Abstract
The pathophysiology of inflammatory bowel disease (IBD) includes leukocyte infiltration, blood and lymphatic remodeling, weight loss and protein enteropathy. The roles of angiopoietin-2 (Ang-2) in initiating gut inflammation, leukocyte infiltration and angiogenesis are not well understood. Several important differences were seen in the development of experimental IBD in Ang-2-/- mice. Although weight change and disease activity differ only slightly in WT and Ang-2-/- + DSS treated mice, leukocyte infiltration, inflammation and blood and lymphatic vessel density is significantly attenuated compared to WT+ DSS mice. Gut capillary fragility and water export (stool blood and form) appear significantly earlier in Ang-2-/- + DSS mice vs. WT. Colon lengths were also significantly reduced in Ang-2-/- and gut histopathology was less severe in Ang-2-/-compared to WT + DSS. Lastly, the decrease in serum protein content in WT + DSS was less severe in Ang-2-/- + DSS, thus protein losing enteropathy (PLE) a feature of IBD is relieved by Ang-2-/-. These data demonstrate that in DSS colitis, Ang-2 mediates inflammatory hemangiogenesis, lymphangiogenesis and neutrophil infiltration to reduce some, but not all clinical features of IBD. The implications for Ang-2 manipulation in the development of IBD and other inflammatory diseases and treatments involving Ang-2 are discussed.
Keywords: MECA-32, VEGFR-3, Neutrophils, Crohn’s disease, Ulcerative colitis
Introduction
IBD is associated with extensive tissue injury and remodeling caused by tissue edema, inflammatory cell infiltrates, loss of epithelial integrity and increased angiogenesis. These features contribute to IBD pathophysiology through several diverse mechanisms. Angiogenesis has also recently been described as a novel contributory cause of IBD tissue injury and not simply an epiphenomenon ascribed to inflammation. For instance, increased levels of vascular endothelial growth factor-A (VEGF-A) released during active episodes of IBD increase endothelial expression of intercellular adhesion molecule-a (ICAM-1) to promote enhanced leukocyte adhesion (15) which drives injury mediated by infiltrated inflammatory cells in colitis. Further, VEGF-A increases blood vascular permeability which intensifies gut edema, a central characteristic of IBD. Lastly, new blood vessels formed in the inflamed gut in response to VEGF-A may exhibit delayed maturation displaying incomplete recruitment of pericytes necessary to stabilize vessels, inhibit endothelial proliferation and reduce vascular leakage.
Endothelial TEK/Tie-2 signaling mediates blood vessel maturation via angiopoietins 1, 2, 3 and 4 (54). While angiopoietins 1 and 4 are agonists of Tie-2 signaling, angiopoietins 2 and 3 are considered to be antagonists of angiopoietin-1 mediated Tie-2 phosphorylation (28;54). Currently, the exact biological activities and pattern of Tie2 signaling by Ang-3 and -4 are less well characterized but shown to function in a cell specific manner (28;54). The final steps in blood capillary maturation involve the induction of angiopoietin-2 and down-regulation of the pro-angiogenic regulator angiopoietin-1 (22;27). Ang-2 is a competitive antagonist for the Tie-2 receptor on endothelial cells (33), which antagonizes the angiopoietin-1 mediated recruitment of microvascular pericytes through expression of leading to the induction and maintenance of a ‘quiescent’ phenotype in capillaries. Ang-2 is an important activator of sprouting angiogenesis (58), and Ang-2 deficient mice show complex defects in vascular development and post-natal vascular remodeling (12;13;56). In addition to blood vessel organization, Ang-2 deficiency also influences the development of lymphatic vessels, particularly those within the gastrointestinal system (8). Ang-2 deficient mice have been reported as a relevant model of intestinal lymphatic dysplasia (49). Because lymphatics play especially important roles in intestinal homeostasis, the Ang-2-/- model might thus be an ideal system for investigating how congenital lymphatic disturbances in the intestine might impact the pathogenesis and course of experimental colitis, a model of human IBD.
Angiogenesis contributes to the development of human and experimental IBD through the remodeling of blood vessels; mediators like VEGF-A, which trigger endothelial proliferation, also cause vascular leakage and leukocyte infiltration (5;17). Ang-2, a competitive ligand with Ang-1 for the TEK/Tie-2 receptor (13) plays a critical role in angiogenesis by dislodging vascular adventitial support cells (e.g. pericytes and smooth muscle) to permit maximal angiogenic responsiveness to mediators possibly enhancing vascular responses to inflammation (59). While angiogenesis has been shown to promote and sustain many events in inflammation, the role that Ang-2 plays in the development of intestinal inflammation is less clear. On one hand, Ang-2-/- mediated lymphodysplasia (e.g. that in the intestine) (8) might intensify injury and edema by interfering with lymphatic transport of interstitial fluid and its components. Alternatively, Ang-2 deficiency should also lead to unopposed Ang-1 vascular hyperstabilization, and might block several important inflammatory events to reduce the extent of inflammation. To answer these questions, we used 3% DSS to induce intense intestinal inflammation in wild type and Ang-2 gene knockout mice as a model of ulcerative colitis. We found that Ang-2 deficiency only slightly altered the course of the weight loss during experimental colitis as was disease activity. In this model histopathological evidence of injury was significantly reduced along with neutrophil infiltration by Ang-2 deficiency. Moreover, the blood vessel and lymphatic vessel densities were significantly elevated in DSS treated mice and were completely blocked in Ang-2 deficient mice. Colon shortening was also increased in DSS treated animals an effect which was partially attenuated by Ang-2 deficiency. These data demonstrate that several parameters of both inflammation induced hemangiogenesis and lymphangiogenesis are reduced by Ang-2 deficiency. Not all evidence of intestinal disease is however eliminated by Ang-2 deficiency, for e.g. weight loss, colon length, occult blood and diarrhea were still worse than WTs. These data are discussed in terms of potential roles of Ang-2 in the etiology of colitis and IBD.
