Abstract
Preclinical models that accurately reproduce specific aspects of human disease etiology are invaluable for the initial development and evaluation of chemopreventive agents. We developed a novel, short-term prevention model, which is particularly useful for assessing a compound’s efficacy to prevent hormonally responsive and non-responsive in situ carcinomas. In this model, carcinogenesis is induced by a high titer of neu-containing, replication-defective retrovirus. The multiplicity and size of the resulting in situ carcinomas are scored in whole-mounted, aluminum carmine-stained mammary glands at 15 days post-infusion.
These in situ carcinomas represent a distinct biological time-point in the development of neu-induced mammary cancer in the rat. They are characterized by high rates of proliferation (40.0%, p<0.0001) and apoptosis (2.8%, p<0.005), compared to mammary carcinomas. The majority of in situ carcinomas regress spontaneously after 20 days post-infusion.
The in situ carcinomas at 15 days post-infusion exhibit hormonal responsiveness. The effects of the chemoprevention agents tamoxifen, celecoxib and targretin on hormonally responsive and non-responsive in situ carcinomas recapitulate those observed on mammary carcinomas at 12 and 18 weeks post-infusion for intact and ovariectomized rats respectively.
Neu-induced in situ carcinomas in the rat represent etiologically relevant intermediate time points of mammary carcinogenesis. Our prevention model represents a cost-efficient in vivo system to determine whether a compound’s preventive effects extend to hormonally non-responsive mammary lesions, for which new chemoprevention approaches are needed.
Keywords: neu-induced retroviral in situ carcinoma rat model, mammary carcinogenesis, chemoprevention, hormonal responsiveness, tamoxifen, celecoxib, targretin
INTRODUCTION
Rodent models of mammary carcinogenesis have been shown to recapitulate histopathological morphology and molecular characteristics of various malignancies of the human breast (1, 2). They are vital for the development and preclinical evaluation of chemopreventive agents. Both rat and mouse mammary cancer models have advanced the field of chemoprevention by demonstrating the efficacy of several pharmacological and natural compounds to prevent mammary cancer in vivo.
Hormonal responsiveness, the ability to prevent mammary tumors by physiological or chemical hormone ablation, is a hallmark of mammary cancer. The NSABP Breast Cancer Prevention Trial (P-1) demonstrated that 49% of invasive breast cancers were prevented by the selective estrogen response modulator (SERM) tamoxifen (3). This corresponds to a 69% prevention rate for estrogen receptor (ER)-positive breast tumors, while no prevention benefit was observed on ER-negative tumors. Despite investigative chemoprevention efforts to target hormonally non-responsive breast tumors, SERMs remain the clinical standard of care for the prevention of breast cancer. Consequently, the population of breast tumors not prevented by SERMs remains a major cause of mortality from this disease. More efforts need to be undertaken to develop chemoprevention agents specifically targeting hormonally non-responsive breast cancer.
The neu-induced retroviral rat mammary carcinogenesis model exists in two distinct hormonal configurations. In intact rats about 50% of mammary carcinomas are hormonally responsive and, thus, can be prevented by treatments with the SERM tamoxifen. In ovariectomized rats, mammary carcinomas are uniformly hormonally non-responsive and tamoxifen treatment is inefficacious for the prevention of these tumors. The model recapitulates the hormonal responsiveness evident in human breast cancers and has been used to investigate the efficacy of multiple chemoprevention agents to prevent the development of hormonally non-responsive mammary carcinomas (4, 5). However, it might be cost-prohibitive to investigate the efficacy of a large number of potential compounds in this long-term prevention model with fully differentiated mammary carcinomas as endpoints. We have therefore developed a short-term prevention model in which hormonally responsive and non-responsive in situ carcinomas are scored as endpoints.
The prevention of precancerous lesions should be a primary effort in the field of chemoprevention. The majority of invasive breast tumors arise from in situ carcinomas (6) and the treatment of ductal carcinomas in situ (DCIS) in women is considered a viable strategy for the prevention of invasive breast carcinomas (7). Because our current understanding of the molecular changes that govern the progression from DCIS to invasive breast cancer is inadequate, the clinical care for DCIS varies greatly, ranging from mastectomy to excision and radiation to excision alone with considerable variation in recurrence rates (8). Preclinical models of early endpoints in mammary carcinogenesis are therefore urgently needed to elucidate the mechanisms by which the progression from in situ carcinoma into mammary carcinoma occurs.
