Abstract
Background:
Current evidence indicates that measurement of pleural fluid N-terminal pro-brain natriuretic peptide (NT-proBNP) levels can aid in distinguishing pleural effusions of cardiac origin from those of noncardiac origin. To date, only one study, to our knowledge, has described simultaneous measurement of pleural fluid brain natriuretic-32 peptide (BNP) and NT-proBNP. The purpose of the present study was to determine pleural fluid BNP and NT-proBNP levels and analyze the relationship between these two measurements. We hypothesized that there would be a positive correlation between pleural fluid NT-proBNP and BNP, whereas NT-proBNP levels would be higher than BNP levels.
Methods:
Levels of pleural fluid NT-proBNP and BNP were measured by enzyme immunoassay in a total of 80 patients: 20 with congestive heart failure, 20 status post-coronary artery bypass graft, 20 with carcinoma, and 20 with pneumonia.
Results:
Comparison of NT-proBNP and BNP concentrations using the Spearman method of statistical analysis revealed a correlation coefficient of 0.572, P < .001. Evaluation of the diagnostic accuracy of BNP and NT-proBNP in patients with pleural effusions of cardiac origin demonstrated an area under the receiver operating characteristic curve of 0.700 (95% CI, 0.569-0.831) and 0.835 (95% CI, 0.721-0.949), respectively.
Conclusions:
Although levels of pleural fluid BNP have a statistically significant correlation with those of NT-proBNP, this relationship only explains 32% of the variance in NT-proBNP levels. Furthermore, when compared with BNP, NT-proBNP is a more accurate diagnostic aid in the evaluation of pleural effusions of cardiac origin.
Despite the clinical usefulness of the well-established Light criteria, differentiating transudative from exudative pleural effusions can still prove difficult in the setting of cardiovascular disease, particularly following the administration of diuretics. In recent years the brain natriuretic peptides have emerged as potential diagnostic markers capable of identifying effusions resulting from congestive heart failure (CHF).
Brain natriuretic peptide-32 (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) are derived from the precursor peptide proBNP and are secreted into the circulation in equimolar concentrations.1-3 Ventricular myocytes secrete these peptides into the circulation in response to increased ventricular wall tension. Clinical studies have revealed that plasma concentrations of both BNP and NT-proBNP are elevated in the setting of CHF; in addition, research has shown that pleural fluid concentrations of NT-proBNP are elevated in the setting of heart failure and may reliably differentiate effusions due to heart failure from those of other causes.4-7
Unfortunately, measuring NT-proBNP levels in the clinical laboratory is difficult and costly, whereas measurement of BNP concentrations is more commonly performed. The purpose of the present study was to compare levels of NT-proBNP and BNP in pleural fluid across a spectrum of diseases to assess the diagnostic usefulness of BNP levels in pleural fluid. We hypothesized that levels of these peptides would closely correlate and would have comparable usefulness in identifying pleural effusions due to CHF.
Materials and Methods
This study was approved by the Institutional Review Board of Saint Thomas Hospital with each patient giving informed consent when pleural fluid was obtained. A total of 80 pleural fluid samples were chosen from a database of 2,245 samples, all of which were obtained via thoracentesis between 1998 and 2004. Fluid used for analysis was collected in ethylenediaminetetraacetic acid-containing tubes (Becton Dickinson; Plymouth, Devon, England), and centrifuged at 3,000g at 4°C. The supernatants were then removed and frozen at −70°C until needed for analysis. Fluid samples undergoing prior freeze-thaw cycles were excluded. Chosen samples were drawn from a database in succession until 20 samples of each disease state had been identified, including 20 samples from patients with pleural effusions secondary to CHF, 20 post-coronary artery bypass grafting (CABG), 20 with pneumonia, and 20 with malignancy.
