p < 0.005.
The DGI score was significantly increased after treatment indicating the achievement of better dynamic balance and gait performances in both Groups A (p < 0.05) and B (p < 0.005) (Table I). Also the self-perceived dizziness handicap was globally reduced as indicated by the total score of DHI (p < 0.005) in both groups. The three sub-scores almost equally contributed to the afore-mentioned global result since this reduction was significant in all cases (physical: p < 0.005; functional: p < 0.005; emotional: p < 0.05) (Table I).
The overall level of self-rated psychological distress significantly decreased after VRT in both groups (p < 0.005); depression score reduction was the decisive factor for psychological improvement (p < 0.005) in both groups while the anxiety score improved less significantly in Group A (p < 0.05) than in Group B (p < 0.005) (Table I).
Group A (VRT alone) versus Group B (VRT + GFC)
The reduction in body sway after VRT, both in EO and EC conditions, was not statistically different in Group B patients with respect to those of Group A (p > 0.05) (Table II). Body sway, on the contrary, during OKS to both sides, was significantly more reduced in Group B than in Group A, as documented by a statistically greater difference between baseline and final values (p < 0.05). VOR asymmetry reduction did not vary between Group B and Group A after treatment (p > 0.05) (Table II).
However, gait and balance dynamic performances, as scored by DGI, showed a greater improvement in patients in Group B, compared to those in Group A (p < 0.05) (Table II).
Patients in Group B showed a greater improvement of self-rated dizziness handicap than patients of Group A as measured by DHI total scores; mean differences were statistically different between the 2 groups (p < 0.001) (Table II). The three subscale scores contributed in different ways to the afore-mentioned difference in the overall self-perceived dizziness handicap, in the two groups. ‘Physical’ score reduction was the most relevant factor that generated the difference between groups (p < 0.001) while the difference in the ‘functional’ score was less relevant (p < 0.05) and no difference was found with respect to the ‘emotional’ score (p > 0.150) (Table II).
Finally, the overall level of self-rated psychological distress was comparable in patients of Groups B and A (p > 0.05). The anxiety subscore was comparable in the two groups (p > 0.05) but the depression subscore was greatly reduced in Group B patients with respect to those in Group A (p < 0.05).
Discussion
The most important observation emerging from this study is that VRT is confirmed to be a valid tool in the improvement of vestibular compensation with respect to both vestibular-ocular and vestibular-spinal impairment also in elderly labyrinthine-defective patients with cerebral vascular disease. Moreover, outcomes of VRT are more than satisfying, not only if evaluated by instrumental or clinical methods, but also from a subjective point of view, as documented by a significant reduction in the self-rated dizziness handicap thus excluding a desynchronization between physical improvement and its mental perception. In addition, this study confirms the recent observation 38 that the achievement of better gait and balance performances is accompanied by a reduction in psychological distress particularly with regard to anxiety, the somatic component of which is not only implicated in the enhancement of autonomic symptoms 39 but also accounts for a deterioration in the quality of life 40. It should be observed that a reduction in anxiety per se might allow labyrinthine-defective patients to achieve better postural control if exposed to moving visual scenes like those generated by optokinetic stimulation 41 on account of an increased capacity to resolve the sensorial mismatch inside the central nervous system. At the same time, the reduction in anxiety may also be the consequence of repeated exposure to a destabilizing environments, like those employed in VRT, which are carefully avoided by patients because of their annoying effect on everyday life. This repeated and prolonged exposure to visual disorientating conditions may actually reduce vertigo-related anxiety by the so-called ‘desensitization’ process which partially overlaps the basic concept of behavioural therapy for panic disorders the key ingredient of which is merely exposure to the feared stimuli 42.
Indeed, the reinforcement of postural control was found to be greater in all the posturographic trials, where both static and moving visual cues are available, than in eyes-closed condition, possibly because VRT, the exercises of which are actually based on visual stimuli, forces the CNS to ‘re-weight’ and rely much more on sensory inputs from the visual system for balance than from the vestibular end-organ the information of which is reduced, absent or misleading. As a consequence, this study confirmed that postural improvement is less appreciable when visual cues are not available.
