Abstract
Background:
Sepsis is a major public health problem. Social factors may affect health behaviors, economic resources, and immune response, leading to hospitalization for infection. This study examines the association between marital status and sepsis incidence and outcomes in a population-based cohort.
Methods:
We analyzed 1,113,581 hospitalizations in New Jersey in 2006. We estimated risk-adjusted incidence rate ratios (IRRs) for sepsis among divorced, widowed, legally separated, single, and married subjects using population data from the American Community Survey. We used multivariable logistic regression to estimate marital status-specific hospital mortality.
Results:
We identified 37,524 hospitalizations for sepsis, of which 40% were among married (14,924), 7% were among divorced (2,548), 26% were among widowed (9,934), 2% (763) were among legally separated, and 26% (9355) were among single subjects. The incidence of hospitalization for sepsis was 5.8 per 1,000 population. The age, sex, and race-adjusted IRR for hospitalization with sepsis was greatest for single (IRR = 3.47; 95% CI, 3.1, 3.9), widowed (IRR = 1.38; 95% CI, 1.2, 1.6), and legally separated (IRR = 1.46; 95% CI, 1.2, 1.8) subjects compared with married (referent). We observed that single men and women and divorced men had greater odds of in-hospital mortality compared with married men; widowed and legally separated men and all ever-married women had no excess mortality during hospitalization for sepsis.
Conclusions:
Hospitalization for sepsis is more common among single, widowed, and legally separated individuals, independent of other demographic factors. Among patients hospitalized for sepsis, single and divorced men and single women experience greater hospital mortality, highlighting the need to characterize the potentially modifiable mechanisms linking marital status to its greater burden of critical illness.
Sepsis is a significant public health problem, with an incidence that increases with age.1 In-hospital mortality from sepsis may exceed 15%, resulting in a large burden on health-care resources.1,2 Many demographic factors are associated with a greater incidence of sepsis, such as age, male sex, and black race.1,3-5 However, the contribution of social factors, such as marital status, to the epidemiology of sepsis is unknown.
The unmarried among both sexes have a greater risk for disease and death.6-9 Transitions in marital status are associated with increased risk of mortality, and multiple transitions may augment risk.10 Among the widowed, divorced, or legally separated, multiple factors, including unhealthy lifestyles,11 reduced economic resources,12 and psychologic distress, contribute to poor outcomes.13
Understanding the social factors that increase patients’ risk for sepsis is a key step toward reducing the burden of disease. Differences in marital status may result in detrimental health behaviors,10,11,14 alternative treatment choices before and during hospital-based care,15,16 and immunosuppression, resulting in a greater risk for pneumonia.7,17,18 These factors may increase the incidence of hospitalization and poor outcomes in sepsis. Thus, we sought to determine the association between marital status and the incidence of hospitalization for sepsis and hospital mortality in a diverse, population-based cohort. We hypothesized that the risk-adjusted incidence of hospitalization for sepsis, as well as hospital mortality, would be greater in unmarried subjects.
Materials and Methods
Study Design and Data Sources
We analyzed a population-based cohort of hospital discharge data from New Jersey using the Healthcare Cost and Utilization Project 2006 State Inpatient Database (SID) to determine: (1) marital status-specific incidence rates of hospitalization for sepsis and (2) the association between marital status and in-hospital mortality in sepsis.19 We chose New Jersey for its racial/ethnic diversity and completeness of marital status data ( < 1% missing). The SID contains data collected by the New Jersey Department of Health on all inpatient and same-day hospital discharges from all general acute care hospitals.19 Age, sex, and race/ethnicity-specific estimates for population denominators were obtained from the 2006 American Community Survey (ACS) of the US Census Bureau,20,21 and were linked to numerator data at the level of 5-year age strata for hospitalizations among persons ≥ 20 years old. This project received exempt review status by the University of Washington Institutional Review Board.
Variables and Definitions
The primary exposure was marital status, defined using the categories: married, widowed, divorced, single, and legally separated. Population census data for married included spouse present and spouse absent. We excluded hospitalizations and population data with marital status coded as missing. The primary outcome was hospitalization for sepsis, defined using clinically validated International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) codes within nine discharge diagnoses in the SID (see online supplement).1 Because of changes in coding for sepsis, we included ICD-9-CM codes 995.91, 995.92, and 785.52 in our definition. Our secondary outcome was in-hospital mortality among patients hospitalized for sepsis, defined by discharge status in the SID.