Materials and Methods
Animals
Mice used in this study were male or female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) or Angiopoietin-2-/- mice (Regeneron Inc., Gale et al., 2002, (8;13). Mice were used in these colitis studies at 8 -12 weeks of age, and had an average initial weight of 20 ± 0.6g (SEM) on day 0. Mice were kept in a controlled environmental room at 25°C with a 12h/12h light/dark cycle in the University of Arizona vivarium and allowed free access to standard pellet diet and tap water. All protocols were approved by the LSUHSC-S and University of Arizona insitutional animal care and use committees (IACUC).
Induction of experimental colitis
Experimental colitis was induced in mice by supplementing drinking water with 3% dextran sulfate sodium (DSS) (as described Soriano et al., 2000), ad libitum (DSS, MW= 36-50 kDa; ICN Biomedicals Inc.); mice in the control group (n = 5) group received tap water (without DSS). 3% DSS administration produces an erosive distal colitis with an initial onset at 3-4 days which is characterized by progressive weight loss, diarrhea, occult blood, leukocyte infiltration, colon shortening, loss of intestinal epithelial barrier and histopathological changes in colon structure (44-46;51). Mice typically lose ~ 20% body weight by day 7 with continuous administration of 3% DSS. Over 7 days, mice were given unrestricted access to pellet diet (Purina), and tap water (control), or tap water containing 3% dextran sulfate sodium (DSS) in “colitis” groups (1;44-46;51). Mouse weight, stool form, occult blood, food (grams) and liquid (mls) consumption were recorded daily. On day 7, mice were sacrificed by cardiac puncture (under ketamine/xylazine anesthesia). Histological samples were fixed in cold 3.7% phosphate buffered formalin; or frozen at -20°C for myeloperoxidase (MPO) and western blotting analysis.
Evaluation of Clinical Colitis
Body weight, stool form and occult blood were scored daily as described (9). Disease activity index (DAI) was determined by averaging scores of weight loss, stool form, and occult blood. Scores were determined as 1) change in weight (0 :< 1%, 1: 1–5%, 2: 5–10%, 3:10-15%, 4 :> 15%), 2) occult blood (0: negative, 2: positive, 4: gross bleeding), and 3) stool form (0: normal, 2: loose stools, 4: diarrhea) as previously described (6;23;44-46). Weight loss was calculated as: percent difference between the original body weight, and body weight on any given day. Stool consistency was scored as 0-4 (0 – small, firm, dry, non-adherent and friable, 1 – small, firm, moist, adherent, 2- larger, soft, very adherent stool, 3 – larger, soft, pliable 4- liquid stool). Occult blood was detected chemically using ‘Coloscreen’ clinical kits, (Helena Labs, Beaumont, TX) and scored as 0-4. In blood score, 0 - indicates no color development, 1 – flecks of color reaction, 2 – consistent blue color, 3 – rust color stools +blue reaction, 4- wet blood +dark blue reaction.
Histopathological analysis
Formalin fixed colon sections were paraffin embedded and 10μm sections stained with hematoxylin and eosin. Slides were analyzed for evidence of histopathological injury using criteria established by Cooper et al., (1993). This system includes edema, extent of injury, leukocyte infiltration, crypt abscesses, and loss of goblet cells (6). In this grading system, inflammation severity is scored on a 0-3 scale, (0-none, 1-slight, 2-moderate, 3-severe), the extent of injury was scored on a 0-3 scale (0-none, 1-mucosal, 2-mucosal + submucosal, 3-transmural), crypt damage was scored on 0-4 scale (0-none, 1- basal 1/3 damaged, 2-basal 2/3 damaged, 3- only surface epithelium intact, 4- loss of entire crypt and epithelium). Each value was multiplied by an extent index from 1-4 which reflects the amount of involvement for each section (1: 0-25%, 2: 26-50%, 3: 51-75%, 4: 76-100%). Atleast 3 sections from each colon were analyzed to produce each score value. A maximum possible histopathological score for this assay is 40.
Measurement of tissue MPO content
MPO activity was measured according to (16) as modified by (18). Samples (20-30 mg) of colon tissue were frozen under N2(l), crushed and freeze-thawed 3X in 0.5% HETAB buffer sonicated for 10s at 50% max power (29), and cleared by centrifugation at 10,000 x g before MPO activity of supernatants was measured (using 0.1% o-dianisidine substrate). MPO activity is defined as the change in absorbance at 650 nm/min/mg tissue.
Immunohistochemistry
Colon sections fixed >12h in 3.7% phosphate buffered formalin were embedded in paraffin. Sections (10μm) were collected onto Superfrost-Excell slides, and deparaffinized prior to antigen retrieval. Slides were incubated in primary antibody (1:125, 1hr), washed in 0.1% BSA and reacted in HRP-conjugated secondary antibody (1:2,500, 1h), and reacted with DAB/peroxide (5 mins). 3.7% phosphate-buffered formaldehyde fixed tissue sections (5 μm) were immunostained using DAB/ peroxidase for vascular endothelial growth factor receptor-3 (VEGFR-3) (lymphatic vessels) and MECA-32 (mouse endothelial cell antigen-32). MECA-32 monoclonal antibody (30;34), a blood vascular specific marker (34), was obtained from Developmental Hybridoma Studies Bank (Iowa City, IA) and prepared on-site. Slides were hematoxylin counterstained (30s) and sealed in Permount.