In both cases, great values of standard deviations would suggest a great inter-individual difference in the improvement of static postural control that could depend on a large variety of variables, ranging from motivation to fatigue and attention 43, the latter being considered a crucial factor for the central processing of information required for the perception and control of orientation 44.
From this point of view, it is not surprising that an additional factor that enhances the efficacy of VRT in patients with reduced cognitive resources is simultaneous treatment with choline alphascerate relieving the learning and memory alterations which are found in the adult-onset of cerebral vascular disorders due to a combination of reduced nicotinic cholinergic neurotransmission, loss of the acetylcholine synthesizing enzyme choline acetyl-transferase and reduction of nicotinic cholinergic receptors 14 45. It is worthwhile pointing out that patients who received the additional treatment with choline alphascerate finally performed better than those not receiving this treatment in those trials (OKS, DGI) which more closely resemble perceptual-motor skills than basic reflex (EO, EC, pendular rotational test) and making a significant demand upon the cortical and subcortical spatial processing resources 9. Since also patients with vestibular disorders and no cognitive impairment present a persistent and/or recurrent vertigo in periods of increased mental activity, fatigue, and stress 47, it is not surprising that the adaptation (that is i.e. a "learning process") to vestibular imbalance promoted by VRT could be reinforced in patients with cognitive decline by those therapeutic modalities that enhance the attentional resources necessary for the more complex orientation activities. Obviously, it is not possible with the design of this study to distinguish the effect of cholinergic stimulation on central nervous system plasticity from its hypothetical direct effect on the vestibular efferent pathways.
These results collectively suggest further indications for the use of choline precursors; first of all, stimulation of cholinergic neurotransmission might be useful in promoting vestibular compensation also in patient affected by an acute labyrinth failure and without cognitive decline.
This hypothesis is also supported by recent studies that provided evidence that human efferent vestibular axons and terminals could be cholinergic and that the receptors receiving this innervation are both nicotinic 46 and muscarinic 47.
Finally, it is worthwhile suggesting that neurocholinergic stimulation could be extended to those labyrinthine-defective patients in whom vertigo recurrences are closely related to increased mental activity and fatigue as precipitating factors 48.
Conclusions
Vestibular rehabilitation treatment is herewith confirmed to be a useful method to promote a more efficient recovery from labyrinthine hypofunction also in elderly patients in whom a cognitive decline of vascular origin is often present and could account for an incomplete vestibular compensation process.
The outcome of vestibular rehabilitation treatment could be enhanced by simultaneous pharmacological stimulation of cholinergic neurotransmission which is considered to be directly involved in vestibular compensation and that is known to improve those cognitive functions (memory, attention, visual-spatial orientation) which vestibular compensation makes significant demand upon.
Acknowledgments
Authors thank Dr. Roberto Gabriele and Dr. Claudio Celi, Medical Division of M.D.M. Srl, Via Volturno 29, 20052 Monza - Milan (Italy) for scientific support in the study and for personally encouraging them to improve clinical trials in the fields of otoneurological sciences.