The diagnosis of CHF was based upon medical history and clinical findings consistent with heart failure. This included chest radiograph and echocardiograph with measurement of left ventricular ejection fraction. Echocardiography was performed by trained personnel, and all findings were evaluated by cardiologists according to institutional protocol at Saint Thomas Hospital. All patients in the CHF group were classified as stage III or IV in accordance with New York Heart Association functional classification system. Pleural effusions were attributed to CABG surgery when they occurred within the first 3 months following the surgical procedure and no other plausible cause existed. A diagnosis of parapneumonic effusion was based on the presence of an effusion in patients with clinical and radiologic evidence of acute pneumonia. Malignant pleural effusions were identified by demonstrating malignant cells via pleural fluid cytology or pleural biopsy. All effusions were attributed to a specific disease process based on the initial evaluation of the involved physicians.
Levels of BNP were measured using the BNP-32 enzyme immunoassay (EIA) kit (Peninsula Laboratories; San Carlos, CA) with detectable levels of BNP-32 ranging from 0 to 25 ng/mL. Levels of NT-proBNP were measured using the BNP Fragment EIA kit (Biomedica; Vienna, Austria) with detectable levels of NT-proBNP ranging from zero to 1,000 fmol/mL. Using the manufacturer-provided molecular weight, the upper limit of this range was converted to 2,400 pg/mL (1 fmol = 2.4 pg). The assay requires a 1:6 dilution of all samples, and all results were multiplied by a factor of six in order to correct for the mandated dilution. Prior investigations using the same EIA kit reported their results in a similar manner.8 Two patients with CHF had values outside of the detectable range despite this dilution and were assigned the upper detectable value. All measured values were converted to pg/mL as this represents the common format used in clinical settings and recommended by current practice guidelines.9
Statistics
Neither concentrations of BNP nor NT-proBNP were normally distributed, thus data are presented as median values with 25th to 75th percentiles and interquartile ranges. We assessed the correlation between BNP and NT-proBNP with Spearman coefficient of rank correlation. We compared levels of both BNP and NT-proBNP among all groups with the nonparametric Kruskal-Wallis H test, whereas we compared values between two groups with the Mann-Whitney U test. A P value of < .05 was considered statistically significant. To evaluate their diagnostic accuracy in the setting of CHF, we constructed receiver operating characteristic (ROC) plots for both BNP and NT-proBNP and calculated the area under the curve (AUC) for comparison of the two diagnostic tests. SPSS 16 software (SPS, Inc; Chicago, IL) was used for statistical analysis, and Confidence Interval Analysis 2.1.2 (Trevor Bryant, University of Southampton; Southampton, England) was used to determine confidence intervals for NT-proBNP cutoff value sensitivity and specificity.
Results
Pleural fluid samples from a total of 80 patients were analyzed, including 20 with effusions due to CHF, 20 with post-CABG effusions, 20 with malignant effusions, and 20 with parapneumonic effusions.
Distribution of NT-proBNP
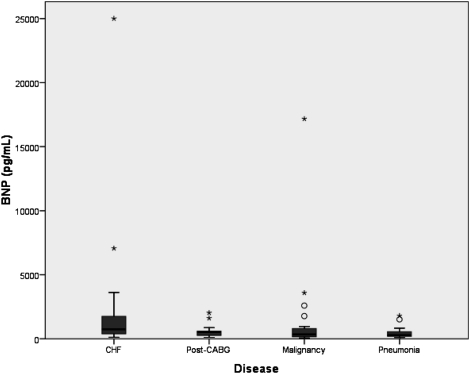
Using the Kruskal-Wallis H test, analysis of the distribution of NT-proBNP levels demonstrated a difference in NT-proBNP levels among disease states (Fig 1). Further analysis with the Mann-Whitney U test revealed NT-proBNP measurement to be useful in identifying patients with pleural effusions due to CHF (Table 1). The median level of NT-proBNP in pleural effusions due to CHF was significantly higher than the median level of the other three disease entities combined (P < .001). Moreover, the median pleural fluid level of NT-proBNP in patients with CHF was significantly higher than that in patients with post-CABG effusions (P = .002), malignant effusions (P < .001), and parapneumonic effusions (P < .001). In addition, there was a significant difference between NT-proBNP when post-CABG was compared with malignancy (P = .021). However, there was no significant difference between post-CABG and pneumonia (P = .157) or between effusions due to malignancy and those due to pneumonia (P = .461).