Measurement of serum albumin
Equivalent volumes (30 μl) of serum were diluted with 30 μl of Laemmli sample buffer (26), and 5μl of each sample were loaded onto 7.5% SDS-PAGE gel and stained with Coomassie blue. The gels were scanned densitometrically and the 66kD serum albumin band was compared in control, Ang-2-/-, DSS and Ang-2-/- + DSS serum samples. Differences in protein band density levels were compared statistically by comparing band density with instat software analysis. Groups were compared to controls using One-way ANOVA with Dunnetts’ post-testing.
Microphotography
Images of immunostained tissue sections were photographed using a Nikon brightfield microscopy station (Olympus CK2) and analyzed for the number of blood and lymphatic vessels and vessel dimensions using Image-J (NIH).
Statistical Analysis
Weight changes and disease activity studies were evaluated using ‘Repeated-measures ANOVA with Dunnett’s post-testing. MPO data were analyzed by one-way ANOVA with Dunnett’s post-testing (Graphpad Instat 3 Software).
Results
Disease activity is increased in Ang-2-/- mice in DSS colitis
Compared to untreated controls, mice treated with 3% DSS showed a progressive weight loss, increase in occult blood, and diarrhea. Ang-2-/- mice treated with 3% DSS also showed greater weight loss and disease activity than WT + DSS treated mice (Fig. 1).
Figure 1. Disease Activity Index (DAI) and weight change.


(a) Disease activity. Ang-2-/-/DSS (KO DSS) was significantly different from baseline at day 2 through day 7; WT + DSS-DAI (WT DSS) was not significantly different from baseline until day 5. (b). Weight change. Weight loss in Ang-2-/- + DSS and WT + DSS were both significantly different (*= p<0.05) from baseline at day 5 through day 7 (repeated measures ANOVA, Dunnett’s post-testing). No difference in weight was observed between. Ang-2-/-/DSS; WT + DSS.
Early appearance of occult blood is seen in Ang-2-/- mice in DSS colitis
Occult blood was detected in the feces of Ang-2-/- + DSS treated mice as early as day 2 and onward until day 7. By comparison, WT + DSS treated mice did not show evidence of fecal blood until day 3 (and onward). No significant presence of occult blood was seen in wild type control or Ang-2-/- mice in the absence of DSS treatment (*=p<0.05 vs. control, **= p < 0.01) (Fig 2).
Figure 2. DSS induced occult blood is exacerbated in Ang-2-/-.

Occult blood was detected in the feces of Ang-2-/- + DSS treated mice as early as day 2 through day 7. WT + DSS treated mice did not show evidence of fecal blood until day 3 (through day 7). No significant presence of occult blood was seen in control (WT) or Ang-2-/- (KO Con) mice. (*= p<0.05 vs. control, ** = p<0.01 vs. control)
Water absorption in colon (stool form) is exacerbated in Ang-2-/- mice in DSS colitis
Evidence of altered intestinal water absorption was seen as early as day 2 in Ang-2-/- + DSS mice (p<0.05). After day 2 Ang-2-/- +DSS mice showed significant stool alterations in fecal integrity until the end of the study. By comparison, in WT + DSS mice, stool form changes were not significant until day 4 (*= p<0.05, and later days) (Fig. 3).
Figure 3. DSS stool form scoring is exacerbated in Ang-2-/-.

Stool form scores were significantly different from baseline by day 2 in Ang-2-/- + DSS. In WT DSS mice significant alterations in stool form were observed from day 3 until day 7 (p<0.05). No significant differnce was observed in WT Con and Ang-/- Con mice.
Inflammation-induced colon shortening
Inflammation of the colon in human IBD and colitis models is characterized by a shortening of the colon (52). Colon length in Ang-2-/- mice (7.3 ± 0.12 cm, SEM) was not significantly different from that in normal controls (7.5 ± 0.13 cm, SEM) but colon length was significantly reduced in WT + DSS-treated colons (to 5.6 ± 0.06 cm, SEM) when compared to WT controls (** p < 0.01). Colon length was also reduced in DSS-treated Ang-2-/- mice and to a higher level of significance vs. its respective control (4.7 ± 0.62 cm, SEM, *** p < 0.001 vs. Ang-2-/-) (Fig. 4).
Figure 4. DSS induced colon shortening is exacerbated in Ang-2-/-.

Colon length in Ang-2-/- mice (7.3 +/-0.25 cm) was not significantly different from that in normal controls (7.45 +/- 0.67 cm). Colon length was significantly reduced in WT + DSS colons (to 5.56 +/- 0.13 cm) compared to WT controls (* p<0.05). Colon length was further reduced in Ang-2-/- mice (4.69 +/- 1.25 cm, **p<0.01 vs. Ang-2-/-).