For multivariable adjustment in incidence rate models, confounders were determined a priori and included age,3 sex, and race/ethnicity.5 We considered additional potential confounders for our mortality analysis, such as urbanicity,5 socioeconomic status (SES),5 comorbidity,4 readmission,22 source of sepsis,4,23 presence of organ dysfunction,4 and type of pathogen.1 We defined race/ethnicity using categorizations in the SID and Census data: white, non-Hispanic; black, non-Hispanic; or Hispanic.24 We excluded numerator and denominator data in other race/ethnicity for consistency between SID and Census coding.5 We used area and individual-level indicators of SES: (1) state median income quartile for ZIP code of residence, and (2) primary and secondary insurance payer of Medicaid.25-27 Urbanicity was dichotomized as metropolitan vs nonmetropolitan from the National Center for Health Statistics county-level scheme in the SID. We defined comorbidity using the Charlson/Deyo score.28,29 We defined additional comorbidities using Clinical Classifications Software and ICD-9-CM codes (online supplement).30,31 We classified hospitalizations that were previously discharged from the same institution within 7 days as readmissions based on reported fields in the SID. Source of sepsis and type of pathogen were categorized using previously published ICD-9-CM codes (online supplement).1,4 We defined severe sepsis as the presence of one or more acute organ dysfunctions in a patient with sepsis (online supplement).1,32
Statistical Analysis
We compared continuous data using one-way analysis of variance or the Kruskal-Wallis test, depending on variable distributions; proportions were compared using the χ2 test. We display age-standardized incidence rates of hospitalization for sepsis among married, widowed, single, legally separated, and divorced individuals. To determine the incidence rates of hospitalization for sepsis among marital groups independent of age, sex, and race/ethnicity, we constructed a multivariable, negative binomial regression model containing the above covariates. We examined for multiplicative interactions between age, sex, race/ethnicity, and marital status guided by past literature and theory.
We constructed multivariable logistic regression models of in-hospital mortality using generalized estimating equations with robust variance estimates and an exchangeable correlation matrix, clustering on the treating hospital. We included marital status, sex, and an interaction between the two, sociodemographic characteristics (age, race/ethnicity, urbanicity, and SES indicators), and comorbidities (Charlson-Deyo score) in the final multivariable model.
First, we assessed the robustness of our findings through several sensitivity analyses. In our incidence rate models, we considered that ICD-9-CM coding practices changed since the clinical validation of our case definition,1 and repeated our primary analysis using only ICD-9-CM code 995.91. Second, we repeated our analysis after excluding hospitalizations coded as readmissions. Third, acknowledging that the count of married individuals in the ACS may be overestimated by including “married: spouse absent,” we repeated our analysis excluding these patients from the population denominators. Because the ACS may have greater sampling error due to a smaller population survey, we repeated our analysis using denominator data from the 2000 Census. We considered readmission, source of sepsis, or type of pathogen as potential confounders or in the causal pathway. Thus, we created models using readmission or source of sepsis/pathogen as adjustment variables. We also considered a hypothetical unobserved confounding variable (lower SES) that would be more frequent among unmarried than among married subjects. We varied the effect of this confounding variable on mortality and its association with marital status to determine how the association between mortality and marital status would change after adjustment.33 All analyses were performed using STATA 10.0 (StataCorp; College Station, TX).
Results
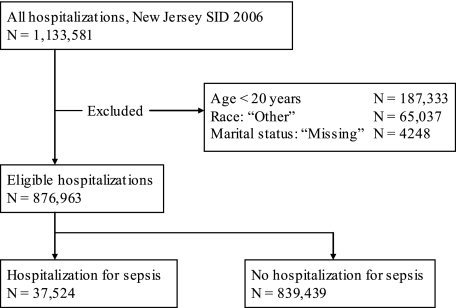
After exclusions, 876,963 adults were hospitalized in 91 hospitals in 2006 (Fig 1), in a population of 6,434,047. We identified 37,524 hospitalizations for sepsis, of which 40% of the patients were married (14,924), 7% were divorced (2,548), 26% were widowed (9,934), 2% (763) were legally separated, and 26% (9,355) were single. Mean age was greatest and female sex most common among widowed patients hospitalized for sepsis (Table 1). Few meaningful differences were present in the characteristics of sepsis, including pathogen type, presence of organ dysfunction, and sepsis source between marital status groups (Table 2). More than half of hospitalizations for sepsis were accompanied by organ dysfunction, irrespective of marital status, with renal dysfunction present in > 30% of subjects. Among survivors of sepsis, reported discharge to skilled nursing facility was significantly greater among widowed and divorced compared with married individuals (P <.01).
Figure 1.
Diagram of cohort construction. SID = State Inpatient Database.
Table 1.