Figure 1.
Box plots showing NT-proBNP levels in pleural fluid among patients with CHF, post-CABG, malignant, and parapneumonic effusions. Box plots demonstrate the median and interquartile range and asterisks represent extreme outliers. CABG = coronary artery bypass graft; CHF = congestive heart failure; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Table 1.
—N-Terminal Pro-Brain Natriuretic Peptide and Brain Natriuretic-32 Peptide Levels Among Effusions of Cardiac and Noncardiac Origin
| Biomarker | Disease | Median (pg/mL) | IQR (pg/mL) | P Value (vs CHF) |
| NT-ProBNP | CHF | 4,189 | 2,346–6,370 | |
| Other (total) | 1,582 | 823–2,159 | < .001 | |
| CABG | 1,782 | 1,516–2,566 | .001 | |
| Malignancy | 1,232 | 634–1,677 | < .001 | |
| Pneumonia | 1,397 | 830–2,094 | < .001 | |
| BNP | CHF | 746 | 380–1872 | |
| Other (total) | 631 | 202–631 | .008 | |
| CABG | 525 | 226–607 | .091 | |
| Malignancy | 346 | 144–880 | .049 | |
| Pneumonia | 284 | 191–607 | .004 |
BNP = brain natriuretic-32 peptide; CABG = coronary artery bypass grafting; CHF = congestive heart failure; IQR = interquartile range; NT-ProBNP = N-terminal pro-brain natriuretic peptide.
Distribution of BNP
Using similar statistical methods, analysis of the distribution of pleural fluid levels of BNP revealed BNP to be less effective than NT-proBNP in differentiating pleural effusions due to CHF (Fig 2, Table 1). Although the median level of BNP in pleural effusions due to CHF was significantly higher than the median level of the other three groups combined (P < .008), the median level in the effusions due to CHF was not significantly higher than that in the post-CABG effusions (P = .091) and only marginally higher than that in the malignant effusions (P = .049). However, the median level of BNP in the effusions due to CHF was significantly higher than that in the parapneumonic effusions (P = .004).
Figure 2.
Box plots showing BNP levels in pleural fluid among patients with CHF, post-CABG, malignant, and parapneumonic effusions. Box plots demonstrate the median and interquartile range and asterisks represent extreme outliers. BNP = brain natriuretic-32 peptide. See Figure 1 legend for expansion of other abbreviations.
Diagnostic Accuracy of BNP and NT-proBNP
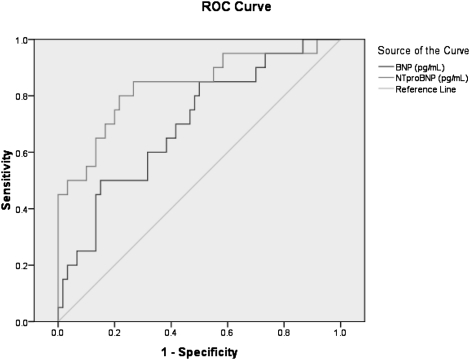
Evaluation of ROC plots in pleural fluid samples taken from patients with effusions of cardiac origin demonstrated that NT-proBNP was superior to BNP (Fig 3). The AUC for NT-proBNP was 0.835 (95% CI, 0.721-0.949), whereas that for BNP was 0.700 (95% CI, 0.569-0.831). An NT-proBNP value of 2,000 pg/mL had a sensitivity of 80% (95% CI, 0.58-0.92) and a specificity of 73% (95% CI, 0.61-0.83), whereas an NT-proBNP cutoff value of 5,000 pg/mL had a sensitivity of 45% (95% CI, 0.26-0.66) and a specificity of 97% (95% CI, 0.89-0.99).
Figure 3.
ROC plot for pleural fluid BNP and NT-proBNP (n = 80) concentrations in pg/mL. The AUC for BNP was 0.700 (95% CI, 0.569-0.831), whereas the AUC for NT-proBNP was 0.835 (95% CI, 0.721-0.949). AUC = area under the curve; ROC = receiver operating characteristic. See Figures 1 and 2 for expansion of other abbreviations.
