Abstract
Chorion melanization is a vital biochemical event for the survival of mosquito eggs in the environment. This study describes the identification and molecular characterization of a prophenoloxidase (proPO) involved in chorion melanization in Aedes aegypti by various biochemical and molecular techniques. Results revealed that transcription of the chorion proPO occurs only in adults, blood feeding greatly stimulated its transcription and haemocytes are responsible for its transcription. Our study provides a solid basis for suggesting an essential role of the isolated proPO in chorion melanization during chorion hardening and also raises fundamental questions regarding its transportation and distribution in the chorion. This study should serve as a useful reference towards functional elucidation of other proPOs in mosquitoes.
Keywords: Aedes aegypti, prophenoloxidase, chorion, chorion melanization, egg development, ovary
Introduction
Melanization is an important physiological and pathological event in insects, being involved in insect cuticular tanning, wound healing and the immune response. All of these are critical for the normal development and survival of insects in the environment. Consequently, phenoloxidase (PO), the enzyme primarily responsible for catalysing the melanization pathway, has attracted considerable attention and there have been numerous studies dealing with various aspects of PO function in insects (Sugumaran, 2002; Cerenius & Soderhall, 2004). It has been accepted that PO is present as an inactive form, commonly termed prophenoloxidase (proPO), and must be activated before being able to mediate the melanization pathway. proPO activation is a rather complicated process and a number of factors, especially proteases, are involved in proPO activation (Chosa et al., 1997; Satoh et al., 1999; Sugumaran, 2002; Jiang et al., 2003; Cerenius & Soderhall, 2004).
In our previous studies dealing with chorion hardening in Aedes aegypti mosquitoes, we determined that PO-mediated melanization is one of the important mechanisms contributing to the formation of a highly protective chorion in mosquitoes (Li, 1994; Li et al., 1996). The chorion of newly oviposited mosquito eggs is white and vulnerable to desiccation. For example, when Ae. aegypti eggs are moved to an extremely dry environment right after oviposition, the developing embryo rapidly dehydrates and dies. However, in a moist environment (such as on water-saturated filter paper under laboratory conditions), the chorion turns black within 2 h after oviposition and becomes highly resistant to desiccation, which was termed chorion hardening in our previous study (Li et al., 1996). In Ae. aegypti, once the chorion turns black, the developed embryo (or first stage larva) within the chorion can survive for months in a dry environment. Because eggs of floodwater mosquitoes, such as Ae. aegypti, hatch only after being flooded following adequate rainfall, the chorion melanization is a vital process for egg survival in the environment.
The physical change of the chorion from white to black suggests the presence of PO in the chorion and its involvement in chorion melanization, but the identity of the PO was unknown. The function of a protein is intimately linked to its local environment, so that the spatial distribution of proPO mRNA and/or its gene product should provide insight essential for its functional elucidation. However, the commonly used methods for mRNA and protein detection are often difficult to apply for the identification of a particular mosquito proPO or PO due to low levels of mRNA transcripts and the presence of multiple proPO sequences sharing high sequence similarity. For example, there are nine individual proPO coding sequences in Anopheles gambiae (Christophides et al., 2004). Four individual proPO clones have been isolated from Armigeres subalbatus and four from Ae. aegypti (Cho et al., 1998; Huang et al., 2001; Taft et al., 2001) (Li et al., unpubl. data). Western and/or histochemical analysis with antiproPO antibodies can verify the presence of proPO in tissues or cells; however, due to cross-reactions, it is difficult to clearly establish the exact identity of the proPO with polyclonal antiproPO antibodies unless monoclonal antibody is available.
Although one Ar. subalbatus proPO has been suggested to be involved in chorion melanization based on the increase in its transcription after blood feeding (Huang et al., 2001), there has been no direct evidence showing the presence of its gene product in developing ovaries or in the chorion of mature eggs. In Anopheles stephensi, a proPO sequence is transcribed in larvae, pupae, males and females and also seems to be present in ovaries at a low level (Cui et al., 2000), but there is no definitive evidence for its involvement in chorion melanization. Through fractionation of chorion proteins by SDS-PAGE and selective in-gel trypsin digestion of protein bands, with molecular weights from 70 to 80 kDa, and subsequent LC/MS/MS of the extracted tryptic peptides, we were able to obtain partial sequence data of a chorion proPO and isolate its cDNA based on the partial amino acid sequences. Our results demonstrate the physical presence of a specific proPO protein in the chorion, which provides a solid basis for suggesting its role in chorion melanization during chorion hardening. In this report, we describe and discuss the cells responsible for the transcription of the proPO, its molecular regulation, and its localization in the chorion. We also discuss the potential problems in determining tissue specific proPO expression by current molecular techniques.
