Abstract
Background
Assessment of asthma via spirometry in children is challenging due to often normal FEV1 values.
Objective
We used Mead’s slope ratio ( ) to analyze the shape of the flow volume loop.
Methods
We analyzed the effects of time, albuterol, and budesonide on FEV1, FEV1/FVC, FEF25-75%, and Mead’s slope ratio, early (between 75% and 50% of FVC, SR61) and late (between 75% and 50% of FVC, SR35) in exhalation, in the Childhood Asthma Management Program cohort at baseline, 4 months, and end of study in participants who received either inhaled placebo or budesonide BID.
Results
In the placebo group, both SR61 and SR35 improved over time. Bronchodilator consistently improved both SR61 and SR35, without change in degree of improvement over time. Similarly, in the budesonide group, time and bronchodilator each independently improved both SR61 and SR35. At 4 months and end of study, budesonide patients had significant improvements in SR61 relative to placebo patients, independent of bronchodilator effect. Budesonide and placebo were not different with respect to pre or post-bronchodilator SR35.
Conclusion
Budesonide-treated patients have less concave flow-volume loops when compared to placebo-treated patients. Time and bronchodilator also make the flow-volume loop less concave. Furthermore, it appears that there are discrete bronchodilator responsive and corticosteroid responsive components of airflow obstruction in pediatric asthma.
Clinical Implications
Changes in flow-volume loop shape in children with asthma may reflect changes in airflow for which slope ratio may be more sensitive than conventional measures.
Keywords: Spirometry, Slope Ratio, Asthma, Pediatric, Children
INTRODUCTION
Asthma is a chronic respiratory disease characterized by partially or completely reversible airflow obstruction, mucus hypersecretion, and increased airway hyperresponsiveness, all of which are felt to result from chronic airway inflammation (1). The Childhood Asthma Management Program (CAMP) trial was designed specifically to evaluate the effects on lung growth as determined by post-bronchodilator FEV1 of three inhaled treatments: budesonide (200 μg twice daily), nedocromil (8 mg twice daily), and placebo over a 4- to 6-year period in children 5 to 12 years of age with mild to moderate asthma (2).
The CAMP results indicated that long-term budesonide treatment was not better than placebo in terms of effect on lung function, as measured by FEV1% predicted post-bronchodilator (3). In contrast, budesonide treatment improved airway responsiveness to methacholine, and resulted in significant reductions in asthma symptoms, beta-agonist use, asthma hospitalizations, urgent care visits, and need for prednisone courses (3). The lack of improvement in FEV1% predicted despite treatment with inhaled corticosteroid and clear clinical benefit in terms of hospitalizations, symptoms, and medication use led to further evaluation of the maximum expiratory flow-volume loop (MEFVL) data obtained in CAMP, in an effort to reconcile these contrasting findings. We hypothesized that there was information about lung function in the MEFVL that was not captured by standard measures (i.e. FEV1%), and further hypothesized that this information might better reflect the improvements in disease burden seen in patients treated with inhaled corticosteroid.
The current study seeks to further define the pulmonary function abnormalities in the CAMP cohort at baseline, and the effects of time in the study, treatment with albuterol, and treatment with budesonide on lung function. After a review of the literature regarding measures of lung function, especially those with relationship to alterations in expiratory flow, we chose to analyze the CAMP cohort’s spirometry data by estimating Mead’s slope ratio in early (between 75% and 50% of FVC, SR61) and late (between 75% and 50% of FVC, SR35) mid-expiration.
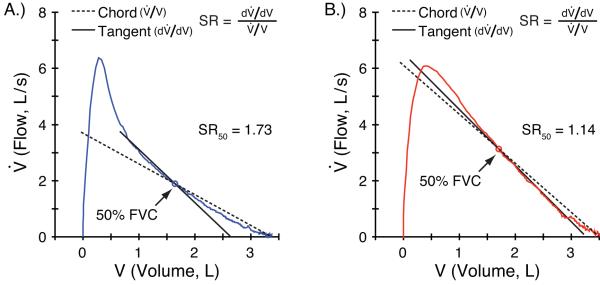
Mead defined the slope ratio as the slope of a tangent to the curve at a given point , divided by the slope of the line drawn through that point and the curve at the residual volume (chord line, ), thus (Figure 1), and described it as a dimensionless ratio sensitive to the shape of the flow-volume curve (4). Here the slope ratios derived from the flow-volume loops are used to analyze the effects of time, bronchodilator, and inhaled corticosteroids on the shape of the MEFVL in patients from CAMP. Some of the results of these studies have been previously reported in the form of an abstract (5).
Figure 1.
Pre- (A) and Post-bronchodilator (B) MEFV curves with tangent and chord lines (broken and solid, respectively) fitted at 50% FVC. The ratio of their slopes, , defines the slope ratio, SR, at 50% FVC. Note that as the MEFV curve becomes more linear post-bronchodilator (B), the two slopes become more similar, and slope ratio decreases.