—Characteristics of Hospitalizations (N = 37,524) for Sepsis by Marital Status in New Jersey in 2006
| Variable | Married | Divorced | Widowed | Legally Separated | Single | P Value |
| No. (%) | 14,924 (40) | 2,548 (7) | 9,934 (26) | 763 (2) | 9,355 (24) | |
| Age, y | 69 ± 14 | 64 ± 13 | 81 ± 9 | 61 ± 14 | 58 ± 19 | <.01 |
| Men | 9,370 (63) | 1,140 (45) | 2,314 (23) | 356 (47) | 4,912 (52) | <.01 |
| Race | ||||||
| White, non-Hispanic | 11,600 (78) | 1,709 (67) | 7,707 (78) | 244 (32) | 4,861 (52) | <.01 |
| Black, non-Hispanic | 1,970 (13) | 561 (22) | 1,544 (16) | 351 (46) | 3,296 (35) | |
| Hispanic | 1,354 (9) | 278 (11) | 683 (7) | 168 (5) | 1,198 (13) | |
| Readmission | 1,614 (16) | 297 (18.5) | 1,063 (16.5) | 74 (16) | 860 (16) | .26 |
| Insurance payer | ||||||
| Primary: Medicaid | 290 (1.9) | 219 (8.6) | 150 (1.5) | 102 (13) | 1,351 (14) | <.01 |
| Secondary: Medicaid | 1,039 (7) | 576 (23) | 1,979 (20) | 247 (32) | 2,229 (24) | <.01 |
| Comorbid conditions | ||||||
| Hypertension | 2,396 (16) | 383 (15) | 1,870 (19) | 142 (19) | 1,210 (13) | <.01 |
| Congestive heart failure | 5,933 (40) | 959 (38) | 5,049 (51) | 285 (37) | 2,904 (31) | <.01 |
| Cancer | 3,302 (22) | 435 (17) | 1,343 (14) | 126 (17) | 1,160 (12) | <.01 |
| COPD | 2,679 (18) | 528 (21) | 2,247 (23) | 127 (17) | 1,278 (14) | <.01 |
| HIV | 145 (1) | 70 (3) | 57 (1) | 57 (7) | 883 (9) | <.01 |
| Diabetes (type 1 or II) | 1,946 (13) | 382 (15) | 1,208 (12) | 116 (15) | 984 (11) | <.01 |
| Charlson/Deyo score | 2.0 ± 1.95 | 2.1 ± 2.0 | 1.8 ± 1.7 | 2.4 ± 2.2 | 2.0 ± 2.3 | <.01 |
| Hospital outcomes | ||||||
| Length of stay, d | 9 [5-17] | 10 [6-18] | 9 [6-16] | 11 [6-19] | 10 [6-18] | <.01 |
| Hospital mortality | 3,126 (21) | 489 (19) | 2,503 (25) | 135 (18) | 1,702 (18) | <.01 |
| Discharge location of survivors | ||||||
| Routine | 4,147 (35) | 590 (29) | 1,234 (17) | 236 (38) | 2,674 (35) | <.01 |
| Skilled nursing facility | 3,915 (33) | 875 (43) | 4,203 (57) | 212 (34) | 2,973 (39) | <.01 |
| Home health care | 1,946 (16) | 279 (14) | 840 (11) | 84 (13) | 736 (10) | <.01 |
| Hospice | 432 (4) | 67 (3) | 379 (5) | 13 (2) | 182 (2) | <.01 |
Data presented as mean ± SD, No. (%), or median [IQR], as appropriate.
Table 2.
—Source, Pathogen, and Organ System Dysfunction Among Hospitalizations for Sepsis
| Covariate | Married | Divorced | Widowed | Legally Separated | Single | P Valuea |
| Pathogen identified | ||||||
| Gram positive | 2,866 (19) | 476 (19) | 1,636 (17) | 148 (19) | 1,855 (20) | <.01 |
| Gram negative | 2,112 (14) | 345 (14) | 1,390 (14) | 103 (14) | 1,095 (12) | |
| Fungal | 295 (2) | 63 (2) | 161 (2) | 18 (2) | 212 (2) | |
| Anaerobic | 109 (1) | 12 (<1) | 75 (1) | 1 (<1) | 36 (<1) | |
| No pathogen coded | 9,542 (64) | 1,652 (65) | 6,672 (67) | 493 (65) | 6,157 (66) | |
| Source of sepsisb | ||||||
| Respiratory | 6,555 (44) | 1,142 (45) | 4,887 (49) | 316 (41) | 4,044 (43) | <.01 |
| Urological | 4,749 (32) | 799 (32) | 4,213 (42) | 219 (29) | 2,788 (30) | <.01 |
| Gastrointestinal | 4,375 (29) | 741 (29) | 2,843 (29) | 208 (27) | 2,571 (27) | .03 |
| Skin, soft tissue, joint | 1,915 (13) | 363 (14) | 1,212 (12) | 109 (14) | 1,351 (14) | <.01 |
| Central nervous system | 62 (0.4) | 15 (0.6) | 10 (0.1) | 10 (1) | 86 (1) | <.01 |
| Cardiovascular | 343 (2) | 58 (2.3) | 157 (2) | 18 (2) | 273 (3) | <.01 |
| Severe sepsis | 8,561 (57) | 1,486 (58) | 5,624 (57) | 451 (59) | 5,483 (59) | .05 |
| No. organ system dysfunctions | ||||||
| 0 | 6,363 (43) | 1,062 (42) | 4,310 (44) | 312 (41) | 3,872 (41) | <.01 |
| 1 | 5,365 (36) | 955 (37) | 3,669 (37) | 290 (38) | 3,459 (37) | |
| 2 | 2,425 (16) | 405 (16) | 1,550 (16) | 126 (17) | 1,505 (17) | |
| 3 | 632 (4) | 110 (4) | 381 (4) | 31 (4) | 429 (5) | |
| 4+ | 139 (1) | 15 (1) | 54 (1) | 0 (0) | 90 (1) | |
| Organ system dysfunctionb | ||||||
| Renal | 5,710 (38) | 1,003 (39) | 3,861 (39) | 324 (42) | 3,583 (38) | .15 |
| Respiratory | 3,776 (25) | 635 (25) | 2,401 (24) | 183 (24) | 2,506 (27) | <.01 |
| Hematologic | 1,101 (7) | 177 (6) | 599 (6) | 43 (6) | 684 (7) | <.01 |
| Cardiac | 642 (4) | 88 (3) | 365 (4) | 21 (3) | 343 (4) | .01 |
| Hepatic | 354 (2) | 55 (2) | 130 (1) | 27 (4) | 230 (2) | <.01 |
| Neurologic | 305 (2) | 59 (2) | 173 (2) | 13 (2) | 249 (3) | <.01 |
| Metabolic | 717 (5) | 128 (5) | 532 (5) | 40 (5) | 527 (6) | .06 |
Data presented as No. (%).