Results
PO activity in mature eggs
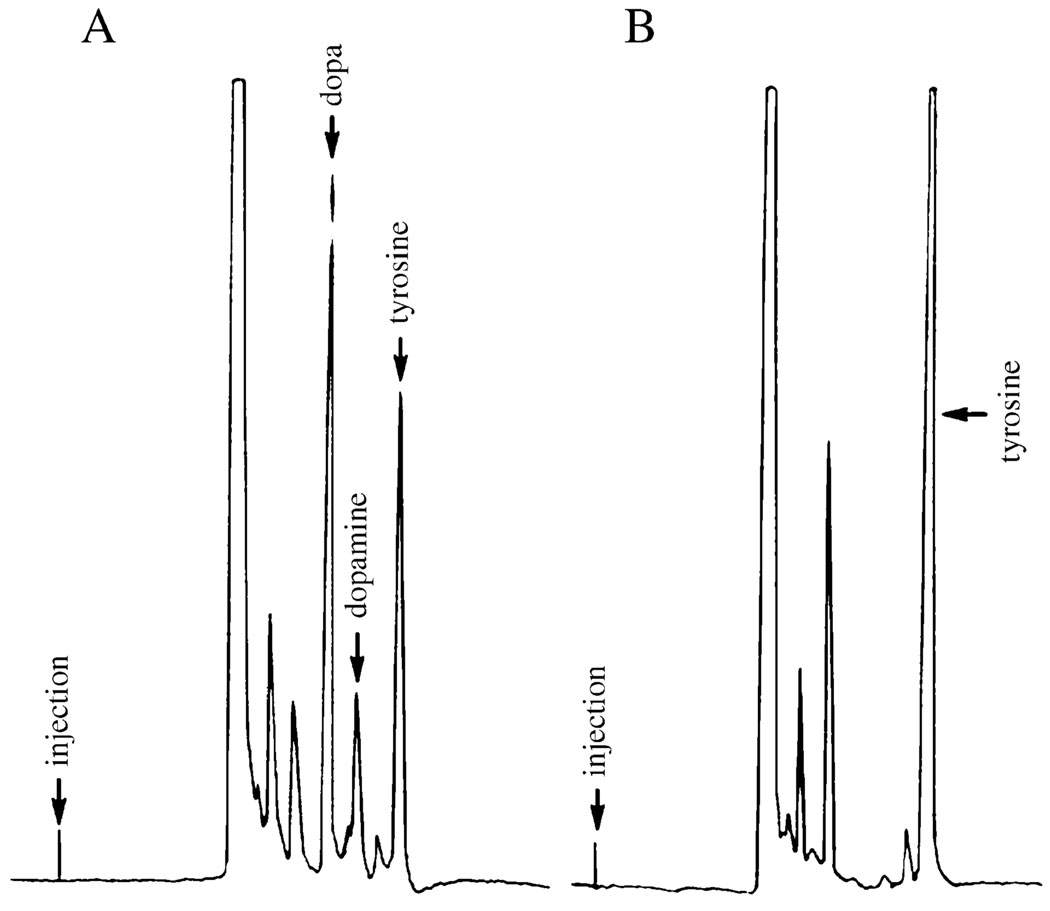
When freshly dissected mature eggs were incubated in 0.1 m phosphate buffer (pH 6.5) at room temperature for 1 h, no colour change of the chorion was observed. However, when the dissected eggs were exposed to 85% ethanol for 1–2 min followed by rapid decanting of the ethanol solution and addition of the same phosphate buffer, formation of black spots on the chorion of individual eggs was observed just a few minutes after incubation. Rapid formation of black spots on the chorion of the individual eggs after a brief ethanol exposure was a highly repeatable event, but the chorion of these eggs never turned completely black as compared to those oviposited on moist substrates, which is likely to be due to damage of the chorion by the organic solvent and release of melanization precursors. Analysis of the phosphate buffer used for incubation of the ethanol exposed eggs at 15 min after incubation indeed resulted in the detection of tyrosine, dopa and dopamine in the buffer (Fig. 1A). When the dissected eggs in phosphate buffer were briefly sonicated, and then incubated for 15 min, release of tyrosine was observed in the supernatant, but essentially no dopa was detected in the supernatant (Fig. 1B). Treatment of the eggs with 0.5% SDS also released tyrosine, but there also was no dopa production in the supernatant after incubation (results not shown). Simultaneous formation of black spots on the chorion of ethanol exposed eggs and detection of dopa and dopamine in the buffer used for incubation of the ethanol treated eggs, in comparison with results of briefly sonicated or SDS treated eggs, provided the basis to suggest that proPO is present in the eggs, chorion melanization starts with proPO activation, and activated proPO catalyses the hydroxylation of tyrosine, thereby initiating chorion melanization reactions.
Figure 1.
Detection of phenoloxidase activity in mature eggs. Dissection of eggs, brief exposure of the eggs in ethanol, incubation of the eggs in phosphate buffer, and subsequent HPLC-ED analysis of supernatant of egg mixture were carried out as described in the Experimental Procedures. (A) Chromatogram showing the detection of tyrosine, dopa and dopamine in phosphate buffer at 15 min after incubation of ethanol-exposed mature eggs. (B) Chromatogram illustrating the relative amount of tyrosine and other electrochemically active compounds in supernatant of a briefly sonicated egg mixture following 15 min incubation. Separation of tyrosine, dopa and dopamine was achieved by reverse phase separation using a reverse phase column (250 × 4.6 mm, Varian, Inc.) with a mobile phase consisting of 0.1 m phosphate buffer (pH 3.0) containing 4% acetonitrile at a flow rate of 0.6 ml per min. The retention times for dopa, dopamine and tyrosine at the applied separation conditions were 7.5, 8.3 and 9.5 min, respectively.
Determination of chorion PO partial sequences
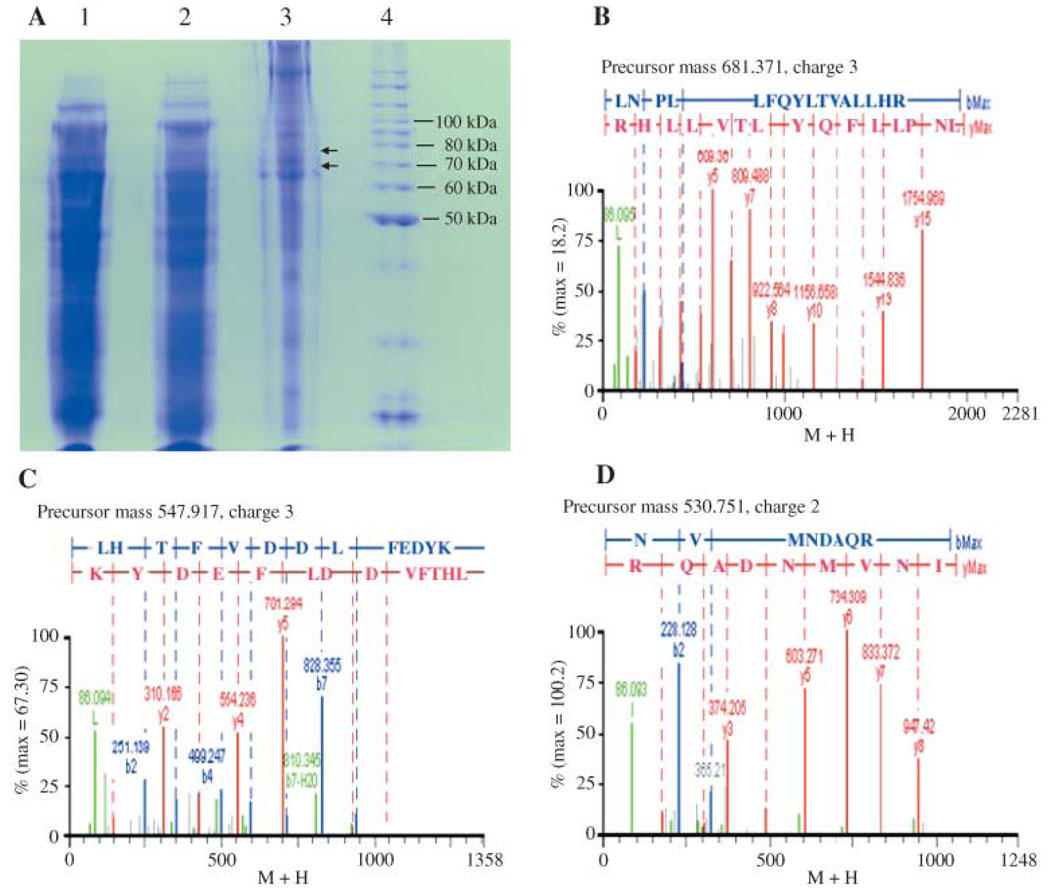
SDS-PAGE analysis of the solubilized embryonic proteins and chorion proteins showed the presence of numerous proteins in the solubilized protein samples (Fig. 2A). Trypsin in-gel digestion of horizontally sectioned gel pieces, de novo sequencing of the collected MS/MS data, and subsequent homology search of de novo sequences resulted in the identification of a number of partial peptide sequences sharing high similarity with mosquito proPO sequences, especially An. gambiae proPO-9. Compared with the protein standards, the proPO-like tryptic peptides were from lanes containing separated chorion proteins with molecular weights of 73 to 74 kDa (Fig. 2A, lane 3 arrow). Figure 2(B–D) illustrates MS/MS spectra and their de novo sequences of the three proPO tryptic peptides. Analysis of the corresponding gel sections containing phosphate and Triton solubilized proteins (with most of them presumably being embryonic proteins) did not lead to the identification of proPO-like fragments. These data provided the basis for suggesting the presence of proPO in Ae. aegypti eggs and its localization in the chorion layer.