METHODS
CAMP Design
Details of the design and methods of the CAMP study, including the characteristics of children at randomization, are published elsewhere (2). 1041 children from 5 through 12 years of age with mild-to-moderate asthma were randomized to receive twice daily dosing of 200 μg of budesonide (n=311), 8 mg of nedocromil (n=312), or placebo (n=418). All patients were treated with albuterol for symptoms, and prednisone for exacerbations. Participants were treated for four to six years (mean +/− SD = 4.3 years, +/− 0.7 years). For purposes of these analyses, only the placebo and budesonide groups were analyzed, as budesonide had the most significant effects on lung function (spirometry measures at 4 months of treatment and methacholine responsiveness throughout the trial) and measures of asthma control (3).
Spirometry
Spirometry was performed before the 28-day screening period and three times yearly during the study, with pre and post-bronchodilator measurements taken at each time point using equipment and testing protocol meeting or exceeding American Thoracic Society standards (6;7). Height, weight, gender, age, and race were recorded so that appropriate predicted normal values (8;9) could be used to calculate spirometric indices as % predicted values. Measurements were taken on study medication.
Interpolation of Slope Ratios
As detailed above, Mead defined the slope ratio as (Figure 1) (4). Permutt later showed that slope ratio at a given point on the flow-volume curve is equivalent to the slope of a log-log plot of flow against volume at that point (10). Thus, the slope ratio at a given point between 50 and 25% FVC is approximately . We used this method to interpolate slope ratios between 25 and 50% of FVC and between 50 and 75% of FVC. Given their derivation from a log-log plot, we will use the midpoint between log 0.25 and log 0.5 (35%) and between log 0.75 and log 0.5 (61%) to place these approximations in expired volume (FVC).
Thus, the SR61 represents the curvature of the expiratory limb of the flow-volume loop between 75 and 50 percent inspired volume remaining, describing early to middle forced expiration. The SR35 represents the curvature of the expiratory limb of the flow-volume loop between 50 and 25 percent inspired volume remaining describing middle to late forced expiration.
Statistical Analysis
The statistical analyses are described in the Supplementary Materials in detail. The degree of change in an outcome measure was determined by subtracting the base-line measurement from the measurement obtained at the last follow-up visit during the treatment period. The difference between the treatment group and the placebo group in each measure of change was determined by multiple linear regression (11), with the change in the measure as the response variable, two indicator variables for the treatment groups, adjusted for covariates. All analyses were performed with SAS software (version 6.12, SAS Institute, Cary, N.C.). The P values presented are two-sided and have not been adjusted for multiple comparisons.
RESULTS
Study Population
The budesonide and placebo groups were similar at baseline in terms of age, race, gender, asthma history, prior asthma therapy, prior morbidity, atopy, and airway hyperresponsiveness as measured by FEV1 methacholine challenge (3) (Table 1). The duration of follow-up was similar as well, with a mean of 4.3 years for each group (Table 1).
Table 1.
Baseline Characteristics*
| CHARACTERISTIC† | BUDESONIDE | PLACEBO |
|---|---|---|
| N | 311 | 418 |
| Age — yr | 9.0±2.1 | 9.0±2.2 |
| Race or ethnic group — no. (%) | ||
| Non-Hispanic white | 201 (64.6) | 292 (69.9) |
| Non-Hispanic black | 44 (14.1) | 56 (13.4) |
| Hispanic | 32 (10.3) | 37 (8.9) |
| Other | 34 (10.9) | 33 (7.9) |
| Sex — no. (%)‡ | ||
| Female | 130 (41.8) | 184 (44.0) |
| Male | 181 (58.2) | 234 (56.0) |
| Age at onset of asthma — yr | 3.1±2.3 | 3.0±2.6 |
| Time since diagnosis of asthma — yr | 5.2±2.6 | 4.9±2.7 |
| Treatments in 6 mo before enrollment — no. of patients (%) | ||
| Cromolyn or nedocromil | 133 (42.8) | 160 (38.3) |
| Inhaled corticosteroid | 126 (40.5) | 150 (35.9) |
| Oral corticosteroid | 107 (34.4) | 162 (38.8) |
| Severity of asthma — no. (%) | ||
| Moderate | 166 (53.4) | 216 (51.7) |
| Mild | 145 (46.6) | 202 (48.3) |
| Hospitalizations for asthma in year before enrollment — no./100 person-yr |
31 | 31 |
| Recordings on daily diary card | ||
| Episode-free days — no./mo§ | 9.7±7.8 | 9.6±7.6 |
| Use of albuterol for symptoms — puffs/wk | 10.4±9.8 | 10.2±9.6 |
| Night awakenings — no./mo | 0.9±1.7 | 0.8±1.5 |
| FEV1 before bronchodilator use — % of predicted | 93.6±14.4 | 94.2±14.0 |
| FEV1 after bronchodilator use — % of predicted | 103.2±13.2 | 103.3±12.2 |
| FEV1/FVC before bronchodilator use — % | 79.4±8.7 | 80.1±8.7 |
| FEV1/FVC after bronchodilator use — % | 85.2±6.6 | 85.8±7.1 |
| Airway responsiveness to methacholine (FEV1PC20) — mg/ml¶ | 1.1±3.3 | 1.1±3.3 |
| Height — percentile | 56.8±28.0 | 55.3±28.8 |
| SR61 before bronchodilator use | 1.33±0.41 | 1.31±0.41 |
| SR61 after bronchodilator use | 1.11±0.38 | 1.11±0.47 |
| SR35 before bronchodilator use | 1.30±0.29 | 1.27±0.32 |
| SR35 after bronchodilator use | 1.19±0.30 | 1.15±0.34 |
Plus–minus values are means ±SD. Not all percentages add to 100, because of rounding or because some children used more than one treatment before enrollment.