χ test for differences.
Proportions not mutually exclusive.
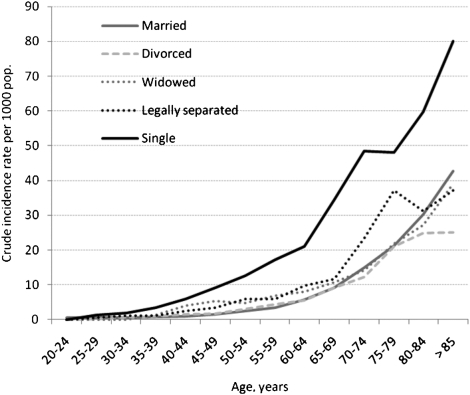
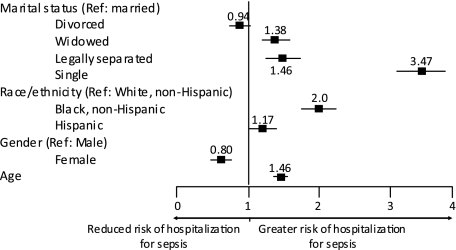
The incidence of hospitalization for sepsis for the study population in 2006 was 5.8 per 1,000, with unadjusted rates greatest among widowed (21.5 per 1,000) compared with single (6.1 per 1,000), separated (5.3 per 1,000), divorced (4.6 per 1,000), and married (4.6 per 1,000) subjects. Incidence rates increased with age, irrespective of marital status (Fig 2). After adjustment for age, race, and ethnicity, single individuals had the greatest incidence rate ratio (IRR) of hospitalization for sepsis compared with married subjects (Table 3). Male sex, advanced age, Hispanic ethnicity, and non-white race were associated with hospitalization for sepsis (Fig 3).
Figure 2.
Crude incidence rate of hospitalization for sepsis among married, widowed, legally separated, divorced, and single subjects across 5-year age groups. Single subjects are shown as black line, legally separated as dotted dark gray line, widowed subjects are depicted as dotted light gray line, divorced subjects as hashed light gray line, and married subjects as solid gray line. Incidence rate is reported per 1,000 population.
Table 3.
—Sensitivity Analyses for IRR of Hospitalization for Sepsis of Widowed, Single, and Legally Separated Subjects Compared With Married Subjects
| Model | Widowed | Single | Legally Separated |
| Base model | 1.38 | 3.5 | 1.5 |
| (1.17, 1.63) | (3.1, 3.9) | (1.2, 1.8) | |
| Alternative case definition: | 1.2 | 3.3 | 1.3 |
| sepsis as ICD-9-CM | (1.02, 1.34) | (2.9, 3.7) | (1.1, 1.6) |
| code 995.91 only | |||
| Alternative numerator: | 1.35 | 3.6 | 1.46 |
| excluding readmission | (1.16, 1.59) | (3.17, 3.99) | (1.21, 1.75) |
| hospitalization | |||
| Alternative denominator: | 1.25 | 3.14 | 1.32 |
| excluding “married: | (1.06, 1.48) | (2.79, 3.54) | (1.09, 1.6) |
| spouse absent” | |||
| Alternative denominator: | 1.36 | 4.8 | 1.38 |
| US Census 2000 | (1.19, 1.55) | (4.2, 5.46) | (1.22, 1.55) |
| population |
All models include adjustment for age, sex, race/ethnicity. Data presented as IRR (95% CI). See the online supplement for details on each model. ICD-9-CM = International Classification of Diseases, 9th edition, Clinical Modification; IRR = incidence rate ratio.
Figure 3.
Incidence rate ratios (IRRs) of hospitalization for sepsis in New Jersey, 2006. Estimates for rate ratios are adjusted for age, race, sex, and marital status. Single, widowed and divorced subjects, black non-Hispanics, Hispanics, and those with advanced age are at greater risk for hospitalization for sepsis. Point estimates for IRRs are displayed above symbols (online supplement), and line constitutes the 95% CI.