A variety of preclinical models for premalignant mammary cancers in the laboratory rat have been developed over the past 40 years. The most widely used are chemical carcinogen-induced models utilizing the polycyclic hydrocarbon 7,12 dimethylbenz(a)anthracene (DMBA) or the direct-acting N-methyl-N-nitrosourea (NMU) (9). Difficulties in adaptation of these models as chemoprevention models arise from the fact that chemical induction of mammary carcinogenesis in the rat results in wide spectrum of premalignant lesions that coexist at the same time (9). This report shows that retrovirally-induced mammary lesions arise faster and result in functionally uniform preventable endpoints.
The neu-induced retroviral in situ carcinoma rat model is a short-term prevention model in which the multiplicity and size of such lesions are scored in whole-mounted, carmine-stained mammary glands. We characterized neu-induced in situ carcinomas as uniform, distinct and transient time points of mammary carcinogenesis in the rat, as well as their potential to develop into mammary carcinomas. The chemopreventive effects of the SERM tamoxifen, the cyclooxygenase-2 inhibitor celecoxib and the rexinoid targretin on hormonally responsive and non-responsive in situ carcinomas are discussed.
MATERIALS AND METHODS
Neu-induced retroviral in situ carcinoma rat model
All animal experiments were performed at our facility under protocols approved by the University of Wisconsin Medical School Animal Care and Use Committee. Virgin Wistar-Furth (WF) female rats were obtained from Harlan Sprague-Dawley, (Indianapolis, IN) at 6 weeks of age. All rats were group housed in suspended wire cages and maintained at a light/dark cycle of 12h, receiving Teklad lab meal (#8604) and acidified water ad libitum. After 1-2 weeks of acclimation, at approximately 50-60 days of age, all rats underwent retroviral infusion with the pJRneu vector, which induces mammary carcinogenesis by expressing the activated Her2/neu oncogene. The construction and generation of the pJRneu retroviral vector have been previously described (10, 11). Details on retroviral gene transfer into the mammary epithelium of the laboratory rat (12) and the application of this technology for chemoprevention in the neu-induced retroviral rat carcinogenesis model (4, 5) have also been published. A 15 μl suspension of replication-defective amphotropic retrovirus containing the activated neu oncogene was infused into the central ducts of the abdominal (fourth) mammary glands. All rats were infused with a viral titer of 1 × 107 CFU/ml. At two days post-infusion, a portion of the rats underwent a bilateral ovariectomy. At four days post-infusion, the rats were randomly assigned to the treatment groups and the administration of the experimental diets was begun. Tamoxifen was administered at a dose of 2 mg/kg diet, celecoxib at 1200 mg/kg diet and targretin at 250 mg/kg diet. The study was terminated 15 days post-infusion. The abdominal mammary glands were excised and whole mounted onto microscope slides. They were fixed in buffered formalin, defatted via acetone treatment, rehydrated through ethanol gradients, stained with aluminum carmine, dehydrated through ethanol gradients and cleared in xylenes. The stained slides were then transferred to mineral oil and photographed. The carmine stain visualized the ductal tree within the mammary gland as well as major anatomic landmarks such as the central duct and lymph nodes within the gland. The in situ carcinomas induced by the retrovirus appeared as nodules on the ductal structure within the mammary gland. Both the number of mammary lesions and the size of the lesions were scored using Image J (NIH, Bethesda, MD), an open source application for data analysis (13).
Chemopreventive agents
Tamoxifen was purchased from Sigma (St. Louis, MO). Celecoxib (LKT Laboratories, Inc, St. Paul, MN) and targretin (Onyx Scientific, Sunderland, United Kingdom) were obtained through the Division of Cancer Prevention Repository. All experimental diets were dry mixed in Teklad 4% fat rodent meal (Harlan Teklad, Madison WI), which was also used as control diet. All diets were prepared fresh weekly and stored at −20°C. Rats were provided fresh diet twice weekly.
Proliferation and Apoptosis
All animals were infused in accordance with the procedure for neu-induced retroviral in situ carcinomas with a retroviral titer of 1 × 107 CFU/ml. Rats were group housed and received control diet (Teklad #8604) and acidified water ad libitum. Twenty-five female WF rats were randomized into five time-point groups, which were sacrificed 15, 18, 21, 24 and 27 days post-infusion. At necropsy, the abdominal mammary glands were excised, fixed in buffered formalin and paraffin embedded. Consecutive slices were stained with hematoxylin and eosin for histological evaluation or used for proliferation and apoptosis assays.