References
- 1.Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vest Res 1995;2:67-107. [PubMed] [Google Scholar]
- 2.Whitaker SR, Parker K, Chandler S, et al. Balance disorders in the elderly. In: Arenberg IK, editor. Dizziness and balance disorders: an interdisciplinary approach to diagnosis, treatment, and rehabilitation. Amsterdam, New York: Kugler Publications; 1993. p. 767-81. [Google Scholar]
- 3.Cawthorne T. Vestibular injuries. Proc R Soc Med 1946;39:270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mc Cabe BF. Experiments on vestibular compensation. Laryngoscope 1969;79:1728-36. [DOI] [PubMed] [Google Scholar]
- 5.Furman JM, Balaban CD, Pollack IF. Vestibular compensation in a patient with a cerebellar infarction. Neurology 1997;48:916-20. [DOI] [PubMed] [Google Scholar]
- 6.Katsarkas A. Dizziness in aging: a retrospective study of 1194 cases. Otolaryngol Head Neck Surg 1994;110:296-301. [DOI] [PubMed] [Google Scholar]
- 7.Andersson G, Hagman J, Talianzadeh R, et al. Dual-task study of cognitive and postural interference in patients with vestibular disorders. Otol Neurotol 2003;24:289-93. [DOI] [PubMed] [Google Scholar]
- 8.Yardley L, Gardner M, Bronstein A, et al. Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiatry 2001;71:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talkowski ME, Redfern MS, Jennings JR, et al. Cognitive requirements for vestibular and ocular motor processing in healthy adults and patients with unilateral vestibular lesions. J Cogn Neurosci 2005;17:1432-4. [DOI] [PubMed] [Google Scholar]
- 10.Hillier SL, Hollohan V. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev 2007;4:CD005397. [DOI] [PubMed] [Google Scholar]
- 11.Whitney SL, Wrisley DM, Marchetti GF, et al. The effect of age on vestibular rehabilitation outcomes. Laryngoscope 2002;112:1785-90. [DOI] [PubMed] [Google Scholar]
- 12.Badke MB, Shea TA, Miedaner JA, et al. Outcomes after rehabilitation for adults with balance dysfunction. Arch Phys Med Rehabil 2004;85:227-33. [DOI] [PubMed] [Google Scholar]
- 13.Cooke DL. Central vestibular disorders. Neurol Rep 1996;20:22-9. [Google Scholar]
- 14.Amenta F, Di Tullio MA, Tomassoni D. The cholinergic approach for the treatment of vascular dementia: evidence from pre-clinical and clinical studies. Clin Exp Hypertens 2002;24:697-713. [DOI] [PubMed] [Google Scholar]
- 15.Doggrell SA, Evans S. Treatment of dementia with eurotransmission modulation. Expert Opin Investig Drugs 2003;12:1633-54. [DOI] [PubMed] [Google Scholar]
- 16.De Lacalle S, Hersh LB, Saper CB. Cholinergic innervation of the human cerebellum. J Comp Neurol 1993;3: 364-76. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima M, Kitahara T, Takeda N, et al. Role of cholinergic mossy fibers in medial vestibular and prepositus hypoglossal nuclei in vestibular compensation. Neuroscience 2001;102:159-66. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res 1975;12:189-98. [DOI] [PubMed] [Google Scholar]
- 19.Magni E, Binetti G, Bianchetti A, et al. Mini-mental State Examination: a normative study in Italian elderly population. Eur J Neurol 1996;3:1-5. [DOI] [PubMed] [Google Scholar]
- 20.Guermazi A, Miaux Y, Rovina-Cañellas A, et al. Neuroradiological findings in vascular dementia. Neuroradiol 2007;49:1-22. [DOI] [PubMed] [Google Scholar]
- 21.Canonico Pl, Nicoletti F, Scapagnini U. Effetti neurochimici e comportamentali di αGFC (Colina Alfascerato). Basi Raz Ter 1990;20:53-4. [Google Scholar]
- 22.Drago F, Nardo L, Freni V, et al. Effetti comportamentali di αGFC in modelli di invecchiamento cerebrale patologico. Basi Raz Ter 1990;20:65-8. [Google Scholar]
- 23.Muratorio A, Bonuccelli U, Nuti A, et al. A neurotropic approach to the treatment of multi-infarct dementia using L-Alpha-Glyceryl-phosphorylcholine. Curr Ther Res 1992;52:741-51. [Google Scholar]
- 24.Ban Ta, Panzarasa RM, Borra S, et al. Choline Alphascerate in elderly patients with cognitive decline due to dementing illness. New Trends Clin Neuropharmacol 1991;5:1-35. [Google Scholar]
- 25.Jongkees LB, Maas JP, Philipszoon AJ. Clinical nystagmo-graphy: a detailed study of electronystagmography in 341 patients with vertigo. Pract Otorhinolaryngol 1962;24:65-93. [PubMed] [Google Scholar]
