Abstract
Background
Mutations in filaggrin (FLG) are associated with atopic dermatitis (AD), and are presumed to provoke a barrier abnormality. Yet, additional acquired stressors may be necessary, since the same mutations can result in a non-inflammatory disorder, ichthyosis vulgaris.
Objective
We examined here whether FLG deficiency alone suffices to produce a barrier abnormality; the basis for the putative abnormality; and its pro-inflammatory consequences.
Methods
Using the flaky-tail (ft/ft) mouse, which lacks processed flg due to a frame-shift mutation in profilaggrin that mimics some mutations in human AD, we assessed whether FLG deficiency provokes a barrier abnormality; further localized the defect; identified its subcellular basis; and assessed thresholds to irritant and hapten-induced dermatitis.
Results
Flaky-tail mice exhibit low-grade inflammation, with increased bidirectional, paracellular permeability of water-soluble xenobiotes due to impaired lamellar body secretion and altered stratum corneum extracellular membranes. This barrier abnormality correlates with reduced inflammatory thresholds to both topical irritants and haptens. Moreover, when exposed repeatedly to topical haptens, at doses that produce no inflammation in +/+ mice, ft/ft mice develop a severe AD-like dermatosis, with a further deterioration in barrier function and features of a th2 immunophenotype (increased CRTH + inflammation, elevated serum IgE levels, and reduced antimicrobial peptide [mBD3] expression).
Conclusions
FLG deficiency alone provokes a paracellular barrier abnormality in mice that reduces inflammatory thresholds to topical irritants/haptens, likely accounting for enhanced antigen penetration in FLG-associated AD.
Keywords: Atopic dermatitis, barrier function, contact dermatitis, filaggrin, flaky tail mouse, lamellar body
INTRODUCTION
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin condition, that displays an associated abnormality in permeability barrier function 1–4. Until recently, AD was viewed as a primary immunologic disorder in which sustained allergen exposure in susceptible hosts leads to TH2-dominant inflammation, which then secondarily provokes defects in barrier function (‘inside-outside’ paradigm) [rev in 5]. However, recent genetic studies have shown a strong association between AD and several loss-of-function mutations in the gene encoding the stratum corneum structural protein, filaggrin (FLG) 6, 7. Moreover, there is a dose-response relationship between FLG-deficiency and disease severity, such that patients with double-allele or compound heterozygote mutations in FLG display more-severe and earlier-onset AD, as well as an increased propensity for AD to persist into adulthood 8–11. This rapidly-growing body of work has led to a paradigm shift in conceptions of AD pathogenesis, with increasing weight being placed on the role of a primary barrier abnormality that then precipitates downstream immunologic abnormalities (“outside-inside” paradigm) 1,12. According to this concept, TH2-dominant inflammation results from sustained epicutaneous access of haptens through a defective skin barrier 13,14. TH2 cytokines then down-regulate expression of TH1 cytokines 5, epidermal antimicrobial peptide expression 15, 16, and epidermal structural proteins [rev. in 1, 2], completing an “outside-inside-(back to) outside” paradigm for AD 2, 17.
FLG is an intracellular protein, with important roles in stratum corneum hydration 18 and as a structural component of the corneocyte cytosol 19. However, normal permeability barrier function is not regulated by the corneocyte, but rather by the lipid-enriched, extracellular matrix of the stratum corneum 20, 21. Indeed, in all inflammatory dermatoses studied to date, including those associated with inherited disorders of corneocyte proteins, the barrier abnormality has been linked to accelerated paracellular water loss 22, 23. Thus, the new-found link between FLG and AD leaves unanswered whether FLG-deficiency alone provokes a barrier abnormality in the epidermis, and if so, the subcellular basis for such an abnormality. While it has been widely hypothesized that a FLG-associated barrier defect facilitates hapten ingress 12, 13, this relationship was only recently demonstrated 24. Yet, the mechanism whereby FLG deficiency compromises barrier function, thereby provoking inflammation, remains unknown. Pertinently, FLG mutations that occur in AD also occur in the inherited, non-inflammatory condition, ichthyosis vulgaris 25, 26, raising questions about whether FLG deficiency alone suffices to provoke inflammation, or whether additional acquired stressors are required to elicit AD 1, 2.