Figure 2.
Determination of partial chorion prophenoloxidase (proPO) sequences. (A) Protein profiles of solubilized embryonic and chorion proteins on SDS-PAGE gel: phosphate buffer and 0.5% Triton-100 solubilized embryonic proteins (lanes 1 and 2), 2.0% SDS solubilized chorion proteins (lane 3), and protein standards (lane 4). The gel section within the two arrows denotes positions corresponding to protein fractions containing chorion proPO. To obtain high quality spectra, the actual amount of either embryonic or chorion protein used for in-gel digestion was about eight-fold more than shown on the SDS-PAGE. (B, C, D) MS/MS spectra of the 2044.113, 1643.751 and 1061.502 proPO tryptic precursor ions. The red, blue and green fragments represent y-ion, b-ion and a-ion, respectively.
Chorion proPO cDNA isolation and its comparison with mass spectral data
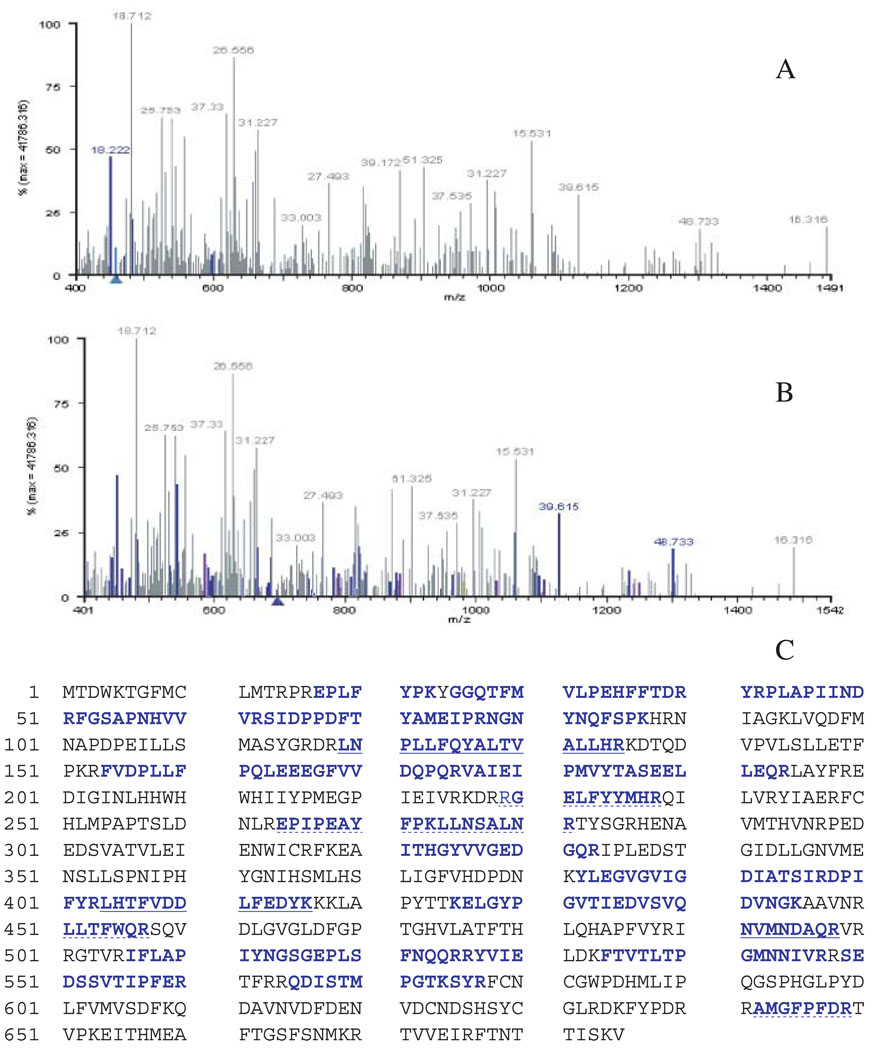
Blast search of the proPO-like tryptic peptides (derived from de novo sequencing) against the Tigr Ae. aegypti database (http://www.tigr.org/tdb/e2k1/aabe/) identified a 730 bp partial EST sequence (aaest_4097) and its deduced sequence matched the SEDSSVTIPFER and QDISTMPGTKSYR tryptic peptides. After cloning and sequence verification of the aaest_4097 sequence as a carboxyl site of a putative proPO sequence, specific primers were synthesized based on this EST sequence for 5-RACE extension, which led eventually to the amplification of a 2.1 kb cDNA with its open reading frame coding for a protein with 685 amino acids residues (NCBI accession #: AY678450). Incorporation of this deduced amino acid sequence into the in-house mosquito database and subsequent ion search of the database using the previous collected MS and MS/MS data matched four and thirty sets of MS and MS/MS spectra to the An. gambiae proPO-9 (Fig. 3A) and the newly isolated Ae. aegypti proPO (Fig. 3B), respectively. Figure 3(C) shows the coverage map of the collected spectral data for the deduced sequence of the isolated chorion proPO clone. Further examination of the four sets of MS and MS/MS data that matched to both An. gambiae proPO-9 (An-proPO9) and the newly isolated Ae. aegypti proPO revealed that their corresponding tryptic peptides (GELFYYMHR, EPIPEAYFPKLLNSALNR, LLTFWQR, AMGFPFDR) are identically present in both An-proPO-9 and Ae-proPO-5. Although the in-house mosquito database contained the other four available Ae. aegypti proPO sequences, none of the MS and MS/MS spectra matched to these Ae. aegypti sequences. These data show the power of the ion search algorithm (see Fig. 3C). The extremely high coverage of the collected MS and MS/MS data to the isolated Ae. aegypti proPO clone provided the verification that it codes for the proPO involved in chorion melanization.