The proliferation index of in situ carcinomas was evaluated by Ki-67 staining (14, 15). Immunohistochemical procedures were performed according to standard protocols using the primary antibody VP-K452 (Vector Labs, Burlingame, CA) to detect the Ki-67 epitope. Primary antibody binding was visualized by horseradish peroxidase conjugated secondary antibody, the VECTASTAIN® ABC Elite System (Vector Labs, Burlingame, CA) and diaminobenzidine (DAB) as the chromogen.
The apoptotic index of mammary carcinomas was evaluated by TdT-mediated nick end-labeling (TUNEL) (16) using the TdT-FragEL™ DNA Fragmentation Detection Kit QIA 33 (Calbiochem, Darmstadt, Germany) Slides were processed according to manufacturer’s recommendations.
KI-67 and TUNEL staining were evaluated by light microscopy. For each time-point twenty in situ carcinomas were randomly chosen for proliferation and apoptosis analysis. Approximately 500 cells in several random fields were evaluated for proliferating or apoptotic cells for each lesion.
Statistical analysis
The statistical analysis of the proliferation and apoptosis analysis, as well as the in situ carcinoma multiplicity and size comparisons, was performed by Wilcoxon rank-sum (Mann-Whitney) test.
RESULTS
The neu-induced retroviral in situ carcinoma rat model
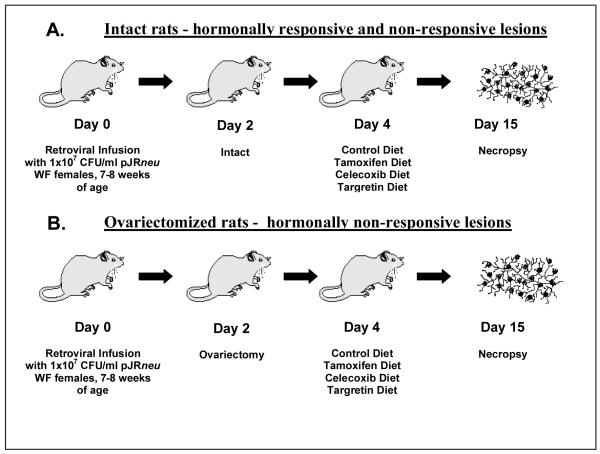
The neu-induced retroviral in situ carcinoma rat model (Figure 1) reproducibly induces in situ carcinomas using a viral titer of 1 × 107 CFU/ml. This high retroviral titer minimizes the number of animals needed and insures reliable statistical power. The number of in situ carcinomas induced in each mammary gland can be regulated by adjusting the retroviral titer, which controls the multiplicity of infection.
Figure 1. The neu-induced retroviral in situ carcinoma rat model.
The neu-induced retroviral in situ carcinoma rat model exists in two distinct hormonal configurations. The in situ carcinomas of intact animals (A) exhibit a mixed hormonal response, whereas those of the ovariectomized configuration (B) are hormonally non-responsive.
Figure 1 outlines the methodology for inducing hormonally responsive and non-responsive in situ carcinomas in our short-term model. Following retroviral infusion, the rats remain untreated for two days after infusion to allow for reverse transcription and stable integration of the retrovirus into the ductal epithelium. After this time period the ovariectomized group undergoes bilateral ovariectomies while the intact group retains normal hormone functions. Ovariectomy in neu-induced rats is associated with reductions of greater than 75% in ER and nearly 90% in progesterone receptor (PR) levels, in the resulting mammary carcinomas (17). Both hormonally responsive and non-responsive in situ carcinomas arise in intact rats, while the in situ carcinomas in ovariectomized animals are uniformly hormonally non-responsive.
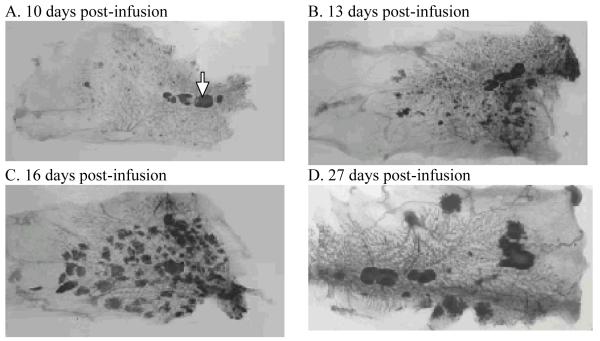
In a preliminary time-course series experiment, early lesions on the ductal structure were visible starting day 10 post-infusion (Figure 2A). The number and size of these lesions increased until approximately day 13 post-infusion (Figure 2B). After day 13, there was no appreciable increase in number of lesions but the lesion size increased steadily through day 16 post-infusion (Figure 2C) and day 19 post-infusion (not shown). At day 27 post-infusion, when tumors are generally palpable in the living rat, the majority of lesions had spontaneously regressed (Figure 2D). Day 15 post-infusion was decided upon as the endpoint in our prevention model as the in situ carcinomas were sufficiently large to be easily counted without the confounding effects of overlapping lesions.