FEV1 denotes the forced expiratory volume in one second, and FEV1PC20 the concentration of methacholine that caused a 20 percent decrease in FEV1
P value for homogeneity among groups=0.02.
An episode-free day was defined as a day with no night awakenings, morning and evening peak flow ≫80 percent of personal best peak flow (determined by algorithm 12), no use of albuterol for symptoms, no use of prednisone, no absence from school or contact with a physician because of asthma symptoms, and no episode of wheezing, coughing, chest tightness, or shortness of breath.
Values are geometric means ±SD.
Baseline Measures of Spirometry and Slope Ratio
At entry into CAMP, the mean pre-bronchodilator slope ratio at 61% FVC (SR61) and slope ratio at 35% FVC (SR35) were greater than one, consistent with convex flow-volume loops. Although FEV1 and FVC were within normal limits, the abnormal FEV1/FVC ratio indicates airflow obstruction (Table 1).
Effects of Bronchodilator and Time on Slope Ratio in the Placebo Group
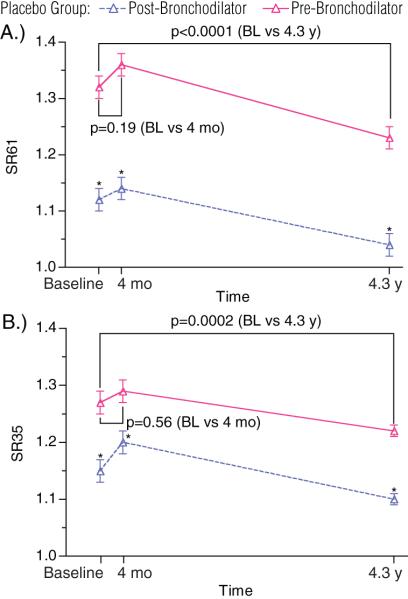
There were no significant changes in the pre-bronchodilator SR61 (E Table 1, Figure 2a) or SR35 (E Table 1, Figure 2b) over the first 4 months in the study. However, by the end of the 4.3 year follow-up, significant improvement occurred in pre-bronchodilator SR61 (Baseline vs. 4.3 y., 1.32 vs. 1.23, p<0.0001) and SR35 (Baseline vs. 4.3 y., 1.27 vs. 1.22, p=0.0002) compared to baseline values (E Table 1).
Figure 2. Effects of Time and Bronchodilator on Slope Ratio in the Placebo Group.
Placebo group SR61 (A) and SR35 (B) pre- and post-bronchodilator are plotted. P-values on graph reflect comparisons of pre-bronchodilator SR61 and SR35 between time points as marked.
*The mean changes Pre- to Post-bronchodilator for SR61 and SR35 are >0 at all time points (p<0.0001).
Bronchodilator improved both SR61 and SR35 at all time points as measured by mean change in slope ratio with bronchodilator ((Post-bronchodilator SR – Pre-bronchodilator SR) vs. 0, p<0.0001, Figure 2). The magnitudes of the improvements with bronchodilator in SR61 (Post-bronchodilator SR61 – Pre-bronchodilator SR61) and SR35 (Post-bronchodilator SR35 – Pre-bronchodilator SR35) did not change significantly over time (P=NS for change over time, E Table 1, Figure 3). Similar to pre-bronchodilator values, there were no significant changes in post-bronchodilator SR61 and SR35 over 4 months, and at end of study there were significant improvements in post-bronchodilator SR61 (baseline vs. 4.3 y., 1.12 vs. 1.04, p=0.001, E Table 1, Figure 2a) and SR35 (baseline vs. 4.3 y., 1.15 vs. 1.1, p=0.002, E Table 1, Figure 2b) compared to baseline values. Despite these significant beneficial effects of both time and bronchodilator on slope ratio, post-bronchodilator SR61 and SR35 in the placebo group remained greater than 1 at end of study, indicating persistence of convexity in the MEFVL (E Table 1, Figure 2).