Correlation
In addition to comparing distributions of BNP and NT-proBNP with regard to disease state, we also assessed for correlation between the two biomarkers (Fig 4). Given the nonnormal distribution of values, correlation was assessed using Spearman ρ. Although the correlation was statistically significant (P < .001), the correlation coefficient of 0.572 indicated that only 32% of the variance in NT-proBNP was explained by its relationship to BNP. In addition, the value of 0.284 for the slope of the regression line indicated that the values for the NT-proBNP were more than three times higher than the values of BNP.
Figure 4.
Correlation of pleural fluid levels of NT-proBNP and BNP. Spearman coefficient of rank correlation is 0.572 (P < .001). See Figures 1 and 2 for expansion of abbreviations.
Discussion
Measurement of the concentrations of NT-proBNP and BNP in pleural effusions of both cardiac and noncardiac origin demonstrated elevated levels of both NT-proBNP and BNP in effusions resulting from CHF. Analysis of these concentrations revealed that NT-proBNP was superior to BNP in its ability to identify effusions due to CHF. Comparison of NT-proBNP and BNP levels demonstrated a statistically significant correlation (r = 0.572), but this only explained 32% of the variance between these biomarkers. In addition, this study demonstrated that levels of NT-proBNP were significantly higher than the levels of BNP in pleural effusions of varying causes.
Previous studies have demonstrated the usefulness of pleural fluid NT-proBNP when attempting to distinguish cardiac from noncardiac pleural effusions.4,7,10 Kolditz et al4 demonstrated elevated levels of NT-proBNP in both serum and pleural fluid of individuals with pleural effusions arising from cardiac disease and also described a high diagnostic accuracy of pleural fluid NT-proBNP with an AUC of 0.98 (95% CI, 0.96-1.00). Additional investigations have confirmed the accuracy of NT-proBNP in identifying CHF-related pleural effusions, with Han et al11 reporting an AUC of 0.996 (95% CI, 0.990-1.003). In our study NT-proBNP was also successful at differentiating cardiac from noncardiac pleural effusions, albeit at a lower AUC of 0.835 (95% CI, 0.721-0.949).
Only one study in addition to our own has evaluated the measurement of BNP in pleural effusions or elucidated the usefulness of this peptide in characterizing effusions. Our study demonstrated the superior ability of NT-proBNP as a diagnostic aid at an AUC of 0.835 (95% CI, 0.721-0.949) compared with an AUC of 0.700 (95% CI, 0.569-0.831) for BNP. In the recent study by Porcel et al,10 NT-proBNP was also more successful than BNP at differentiating cardiac from noncardiac pleural effusions with an AUC of 0.96 (95% CI, 0.94-0.99) for NT-proBNP and 0.90 (95% CI, 0.86-0.95) for BNP. The study by Porcel et al10 demonstrated a high degree of correlation between NT-proBNP and BNP, with r = 0.78 and P < .001, whereas our analysis revealed a positive but less impressive correlation, with r = 0.572 and P < .001.
Porcel et al10 suggested two potential cutoff values for NT-proBNP, 1,300 and 1,500 pg/mL. A cutoff value of 1,300 provided a sensitivity of 95.6% (95% CI, 0.89-0.988) and a specificity of 87.9% (95% CI, 0.794-0.938), whereas a cutoff value of 1,500 pg/mL demonstrated a sensitivity of 93.3% (95% CI, 0.861-0.975) and a specificity of 89% (95% CI, 0.807-0.946). Previously recommended cutoff values vary from 1,176 pg/mL to 4,000 pg/mL for pleural fluid NT-proBNP.11 We found an NT-proBNP value of 2,000 pg/mL to have a sensitivity of 80% (95% CI, 0.58-0.92) and a specificity of 73% (95% CI, 0.61-0.83), whereas a cutoff value of 5,000 pg/mL had a sensitivity of 45% (95% CI, 0.26-0.66) and a specificity of 97% (95% CI, 0.89-0.99). The discrepancy between these studies regarding both the degree of correlation as well as sensitivity and specificity may relate to specific immunoassay. The BNP Fragment EIA kit from Biomedica has been compared with an immunoassay kit offered by Roche Diagnostics, and a significant degree of disagreement was identified between the two methods.12 Each assay targets different regions of the NT-proBNP molecule, and the dual antibodies used in the Roche assay may provide superior recognition of NT-proBNP. The kit used in the study uses a single antibody directed at the N-terminal region of NT-proBNP (amino acids 8-29). These findings certainly suggest the need for further investigation of both NT-proBNP and BNP measurement in pleural fluid and standardization of assay use prior to routine measurement of these biomarkers.