Figure 3.
Identification of chorion prophenoloxidase (proPO) by an ion search algorithm. Collected MS and MS/MS data of an in-gel digested chorion protein fraction (about 73–74 Kd, see Fig. 2A) were ion searched against the in-house mosquito protein database. Coloured precursor ions matched to An-proPO-9 (A) and Ae-proPO-5 (B), respectively. The number above each fragment indicates the retention time of the peptide ion. Coverage map shows unambiguous match (blue residues) of the MS and MS/MS data to Ae-proPO-5 (NCBI accession #: AY678450). The tryptic fragments corresponding to three MS/MS spectra in Fig. 2 are underlined. Tryptic fragments matched to both Ae-proPO-9 and Ae-proPO-5 are dot-underlined.
Sequence comparison
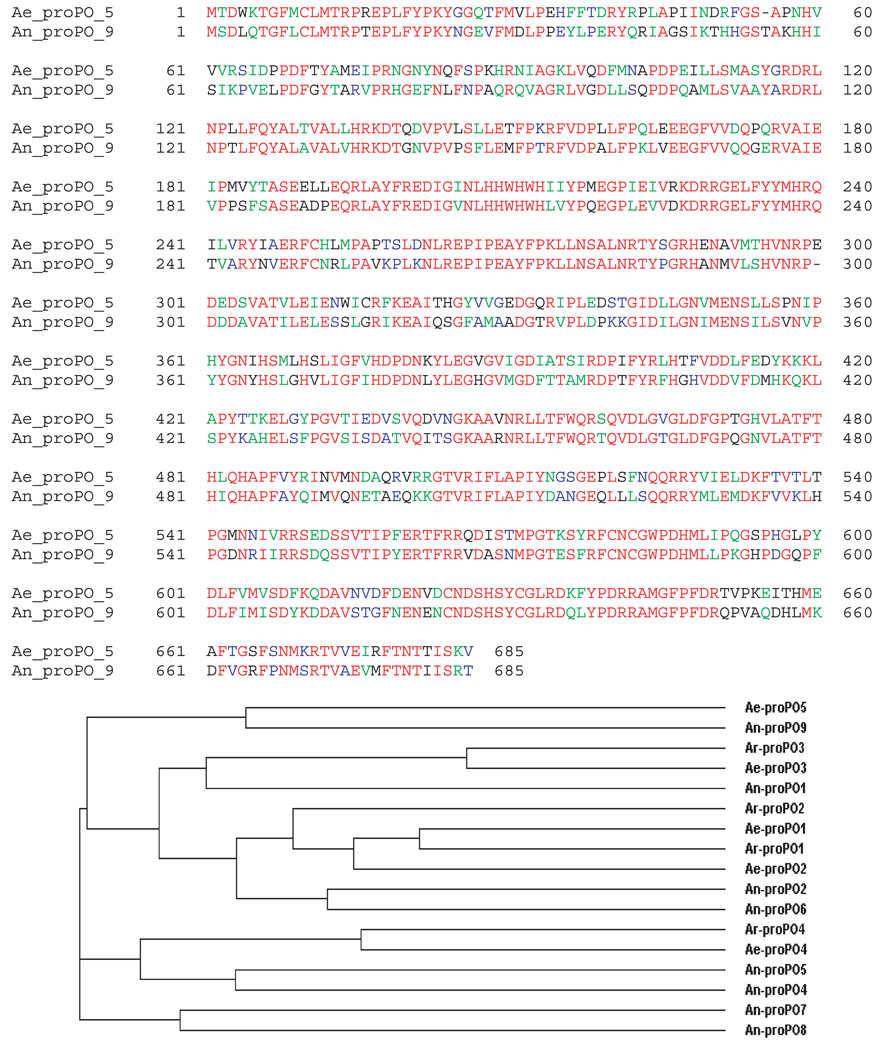
Sequence comparison revealed that the newly isolated cDNA shares 47–63% sequence similarity with An. gambiae proPO sequences, 44–45% similarity with proPO sequences of Drosophila melanogaster, 51–53% similarity with the four available Ae. aegypti proPO sequences, 47–53% similarity with proPO sequences of Ar. subalbatus, and 35–52% similarity with available putative proPO sequences from other insect species. Based on the number of available Ae. aegypti proPO sequences, this isolated chorion proPO was arbitrarily termed Ae. aegypti proPO-5 (Ae-proPO-5). Ae-proPO-5 shares the highest similarity (63%) with An. gambiae proPO-9 (Fig. 4A). Phylogenetic analysis of the available Ae. aegypti, Ar. subalbatus and An. gambiae proPO sequences shows a close relation between Ae-proPO-5 and An. gambiae proPO-9 (Fig. 4B), suggesting that An. gambiae proPO-9 is likely a counterpart of Ae-proPO-5 and may be involved in An. gambiae chorion melanization.
Figure 4.
Comparison of chorion prophenoloxidase (proPO) with other mosquito proPO sequences. (A) Sequence alignment of Ae-proPO-5 with An-proPO-9. Residues in red are those conserved in both sequences. (B) Phylogenetic analysis of Ae-proPO-5 with other mosquito proPO sequences using the CLUSTALW program. Ar-, Ae- and An- represent sequences from Armigeres subalbatus, Aedes aegypti and Anopheles gambiae, respectively.