Figure 3. Effects of Bronchodilator on Slope Ratio.
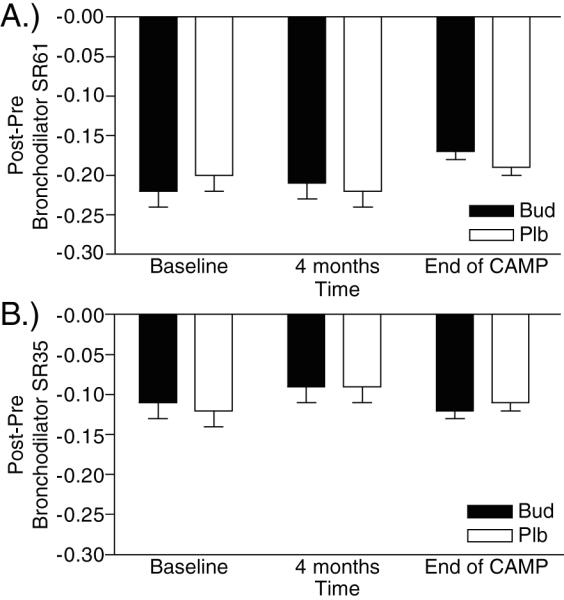
Changes in (A) SR61 and (B) SR35 with bronchodilator ((Post-bronchodilator SR) – (Pre-bronchodilator SR)) for budesonide treated and placebo treated patients. Comparisons between placebo and budesonide groups at marked time points were all statistically insignificant (p=NS) for both Post-Pre-BD SR61 and SR35. The mean Post-Pre-BD SR61 and SR35 are >0 at all time points (p<0.0001).
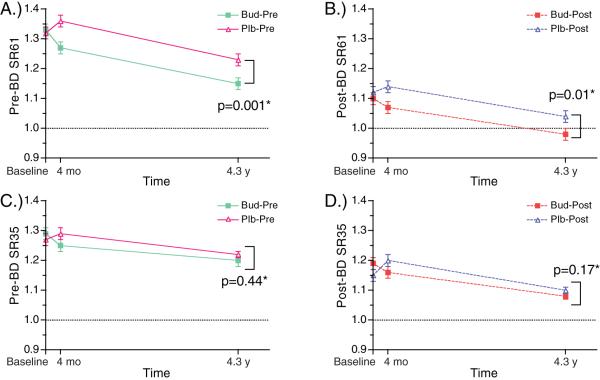
Effects of Budesonide on Slope Ratio
Over the duration of the study, both pre- and post-bronchodilator SR61 improved in budesonide treated patients significantly more than in patients treated with placebo (Figure 4a, 3b, E Table 2). However, budesonide did not significantly affect the pre- or post-bronchodilator SR35 at either time point (Figure 4c, 3d, E Table 2).
Figure 4. Effects of Time, Bronchodilator, and Budesonide on Slope Ratio.
Pre-bronchodilator values (—) for SR61 (A), and SR35 (C); and Post-bronchodilator values (---) for SR61 (B) and SR35 (D) are plotted for both budesonide treated (∎) and placebo treated (▵) patients.
*P-values reflect significance of comparison at end of study between budesonide and placebo treated patients, adjusted for changes from baseline.
Similar to the results in the placebo group, bronchodilator significantly improved both SR61 and SR35 as measured by (Post-bronchodilator SR – Pre-bronchodilator SR). The magnitude of the change in both SR61 and SR35 with bronchodilator in the budesonide treated patients was not significantly different from that seen in placebo treated patients, and did not change over time (Figure 4a, 3b, E Table 3).
At end of study, the post-bronchodilator SR61 in the budesonide group was significantly lower than that in the placebo group (post-bronchodilator SR61 budesonide vs. placebo, 0.98 v 1.04, p=0.01, E Table 2, Figure 4b), and was less than and nearly equal to 1, indicating a near-linear MEFVL. Although pre- and post-bronchodilator SR35 in the budesonide treated patients did improve over time and with bronchodilator, at end of study there were no significant differences in pre- or post- bronchodilator SR35 when comparing the budesonide treated patients to those receiving placebo (Pre-bronchodilator SR35 budesonide vs. placebo, 1.2 vs. 1.22, p=0.44, Figure 4c; Post-bronchodilator SR35 budesonide vs. placebo, 1.08 vs. 101, p=0.17, Figure 4d; E Table 2). Further, analysis of baseline slope ratios finds no relationship between baseline slope ratios and effects of time, bronchodilator, or budesonide on slope ratio (E Table 4).