There are several limitations to the present study. First, one must consider the possibility that samples may have been originally misclassified. Because the data were evaluated retrospectively the complexity of the clinical situation surrounding each effusion cannot be ascertained. Complex cases would likely derive the most benefit from the availability of diagnostic biomarkers. Although the primary intention of this study was to evaluate the correlation between BNP and NT-proBNP, additional information gathered regarding post-CABG effusions may offer insight into misclassification. Of the effusions classified as exudates in the post-CABG setting, seven of 20 samples exhibited NT-proBNP levels > 2,000 pg/mL (cutoff value with 80% sensitivity and 73% specificity). Although the sample size is exceedingly small, two of four post-CABG samples obtained within 15 days postoperatively demonstrated NT-proBNP values > 4,000 pg/mL. These data warrant further investigation of this biomarker in the setting of CABG surgery where it may be useful in unclear scenarios. Overall, of the 60 noncardiac effusions, 16 of these demonstrated NT-proBNP levels > 2,000 pg/mL.
Ideally, these variables would be measured in a prospective fashion and compared simultaneously. Overall, NT-proBNP appears to be a more stable molecule following the sampling process and can be maintained for greater duration of time in an in vitro setting. The instability of BNP in blood has influenced the creation of recommendations regarding its collection and storage.13,14 However, the stability of neither NT-proBNP nor BNP in pleural fluid has been well established. As such, it is difficult to determine if the levels of BNP in pleural fluid were negatively impacted by an extended duration of storage. It is possible that prolonged storage, along with the process of sample thawing, may impact BNP levels significantly. Again, immediate measurement of sample BNP levels would diminish this variable. Further investigation of the effects of prolonged storage should be undertaken if samples will continue to be evaluated in a retrospective fashion.
In conclusion, BNP cannot be recommended to diagnose pleural effusions due to CHF, whereas NT-proBNP appears to be useful in differentiating between pleural effusions of cardiac and noncardiac origin within the population of patients studied. In order to recommend use of either peptide in the analysis of pleural effusions standardization of assay use and consistent cutoff values should be carefully determined as variation in immunoassay and use of stored samples may impact observed diagnostic characteristics. Although pleural fluid NT-proBNP has been evaluated prospectively, data of this nature are lacking for BNP and will be essential for determining its clinical usefulness as a diagnostic marker in pleural fluid. At this juncture the use of BNP cannot be readily promoted.
Acknowledgments
Author contributions: Dr Long: contributed to article design, analysis and interpretation of the data, collection and assembly of data, measurement of biomarkers, and drafting of the article.
Dr O’Neal: contributed to article design, analysis and interpretation of the data, statistical expertise, and drafting of the article.
Dr Peng: contributed to collection and assembly of data and measurement of biomarkers.
Dr Lane: contributed to collection and assembly of data.
Dr Light: contributed to article design, analysis and interpretation of the data, collection and assembly of data, measurement of biomarkers, and drafting of the article.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This work was performed at Vanderbilt University Medical Center, Nashville, TN.