Figure 2. Time-course following the development and progression of neu-induced mammary lesions.
Neu-induced mammary lesions in intact WF rats are visualized in whole-mounted, carmine-stained abdominal mammary glands at 10 days post-infusion (A), 13 days post-infusion (B), 16 days post infusion (C) and 27 days post infusion (D). Lymph nodes are marked in panel A (arrow).
Histopathological evaluation of neu-induced in situ carcinomas
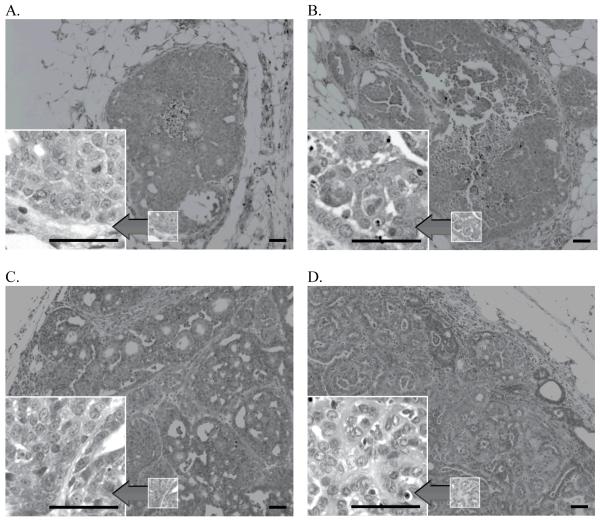
The neu-induced in situ carcinomas of our short-term prevention model display varying degrees of nuclear atypia at 15 days post infusion (Figure 3A and 3B). They exhibit solid, acinar, and papillary growth patterns and are frequently characterized by areas of central necrosis. Stable tumors at 27 days post-infusion (Figure 3C) are larger, with an overall more pronounced degree of architectural derangement and cellular atypia but in general exhibit similar morphologic features. Mammary carcinomas at 12 weeks post-infusion (Figure 3D) exhibit a range of morphological features, including papillary and acinar patterns. The general morphology of neu-induced in situ carcinomas and mammary carcinomas does not appear to be affected by ovariectomy or chemoprevention regimen.
Figure 3. Histopathological morphology of neu-induced mammary lesion.
The histopathological morphology of in situ carcinomas at 15 days post-infusion (3A, 3B), stable tumors at 27 days post-infusion (3C) and mammary carcinomas at 12 weeks post-infusion (3D) is shown. (Reference line = 50 μm)
Proliferation and apoptotic analysis
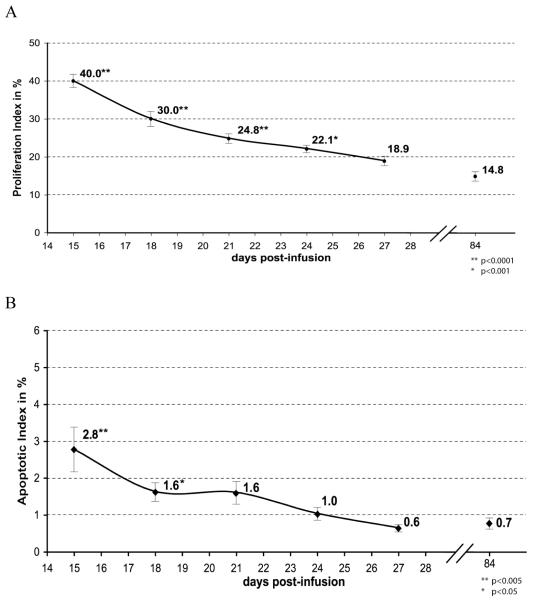
Neu-induced in situ carcinomas at day 15 post-infusion represent a distinct intermediate time-point in the mammary carcinogenesis process, characterized by high levels of both proliferation and apoptosis. Proliferation rates of in situ carcinomas averaged 40.0% at 15 days post infusion (Figure 4A). In this time-course analysis, the mean proliferation rate decreased steadily after 15 days until, by day 27 post-infusion, it approached levels similar to those of mammary carcinomas at 12 weeks post-infusion. The mean apoptotic index of in situ carcinomas at day 15 post-infusion was 2.8% (Figure 4B). Following the 15 day post-infusion time point apoptotic levels sharply decreased over time. By day 27 post-infusion, the apoptotic index had normalized to that of mammary carcinomas at 12 weeks post-infusion.