Effects of Time and Bronchodilator on Other Spirometric Measures in the Placebo Group
For comparison to analyses of slope ratios during CAMP, standard spirometry measures are included (E Table 1). In the placebo group, over the 4.3 years of the study, neither pre- nor post-bronchodilator values for either FEV1 %predicted or FVC %predicted changed significantly. FEV1/FVC ratio decreased both pre- and post-bronchodilator in the placebo group (Baseline vs. 4.3 y. FEV1/FVC: Pre-bronchodilator, 79.9 vs. 78, p<0.0001; Post-bronchodilator, 85.6 vs. 83.9, p<0.0001). FEF25-75% predicted also decreased both pre- and post-bronchodilator in the placebo group (Baseline vs. 4.3 y. FEF25-75 %predicted: Pre-bronchodilator, 67.9 vs. 64.2, p=0.0004; Post-bronchodilator, 87.7 vs. 80.4, p<0.0001).
In the placebo group, FEV1% reversibility [(Post-bronchodilator FEV1 – Pre-bronchodilator FEV1)/Pre-bronchodilator FEV1]% did not change significantly at 4 months, but decreased by end of the study (10.6 vs. 9.2, p=0.03).
Effects of Budesonide on Standard Spirometric Measures
FEV1%predicted, FVC%predicted, FEF25-75% predicted, and FEV1/FVC ratio improved significantly in budesonide treated patients in comparison to placebo treated patients both pre- and post-bronchodilator at 4 months (E Table 2). The pre-bronchodilator improvements with budesonide treatment in these spirometric measures were still significant at end of study. Post-bronchodilator, FEV1%predicted, FVC%predicted, and FEV1/FVC ratio were not significantly different between budesonide and placebo treated patients. Of the spirometric measures, only FEF25-75% showed a difference between the budesonide and placebo groups at end of study (budesonide vs. placebo, 82.5 vs. 80.0, p=0.04, E Table 2).
FEV1 reversibility in the budesonide group was lower than in the placebo group at 4 months (p<0.001), and at the end of the study (p=0.04, E Table 2).
DISCUSSION
Assessment of Airflow Limitation in Asthma
In 1949, Tiffeneau and colleagues (12) first emphasized the FEV1 as an index of airflow limitation in COPD. Subsequently, the development of the MEFVL in 1958 by Hyatt and Fry (13) led to analysis of flow, volume, and time relationships in an effort to gather information about the sources and mechanisms of airflow limitation in both COPD and asthma. Results of this work include the use of FEV1 and FEF25-75 (14) as measures of airflow limitation.
In healthy adult subjects, the expiratory flow-volume relationship, especially during mid-expiration, is linear, consistent with a lung that empties as a single compartment. This linear emptying allows for the use of FEV1 and FEF25-75 as measures of airflow limitation indicative of the entire course of expiration. However, as Pride et al (10) and Mead et al (4) continued their evaluation of the expiratory flow-volume relationship and considerations of lung physiology in general, the limitations of a single compartment model of the lung in modeling disease became apparent.
In a normal lung, the rate constants of emptying will be identical throughout the forced expiration and would be expressed as a linear expiratory flow-volume loop, as is seen in healthy adults. However, in diseased lungs, it is well recognized that emptying is non-uniform throughout volume, resulting in a curvilinear flow-volume relationship as seen in COPD (15;16), cystic fibrosis (17), primary pulmonary hypertension (18), and asthma (17;19;20). This non-linearity is felt to reflect either the existence of multiple compartments in the lung with different rate constants of emptying or a dynamic obstruction applied uniformly across the lung units, also referred to as parallel dynamic airway compression. Although FEV1 accurately measures airflow and can detect airflow limitation, these changes in MEFVL shape precede abnormalities in FEV1 in the course of airway disease (21).
The relative insensitivity of FEV1 to changes in expiratory flow over the entire course of lung emptying led to further analysis of forced expiratory flow within the time domain via time constants (22), and analysis of the geometric configuration of the expiratory flow-volume loop via Mead’s slope ratio (21). Both time constants and Mead’s slope ratios were found to be more sensitive than FEV1 or MMEF to early airway changes as seen in asymptomatic smokers compared to asymptomatic non-smokers (10).
Present Findings
Natural History of SR in Childhood Asthma
In the analyses of the placebo group over time, we found that there is an improvement over time in convexity of the flow-volume loop as measured by both SR61 and SR35. This improvement is not seen at 4 months, but is significant at end of study both pre and post-bronchodilator (E Table 1). Thus, despite these patients not having received continuous budesonide therapy, they still had an improvement in their airflow limitation, independent of bronchodilator treatment. The improvement in slope ratios over time may be caused by increase in airway caliber due to growth, which confers protection from factors that produced airflow obstruction in smaller airways. A second explanation is that continued alveolarization of lung units lead to a lung with an overall slower, but more homogeneous emptying. Notable in this change over time is the lack of significant improvement in any of the traditional metrics of lung function. Lack of improvement in FEV1 relative to normal children while slope ratios improve suggests that FEV1%predicted is not sensitive to changes in the rate of lung emptying.