In sensitivity analyses (Table 3), sepsis defined using ICD-9-CM code 995.91 resulted in 13,570 hospitalizations for analysis. Our results were similar using this alternative definition (online supplement). Our findings were also similar after exclusion of sepsis hospitalizations coded as readmissions (n = 3,907) (online supplement). We used two alternative population denominators, exclusion of “married: spouse absent” and US Census 2000, and observed greater IRRs for hospitalization for sepsis among all groups compared with married subjects (online supplement).
Single and divorced men and single women were at greater odds of hospital mortality compared with married men after accounting for sociodemographic factors and subject comorbidity (Table 4). In sensitivity analyses, we excluded readmissions (online supplement) and observed similar results. We included the source of sepsis and gram-positive infection as covariates and observed no change in our model (online supplement). We considered that unmeasured confounding due to SES accounted for the greater odds of hospital mortality among unmarried subjects. Our analyses demonstrated, for example, that if the prevalence of an unobserved confounder among divorced men was 25% compared with 15% among married men, the odds of death among men with the confounder would need to be three times that of men without the confounder for the risk of death associated with divorce to appear equivalent to that of marriage.
Table 4.
—Multivariable Logistic Regression Model for Mortality Among Sepsis Hospitalizations
| Model A |
Model B |
|||
| Covariate | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
| Women | ||||
| Married | 0.86 | <.01 | 0.95 | .2 |
| (0.8, 0.95) | (0.87, 1.03) | |||
| Divorced | 0.84 | <.01 | 0.90 | .28 |
| (0.79, 0.93) | (0.74, 1.09) | |||
| Widowed | 1.38 | <.01 | 1.0 | .97 |
| (1.28, 1.50) | (0.93, 1.08) | |||
| Single | 0.98 | .74 | 1.16 | .01 |
| (0.88, 1.09) | (1.03, 1.3) | |||
| Legally separated | 0.81 | .11 | 0.96 | .76 |
| (0.63, 1.05) | (0.73, 1.26) | |||
| Men | ||||
| Married | 1.0 (ref) | 1.0 (ref) | … | |
| Divorced | 1.02 | .8 | 1.19 | .02 |
| (0.88, 1.18) | (1.03, 1.40) | |||
| Widowed | 1.29 | <.01 | 1.0 | .96 |
| (1.17, 1.42) | (0.91, 1.10) | |||
| Single | 0.78 | <.01 | 1.13 | <.01 |
| (0.73, 0.85) | (1.03, 1.24) | |||
| Legally separated | 0.89 | .4 | 1.13 | .4 |
| (0.68, 1.17) | (0.86, 1.5) | |||
| Age, y | … | … | 1.03 | <.01 |
| (1.02, 1.03) | ||||
| Race/ethnicity | ||||
| White, non-Hispanic | … | … | 1.0 (ref) | … |
| Black, non-Hispanic | … | … | 0.84 | <.01 |
| (0.77, 0.91) | ||||
| Hispanic | … | … | 0.84 | <.01 |
| (0.74, 0.96) | ||||
| Urbanicity | ||||
| Nonmetropolitan | … | … | 1.0 (ref) | … |
| Metropolitan | … | … | 0.91 | .23 |
| (0.79, 1.06) | ||||
| Quartile of median income by zip code | ||||
| Lowest | … | … | 1.0 (ref) | … |
| Second | … | … | 0.98 | .71 |
| (0.86, 1.11) | ||||
| Third | … | … | 0.96 | .4 |
| (0.87, 1.06) | ||||
| Highest | … | … | 0.98 | .67 |
| (0.89, 1.08) | ||||
| Payer | ||||
| Primary: | … | … | 1.0 | .9 |
| Medicaid | (0.87, 1.15) | |||
| Secondary: | … | … | 0.90 | <.01 |
| Medicaid | (0.84, 0.97) | |||
| Charlson-Deyo score | … | … | 1.12 | <.01 |
| (per point) | (1.10, 1.13) | |||
OR = odds ratio; ref = referent category.
Discussion
In a population-based cohort study, we observed that widowed, single, and legally separated subjects have a greater risk-adjusted incidence of hospitalization for sepsis than married individuals. Our findings were robust to alternative specifications for the definition of sepsis, marriage, and the source of population data. After adjustment for demographic factors and comorbid conditions, single and divorced men and single women have greater odds of in-hospital mortality than married subjects hospitalized with sepsis. Our results suggest that social factors, such as marital status, have important associations with the incidence and outcomes of sepsis.