Histopathological scoring
Histopathological scoring based on hematoxylin and eosin stained sections from fixed colons under light microscopy was scored by a pathologist blind to experimental conditions. Compared to control mice, 3% DSS treated mice showed significantly increased histopathologic scoring of disease. On a range of 40 (where 0 score is control, and 40 is the maximal injury) (9), 3% DSS treated WT mice received an injury score of 21.71 ± 1.10; whereas, WT controls were only 3 ± 1.22. Ang-2-/-+ DSS had an injury score of 15.54 ± 2.06 which was significantly reduced (28.52% lower) compared to WT + DSS treated specimens. Ang-2-/- mice not treated with DSS showed a score of 3 ± 1.16. (p<0.05 is significant, Fig. 5a). DSS treated mice showed extensive loss of epithelial and goblet cells and a dense infiltration of neutrophils (Fig. 5b). No differences in the histopathology were observed in WT and Ang-2-/- controls. This form of injury was also evident in Ang-2-/- mice treated with 3% DSS which also exhibit epithelial disturbances and blunting, less edema, and slightly improved histology.
Figure 5. Effects of Ang-2-/- status on histopathological scoring in DSS colitis.


(a) Histopathology scores. WT + DSS had a cumulative histopathology score of 21.71±1.10 (Normal score=0, max injury=40), whereas, WT controls were 3±1.22 Ang-2-/-/DSS (15.54±2.06) was significantly reduced (by 28.52%) compared to WT + DSS. Ang-2-/- showed a score of 3 ±1.16. (One-way ANOVA with Bonferroni post-testing. (b). Representative images of hematoxylin/eosin stained tissue from WT Control, Ang-2 KO Con, WT DSS and Ang-2-/- + DSS treated colons.
Changes in colon hemangiogenesis
Angiogenesis is an important component of colon inflammation (7;17), which was analyzed by immunostaining for the mouse endothelial-specific antigen MECA-32 (38). Hemangiogenesis was measured by counting the number of MECA-32+ vessels in colon cross-sections after immunostaining (by 2 individuals separately, blind to the experimental groups). MECA-32+ immunostained vessels were significantly increased in the colons of WT + DSS mice compared to WT-Con (27.2 ± 8.4 vs. 12.4 ± 4.3 vessels; a 2.19 fold increase; p<0.001). This inflammation induced hemangiogenesis was significantly reduced in Ang-2-/- + DSS compared to untreated controls or to Ang-2 controls (8.9 ± 3.1 vs. 8.667 ± 4.22 vessels; p<0.001) (Fig. 6a)
Figure 6. Effects of Ang-2-/- status on blood and lymphatic vessel density scoring in DSS colitis.
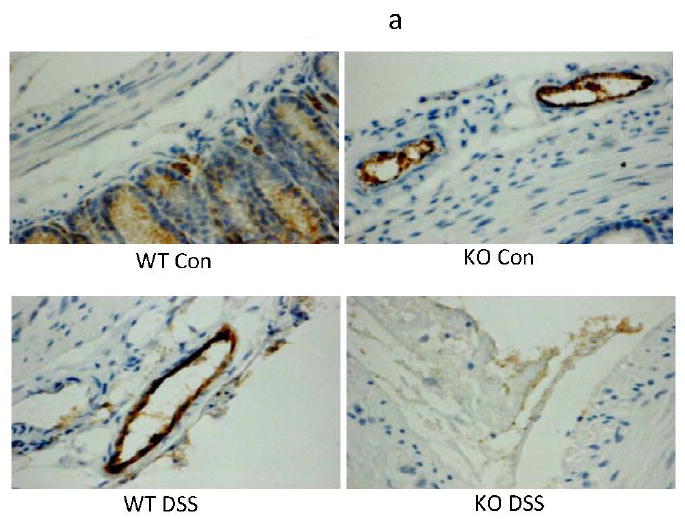


(a) Blood vessel (MECA-32+) immunostained vessels were significantly increased in the colon of WT + DSS (2.19 fold increase over the WT controls). (b) Similarly, lymphatic (VEGFR-3+) vessels showed a 4.9 fold increase from WT to WT + DSS. Increased lymphangiogenesis was significantly reduced in Ang-2-/- and Ang-2-/-/DSS. (c) Histogram of # vessels/section (One-way ANOVA w/Bonferroni post-testing). (Magnification = 200X).
Changes in colon lymphangiogenesis
Lymphangiogenesis, another form of vascular remodeling, accompanies gut inflammation (14;40) and was analyzed by immunostaining for VEGFR-3, a growth factor receptor highly enriched on lymphatic endothelium (20). Lymphangiogenesis was measured by counting the number of VEGFR-3+ vessels in colon cross-sections after immunostaining (by 2 individuals separately, blind to the experimental groups). VEGFR-3+ vessels were dramatically increased in WT + DSS treated colons, (to 57.4 ± 10.8 vessels), 4.9-fold greater than wild type controls, (11.7 ± 7.7 vessels) (p<0.001); VEGFR-3+ vessels in Ang-2-/- controls (10.1 ± 3.6 vessels) were similar to wild type controls. This WT + DSS induced increased lymphangiogenesis was significantly reduced in Ang-2-/- + DSS (6.9 ± 10.1 vessels, p<0.001)(Fig. 6b).
Comparison of colon hemangiogenesis and lymphangiogenesis
The Increases in lymphatic density paralleled changes in blood vessel density. The number of both blood vessels and lymphatic vessels increased significantly in the colons of WT + DSS vs. WT-Control or Ang-2-/- (***p<0.001 vs. WT or Ang-2-/- controls). The increased hemangiogenesis and lymphangiogenesis seen in WT + DSS was significantly reduced in Ang-2-/- + DSS colons (not significant compared to WT or Ang-2-/-, ***p<0.001 vs. WT + DSS). The density of blood and lymphatic vessels in Ang-2-/- + DSS was not significantly different from the densities in Ang-2-/- and WT control (Fig 6c). Although non-inflamed colons had similar numbers of blood and lymphatic vessels per section (12.4 ± 4.3 and 11.7 ± 7.7 respectively), the increase in the abundance of colon lymphatic vessels during inflammation was just over twice (2.11X) that of blood vessels density induced by inflammation.