Effects of Bronchodilator on SR
Albuterol is a short-acting beta-agonist that has been used to treat asthma since the late 1960s (23-25), primarily through its well-established effects on bronchial smooth muscle. By relaxing bronchial smooth muscle, airway diameter increases and airflow limitation is improved. Although albuterol has been noted to improve lung function as measured by spirometry in children with asthma since 1969 (21), no studies have been done on the effects of albuterol on slope ratio. O’Donnell and colleagues (20) studied the effects of isoproterenol on slope ratio in asthma, and noted that the configuration of the MEFVL does change towards linearity with isoproterenol use. Similarly, in the CAMP cohort there was a consistent improvement of approximately −0.2 in SR61 and of −0.1 in SR35 with albuterol administration, regardless of time or treatment group (p<0.0001).
Effects of Budesonide on SR
In the original analysis of the CAMP data (3), the authors make the unexpected finding that budesonide did not improve lung function as measured by FEV1 % predicted post-bronchodilator at the end of the 4.3-year treatment interval when compared to placebo treated patients. In contrast to this finding, post-bronchodilator SR61 is significantly improved in the budesonide group compared to the placebo group (E Table 1), reflecting linearization of the flow-volume relationship early in exhalation, consistent with a reduction of airflow limitation.
In contrast, we did not find a significant difference in SR35 at the end of the study between budesonide and placebo patients pre or post-bronchodilator (pre-bronchodilator 1.20 vs 1.22, p=0.44, post-bronchodilator 1.08 vs 1.10, p=0.17). This may reflect poor delivery of drug to late emptying airways, the presence of a non-steroid responsive pathology in these airways, or other mechanisms not yet elucidated. Further study of the CAMP cohort, including evaluation of the differences between patients who did have improvement in SR35 and those who did not may help elucidate mechanisms of airflow limitation in late expiration and provide further guidance for development of new strategies to improve end expiratory flow delay.
Differing effects of time, bronchodilator, and budesonide
As reported above, both time and bronchodilator treatment improve SR61 and SR35 in the placebo group. Given that SR61 and SR35 both pre and post-bronchodilator improve significantly in placebo treated patients over the course of the study (E Table 1), and that the effects of bronchodilator on SR61 and SR35 do not change significantly over the 4.3 years of the study (E Table 2), these data suggest that the effects of time and bronchodilator are both independent and additive. Given that there is a significant difference in post-bronchodilator SR61 in placebo and budesonide treated patients (E Table 3), and the lack of significant difference in pre-post-bronchodilator SR61 between placebo and budesonide treated patients (E Table 2), the effects of budesonide and bronchodilator are both independent and additive. While our findings do not conclusively prove that time (reflecting lung growth, development and remodeling), response to bronchodilator (reflecting relaxation of airway smooth muscle), and inhaled corticosteroids (reflecting reduction in airway inflammation) work through different mechanisms, it is plausible that they do.
Although inhaled corticosteroid treatment did not significantly change SR35, bronchodilator does consistently. As SR35 reflects the time rate of lung emptying and thus airflow limitation late in expiration, and as asthma is classically described as a disease of “peripheral airways” (26;27) generally thought to empty late, one of the more intriguing findings of this study is the finding that while budesonide treatment does not appear to improve MEFVL convexity late in expiration, albuterol does. This may reflect either different mechanisms of action or may indicate the lack of deposition of the inhaled corticosteroids in the lung periphery.
Comparison between SR and FEF25-75
Slope ratio measures MEVFL shape in terms of deviation from perfect single exponential emptying of the lung. The FEF25-75 has long been described as a measure that reflects the behavior of distal or “small” airways airflow obstruction (14). In the CAMP cohort, budesonide treatment improved FEF25-75% predicted and SR61, but not SR35. This indicates that the observed improvements in FEF25-75% predicted can be related only to changes in early but not late emptying, reflecting an important limitation of the FEV25-75 in assessing the site of impact of therapy.
Limitations of the Present Findings
A limitation of this study is the unavailablility of longitudinally measured normal values for SR61 and SR35. Without these data, we cannot compare the expected values or changes over time in SR in normal children with our asthma cohort. However, given the improvements seen with bronchodilator and budesonide, both consistent with their known mechanisms of action in asthma, these results provide a starting point for the evaluation of the shape of the MEFVL by slope ratio in asthma, other lung diseases, and in healthy individuals. Another limitation is the use of Permutt’s interpolation of slope ratio, which may not be precisely the same as using a single point along the flow-volume tracing. Therefore, we were limited to measuring slope ratios in the log-transformed mid-points of the second and third quarters of expiration.