Multiple factors may contribute to the greater incidence of hospitalization for sepsis among single, widowed, and separated individuals. The loss of a spouse is a well-known risk factor for death.34 The “widowhood effect” may also increase detrimental health habits, such as smoking, alcohol consumption, and reduced physical activity, altering the occurrence of disease.11,14 Psychiatric well-being is also influenced by spousal loss, with greater depression and anxiety during and after the period of bereavement among widowed compared with married individuals.35 Widowhood may also have untoward immunomodulatory effects. For example, peak antibody responses 1 month after influenza immunization were reduced in bereaved elders compared with married elders.36 Combined with age-related changes to the human immune system,37 these immunologic changes may render the widowed elderly particularly more susceptible to sepsis. The greater incidence of sepsis hospitalization among widowed individuals in our cohort dovetails with the literature that death from sepsis or infection is greater after spousal loss.38 Other biologic mechanisms for greater incidence of sepsis among unmarried individuals may include altered neuroendocrine function and immune response during marital discord.17,39 Single/never married subjects also have more frequent detrimental health habits and are at socioeconomic disadvantage, mechanisms likely contributing to the greater incidence of sepsis in this group.40
Studies of sex-specific differences in the risk-adjusted mortality from sepsis have produced conflicting results.1,2,4,41 Authors implicate differences in sex hormone levels or the use of invasive procedures as mechanisms by which men experience greater mortality in sepsis.4,41,42 Yet, epidemiologic studies report variable odds of death for men compared with women hospitalized with infection.41,43 We observed that sex-specific differences in sepsis mortality were present, modified by marital status, and were independent of multiple confounders. Our findings of greater odds of mortality in single and divorced men and single women complement broader investigations, such as the National Longitudinal Mortality Study, in which the unmarried of both sexes were at increased risk for pneumonia and influenza deaths relative to married.7 A similar association between unmarried, male sex, and greater mortality is observed in cardiovascular disease8,44,45 and various cancers.46,47 Unmeasured health behaviors may contribute to this excess mortality and may be more common among men; all unmarried categories of both sexes use tobacco more frequently than married,40 whereas separated, divorced, and widowed men have greater alcohol abuse,8,45 an independent risk factor for greater morbidity and mortality in sepsis.48 Our mortality estimates may also be influenced by alternative choices for life-sustaining procedures and critical care interventions by individuals of varying ages when hospitalized for sepsis.
Sepsis is generally understood as a significant burden for patients, clinicians, and the US health-care system.2 Although hospital-based treatment strategies have reduced mortality from sepsis,49 further gains in clinical outcomes may require preventive strategies. The general public’s awareness of and knowledge about sepsis is poor,50 and prevention may necessitate targeting of at-risk populations by clinicians in office-based practices. Greater characterization of the social factors that increase the risk for sepsis hospitalization, the mechanisms that explain this association, and how it changes over time (life-course perspective)51 may better inform these clinicians, in the same way that knowledge of other risk factors with limited modifiability (eg, family history) can clearly contribute to patient care. Once patients are admitted to the ICU with sepsis, we hypothesize that the greater odds of mortality among single men and women and divorced men may be evidence that unmarried subjects present with greater levels of undiagnosed comorbidity due to worse self-care or may present late or atypically. Early interventions by social workers may be needed to ensure adequate support during intensive care. Social isolation of unmarried sepsis survivors may also result in a greater use of post-discharge services, such as skilled nursing facilities; the clinical and financial impact of these transfer are currently unknown. Our results support the assessment of social factors, such as marital status, as potential covariates and confounders in future observational studies of sepsis, whereas interventions trials may benefit from balance of social factors between treatment groups.
Our results have several limitations. First, the incidence rates in our cohort derive from an administrative case definition. To define sepsis, we included new ICD-9-CM codes in addition to our previously validated case definition.1 We explored if these new codes represented different patients by conducting a sensitivity analysis of our incidence rate model, and found no difference in our main effect. Because we used multiple data sources to derive IRRs misclassification of marital status may introduce bias into our results.52 Yet, we observed consistently worse outcomes for single individuals in both the incidence and mortality analyses, which supports a true association, not misclassification bias, as the main effect. Second, in any observational study, unmeasured confounding may be present. We performed quantitative bias analysis to determine the magnitude of this potential confounder necessary to abrogate our results.33 Using divorced men as an example, we found that the association between divorce, male sex, and mortality from sepsis was robust to a wide range of plausible assumptions. We also considered multiple confounders, which arguably may participate in the causal pathway between marital status and mortality from sepsis, and found no change in our estimates. Third, neighborhood and community factors may be associated with health status and risk for hospitalization independent of marital status.53,54 Limitations in our data prevent the incorporation of neighborhood structural contexts in our analysis; however, we adjusted for community-level SES indicators as well as the nonindependence of mortality within hospitals in our multivariable analysis. Also, the timing of marital transitions was unavailable, such that we could not study if the proximity to divorce, death of a spouse, or separation was associated with greater risk of hospitalization or death from sepsis.10,11,14,51 Finally, we were unable to adjust for differences in hospital-based care, such as ICU use, end-of-life decisions, or do-not-resuscitate orders, because these variables were not available in our data set. Yet, changes in goals of care may be facilitated by the presence of spouses,55 adding a conservative bias to our analyses.
Conclusions
Hospitalization for sepsis is more common among widowed, single, and legally separated individuals, independent of other demographic factors. Among patients hospitalized for sepsis, single men and women and divorced men experience greater hospital mortality. This study suggests that social factors contribute to the epidemiology and outcomes of sepsis, and highlights the need to characterize the underlying, potentially modifiable mechanisms linking marital status to its greater burden of critical illness.