Chorion proPO transcription
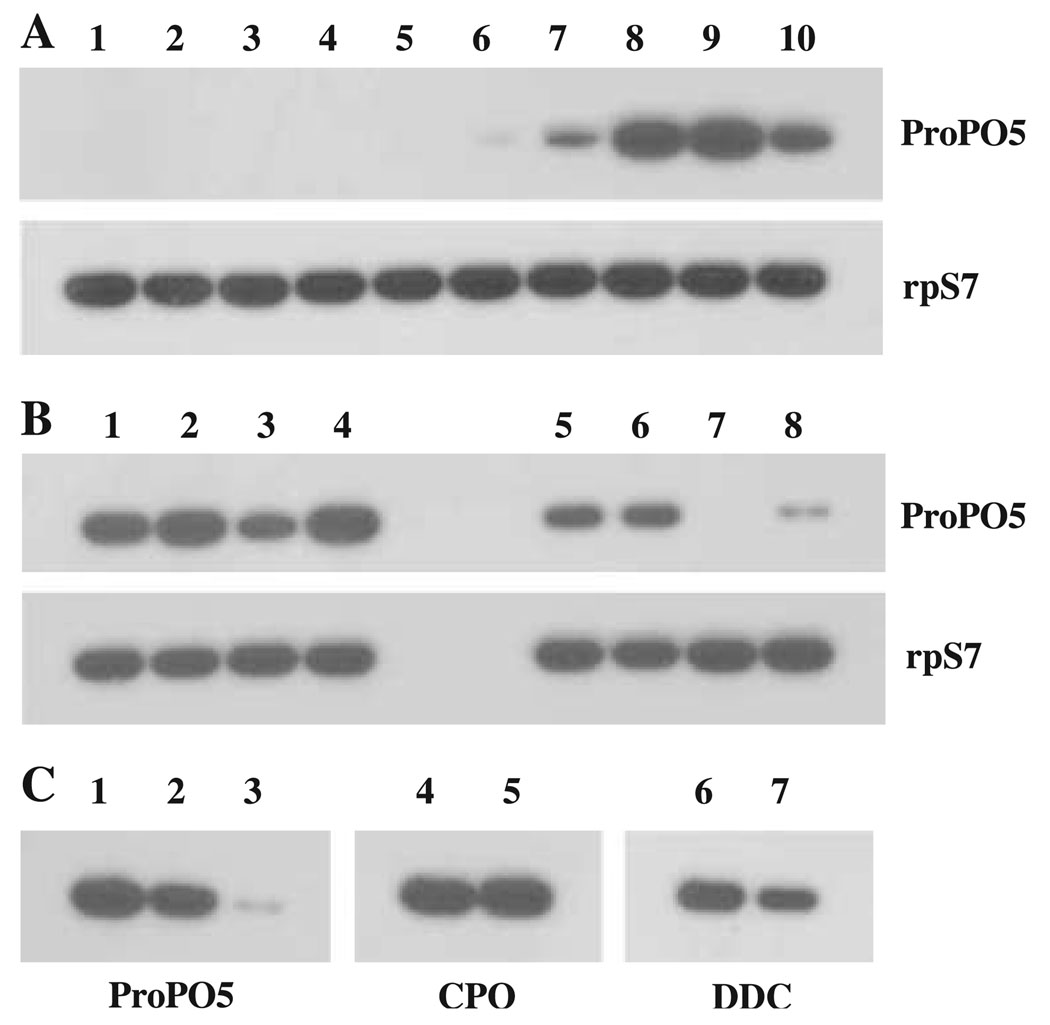
RT-PCR amplification of Ae-proPO-5 fragment from total RNA samples, extracted from different stage larvae and pupae, newly emerged adult males and females, and adult females at 24, 36 and 48 h after blood feeding, revealed that Ae-proPO-5 was only transcribed in adults, it was transcribed in female mosquitoes at extremely low levels, but transcript abundance increased significantly after blood feeding (Fig. 5A). When total RNA from different body parts of 24 h blood-fed females was used as a template for RT-PCR, Ae-proPO-5 was amplified from total RNA extracted from head, thorax, abdomen and ovaries of blood-fed females (Fig. 5B). However, when different body parts were extensively washed in DEPC-treated PBS before total RNA isolation, the relative intensities of the amplified Ae-proPO-5 cDNA fragment from the same amount of head, thorax, abdomen and ovaries total RNA were either greatly decreased or barely visible (Fig. 5B). When total RNA from haemolymph were used as template, Ae-proPO-5 was easily amplified, which, in conjunction with the relative intensity of Ae-proPO-5 bands amplified from total RNA of washed and unwashed body parts, suggested that amplified Ae-proPO-5 fragment from head, thorax, abdomen and ovaries total RNA could be due to haemocyte contamination. When the same total RNA samples that were extracted from either extensively washed or not washed ovaries were used to amplify DNA fragments for dopa decarboxylase (DDC), chorion peroxidase (CPO) and Ae-proPO-5, using their corresponding specific primers, there were no noticeable differences in relative intensity of CPO DNA fragment between the washed and unwashed samples, there was some decrease in the relative intensity of the DDC fragment in washed sample as compared to unwashed sample, but the relative intensity of the Ae-proPO-5 band was barely visible in the washed samples (Fig. 5C). These data suggest that the decrease in the intensity of the amplified Ae-proPO-5 is not due to its mRNA degradation.
Figure 5.
RT-PCR analysis. (A) Lanes 1, 2 and 3, total RNA from 1-, 3- and 5-day old larvae; lanes 4 and 5, total RNA from 0.5 and 12 h pupae; lane 6, total RNA from males; lane 7, total RNA from females; lanes 8, 9 and 10, total RNA from females at 24, 36 and 48 h after blood feeding. (B) Lanes 1, 2, 3 and 4, total RNA from unwashed head, thorax, abdomen and ovaries, and lanes 5, 6, 7 and 8, total RNA from head, thorax, abdomen and ovaries that had been extensively washed before total RNA isolation. (C) Lane 1, total RNA from haemocytes; lanes 2 and 3, total RNA from unwashed and washed ovaries, respectively; lanes 4 and 5, RT-PCR results for chorion peroxidase (CPO) amplified from total RNA of unwashed and washed ovaries; lanes 6 and 7, RT-PCR results for dopa decarboxylase (DDC) amplified from total RNA of unwashed and washed ovaries. In (C), the same unwashed or washed sample was used for amplification of Ae-proPO-5, CPO and DDC.
Discussion
Based on results of our previous studies, we suggested that PO-mediated chorion melanization is an important biochemical event contributing to the formation of a hardened chorion in Ae. aegypti (Li, 1994; Li et al., 1996). Limitation in the amount of chorion proteins available from mature eggs makes it extremely difficult to physically isolate the enzyme for characterization. Based on partial sequence data derived from LC/MS/MS of trypsin digested chorion proteins, however, we were able to identify a number of PO tryptic peptides and isolate chorion proPO cDNA based on the partial sequence data. RT-PCR data provide rather convincing evidence for the transcription of Ae-proPO-5 in haemocytes, which is intriguing and links the function of haemocytes with chorion formation and hardening. This study should serve as a useful reference towards identification and characterization of other Ae. aegypti proPO sequences, especially those involved in chorion melanization in other mosquitoes.