Abbreviations
- AUC
area under the curve
- BNP
brain natriuretic-32 peptide
- CABG
coronary artery bypass graft
- CHF
congestive heart failure
- EIA
enzyme immunoassay
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- ROC
receiver operating characteristic
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grant HL087738, Clinical and Transitional Research Training Grant]; and the Baton Rouge Area Foundation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 suppl):S81–S83. doi: 10.1016/j.cardfail.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Hunt PJ, Espiner EA, Nicholls MG, et al. The role of the circulation in processing pro-brain natriuretic peptide (proBNP) to amino-terminal BNP and BNP-32. Peptides. 1997;18(10):1475–1481. doi: 10.1016/s0196-9781(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ. 2006;175(6):611–617. doi: 10.1503/cmaj.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolditz M, Halank M, Schiemanck CS, Schmeisser A, Höffken G. High diagnostic accuracy of NT-proBNP for cardiac origin of pleural effusions. Eur Respir J. 2006;28(1):144–150. doi: 10.1183/09031936.06.00113205. [DOI] [PubMed] [Google Scholar]
- 5.Porcel JM, Chorda J, Cao G, Esquerda A, Ruiz-González A, Vives M. Comparing serum and pleural fluid pro-brain natriuretic peptide (NT-proBNP) levels with pleural-to-serum albumin gradient for the identification of cardiac effusions misclassified by Light’s criteria. Respirology. 2007;12(5):654–659. doi: 10.1111/j.1440-1843.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 6.Porcel JM, Vives M, Cao G, Esquerda A, Rubio M, Rivas MC. Measurement of pro-brain natriuretic peptide in pleural fluid for the diagnosis of pleural effusions due to heart failure. Am J Med. 2004;116(6):417–420. doi: 10.1016/j.amjmed.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Tomcsányi J, Nagy E, Somlói M, et al. NT-brain natriuretic peptide levels in pleural fluid distinguish between pleural transudates and exudates. Eur J Heart Fail. 2004;6(6):753–756. doi: 10.1016/j.ejheart.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Liao H, Na MJ, Dikensoy O, Lane KB, Randal B, Light RW. Diagnostic value of pleural fluid N-terminal pro-brain natriuretic peptide levels in patients with cardiovascular diseases. Respirology. 2008;13(1):53–57. doi: 10.1111/j.1440-1843.2007.01191.x. [DOI] [PubMed] [Google Scholar]
- 9.Apple FS, Wu AH, Jaffe AS, et al. National Academy of Clinical Biochemistry. IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine practice guidelines: Analytical issues for biomarkers of heart failure. Circulation. 2007;116(5):e95–e98. doi: 10.1161/CIRCULATIONAHA.107.185266. [DOI] [PubMed] [Google Scholar]
- 10.Porcel JM, Martinez-Alonso M, Cao G, et al. Biomarkers of heart failure in pleural fluid. Chest. 2009;136(3):671–677. doi: 10.1378/chest.09-0270. [DOI] [PubMed] [Google Scholar]
- 11.Han CH, Choi JE, Chung JH. Clinical utility of pleural fluid NT-pro brain natriuretic peptide (NT-proBNP) in patients with pleural effusions. Intern Med. 2008;47(19):1669–1674. doi: 10.2169/internalmedicine.47.1276. [DOI] [PubMed] [Google Scholar]
- 12.Mueller T, Gegenhuber A, Poelz W, Haltmayer M. Comparison of the Biomedica NT-proBNP enzyme immunoassay and the Roche NT-proBNP chemiluminescence immunoassay: implications for the prediction of symptomatic and asymptomatic structural heart disease. Clin Chem. 2003;49(6 pt 1):976–979. doi: 10.1373/49.6.976. [DOI] [PubMed] [Google Scholar]
- 13.Azzazy HM, Christenson RH, Duh SH. Stability of B-type natriuretic peptide (BNP) in whole blood and plasma stored under different conditions when measured with the Biosite Triage or Beckman-Coulter Access systems. Clin Chim Acta. 2007;384(1-2):176–178. doi: 10.1016/j.cca.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Apple FS, Panteghini M, Ravkilde J, et al. Committee on Standardization of Markers of Cardiac Damage of the IFCC Quality specifications for B-type natriuretic peptide assays. Clin Chem. 2005;51(3):486–493. doi: 10.1373/clinchem.2004.044594. [DOI] [PubMed] [Google Scholar]