- 26.Norrè ME, Forrez GH, Beckers AM. Vestibular compensation evaluated by rotation tests and posturography. Arch Otolaryngol Head Neck Surg 1987;113:533-5. [DOI] [PubMed] [Google Scholar]
- 27.Honrubia V. Quantitative vestibular function tests and the clinical examination. In: Herdman SJ, ed. Vestibular rehabilitation. Philadelphia: FA Davis; 1994. p. 113-64. [Google Scholar]
- 28.Bles W, Vianney de Jong JM, de Wit G. Compensation for labyrinthine defects examined by use of a tilting room. Acta Otolaryngol (Stockh) 1983;95:576-9. [DOI] [PubMed] [Google Scholar]
- 29.Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124:1646-56. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson M, Pykkö I, Jäntti U. Effect of alertness and visual attention on optokinetic nystagmus in humans. Am J Otolaryngol 1985;6:419-25. [DOI] [PubMed] [Google Scholar]
- 31.Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil 2006;28:789-95. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson G, Newman C. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg 1990;116:424-7. [DOI] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The Hospital Anxiety And Depression Scale. Acta Psychiatry Scand 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
- 34.Monzani D, Casolari L, Guidetti G, et al. Psychological distress and disability in patients with vertigo. Psychosom Res 2001;50:319-23. [DOI] [PubMed] [Google Scholar]
- 35.Vitte E, Semont A, Berthoz A. Repeated optokinetic stimulation in conditions of active standing facilitates recovery from vestibular deficits. Exp Brain Res 1994;102:141-8. [DOI] [PubMed] [Google Scholar]
- 36.Monzani D, Setti G, Marchioni D, et al. Repeated visually-guided saccades improves postural control in patients with vestibular disorders. Acta Otorhinolaryngol Ital 2005;25:224-32. [PMC free article] [PubMed] [Google Scholar]
- 37.Isableu B, Vuillerme N. Differential integration of kinaesthetic signals to postural control. Exp Brain Res 2006;174:763-8. [DOI] [PubMed] [Google Scholar]
- 38.Yardley L, Beech S, Zander L, et al. A randomized controlled trial of exercise therapy for dizziness and vertigo in primary care. Br J Gen Pract 1998;48:1136-40. [PMC free article] [PubMed] [Google Scholar]
- 39.Meli A, Zimatore G, Badaracco C, et al. Effects of vestibular rehabilitation therapy on emotional aspects in chronic vestibular patients. J Psychosom Res 2007;63:185-90. [DOI] [PubMed] [Google Scholar]
- 40.Rief W, Shaw R, Fichter MM. Elevated levels of psychophysiological arousal and cortisol in patients with somatisation syndrome. Psychosom Med 1998;60:198-203. [DOI] [PubMed] [Google Scholar]
- 41.Monzani D, Marchioni D, Bonetti S, et al. Anxiety affects vestibulo-spinal function of labyrinthine-defective patients during optokinetic stimulation. Acta Otorhinolaryngol Ital 2004;24:117-24. [PubMed] [Google Scholar]
- 42.Ballenger JC, Lydiard RB, Turner SM. Panic disorder and agoraphobia. In: Gabbard GO, editor. Treatment of psychiatric disorders. Washington, DC: American Psychiatric Association; 1995. p. 1421-52. [Google Scholar]
- 43.Redfern MS, Jennings JR, Martin C, et al. Attention influences sensory integration for postural control in older adults. Gait Posture 2001;14:211-8. [DOI] [PubMed] [Google Scholar]
- 44.Gottfries CG. Neurochemical aspects of Dementia Disorders. Dementia 1990;1:56-64. [Google Scholar]
- 45.Baddeley AD. Working memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- 46.Ishiyama A, Lopez I, Wackym PA. Distribution of efferent cholinergic terminals and alpha-bungarotoxin binding to putative nicotinic acetylcholine receptors in the human vestibular end-organs. Laryngoscope 1995;105:1167-72. [DOI] [PubMed] [Google Scholar]
- 47.Wackym PA, Chen CT, Ishiyama A, et al. Muscarinic acetylcholine receptor subtype mRNAs in the human and rat vestibular periphery. Cell Biol Int 1996;20:187-92. [DOI] [PubMed] [Google Scholar]
- 48.Andersson G, Hagnebo C, Yardley L. Stress and symptoms of Ménière’s disease: a time series analysis. J Psycosom Res 1997;43:595-603. [DOI] [PubMed] [Google Scholar]