The above questions are difficult to address within the context of human AD, where multiple genetic and environmental factors intertwine to provoke disease pathogenesis. Thus, we employed a pre-existing model, the flaky tail (ft/ft) mouse, which exhibits very reduced levels of flg 27. Flaky tail mice possess a homozygous frameshift mutation in profilaggrin that prevents the processing of profilaggrin into flg 24, such that ft/ft mice simulate double allele, loss-of-function mutations in certain human AD patients that also result in reduced levels of processed FLG protein 6, 28. The ft/ft mutation was shown previously to yield a phenotype of diffuse, mild flaking, analogous to human ichthyosis vulgaris 27. Utilizing the ft/ft model, we demonstrate that ft/ft mice display: 1) abnormal barrier function and low-grade inflammation at baseline, confirming recent observations of Fallon, et al. 24; and 2) enhanced bidirectional, paracellular penetration of water-soluble tracers. We demonstrate further that: 1) Increased penetration through the stratum corneum can be attributed to structural defects in the lamellar body secretory system; 2) flg deficiency alone reduces inflammatory thresholds to both irritants and allergens; and 3) ft/ft mice develop a hapten-induced, AD-like dermatosis at lower challenge doses than do +/+ mice, mirroring recent work in ft/ft mice exposed to a complete antigen 24. Thus, flg deficiency yields a paracellular barrier abnormality that likely also favors elicitation of AD by allowing enhanced/sustained hapten access.
METHODS (SECTION MOVED FROM AFTER DISCUSSION SECTION)
Materials and animals
Studies were conducted in maft/maft/J(ft/ft) mice (new designation: a/a ma ft/ma ft/JSun JR#9078), and age/sex-matched C57Bl/6J+/+ mice (The Jackson Lab, Bar Harbor, ME), housed under pathogen-free conditions. Flaky tail mice are compound mutants at two loci, both matted (ma/ma) and flaky tail (ft/ft). Matted confers a stable, lifelong, and readily-identifiable phenotype of subtle hair coarseness that was introduced by Jackson to facilitate identification of mutants without reliance on genotyping 29. Young ft/ft and +/+ mice were between 6–12 weeks of age, while old mice were 50–52 weeks of age. Ethanol, acetone and lanthanum nitrate were purchased from Fisher Scientific (Fairlane, NJ). Oxazolone (Ox) and calcium green were purchased from Sigma Chemical Co. (St Louis, MO). Affinity-purified, rabbit anti-mouse antibodies to both proflg and flg were purchased from BabCo (Richmond, CA). Rabbit anti-mouse antibody against the prostaglandin D2 receptor, CRTH2/DP2, which is expressed solely on th2-bearing lymphocytes and mast cells, was purchased from Cayman Chemical (Ann Arbor, MI). Rabbit anti-mouse antibody against CD3 was purchased from BD Biosciences (San Jose, CA).
Experimental protocols and functional studies
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Basal stratum corneum hydration and surface pH were measured (CM 825/PH 900, Courage & Khazaka, Germany) on shaved back skin in ft/ft and +/+ mice (n = 4–6 in all experimental groups, except where noted in figure legends). Transepidermal water loss (TEWL) measurements were taken under basal conditions, using an electrolytic water analyzer (Meeco, Warrington, PA), as well as 2 and 4 hours after a tape stripping-induced, 3-fold increase in TEWL. Barrier recovery was calculated as percent barrier recovery from ‘0’ levels, immediately after acute disruption 30–37.
Irritant and acute allergic contact dermatitis were induced in ft/ft and +/+ mice using TPA and Ox, respectively, as described previously 38. TPA (either 0.3% or 0.1% in acetone) was applied once to the inner and outer surface of the ear. Challenge doses of Ox (either 0.02% or 0.5% in acetone) were applied once following initial Ox sensitization (2% Ox). In all cases, ear thickness was measured 2 hrs post-treatment.
To induce an AD-like dermatosis in mice, ft/ft and +/+ mice were sensitized with 2% Ox, as above. One week later, shaved areas on the flanks of ft/ft and +/+ mice were treated every other day topically with 60 µl of 0.5% or 0.02% Ox for an additional 3 weeks (10 challenges). Other groups of ft/ft and +/+ mice were treated with ethanol alone as the vehicle control group (n = 10–12 in each group). At the end of treatments, basal TEWL and stratum corneum hydration were measured again.
Immunohistochemistry
Changes in overall morphology were visualized after hematoxylin and eosin staining of 5 µm paraffin-embedded tissue samples. Both the number of epidermal nucleated cell layers and the density of the inflammatory infiltrate were measured in 20–30 randomly-chosen fields from ft/ft and +/+ mice before and after Ox challenge doses, as above. Immunohistochemical assessment of changes in profilaggrin/flg, CTRH2, and CD3 were performed, as described previously 39. Briefly, 5 µm paraffin sections were incubated with the primary antibodies overnight at 4°C. After washing, sections were incubated with the secondary antibody for 30 min. Staining was detected with ABC-peroxidase kit from Vector Lab. Frozen sections were examined with a Zeiss fluorescence confocal microscope (Jena, Germany), and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). A similar sequence was employed to assess two antimicrobial peptides in 5 µm frozen sections [primary antibodies to mBD3 from Alpha Diagnostics; mouse cathelicidin (CAMP) antibody was a gift from Dr. Richard Gallo (UCSD)].