Figure 4. Proliferation and apoptotic indices during the progression from in situ carcinomas into mammary carcinomas.
Proliferation indices (A) and apoptotic indices (B) decrease rapidly during the progression from in situ carcinomas at 15 days post-infusion into early mammary carcinomas at 27 days post-infusion, approaching the rates of mammary carcinomas at 84 days post-infusion. Averages are labeled. Error bars denote standard error of means. Asterisks indicate significant differences from 84-day value. The 84 days post-infusion values have been published previously (4).
Progression rates of neu-induced in situ carcinomas
We compared the multiplicity of in situ carcinomas at day 15 post-infusion to the multiplicity of mammary carcinomas at 84 days post-infusion in intact rats and at 126 days post-infusion for ovariectomized rats. The retroviral titers were identical for each corresponding short-term and long-term model. In intact animals, the proportion of mammary carcinomas present at day 84 post-infusion compared to in situ carcinomas present at day 15 post-infusion is approximately 5% (Table 1). In ovariectomized rats, the ratio of mammary carcinoma at 126 days post-infusion to in situ carcinomas present at day 15 post-infusion is approximately 1% (Table 1).
Table 1.
Multiplicity of intermediate in situ carcinomas and mammary carcinomas induced by identical retroviral titers
| Model | INT/ OVX* |
Endpoint | Viral Titer | Duration | # rats | Multiplicity |
|---|---|---|---|---|---|---|
| Short- term |
INT | in situ carcinomas | 7.5 × 104 CFU | 15 days | 12 | 8.7 lesions/gland |
| Long- term |
INT | carcinomas | 7.5 × 104 CFU | 84 days | 15 | 0.43 carcinomas/gland |
| Short- term |
OVX | in situ carcinomas | 5 × 105 CFU | 15 days | 12 | 22.7 lesions/gland |
| Long- term |
OVX | carcinomas | 5 × 105 CFU | 126 days | 25 | 0.24 carcinomas/gland |
INT = Intact rat model, OVX= ovariectomized rat model
Chemopreventive effects of tamoxifen, celecoxib and targretin on neu-induced in situ carcinomas
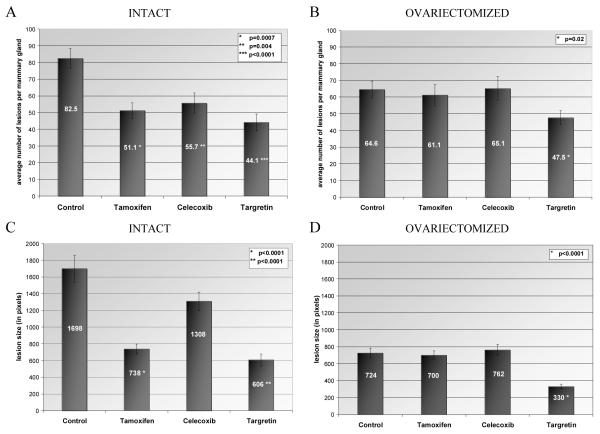
The chemopreventive effects of tamoxifen were limited to hormonally responsive in situ carcinomas arising in intact rats. Dietary tamoxifen reduced the multiplicity of in situ carcinomas in intact rats by 38% (Figure 5A, p=0.0007) and lesion size was reduced by 57% (Figure 5C, p<0.0001). No changes in multiplicity (Figure 5B) or size (Figure 5D) of in situ carcinomas were observed in the ovariectomized, tamoxifen-treated animals.
Figure 5. Chemoprevention of in situ carcinomas using tamoxifen, celecoxib and targretin.
The multiplicity of in situ carcinomas within the abdominal mammary glands for each treatment is shown in (A) intact and (B) ovariectomized rats. The average size of in situ carcinomas, as measured by pixels within lesion area, is shown for each treatment in (C) intact and (D) ovariectomized rats. Group means are indicated. Error bars denote standard error of means.