Conclusion
These data suggest several interesting findings. First is the finding that over time, the natural history of the shape of the MEFVL in children with asthma is one of improvement. That post-bronchodilator FEV1 % predicted did not improve in CAMP suggests that improvement in MEFVL convexity does not necessarily translate into improved peak expiratory flow. Further longitudinal study and comparison to healthy individuals will be necessary to determine the natural history and significance of changes in slope ratio over time.
Second is the finding that inhaled corticosteroids appear to improve lung function via different mechanisms than bronchodilators, and that those effects can be measured independently of one another by use of the slope ratio.
Third is that slope ratio is a more sensitive index for assessment of flow dynamics and its response to treatment in asthmatic children than the current gold standard, FEV1 %predicted.
Similarly, although the FEF25-75 has been long held as a measure of “small airways” obstruction, the improvements in FEF25-75 seen with budesonide and bronchodilator in the CAMP cohort are due to improvements in early flow as measured by SR61, rather than late flow as measured by SR35. This finding is important because although by definition, FEF25-75 measures the average flow over mid-expiration (from 25% through 75% of expired volume); it has been described as reflecting distal airways and late flow events. Our data show that FEF25-75 can reflect changes primarily in early flow rather than late flow.
To fully determine the significance of these findings, there needs to be study of normal children and adults to define the natural history of the normal time distribution of expiratory flow. Also, slope ratio analysis combined with FEV1 % predicted might allow for improved prediction of asthma outcomes, allowing for refined treatment guidelines. Other diseases of airway obstruction (i.e. cystic fibrosis, COPD) would also be amenable to slope ratio analysis of MEFVL.
Questions of these sorts would be best answered by studies where the spirometry equipment used captures moment to moment flow, time, and volume data digitally, so that accurate slope ratios of Mead can be calculated directly, rather than being estimated. Furthermore, such measurement would also allow for other, potentially more accurate measurements of the time distribution of expiratory flow, such as moments analysis, to be carried out. Although FEV1 reliably reflects airflow limitation and is well known to predict asthma outcomes, our findings show that measurements of MEFVL shape can provide other information about the state of the lung in asthma. Furthering the work of Mead, Permutt, and others in understanding the distribution of expiratory flow over volume and time in airway diseases may provide a better understanding of these diseases.
Capsule Summary.
Analysis of shape of flow volume loops over an extended period of treatment with budesonide compared to placebo in children with mild to moderate asthma demonstrated discrete bronchodilator- and corticosteroid-responsive components of airflow obstruction.
Supplementary Material
Acknowledgments
The authors thank the members of the CAMP Research Group (see Supplementary Materials) for recruitment and retention of the children with asthma and the collection of high quality data for use in these analyses.
Support: This work was supported by T32 HL007317 from the NHLBI. The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources.
Abbreviations/Acronyms
- CAMP
Childhood Asthma Management Program
- MEFVL
Maximal Expiratory Flow-Volume Loop
- SRN
Slope Ratio of Mead at N % of FVC
- FEV1
Forced Expiratory Volume in 1 second
- FVC
Forced Vital Capacity
- FEV1 %pred
% of predicted Forced Expiratory Volume in 1 second
- FVC %pred
% of predicted Forced Vital Capacity
- FEF25-75
Forced Expiratory Flow from 25% to 75% of expired volume (aka mid-maximal expiratory flow, MMEF)
- COPD
Chronic Obstructive Pulmonary Disease
- FEFN
Forced Expiratory Flow at N% of FVC
References
- (1).Walter MJ, Holtzman MJ. A centennial history of research on asthma pathogenesis. Am J Respir Cell Mol Biol. 2005;32(6):483–9. doi: 10.1165/rcmb.F300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999;20(1):91–120. [PubMed] [Google Scholar]
- (3).Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343(15):1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- (4).Mead J. Analysis of the configuration of maximum expiratory flow-volume curves. J Appl Physiol. 1978;44(2):156–65. doi: 10.1152/jappl.1978.44.2.156. [DOI] [PubMed] [Google Scholar]