MPO content (neutrophil infiltration)
Neutrophil infiltration, a prominent feature of Crohn’s disease and also of DSS colitis measured using MPO activity in colon tissue specimens, was significantly elevated in WT + DSS samples compared to control tissues, and was significantly decreased in Ang-2-/- + DSS mice compared to WT + DSS mouse colons (Fig. 7).
Figure 7. Ang-2 regulates gut neutrophil infiltration in DSS colitis.

The level of neutrophil invasion, measured by myeloperoxidase (MPO) activity of the colonic tissue, was significantly elevated in WT + DSS vs. WT, and was not significantly affect in Ang-2-/- /DSS vs. WT + DSS.
Decreased serum protein content
The abundance of albumin in serum was densitometrically analyzed using NIH image-J, and the scan density values compared statistically (using Graphpad Instat 3). Compared to WT controls (reflects the physiological levels of serum albumin). There was a significant decrease in the serum albumin content (24.8% reduction) observed in the WT + DSS mice compared to WT controls (75.16 ± 7.01 vs. 100 ± 5.32) which represents, a large loss of protein and parallels the pathological features protein- losing enteropathy in of IBD/Crohns disease (p<0.01). There was no significant change in serum albumin content in Ang2-/- controls to that of WT controls (101.17 ± 6.56% vs. 100 ± 5.32) control). Ang2-/- + DSS treated mice (91.01 ± 9.56) had only small changes (not significant vs. Ang2-/-control or WT controls)) in serum albumin (Fig. 8).
Figure 8. Ang-2-/- status affects serum albumin content in DSS colitis.

A significant decrease in the serum protein content was observed in the WT + DSS (p<0.05 vs.controls). There was no significant loss of serum protein content in Ang-2-/- or. Ang-2-/-/DSS compared to control. (One-way ANOVA with Bonferroni post-testing).
Discussion
Ang-2 is a classical antagonist of Ang-1:Tie-2 system that may have both beneficial or detrimental effects on inflammation depending on the particular physiological and pathological settings being considered (11;24;35;42;53) Activation of Tie-2 by Ang-1 is well known to suppress endothelial migration and enhance survival, in contrast to Ang-2 which results in rapid destabilization of endothelium, inducing inflammation and solute permeability (11;24;31;39;42;48). Several reports have shown that inhibition of Ang-2 signaling is beneficial in pathologic conditions such as sepsis, acute lung injury, systemic lupus erythematosus (SLE), and inflammatory synovitis (25;55). It is possible that by preventing the vascular tissue remodeling in these conditions controlled by the Ang-1/Ang-2, some features of injury might be attenuated. In our present study we have observed both beneficial and detrimental (pathologic) effects of Ang-2 genetic deficiency, which is due to the highly complex pathological environment in experimental and human IBD and the participation of Ang-2 in lymphatic and blood vascular integrity.
Histopathological analysis
Wild-type colons showed a normal epithelial surface with no evidence of inflammatory infiltrates or edema; occasional regenerative atypia could be observed in a few sections. Untreated Ang-2-/- colons also showed normal colon histological architecture, and were essentially indistinguishable from normal controls. By comparison, colons from DSS-treated WT mice showed extensive interruptions of the epithelial surface, with submucosal edema and chronic inflammatory cell infiltrates which consisted of dense lymphoid aggregates which were devoid of germinal centers (Fig-5). Ang-2 deficiency was seen to alter the histopathological character of gut injury provoked by 3% DSS in this model. Colons from Ang-2-/- mice treated with DSS showed some evidence of reduced tissue injury; samples from this group retained a more relatively intact epithelial surface with little inflammation in the intestinal glands. There was evidence of some sub-serosal inflammation and submucosal edema. Compared to WT + DSS, Ang-2-/- showed an attenuated inflammatory response, and fewer cell infiltrates which consisted of lymphocytes, monocytes and neutrophils. Ang-2-/- mice treated with DSS also showed the presence of numerous enlarged colon mucous vacuoles which could be microinspisated.
Decreased neutrophil infiltration and inflammation in Ang-2-/- mice
Recent reports have clearly shown that neutrophil infiltration, an important facet of inflammation, may be triggered by angiogenesis and that these events are causally associated (5;7;36). Although Ang-2 participates in angiogenesis, the role of Ang-2 in mediating inflammatory responses during IBD is not well understood. In the present study we observed a highly significant reduction in neutrophil infiltration, hisopathological evidence of inflammation, angiogenesis and lymphangiogenesis in Ang-2-/-+ DSS mice compared to WT + DSS mice indicating that Ang-2 plays a significant role in mediating immune responses involving the migration of leukocytes especially neutrophils into the inflamed intestine/colon in IBD. Neutrophils that infiltrate the inflamed gut in IBD provide many of the cytokines, growth factors, proteolytic enzymes and oxidants which are significant contributors to injury and inflammatory angiogenesis. These newly formed blood vessels supply the inflamed tissue with oxygen and nutrients thereby allowing the transport of immune cells into the inflamed region (3;4;37). The decreased neutrophil content, tissue inflammation, and inflammatory angiogenesis seen in the tissues of Ang-2-/- mice clearly points out some of the detrimental effects mediated by Ang-2 in the intestine during IBD. It has becomes clear that Ang-2’s role in various pathologic conditions is context dependent, and that Ang-2 might have many more functions than previously thought. Despite these examples of relative tissue protection against DSS in Ang-2-/-mice, we found that weight loss, appearance of occult blood and disease activity, and changes in stool form were significantly exacerbated in Ang-2-/- + DSS mice. Further increased colon shortening and early evidence of stool blood in the Ang-2-/- mice indicate that Ang-2-/- actually aggravates IBD and inhibiting Ang-2 might only attenuate certain pathologic features of the disorder.