Ae-proPO-5 is localized in the chorion layer of mature eggs and primarily responsible for initiating the chorion melanization pathway. Dramatic change of the chorion from white to black provides the basis for suggesting the involvement of PO in chorion melanization, but to reach a definitive conclusion, one has to establish that the enzyme is indeed present in the chorion and is indispensable for the melanization reaction, because a chorion peroxidase that is present in the chorion (Li et al., 2004) also could potentially mediate the melanization pathway if dopa or dopamine is present in the chorion. Although absence of dopa or dopamine in mature eggs (Fig. 1A) does not completely rule out the involvement of peroxidase from participating in chorion melanization reactions, it apparently excludes it from initiating the chorion melanization pathway, because peroxidase cannot catalyse tyrosine hydroxylation. Accumulation of dopa following ethanol treatment of mature eggs provides the basis for suggesting the presence of a proPO in the mature eggs and its involvement in chorion melanization, while high coverage of Ae-proPO-5 from collected MS and MS/MS data from a trypsin-digested chorion protein fraction, and absence of PO or proPO fragments from the collected MS and MS/MS data in trypsin digested, corresponding SDS PAGE separated embryonic protein fractions, provide the basis for the localization of Ae-proPO-5 in the egg chorion.
Based on the number of available putative proPO sequences, it seems clear that mosquitoes have more proPO coding sequences (Cho et al., 1998; Huang et al., 2001; Taft et al., 2001; Christophides et al., 2004; Li et al., unpubl. data) than other model insect species, such as D. melanogaster and Manduca sexta (Fujimoto et al., 1995; Asada et al. 2003; Kanost et al., 2004). Conceivably, individual proPOs may have specific biological functions or may be multifunctional, but with a particular one as its primary function. Based on sequence similarity, it is quite possible that different PO proteins have similar biochemical characteristics and the specific function of individual proPOs may be dictated by their activating pathway and their spatial localization. Although there has been a number of studies dealing with the functional elucidation of mosquito proPOs, the specific physiological function for an individual proPO in vivo, has rarely been clearly established. One exception is that Shiao et al. (2001) were able to use gene knock-down strategies in vivo to verify that Ar. subalbatus proPO-1 is responsible for melanization immune response against filarial worms. One of the major challenges in mosquito proPO research is to unambiguously establish the specific function for individual proPO proteins.
Haemocytes are likely responsible for Ae-proPO-5 expression. Compared to the spatial and temporal profiles of chorion-hardening related enzymes, the transcription of Ae-proPO-5 is intriguing. For example, DDC is involved in the same chorion melanization pathway and blood feeding stimulates its transcription in ovary follicular cells (Ferdig et al., 1996). Peroxidase is another enzyme involved in mosquito chorion hardening, and the enzyme is also specifically transcribed in the developing eggs after a blood meal (Li et al., 2004). Based on the DDC and chorion peroxidase studies, the transcription and translation of the chorion proPO was initially considered to occur in follicular epithelial cells. With that notion, ovary total RNA extracted from 24 h ovaries were actually used for the initial amplification of the partial Ae-proPO-5 and its subsequent 5-RACE extension. Based on subsequent RT-PCR analysis of the total RNA from different body parts, it seems apparent that transcription of Ae-proPO-5 occurs primarily in haemocytes and amplification of the proPO from ovary total RNA likely is due to haemocyte contamination. The transcription and translation of chorion proPO by haemocytes raise some fundamental questions regarding the transport of proPO to the developing ovaries and the mechanism for uniform distribution of the pro-enzyme in the chorion. Extensive experimentation will be required to answer these questions.
In mosquitoes, only haemocytes or haemocyte-like cells may be responsible for proPO transcription. Based on available data about mosquito proPO transcription, it seems that the transcription of most proPO sequences occurs primarily in haemocytes. In An. gambiae, a haemocyte-like cell line was able to express six different proPOs under ecdysone stimulation (Muller et al., 1999). All four cloned Ar. subalbatus proPO sequences are transcribed in haemocytes (Cho et al., 1998; Huang et al., 2001). Transcription of Ae. aegypti proPO-1 also has been demonstrated in haemocytes (Taft et al., 2001). The transcription of Ae. aegypti proPO-2 and An. gambiae proPO-1 has been demonstrated in 14-day old Ae. aegypti eggs (Taft et al., 2001) and 2-day old An. gambiae eggs (Muller et al., 1999), respectively, but their mRNA still might be transcribed by haemocytes or haemocyte-like cells, because embryonation of the oviposited eggs takes about 3 days depending upon temperature and a number of different cells, including haemocytes, should have been derived from their corresponding germ-line cells. Haemocytes play important roles in insect immune responses (Hillyer et al., 2003), and proPO and its activation cascade also play an important part in the responses (Cerenius & Soderhall, 2004). Therefore, it seems logical that haemocytes express proPOs involved in innate immunity. However, egg development is an integral part of the normal life cycle in mosquitoes as well as in other insects; accordingly, there is no apparent connection between egg development and immune responses. Consequently, the expression of Ae-proPO-5 involved in chorion melanization by haemocytes raises an interesting question: are haemocytes responsible for the expression of all mosquito proPO sequences in mosquitoes?
Results of RT-PCR amplification of Ae-proPO-5 indicate that contamination of haemocytes during RNA isolation could be a major problem when assessing tissue- or cell-specific proPO transcription. In Ae. aegypti, the relative amount of transcripts for some proPO sequences, including Ae-proPO-5, is relatively low, which makes it difficult to assess their spatial and temporal profiles by in situ hybridization. This is particularly true for haemocyte-derived proPO, because haemocytes are in close proximity with many tissues or cells and easily break during sample preparation. RT-PCR is highly sensitive, so it has often been used for detection of low abundance mRNA (including proPO sequences) in different tissues or cells. Our data indicate that it is essential to take necessary precautions in assessing spatial profiles of gene transcription when haemocytes may be part of the cells responsible for its transcription. In the case of Ae-proPO-5, it was the difference in its tissue- or cell-specific transcription and its product localization that prompted our further RT-PCR analysis of Ae-proPO-5. Otherwise, it might have been concluded that this gene was transcribed in head, thorax, abdomen and ovaries. The RT-PCR results for Ae-proPO-5 could serve as a useful reference for assessing the spatial and temporal profiles for other Ae. aegypti proPO sequences.