Dietary treatment with the cyclooxygenase-2 inhibitor celecoxib resulted in a 32% (Figure 5A, p=0.004) reduction of in situ carcinoma multiplicity in intact rats. While a marginal reduction of in situ carcinoma size might be discerned in intact rats following celecoxib treatment (Figure 5C), it failed to reach levels of statistical significance. Celecoxib treatment did not result in modulation of multiplicity (Figure 5B) or size (Figure 5D) of in situ carcinomas in ovariectomized rats.
The RXR-selective retinoid targretin was efficacious for the prevention of both hormonally responsive and non-responsive in situ carcinomas. Dietary targretin decreased the multiplicity of in situ carcinomas by 47% (Figure 5A, p<0.0001) and 26% (Figure 5B p=0.02) in intact and ovariectomized rats, respectively. Targretin treatment also reduced lesion size by 64% (Figure 5C, p<0.0001) in intact rats and by 54% (Figure 5D, p<0.0001) in ovariectomized animals.
DISCUSSION
The neu-induced in situ carcinoma model presents a reliable and convenient methodology to induce distinct, transient intermediate endpoints of mammary carcinogenesis in the rat. Aside from sharing histopathological features with their human DCIS counterparts and their ability to spontaneously regress or progress into mammary carcinomas, neu-induced in situ carcinomas have molecular characteristics relevant for the study of human breast cancer development. For instance, cyclooxygenase-2 expression is upregulated in neu-induced in situ carcinomas (5), compared to mammary carcinomas in our long-term model and only low baseline levels in normal mammary gland tissue. This recapitulates a feature of human Her-2/neu positive DCIS, in which cyclooxygenase-2 overexpression is found more frequently than in invasive breast carcinomas (18), while it is virtually absent from normal breast parenchyma (19). In addition, we provided evidence that hormonal responsiveness, a highly relevant characteristic of human breast cancer etiology, can be accurately assessed in this short-term chemoprevention model.
The chemopreventive effects of the SERM tamoxifen are well established in the literature. We observed a 38% reduction in multiplicity of in situ carcinomas in our short-term model, which is consistent with reductions of 33% to 49% in mammary carcinoma multiplicity in our long-term prevention model published previously (5). Tamoxifen was not efficacious for the prevention of hormonally non-responsive in situ carcinomas in ovariectomized rats, which is also reflected in our long-term prevention studies (4, 5). The significant reduction in size of hormonally responsive in situ carcinomas following short-term treatment with tamoxifen is also consistent with observations in long-term models of neu-induced (4, 5) and chemically-induced (20) mammary carcinomas.
The significant reduction in multiplicity of in situ carcinomas associated with celecoxib treatment in intact rats is in agreement with data from our long-term prevention model (5) and those of other carcinogen-induced rat models (21, 22) in which tumors tend to be uniformly hormonally responsive. The lack of effect of celecoxib on in situ carcinoma size in intact rats is also consistent with our observations in neu-induced mammary carcinomas where it is accompanied by failure to modulate proliferation or apoptotic rates (5). The effects of celecoxib on tumor size in carcinogen-induced rat models appear to be less consistent. While one study using the DMBA rat model reported no effects on tumor volume (22), another found an 81% reduction of tumor volume following celecoxib treatment at the same dose of 1500 ppm (21). Consistent with our previous findings that efficacy of celecoxib is limited to hormonally responsive mammary carcinomas (5) we observed no effect of celecoxib treatment on lesion multiplicity or size in ovariectomized rats. While the literature cites celecoxib-modulated reductions in tumor multiplicity but not size in a ER-negative mouse model of mammary carcinogenesis (23), these findings must be understood in context, as tamoxifen treatment also causes significantly decreases in tumor multiplicity in this preclinical model (24). Importantly, a recent randomized, placebo controlled prevention trial with celecoxib in women at high risk for breast cancer showed no modulation of the proliferation maker Ki-67 in breast epithelial cells, following celecoxib treatment (25). This result is consistent with our findings that proliferation rates are not affected by celecoxib treatment (5) and might explain why the size of celecoxib-treated mammary carcinomas and in situ carcinomas appears unmodulated in our experiments, despite reductions in multiplicity of these endpoints.