Decreased inflammatory angiogenesis/ neo-lymphangiogenesis in Ang-2-/- mice
Many pathological conditions, including inflammation, immune responses, tumorigenesis, trauma, infections, surgery, and irradiation, affect lymphatic structure and function often triggering neo-lymphangiogenesis (32). Many of the known regulators of developmental lymphangiogenesis have also been implicated in neo-lymphangiogenesis and are active in pathological conditions. Our results have shown that absence of Ang-2 aggravates some features of IBD like increased weight loss and stool form despite improvements in tissue histology and leukocyte infiltration; its role in lymphatic maturation resulted in the dysregulation of lymphatic growth which may be detrimental in IBD. We also observed a significant increase in inflammatory lymphangiogenesis and angiogenesis in the WT + DSS compared to the WT-controls. This stimulus to hemangio/lymphangiogenesis was greatly reduced in Ang-2-/- mice (vs. WT + DSS). Though reduction of inflammatory angiogenesis is beneficial in the pathology of IBD, the lower abundance and compromised function of lymphatics might suggest that Ang-2 gene deficiency may also block lymphatic expansion which may also drive the pathologic changes. Further, the exact role of Ang-2 in modulating the function of lymphatics under normal and pathological conditions is still not understood. Parallel to the mutually interacting overlap among inflammation, immune responses and hemangiogenesis; inflammation and immune responses also exhibit an intimate association with increased lymphangiogenesis (32;41). Lymphatic vessels actively regulate inflammatory responses by transporting not only interstitial fluid and antigens, but also leukocytes of different types from sites of inflammation to secondary lymphoid organs (32;47). The decrease in the neutrophil content in the gut of Ang-2-/- + DSS mice might reflect decreased vascular penetration or lymphatic clearance of leukocytes. Leukocyte products, including cytokines, proteases, and oxidants due to prolonged leukocyte residence in tissues may explain the increased injury in this model. Although angiogenesis contributes greatly to the inflammation seen in IBD, it also serves an important role in the repair of tissue and blood vessel injury (2). The inability to initiate repair process (due to vessel hyperstabilization) in Ang-2-/- mice might account for the appearance of occult blood in the Ang-2-/- + DSS mice at earlier time points, and the inability of the gut to reabsorb water possibly due to lymphatic dysfunction.
Protein losing enteropathy as a diagnostic marker for IBD
The pathobiology of gut injury in inflammatory bowel disease is multifactorial. The primary function of the lymphatic system is to control tissue fluid homeostasis by transporting lymph from tissues and organs back to the blood vascular system for recirculation (43). It has been previously reported that hypoproteinemia in ulcerative colitis is mainly due to loss of protein from the bowel, rather than malabsorption which can be accompanied by lymphocytopenia/lymphopenia (loss of lymph in the bowel) reflecting a lymphatic malfunction. Clinical features of IBD include lymphopenia, hypoproteinemia (hypoalbuminemia) hypo-γ-globulinemia and hypochromic anemia (19). An important clinical feature in IBD patients is loss of protein reflecting a lymphatic malfunction or protein losing enteropathy. Measuring the serum protein content in IBD patients provides one important diagnostic indicator of IBD. Protein losing enteropathy (PLE) is a disorder of lymphatic insufficiency and/or obstruction seen in several pathological conditions including Fontan repair, follicular lymphomas, lupus, Crohn’s disease and Whipple disease (10;21;50;57). This clinical feature parallels our PLE results indicating that the IBD of DSS is also characterized as a form of protein losing enteropathy (10). Our results demonstrating the decreased serum protein content in WT + DSS mice over WT controls is most consistent with lymphatic insufficiency to relieve edema in DSS colitis, provoking a loss of plasma (lymph) protein from the gut. Although DSS causes PLE, PLE was not observed in Ang-2-/- + DSS treated mice. It is interesting to note that the serum albumin/protein levels in the Ang-2-/- and Ang-2-/- + DSS mice were nearly normal. These data may be interpreted on the relative ratios of blood and lymphatic vessels, and their relative states of inflammation. For example, the normal colons contain blood and lymphatic vessels, at a ratio of 1.06. In DSS treated colons, the numbers of both blood and lymphatic vessels is increased, but there is still an overall higher number of lymphatic vessels, and the blood:lymphatic vessel ratio o is 0.47, consistent with a greater lymphatic expansion in response to and compensating for leaky blood vessels. By comparison, Ang-2-/- mice have nearly normal ratios of blood and lymphatic vessels (0.89), and this ratio is slightly increased by DSS treatment, giving a blood vessel to lymphatic vessel ratio of 1.26. Therefore the trend for lymphatic compensation seen in normal mice during inflammation is absent or reversed in Ang-2-/- mice
Considering that Ang-2-/- mice have normal levels (101.17 ± 6.56%) of serum albumin under control conditions, (i.e. without inflammation), it is clear that Ang-2-/- mice do not basally exhibit evidence of protein losing enteropathy (PLE) or at least are in a compensated state by increased protein synthesis. In the human condition of lymphatic hypoplasia, lymphatic vessels are nearly absent in the intestine, while blood vessels are present (60). This condition is characterized by a blood to lymphatic vessel ratio which far exceeds that of Ang-2-/- controls, normal controls or Ang-2-/-+ DSS mice. Lymphatic hypoplasia, a form of enteric protein loss (PLE) is characterized by marked lymphedema due to insufficient numbers of lymphatics, with normal blood vessel numbers leading to lymphatic stasis, since ISF cannot be relieved by lymphatics.