- (5).Patel AC, Van Natta M, Wise R, Strunk RC. Effects of Budesonide on Ventilatory Inhomogeneity in Children with Asthma. Proceedings of the American Thoracic Society. 2005;2:A774. (Abstract) [Google Scholar]
- (6).Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- (7).Johns Hopkins Univ. BMDCfCT. Childhood Asthma Management Program (CAMP) Spirometry Manual. Version 3.0 United States, National Heart, Lung, and Blood Inst.; Bethesda, MD: Oct 26, 1994. [Google Scholar]
- (8).Coultas DB, Howard CA, Skipper BJ, Samet JM. Spirometric prediction equations for Hispanic children and adults in New Mexico. Am Rev Respir Dis. 1988;138(6):1386–92. doi: 10.1164/ajrccm/138.6.1386. [DOI] [PubMed] [Google Scholar]
- (9).Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- (10).Permutt S, Menkes HA. Spirometry: Analysis of Forced Expiration Within the Time Domain. In: Macklem PT, Permutt S, editors. The Lung in the transition between health and disease. M. Dekker; New York: 1979. pp. 113–52. [Google Scholar]
- (11).McCullagh P, Nelder JA. Generalized linear models. 2nd ed Chapman and Hall; London: 1989. [Google Scholar]
- (12).Tiffeneau R, Rousser J, Drutel R. Capacite vitale et capacite pulmonaire utilisable a l’effort; criteres statique et dynamique de la ventilation pulmonaire. Paris Med. 1949;137:543–7. [PubMed] [Google Scholar]
- (13).Hyatt RE, Schilder DP, Fry DL. Relationship between maximum expiratory flow and degree of lung inflation. J Appl Physiol. 1958;13(3):331–6. doi: 10.1152/jappl.1958.13.3.331. [DOI] [PubMed] [Google Scholar]
- (14).McFadden ER, Jr., Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52(6):725–37. doi: 10.1016/0002-9343(72)90078-2. [DOI] [PubMed] [Google Scholar]
- (15).Healy F, Wilson AF, Fairshter RD. Physiologic correlates of airway collapse in chronic airflow obstruction. Chest. 1984;85(4):476–81. doi: 10.1378/chest.85.4.476. [DOI] [PubMed] [Google Scholar]
- (16).Jayamanne DS, Epstein H, Goldring RM. Flow-volume curve contour in COPD: correlation with pulmonary mechanics. Chest. 1980;77(6):749–57. doi: 10.1378/chest.77.6.749. [DOI] [PubMed] [Google Scholar]
- (17).Landau LI, Taussig LM, Macklem PT, Beaudry PH. Contribution of inhomogeneity of lung units to the maximal expiratory flow-volume curve in children with asthma and cystic fibrosis. Am Rev Respir Dis. 1975;111(6):725–31. doi: 10.1164/arrd.1975.111.6.725. [DOI] [PubMed] [Google Scholar]
- (18).Meyer FJ, Ewert R, Hoeper MM, Olschewski H, Behr J, Winkler J, et al. Peripheral airway obstruction in primary pulmonary hypertension. Thorax. 2002;57(6):473–6. doi: 10.1136/thorax.57.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kraan J, van der Mark TW, Koeter GH. Changes in maximum expiratory flow-volume curve configuration after treatment with inhaled corticosteroids. Thorax. 1989;44(12):1015–21. doi: 10.1136/thx.44.12.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).O’Donnell CR, Castile RG, Mead J. Changes in flow-volume curve configuration with bronchoconstriction and bronchodilation. J Appl Physiol. 1986;61(6):2243–51. doi: 10.1152/jappl.1986.61.6.2243. [DOI] [PubMed] [Google Scholar]
- (21).Hyatt RE, Mead J, Rodarte JR, Wilson TA. Changes In Lung Mechanics: Flow-Volume Relationships. In: Macklem PT, Permutt S, editors. The Lung in the transition between health and disease. M. Dekker; New York: 1979. pp. 73–112. [Google Scholar]
- (22).Tockman M, Menkes H, Cohen B, Permutt S, Benjamin J, Ball WC, Jr., et al. A comparison of pulmonary function in male smokers and nonsmokers. Am Rev Respir Dis. 1976;114(4):711–22. doi: 10.1164/arrd.1976.114.4.711. [DOI] [PubMed] [Google Scholar]
- (23).Hartley D, Jack D, Lunts LH, Ritchie AC. New class of selective stimulants of beta-adrenergic receptors. Nature. 1968;219(156):861–2. doi: 10.1038/219861a0. [DOI] [PubMed] [Google Scholar]
- (24).Nelson HS. Beta-adrenergic bronchodilators. N Engl J Med. 1995;333(8):499–506. doi: 10.1056/NEJM199508243330807. [DOI] [PubMed] [Google Scholar]
- (25).Palmer KN, Diament ML. Effect of salbutamol on spirometry and blood-gas tensions in bronchial asthma. Br Med J. 1969;1(635):31–2. doi: 10.1136/bmj.1.5635.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kraft M. Part III: Location of asthma inflammation and the distal airways: clinical implications. Curr Med Res Opin. 2007;23(Suppl 3):S21–S27. doi: 10.1185/030079907X226177. [DOI] [PubMed] [Google Scholar]
- (27).Tashkin DP. The role of small airway inflammation in asthma. Allergy Asthma Proc. 2002;23(4):233–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.