The significant efficacy of the rexinoid targretin to prevent both hormonally responsive and non-responsive in situ carcinomas in our short-term model mirrors the effects of targretin in our neu-induced mammary carcinoma prevention models (4), albeit with slightly lower magnitudes. This is consistent with the literature, which reports that both hormonally responsive mammary carcinomas in carcinogen-induced rat models (26, 27) and ER-negative mammary tumors in transgenic mice (28, 29) are preventable by targretin treatment. The targretin-mediated reductions of lesion size in intact rats were mirrored in the results of our long-term prevention model (4). While targretin treatment showed no significant effect on tumor volume in ovariectomized rats in our long term model, targretin caused strong reductions in proliferation rates and significant increases in apoptotic rates in hormonally non-responsive mammary carcinomas (4). We postulated that the 84% reduction in tumor multiplicity resulted in too few carcinomas included in the tumor size analysis to unveil a significant effect of targretin treatment on the size of mammary carcinomas in ovariectomized rats. In our short-term model, targretin treatment resulted in a 54% reduction in the size of hormonally non-responsive in situ carcinomas in ovariectomized rats. In a randomized, placebo-controlled trial, the retinoid fenretinide has demonstrated a protective effect against the development of secondary breast cancers, regardless of the ER status of the primary tumor in pre-menopausal women (30). These data are suggestive of a role for retinoids in the prevention of hormonally non-responsive breast cancer consistent with our findings.
Our report suggests that the progression from in situ carcinomas at 15 days post-infusion into stable, palpable mammary carcinomas with proliferation and apoptotic rates equivalent to those of mammary carcinomas in our long-term models takes a mere 12 days. This short and reproducible duration, along with the ability to adjust the multiplicity of arising lesions to a desired level, could make the model adaptable for the study of molecular mechanisms underlying the progression from in situ carcinoma to carcinoma stage. With recent advances in micro-imaging technology (31), it might be possible to identify progressing and regressing lesions at various stages of progression and characterize them molecularly.
It should be stated that the high rate of in situ carcinoma regression is most likely related to the fact that these lesions are hyperproliferative and eventually outgrow their blood supply within the mammary gland. No regression was observed in the three days preceding or following the 15 days post-infusion endpoint. Our data contains no unequivocal evidence ruling out the possibility that a compound could affect progressing and regressing lesions differentially. However, the fact that the unique prevention patterns of the three compounds in regard to their effects on multiplicity and size across hormonal configuration mirror one another for the two distinct endpoint, suggests the absence of such an artifact and is consistent with the notion that the mechanisms for the prevention of in situ carcinomas and mammary carcinomas are functionally related.
Based on the evidence presented, the use of intermediate in situ carcinomas as preventable endpoints in this short-term model is a valid initial methodology for assessing a compound’s efficacy for preventing hormonally responsive and non-responsive mammary cancer. As potential chemopreventive agents for breast cancer are being developed, especially by high throughput methodologies in in vitro models (32), cost-effective in vivo prevention models are vital for their initial pre-clinical efficacy assessment. Its short duration, the use of fewer animals and high statistical power should make the neu-induced retroviral in situ carcinoma model an excellent choice for this undertaking. In addition, the amount of chemopreventive agent required for efficacy testing is significantly less in our short-term model compared to other preclinical models, an important economical consideration, especially for testing precious quantities of novel agents in drug development.
ACKNOWLEDGEMENTS
This work was supported by the NIH grants R01-CA101201, P30-CA014520 and the DOD grant W81XWH-04-1-0312.