In the Ang-2-/- model, blood and lymphatic vessels are present, but hyperstabilized by the absence of Ang-2-/-. Therefore despite the presence of stimuli that would provoke blood vascular leakage (seen in DSS + WT mice), neither blood vessel nor lymphatic vessel remodeling occurs, preventing any vascular leakage that would drive this path to tissue injury. Ang-2 + DSS mice have (91.09 ± 9.56%) of normal albumin levels, compared to (75.16 ± 7.02%) normal albumin in DSS + WT mice. Based on these results enteric protein loss in this model of colitis appears to involve Ang-2 mediated blood vessel leakage and reorganization, and lymphatic expansion is a physiological compensating response for leaky blood vessels and increased lymph formation.
Summary/ Conclusions
Despite reducing several pathological aspects of IBD, (including angiogenesis) Ang-2-/- mice still exhibit reduced lymphatic density and lymphangiogenesis an effect that may contribute to pathology in this model. Ang-2-/-+ DSS treated mice showed advanced, (albeit mild) weight loss, earlier evidence of increased vascular fragility (shown by the early appearance of occult blood), evidence of early and more severe intestinal barrier failure (shown by changes in stool form), and an overall accelerated course of disease activity. Our results indicate that absence of Ang-2 might exert a beneficial effect (by reducing inflammatory angiogenesis and neutrophil infiltration) but that its role in creating a defective lymphatic vasculature might also compromise the beneficial effects of Ang-2 deficiency on blood vessels. Clearly the roles of Ang-2 in inflammation are highly complex, likely to be model specific, and further studies are required to elucidate the multifaceted context dependent roles of Ang-2 in various forms of vascular and tissue injury.
Supplementary Material
Acknowledgments
The authors would like to acknowledge NIH grant DK43785, American Heart Association, Louisiana Gene Therapy Program and Feist Cardiovascular Research Program for supporting this work
Financial Support. This work is supported by NIH grant DK43785, American Heart Association, Louisiana Gene Therapy Program and Feist Cardiovascular Research Program.
Reference List
- 1.Ando T, Jordan P, Wang Y, et al. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258–264. doi: 10.1097/01.mib.0000160807.53858.1c. [DOI] [PubMed] [Google Scholar]
- 2.Bao P, Kodra A, Tomic-Canic M, et al. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli R, Albini A, Noonan D. Neutrophils and angiogenesis: potential initiators of the angiogenic cascade. Chem Immunol Allergy. 2003;83:167–181. doi: 10.1159/000071560. [DOI] [PubMed] [Google Scholar]
- 4.Benelli R, Lorusso G, Albini A, et al. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des. 2006;12:3101–3115. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- 5.Chidlow JH, Jr, Langston W, Greer JJ, et al. Differential angiogenic regulation of experimental colitis. Am J Pathol. 2006;169:2014–2030. doi: 10.2353/ajpath.2006.051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 7.Danese S, Sans M, de la MC, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieleman LA, Pena AS, Meuwissen SG, et al. Role of animal models for the pathogenesis and treatment of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1997;223:99–104. [PubMed] [Google Scholar]
- 10.Ferrante M, De HG, Penninckx F, et al. Protein-losing enteropathy in Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:A25. doi: 10.1016/s1542-3565(05)00243-0. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27:552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gale NW, Thurston G, Davis S, et al. Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harb Symp Quant Biol. 2002;67:267–273. doi: 10.1101/sqb.2002.67.267. [DOI] [PubMed] [Google Scholar]
- 13.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 14.Geleff S, Schoppmann SF, Oberhuber G. Increase in podoplanin-expressing intestinal lymphatic vessels in inflammatory bowel disease. Virchows Arch. 2003;442:231–237. doi: 10.1007/s00428-002-0744-4. [DOI] [PubMed] [Google Scholar]
- 15.Goebel S, Huang M, Davis WC, et al. VEGF-A stimulation of leukocyte adhesion to colonic microvascular endothelium: implications for inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G648–G654. doi: 10.1152/ajpgi.00466.2005. [DOI] [PubMed] [Google Scholar]
- 16.Grisham MB, Granger DN, Lefer DJ. Modulation of leukocyte-endothelial interactions by reactive metabolites of oxygen and nitrogen: relevance to ischemic heart disease. Free Radic Biol Med. 1998;25:404–433. doi: 10.1016/s0891-5849(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum OA, Heidemann J, Binion DG. The intestinal microvasculature as a therapeutic target in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:78–97. doi: 10.1196/annals.1326.003. [DOI] [PubMed] [Google Scholar]
- 18.Hausmann M, Obermeier F, Paper DH, et al. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2007;148:373–381. doi: 10.1111/j.1365-2249.2007.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iljin K, Karkkainen MJ, Lawrence EC, et al. VEGFR3 gene structure, regulatory region, and sequence polymorphisms. FASEB J. 2001;15:1028–1036. doi: 10.1096/fj.00-0383com. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko H, Yamashita M, Ohshiro M, et al. Protein-losing enteropathy in a case of nodal follicular lymphoma without a gastrointestinal mucosal lesion. Intern Med. 2008;47:2171–2173. doi: 10.2169/internalmedicine.47.1189. [DOI] [PubMed] [Google Scholar]