In summary, LC/MS/MS of tryptic peptides of chorion proteins resulted in the identification of a number of tryptic chorion proPO peptides, which provided essential evidence for subsequent identification of Ae-proPO-5 as the gene coding for the chorion proPO. Although the presence of low levels of Ae-proPO-5 in non-blood-fed females questions whether chorion melanization is its only physiological role in mosquitoes, its up-regulation after blood feeding and its primarily localization in the chorion of mature eggs provide practical evidence for suggesting chorion melanization as its primary function, or at least one of its primary functions. Absence of Ae-proPO-5 mRNA in developing ovaries indicates that the enzyme is unlikely expressed in the ovaries. RT-PCR data provide a reasonable basis for suggesting its transcription and translation in haemocytes. Like other Ae. aegypti proPO sequences, Ae-proPO-5 has no apparent secretion or signal peptide based on commonly used programs for signal peptide analysis. Consequently, the secretion of the protein, its transportation to, and its uniform distribution in the chorion are some of the fundamental questions that must be answered if we are to gain a comprehensive understanding of Ae-proPO-5 in Ae. aegypti.
Experimental procedures
Mosquito rearing
Aedes aegypti used in this study were reared in an environmental chamber at 26 ± 0.5 °C and 70 ± 5% relative humidity with a 16 h light/8 h dark cycle.
Detection of PO activity in mosquito eggs
Mosquito ovaries containing mature eggs were dissected from females 72 h after a blood meal. Dissected ovary pairs were transferred into 1.5 ml microfuge tubes at ten pairs per tube and 0.6 ml 95% ethanol was added to each tube. The eggs were incubated in the ethanol solution for 90 s on ice (with brief, gentle hand shaking of the egg sample twice during the incubation), followed by rapid decanting of the ethanol solution and addition of 0.25 ml 100 mm phosphate buffer. At 15 min after incubation at 30 °C, 100 µl supernatant of the egg mixture was withdrawn and mixed with an equal volume of 0.8 m formic acid. After centrifugation (20 000 g for 15 min at 4 °C), 10 µl supernatant was injected for reverse-phase HPLC with electrochemical detection (HPLC-ED) to determine the potential production of intermediates in the melanization pathway. The HPLC-ED system consisted of a Hitachi LC system (San Jose, CA) fitted with a Bioanalytical System LC-4C detector (Lafayette, IN). A potential of 850 mV (vs. an Ag/AgCl/KCl reference electrode) was applied to the working electrode and the sensitivity was set at 20 nA. Dissected ovary pairs, either sonicated briefly in 0.1 m phosphate buffer using a low energy sonicator or treated with 0.5% SDS, served as controls. These control samples were incubated and analysed in the same manner.
Isolation of chorion proteins
Mosquito ovaries were collected from females 72 h after blood feeding. The collected eggs were homogenized in 50 mm phosphate, pH 6.5, containing 0.1 m NaCl and 2 mm PMSF. Chorion sediments were obtained by centrifugation and washed twice with the same buffer. The chorion sediments were washed twice with 0.5% Triton-100 solution and then treated with 2% SDS plus sonication. Proteins solubilized by 2% SDS plus sonication were considered to be chorion proteins.
SDS-PAGE of chorion proteins and LC/MS/MS of trypsin digested chorion proteins
SDS-solubilized chorion proteins were loaded into several wells on a polyacrylamide gel and electrophoresis was conducted at 4 °C. Proteins solubilized by phosphate buffer alone and by phosphate buffer containing 0.5% Triton-100, separated under identical conditions, served as controls. After electrophoresis, the gel was stained with Coomassie blue. Individual lanes containing separated proteins corresponding to relative molecular weights of 68–78 kDa on the gel were horizontally sectioned, and proteins in the sectioned gel pieces were in-gel digested with trypsin according to a in-gel digestion protocol (http://www.pharma.ethz.ch/bmm/protocols/ingeldig.html). Tryptic peptides extracted from individual gel pieces were sequentially analysed by liquid chromatography and tandem mass spectrometry (LC/MC/MS). The LC/MS/MS system consisted of a Waters capillary HPLC interfaced with a Waters Q-TOF-micro (Waters Corporation, Milford, MA) through electrospray ionization (ESI). An in-house mosquito protein database (with sequences downloaded from NCBI protein databases), containing all predicted An. gambiae protein sequences, several hundred available Ae. aegypti protein sequences (including four Ae. aegypti proPO sequences) and four Ar. subalbatus proPO sequences, was prepared and used for the database search. Collected MS/MS data were either first de novo sequenced and subsequently searched for homology against the in-house-built mosquito protein database or ion searched the same in-house database using ProteinLynx software from Waters Company.
cDNA cloning and sequencing
De novo sequencing of the collected MS/MS data and subsequent homology searches of the in-house mosquito protein database identified eight PO-like partial sequences. Blast search of the Ae. aegypti EST database using the de novo sequences matched the DSSVTIPFER and QDISTMPGTKSYR tryptic sequence to a 730 bp Ae. aegypti EST sequence (aaest_4097, http://www.tigr.org/tdb/e2k1/aabe/). The aaest_4097 was amplified from first strand cDNAs synthesized from ovary total RNA, collected from females at 24 h after blood feeding, and its DNA sequence was verified by DNA sequencing. Sequence analysis indicated that aaest_4097 codes for the carboxyl end of a putative proPO and contains the stop codon. To determine its entire open reading frame (ORF), 5′-RACE was performed using an Invitrogen 5′ RACE kit. Three antisense primers (5′-GCGATCGTTGCCAGAACGTAAGCAA-3′, 5′-CCGTTGACGTC TTGCACGGAAACATC-3′ and 5′-GCTCTTTGGTCGTGTATGGTGCAAGTT-3′ as GSP1, GSP2 and GSP3, respectively) were synthesized based on the confirmed 730 bp EST sequence. First strand cDNA was synthesized using the gene specific primer (GSP1) from 24 h ovary total RNA. The addition of a polymeric C tail to first strand cDNA, PCR amplification using the specific GSP2 primer and an abridged anchor primer (5′-GGCCACGCGTCGACTAGTACGGGI IGGGIIGGGIIG-3′), and subsequent nested PCR amplification of its PCR product, using GSP3 specific primer and an abridged universal amplification primer (GGCCACGCGTCGACTAGT AC), were done based on the manufacturer’s protocol. The amplified PCR fragments were subcloned into pGEM-T easy vector (Promega, Madison, WI) and subjected to DNA sequencing. Sequence data were analysed using the Biology WorkBench 3.2 program (http://workbench.sdsc.edu).
Sequence analysis
The 5′-RACE extended clone included the entire ORF and 5′-end untranslated region. Its deduced sequence was added to the in-house mosquito protein database and the collected spectral data were ion searched against the database. After the proPO identity of the isolated clone was confirmed, its primary sequence was compared with other available proPO sequences from Ae. aegypti, An. gambiae, Ar. subalbatus and other insects.