REFERENCES
- 1.Singh M, McGinley JN, Thompson HJ. A comparison of the histopathology of premalignant and malignant mammary gland lesions induced in sexually immature rats with those occurring in the human. Lab Invest. 2000;80:221–31. doi: 10.1038/labinvest.3780025. [DOI] [PubMed] [Google Scholar]
- 2.Green JE, Hudson T. The promise of genetically engineered mice for cancer prevention studies. Nat Rev Cancer. 2005;5:184–98. doi: 10.1038/nrc1565. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Woditschka S, Haag JD, Waller JL, et al. Neu-induced retroviral rat mammary carcinogenesis: a novel chemoprevention model for both hormonally responsive and nonresponsive mammary carcinomas. Cancer Res. 2006;66:6884–91. doi: 10.1158/0008-5472.CAN-05-1823. [DOI] [PubMed] [Google Scholar]
- 5.Woditschka S, Haag JD, Mau B, Lubet RA, Gould MN. Chemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neu-induced retroviral rat model. Breast Cancer Res. 2008;10:R18. doi: 10.1186/bcr1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med. 2004;350:1430–41. doi: 10.1056/NEJMra031301. [DOI] [PubMed] [Google Scholar]
- 7.Cady B, Chung MA. The prevention of invasive breast carcinoma. Cancer. 2004;101:2147–51. doi: 10.1002/cncr.20620. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M. The certainties and the uncertainties of ductal carcinoma in situ. J Natl Cancer Inst. 2004;96:424–5. doi: 10.1093/jnci/djh088. [DOI] [PubMed] [Google Scholar]
- 9.Thompson HJ, Singh M. Rat models of premalignant breast disease. J Mammary Gland Biol Neoplasia. 2000;5:409–2. doi: 10.1023/a:1009582012493. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Carcinoma induction following direct in situ transfer of v-Ha-ras into rat mammary epithelial cells using replication-defective retrovirus vectors. Cancer Res. 1991;51:2642–8. [PubMed] [Google Scholar]
- 11.Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Frequent induction of mammary carcinomas following neu oncogene transfer into in situ mammary epithelial cells of susceptible and resistant rat strains. Cancer Res. 1991;51:5649–54. [PubMed] [Google Scholar]
- 12.Thompson TA, Gould MN. Direct gene transfer into the mammary epithelium in situ using retroviral vectors. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. KluwerAcademic/Plenum Publ; New York: 2000. pp. 245–57. [Google Scholar]
- 13.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 14.Gerdes J, Lemke H, Baisch H, Wacker WW, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 15.Gerlach C, Golding M, Larue L, Alison M, Gerdes J. Ki-67 immunoexpression is a robust marker of proliferative cells in the rat. Lab Invest. 1997;77:697–8. [PubMed] [Google Scholar]
- 16.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Kennan WS, Yasukawa-Barnes J, Lindstrom MJ, Gould MN. Difference in the response of neu and ras oncogene-induced rat mammary carcinomas to early and late ovariectomy. Cancer Res. 1992;52:4102–5. [PubMed] [Google Scholar]
- 18.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;2(Suppl 7):22–9. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 19.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 20.Bischoff ED, Gottardis MM, Moon TE, Heyman RA, Lamph WW. Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRETIN) causes complete regression of mammary carcinoma. Cancer Res. 1998;58:479–84. [PubMed] [Google Scholar]
- 21.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–3. [PubMed] [Google Scholar]
- 22.Jang TJ, Jung HG, Jung KH, O MK. Chemopreventive effect of celecoxib and expression of cyclooxygenase-1 and cyclooxygenase-2 on chemically-induced rat mammary tumours. Int J Exp Pathol. 2002;83:173–82. doi: 10.1046/j.1365-2613.2002.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanza-Jacoby S, Miller S, Flynn J, et al. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev. 2003;12:1486–91. [PubMed] [Google Scholar]
- 24.Nanni P, Nicoletti G, De Giovanni C, et al. Prevention of HER-2/neu transgenic mammary carcinoma by tamoxifen plus interleukin 12. Int J Cancer. 2003;105:384–9. doi: 10.1002/ijc.11092. [DOI] [PubMed] [Google Scholar]
- 25.Fabian CJ, Kimler BF, Mayo MS, Zalles CM. Phase II biomarker prevention trial of celecoxib vs. placebo in women at high risk for development of breast cancer [abstract]; Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; San Diego, CA. Philadelphia (PA). 2008; AACR; Apr 12-16, 2008. Abstract nr 4189. [Google Scholar]
- 26.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR selective ligand. Cancer Res. 1996;56:5566–70. [PubMed] [Google Scholar]
- 27.Lubet RA, Christov K, Nunez NP. Efficacy of targretin on methylnitrosourea-induced mammary cancers: prevention and therapy dose-response curves and effects on proliferation and apoptosis. Carcinogenesis. 2005;26:441–8. doi: 10.1093/carcin/bgh338. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Kim HT, Rodriquez JL, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–74. [PubMed] [Google Scholar]
- 29.Wu K, Zhang Y, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 30.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–56. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 31.Wessels JT, Busse AC, Mahrt J, Dullin C, Grabbe E, Mueller GA. In vivo imaging in experimental preclinical tumor research--a review. Cytometry A. 2007;71:542–9. doi: 10.1002/cyto.a.20419. [DOI] [PubMed] [Google Scholar]
- 32.Kinghorn AD, Su BN, Jang DS, et al. Natural inhibitors of carcinogenesis. Planta Med. 2004;70:691–705. doi: 10.1055/s-2004-827198. [DOI] [PubMed] [Google Scholar]