- 22.Karkkainen MJ, Alitalo K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin Cell Dev Biol. 2002;13:9–18. doi: 10.1006/scdb.2001.0286. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol. 1992;27:529–537. doi: 10.3109/00365529209000116. [DOI] [PubMed] [Google Scholar]
- 24.Kim I, Kim JH, Moon SO, et al. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 25.Kumpers P, David S, Haubitz M, et al. The Tie2 receptor antagonist Angiopoietin-2 facilitates vascular inflammation in Systemic Lupus Erythematosus. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lauren J, Gunji Y, Alitalo K. Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol. 1998;153:1333–1339. doi: 10.1016/S0002-9440(10)65717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Cho CH, Hwang SJ, et al. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 29.Lee YT, Sung JJ, Poon P, et al. Association of HLA class-II genes and anti-neutrophil cytoplasmic antibodies in Chinese patients with inflammatory bowel disease. Scand J Gastroenterol. 1998;33:623–627. doi: 10.1080/00365529850171909. [DOI] [PubMed] [Google Scholar]
- 30.Leppink DM, Bishop DK, Sedmak DD, et al. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation. 1989;48:874–877. doi: 10.1097/00007890-198911000-00032. [DOI] [PubMed] [Google Scholar]
- 31.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maby-El HH, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- 33.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 34.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochizuki Y, Nakamura T, Kanetake H, et al. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 36.Noonan DM, De Lerma BA, Vannini N, et al. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 37.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orosz CG, van BA, Sedmak DD, et al. Role of the endothelial adhesion molecule VCAM in murine cardiac allograft rejection. Immunol Lett. 1992;32:7–12. doi: 10.1016/0165-2478(92)90191-p. [DOI] [PubMed] [Google Scholar]
- 39.Oshima Y, Oshima S, Nambu H, et al. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- 40.Pedica F, Ligorio C, Tonelli P, et al. Lymphangiogenesis in Crohn’s disease: an immunohistochemical study using monoclonal antibody D2-40. Virchows Arch. 2008;452:57–63. doi: 10.1007/s00428-007-0540-2. [DOI] [PubMed] [Google Scholar]
- 41.Ristimaki A, Narko K, Enholm B, et al. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 42.Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 43.Saharinen P, Petrova TV. Molecular regulation of lymphangiogenesis. Ann N Y Acad Sci. 2004;1014:76–87. doi: 10.1196/annals.1294.008. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki M, Bharwani S, Jordan P, et al. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radic Biol Med. 2003;35:1679–1687. doi: 10.1016/j.freeradbiomed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki M, Bharwani S, Jordan P, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther. 2003;305:78–85. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki M, Mathis JM, Jennings MH, et al. Reversal of experimental colitis disease activity in mice following administration of an adenoviral IL-10 vector. J Inflamm (Lond) 2005;2:13. doi: 10.1186/1476-9255-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scavelli C, Weber E, Agliano M, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. 2004;204:433–449. doi: 10.1111/j.0021-8782.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharpfenecker M, Fiedler U, Reiss Y, et al. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 49.Shimoda H, Bernas MJ, Witte MH, et al. Abnormal recruitment of periendothelial cells to lymphatic capillaries in digestive organs of angiopoietin-2-deficient mice. Cell Tissue Res. 2007;328:329–337. doi: 10.1007/s00441-006-0360-8. [DOI] [PubMed] [Google Scholar]
- 50.Silvilairat S, Cabalka AK, Cetta F, et al. Protein-losing enteropathy after the Fontan operation: associations and predictors of clinical outcome. Congenit Heart Dis. 2008;3:262–268. doi: 10.1111/j.1747-0803.2008.00200.x. [DOI] [PubMed] [Google Scholar]
- 51.Soriano A, Salas A, Salas A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 52.Taniguchi T, Tsukada H, Nakamura H, et al. Effects of the anti-ICAM-1 monoclonal antibody on dextran sodium sulphate-induced colitis in rats. J Gastroenterol Hepatol. 1998;13:945–949. doi: 10.1111/j.1440-1746.1998.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 53.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 54.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 55.van der HM, van Nieuw Amerongen GP, Chedamni S, et al. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets. 2009;13:39–53. doi: 10.1517/14728220802626256. [DOI] [PubMed] [Google Scholar]
- 56.Veikkola T, Alitalo K. Dual role of Ang2 in postnatal angiogenesis and lymphangiogenesis. Dev Cell. 2002;3:302–304. doi: 10.1016/s1534-5807(02)00231-9. [DOI] [PubMed] [Google Scholar]
- 57.Wang YF, Tseng KC, Chiu JS, et al. Outcome of surgical resection for protein-losing enteropathy in systemic lupus erythematosus. Clin Rheumatol. 2008;27:1325–1328. doi: 10.1007/s10067-008-0929-6. [DOI] [PubMed] [Google Scholar]
- 58.Xue Y, Cao R, Nilsson D, et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc Natl Acad Sci U S A. 2008;105:10167–10172. doi: 10.1073/pnas.0802486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan HT, Khankin EV, Karumanchi SA, et al. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng Y, Wang F, Williams ED, et al. Lymphatics in the alimentary tract of children in health and disease: study on mucosal biopsies using the monoclonal antibody d2-40. Pediatr Dev Pathol. 2005;8:541–549. doi: 10.1007/s10024-005-0023-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