Reverse transcription PCR (RT-PCR)
Total RNA was extracted from larvae (1–6 days), pupae (0.5 and 12 h), adult males and females at 3 days after emergence, bloodfed and unfed females using Trizol reagent (Invitrogen, Carlsbad, CA) and then treated with DNase I. Treated RNA samples (1 µg per sample) were used for cDNA synthesis with oligo (dt) primer and Super Script II reverse transcriptase (Invitrogen). PCR amplifications were performed using a pair of specific primers (5′-GGCATCCGAGGAGTTGCTGGAACAA-3′ and 5′-GCTCTTTGGTCGTGTATGGTGCAAGT-3′) under the following conditions: 94 °C for 30 s, 62 °C for 45 S, and 72 °C for 1 min for 32 cycles with final extension at 72 °C for 10 min. Initial results showed that the Ae. aegypti proPO was female specific and greatly upregulated in females after blood feeding. To determine its relative spatial and temporal profiles, total RNA from different body parts of 24 h blood-fed mosquitoes, including head, thorax, abdomen, ovary and ovary dissected abdomen, were extracted and used as templates for RT-PCR using the same pair of specific proPO primers. To assess the integrity of the mRNA templates, three specific primer pairs with one for ribosomal protein S7 (RPS7: 5′-TTCGGATCAAAGGTGAT CAAG-3′ and 5′-GTGAAGGTGTCGACCTTGTGT-3′), one for chorion peroxidase (CPO: 5′-CCTCACTTACGGTCTAACACG-3′ and 5′-TAAAATCCTTCCTCTCGCCATAG-3′) and the other for dopa decarboxylase (DDC: 5′-TGTCTGTCCAGAATATCGTCATCT-3′ and 5′-TACTGCCATACGCAAGAAGTAGAC-3′), respectively, were also used for RT-PCR. PCR products were separated on a 1% agarose gel, transferred on a Hybond-P membrane (Amersham Biosciences) with 0.4 N NaOH, cross-linked to the membrane using a Bio-Rad UV cross-linker, and hybridized with a 550 bp [α-32P] dCTP labelled specific probes. After hybridization at 55 °C for 16 h, the membranes were washed twice with 2× SSC containing 0.1% SDS at 25 °C for 20 min and two times with 0.1× SSC containing 0.1% SDS at 68 °C for 20 min, and exposed to X-ray film (Kodak, Rochester, NY) at −80 °C.
Acknowledgements
This work was supported by NIH grant AI37789 to JL and NIH grant AI19769 to BMC.
References
- Asada N, Yokoyama G, Kawamoto N, Norioka S, Hatta T. Prophenol oxidase A3 in Drosophila melanogaster: activation and the PCR-based cDNA sequence. Biochem Genet. 2003;41:151–163. doi: 10.1023/a:1023325610300. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Cho WL, Liu HS, Lee CH, Kuo CC, Chang TY, Liu CT, Chen CC. Molecular cloning, characterization and tissue expression of prophenoloxidase cDNA from the mosquito Armigeres subalbatus inoculated with Dirofilaria immitis microfilariae. Insect Mol Biol. 1998;7:31–40. doi: 10.1046/j.1365-2583.1998.71049.x. [DOI] [PubMed] [Google Scholar]
- Chosa N, Fukumitsu T, Fujimoto K, Ohnishi E. Activation of prophenoloxidase A1 by an activating enzyme in Drosophila melanogaster. Insect Biochem Mol Biol. 1997;27:61–68. doi: 10.1016/s0965-1748(96)00070-7. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Vlachou D, Kafatos FC. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol Rev. 2004;198:127–148. doi: 10.1111/j.0105-2896.2004.0127.x. [DOI] [PubMed] [Google Scholar]
- Cui L, Luckhart S, Rosenberg R. Molecular characterization of a prophenoloxidase cDNA from the malaria mosquito Anopheles stephensi. Insect Mol Biol. 2000;9:127–137. doi: 10.1046/j.1365-2583.2000.00169.x. [DOI] [PubMed] [Google Scholar]
- Ferdig MT, Li J, Severson DW, Christensen BM. Mosquito dopa decarboxylase cDNA characterization and blood-meal-induced ovarian expression. Insect Mol Biol. 1996;5:119–126. doi: 10.1111/j.1365-2583.1996.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Okino N, Kawabata S, Iwanaga S, Ohnishi E. Nucleotide sequence of the cDNA encoding the proenzyme of phenol oxidase A1 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1995;92:7769–7773. doi: 10.1073/pnas.92.17.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer JF, Schmidt SL, Christensen BM. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003;313:117–127. doi: 10.1007/s00441-003-0744-y. [DOI] [PubMed] [Google Scholar]
- Huang LH, Christensen BM, Chen CC. Molecular cloning of a second prophenoloxidase cDNA from the mosquito Armigeres subalbatus: prophenoloxidase expression in blood-fed and microfilariae-inoculated mosquitoes. Insect Mol Biol. 2001;10:87–96. doi: 10.1046/j.1365-2583.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu XQ, Zhu Y, Kanost M. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol. 2003;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Li J. Egg chorion tanning in Aedes aegypti mosquito. Comp Biochem Physiol A Physiol. 1994;109:835–843. doi: 10.1016/0300-9629(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Li J, Hodgeman BA, Christensen BM. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochem Mol Biol. 1996;26:309–317. doi: 10.1016/0965-1748(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Li JS, Kim SR, Li JY. Insect Biochem Mol Biol. 2004;157:17–26. [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Satoh D, Horii A, Ochiai M, Ashida M. Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori. Purification, characterization, and cDNA cloning. J Biol Chem. 1999;274:7441–7453. doi: 10.1074/jbc.274.11.7441. [DOI] [PubMed] [Google Scholar]
- Shiao SH, Higgs S, Adelman Z, Christensen BM, Liu SH, Chen CC. Effect of prophenoloxidase expression knockout on the melanization of microfilariae in the mosquito Armigeres subalbatus. Insect Mol Biol. 2001;10:315–321. doi: 10.1046/j.0962-1075.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- Sugumaran M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 2002;15:2–9. doi: 10.1034/j.1600-0749.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- Taft AS, Chen CC, Li J, Christensen BM. Molecular cloning of two prophenoloxidase genes from the mosquito Aedes aegypti. Insect Mol Biol. 2001;10:97–103. doi: 10.1046/j.1365-2583.2001.00242.x. [DOI] [PubMed] [Google Scholar]