Supplementary Material
Acknowledgments
Author contributions: Dr Seymour: contributed to concept design and study hypotheses, data analysis, interpretation of results, and drafting of the manuscript.
Dr Iwashyna: contributed to concept design and study hypotheses, data analysis, interpretation of results, and drafting of the manuscript.
Dr Cooke: contributed to data analysis, interpretation of results, and drafting of the manuscript.
Dr Hough: contributed to interpretation of results and drafting of the manuscript.
Dr Martin: contributed to concept design and study hypotheses, interpretation of results, and drafting of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This work was performed at the University of Washington, Division of Pulmonary and Critical Care Medicine.
Abbreviations
- ACS
American Community Survey
- ICD-9-CM
International Classification of Diseases, 9th edition, Clinical Modification
- IRR
incidence rate ratio
- SES
socioeconomic status
- SID
State Inpatient Database
This abstract was submitted for presentation at the American Thoracic Society International Meeting, New Orleans, LA, in May 2010.
Funding/Support: This study was supported in part by an extramural training grant from the National Institutes of Health [Grant T32 NIH/HL07287].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 4.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzoli L, Villari P, Pirone GM, Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med. 2007;64(1):77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol. 2000;10(4):224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 8.Rosengren A, Wedel H, Wilhelmsen L. Marital status and mortality in middle-aged Swedish men. Am J Epidemiol. 1989;129(1):54–64. doi: 10.1093/oxfordjournals.aje.a115124. [DOI] [PubMed] [Google Scholar]
- 9.Gove WR. Sex, marital status, and mortality. AJS. 1973;79(1):45–67. doi: 10.1086/225505. [DOI] [PubMed] [Google Scholar]
- 10.Dupre ME, Beck AN, Meadows SO. Marital trajectories and mortality among US adults. Am J Epidemiol. 2009;170(5):546–555. doi: 10.1093/aje/kwp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eng PM, Kawachi I, Fitzmaurice G, Rimm EB. Effects of marital transitions on changes in dietary and other health behaviours in US male health professionals. J Epidemiol Community Health. 2005;59(1):56–62. doi: 10.1136/jech.2004.020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holden KC, Kuo HH. Complex marital histories and economic well-being: the continuing legacy of divorce and widowhood as the HRS cohort approaches retirement. Gerontologist. 1996;36(3):383–390. doi: 10.1093/geront/36.3.383. [DOI] [PubMed] [Google Scholar]
- 13.Zivin K, Christakis NA. The emotional toll of spousal morbidity and mortality. Am J Geriatr Psychiatry. 2007;15(9):772–779. doi: 10.1097/JGP.0b013e318050c9ae. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Cho E, Grodstein F, Kawachi I, Hu FB, Colditz GA. Effects of marital transitions on changes in dietary and other health behaviours in US women. Int J Epidemiol. 2005;34(1):69–78. doi: 10.1093/ije/dyh258. [DOI] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med. 2003;57(11):2137–2147. doi: 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 16.Gordon HS, Rosenthal GE. Impact of marital status on outcomes in hospitalized patients. Evidence from an academic medical center. Arch Intern Med. 1995;155(22):2465–2471. [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Malarkey WB, Chee M, et al. Negative behavior during marital conflict is associated with immunological down-regulation. Psychosom Med. 1993;55(5):395–409. doi: 10.1097/00006842-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Farr BM, Bartlett CL, Wadsworth J, Miller DL. British Thoracic Society Pneumonia Study Group Risk factors for community-acquired pneumonia diagnosed upon hospital admission. Respir Med. 2000;94(10):954–963. doi: 10.1053/rmed.2000.0865. [DOI] [PubMed] [Google Scholar]
- 19.State Inpatient Databases HCUP (SID) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 20.King M, Ruggles S, Alexander T, Leicach D, Sobek M. Integrated Public Use Microdata Series, Current Population Survey: Version 2.0. Minneapolis, MN: Minnesota Population Center [producer and distributor]; 2009. [machine-readable database] [Google Scholar]
- 21.American Community Survey (ACS) Public Use Microdata Sample: US Census Bureau-ACS. 2006. [Accessed June 1, 2009]. http://usa.ipums.org/usa/sda.
- 22.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 23.Sevransky JE, Martin GS, Mendez-Tellez P, et al. Pulmonary versus non-pulmonary sepsis and mortality in acute lung injury. Chest. 2008;134(3):534–538. doi: 10.1378/chest.08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez AD, Hirschman C. The changing racial and ethnic composition of the US population: emerging American identities. Popul Dev Rev. 2009;35(1):1–51. doi: 10.1111/j.1728-4457.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canto JG, Rogers WJ, French WJ, Gore JM, Chandra NC, Barron HV. Payer status and the utilization of hospital resources in acute myocardial infarction: a report from the National Registry of Myocardial Infarction 2. Arch Intern Med. 2000;160(6):817–823. doi: 10.1001/archinte.160.6.817. [DOI] [PubMed] [Google Scholar]
- 26.Winkleby MA, Cubbin C. Influence of individual and neighbourhood socioeconomic status on mortality among black, Mexican-American, and white women and men in the United States. J Epidemiol Community Health. 2003;57(6):444–452. doi: 10.1136/jech.57.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadley J, Steinberg EP, Feder J. Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome. JAMA. 1991;265(3):374–379. [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 29.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.Clinical Classifications Software (CCS) for ICD-9-CM . Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; pp. 2000–2003. [Google Scholar]
- 31.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 32.Bone RC, Balk RA, Cerra FB, et al. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 33.Lash TLFM, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York, NY: Springer; 2009. [Google Scholar]
- 34.Parkes CM, Benjamin B, Fitzgerald RG. Broken heart: a statistical study of increased mortality among widowers. BMJ. 1969;1(5646):740–743. doi: 10.1136/bmj.1.5646.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vink D, Aartsen MJ, Comijs HC, et al. Onset of anxiety and depression in the aging population: comparison of risk factors in a 9-year prospective study. Am J Geriatr Psychiatry. 2009;17(8):642–652. doi: 10.1097/jgp.0b013e3181a65228. [DOI] [PubMed] [Google Scholar]
- 36.Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20(3):279–289. doi: 10.1016/j.bbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(suppl 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 38.Christakis NA, Allison PD. Mortality after the hospitalization of a spouse. N Engl J Med. 2006;354(7):719–730. doi: 10.1056/NEJMsa050196. [DOI] [PubMed] [Google Scholar]
- 39.Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosom Med. 1994;56(1):41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Maselko J, Bates LM, Avendano M, et al. The intersection of sex, marital status, and cardiovascular risk factors in shaping stroke incidence: results from the Health and Retirement Study. J Am Geriatr Soc. 2009;57(12):2293–2299. doi: 10.1111/j.1532-5415.2009.02555.x. [DOI] [PubMed] [Google Scholar]
- 41.Adrie C, Azoulay E, Francais A, et al. OutcomeRea Study Group Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132(6):1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 42.Berkowitz DM, Martin GS. Sepsis and sex: can we look beyond our hormones? Chest. 2007;132(6):1725–1727. doi: 10.1378/chest.07-2001. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree TD, Pelletier SJ, Gleason TG, Pruett TL, Sawyer RG. Gender-dependent differences in outcome after the treatment of infection in hospitalized patients. JAMA. 1999;282(22):2143–2148. doi: 10.1001/jama.282.22.2143. [DOI] [PubMed] [Google Scholar]
- 44.Ebrahim S, Wannamethee G, McCallum A, Walker M, Shaper AG. Marital status, change in marital status, and mortality in middle-aged British men. Am J Epidemiol. 1995;142(8):834–842. doi: 10.1093/oxfordjournals.aje.a117723. [DOI] [PubMed] [Google Scholar]
- 45.Molloy GJ, Stamatakis E, Randall G, Hamer M. Marital status, gender and cardiovascular mortality: behavioural, psychological distress and metabolic explanations. Soc Sci Med. 2009;69(2):223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Shlomo Y, Smith GD, Shipley M, Marmot MG. Magnitude and causes of mortality differences between married and unmarried men. J Epidemiol Community Health. 1993;47(3):200–205. doi: 10.1136/jech.47.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito-Nakaya K, Nakaya N, Akechi T, et al. Marital status and non-small cell lung cancer survival: the Lung Cancer Database Project in Japan. Psychooncology. 2008;17(9):869–876. doi: 10.1002/pon.1296. [DOI] [PubMed] [Google Scholar]
- 48.Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis. 2005;41(suppl 7):S490–S497. doi: 10.1086/432003. [DOI] [PubMed] [Google Scholar]
- 49.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 50.Rubulotta FM, Ramsay G, Parker MM, Dellinger RP, Levy MM, Poeze M. Surviving Sepsis Campaign Steering Committee; European Society of Intensive Care Medicine; Society of Critical Care Medicine An international survey: public awareness and perception of sepsis. Crit Care Med. 2009;37(1):167–170. doi: 10.1097/ccm.0b013e3181926883. [DOI] [PubMed] [Google Scholar]
- 51.Williams K, Umberson D. Marital status, marital transitions, and health: a gendered life course perspective. J Health Soc Behav. 2004;45(1):81–98. doi: 10.1177/002214650404500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korenman S, Goldman N, Fu H. Misclassification bias in estimates of bereavement effects. Am J Epidemiol. 1997;145(11):995–1002. doi: 10.1093/oxfordjournals.aje.a009068. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian SV, Elwert F, Christakis N. Widowhood and mortality among the elderly: the modifying role of neighborhood concentration of widowed individuals. Soc Sci Med. 2008;66(4):873–884. doi: 10.1016/j.socscimed.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen M, Christakis NA. Neighborhood effects on posthospitalization mortality: a population-based cohort study of the elderly in Chicago. Health Serv Res. 2005;40(4):1108–1127. doi: 10.1111/j.1475-6773.2005.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maksoud A, Jahnigen DW, Skibinski CI. Do not resuscitate orders and the cost of death. Arch Intern Med. 1993;153(10):1249–1253. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.