Ultrastructural Lipase Detection
Ultrastructural cytochemistry of lipase activity has been employed as a content marker for epidermal lamellar bodies, allowing assessment of the efficacy of organelle secretion 40. Briefly, samples were incubated in 5% Tween 85 (1 ml) in 0.2M HEPES buffer (2.5 ml), 2.5% sodium taurocholate (2 ml) and 10% calcium chloride (1 ml) in 25 ml distilled water (pH 7.4). Microwave incubations were carried out twice for 30 sec at 2450 MHz, and the water bath was changed between the pulses, in order to maintain temperatures below 40°C 41. After incubation in the same medium for an additional 30 min at 37°C, all samples were incubated further in 0.1M cacodylate buffer containing 0.2% Tween 85 with our without 200 µm of the lipase inhibitor, tetrahydrolipstatin, as we have described 42.
Tracer Penetration
To assess the outward permeation of water-soluble markers, freshly-obtained explants of back skin from untreated ft/ft and +/+ mice were exposed dermis-side-downward (30 min-2 hrs) to the low-molecular weight tracer, colloidal lanthanum nitrate (4%) in Karnovsky’s solution. To assess permeation from the outside, we placed 3 +/+ control and 3 ft/ft mice in Petri dishes, while under general anesthesia, with one side of each mouse immersed in 4% colloidal lanthanum, in a 37°C incubator for 2 hr. Lanthanum nitrate was prepared in 4% w/v sucrose in 0.05 M Tris buffer, pH 7.5. After immersion, biopsy samples were taken and processed as below. In parallel studies, a 20 µM solution of calcium green 5N (Invitrogen) in nanopure water was applied on the stratum corneum side of ft/ft and wt mice for 10 and 75 min. Ten mm biopsies were mounted immediately on microscope coverslips, washed in nanopure water, and imaged in a Zeiss 510 meta NLO (Zeiss Germany) at the Live Cell Analysis Core Facility at UCSF. An optically-pumped (Coherent) mode-locked Mira 900 Ti:Saph laser (Coherent), was used as the two-photon excitation source at a wavelength of 800 nm. A 500–550 nm band pass filter was placed in the detection path in order to minimize sample autofluorescence. Under these experimental conditions, we estimated the contribution of autofluorescence to the total signal to be <5%. Z-stacks of 235×235×20 µm3 images were acquired, beginning with the outer surface of the stratum corneum at z increments of 1 µm using a 40× oil immersion objective with a 1.3 numerical aperture (Zeiss, Germany). Matlab software (The Math Works) was used to quantitate image intensity at each z-stack, and to calculate mean penetration depths.
Electron Microscopy
Aldehyde-fixed biopsies were post-fixed with either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide, containing 1.5% potassium ferrocyanide, as described previously 43. Ultrathin sections were examined using an electron microscope (Zeiss 10A, Carl Zeiss, Thornwood, NY) operated at 60 kV. Images were captured using Digital Micrograph 3.10.0 software from Gatan, Inc. (Pleasanton, CA).
Serum IgE
Blood samples were collected from ft/ft and wt mice under basal conditions, and after prior Ox sensitization, followed by repeated treatments with either Ox or vehicle. Serum IgE concentrations were determined with a mouse IgE ELISA quantitation kit from Bethyl Laboratories (Montgomery, TX), following instructions provided by the manufacturer.
In these, and all other studies, statistical analyses were performed using a Student’s t test to compare differences between two groups, with a further ANOVA analysis when three or more groups were compared.
RESULTS
Filaggrin Deficiency Results in Abnormal Barrier Function
Prior studies have shown that ft/ft mice display markedly-reduced F-type keratohyalin granules, with an absence of flg 24, due to a recently-identified, frame-shift mutation in profilaggrin 24. Using a consensus antibody that recognizes both profilaggrin and flg, we found intense, positive immunostaining restricted to the stratum granulosum (granular layer), with no residual staining of flg in the stratum corneum in ft/ft mice (suppl Fig. 1, green staining). In contrast, +/+ epidermis displayed immunostaining in the granular layer, as well as diffuse staining of the stratum corneum. Thus, the mutation in profilaggrin in ft/ft mice results in reduced flg in the SC, analogous to some humans with FLG-associated AD 6, 28.
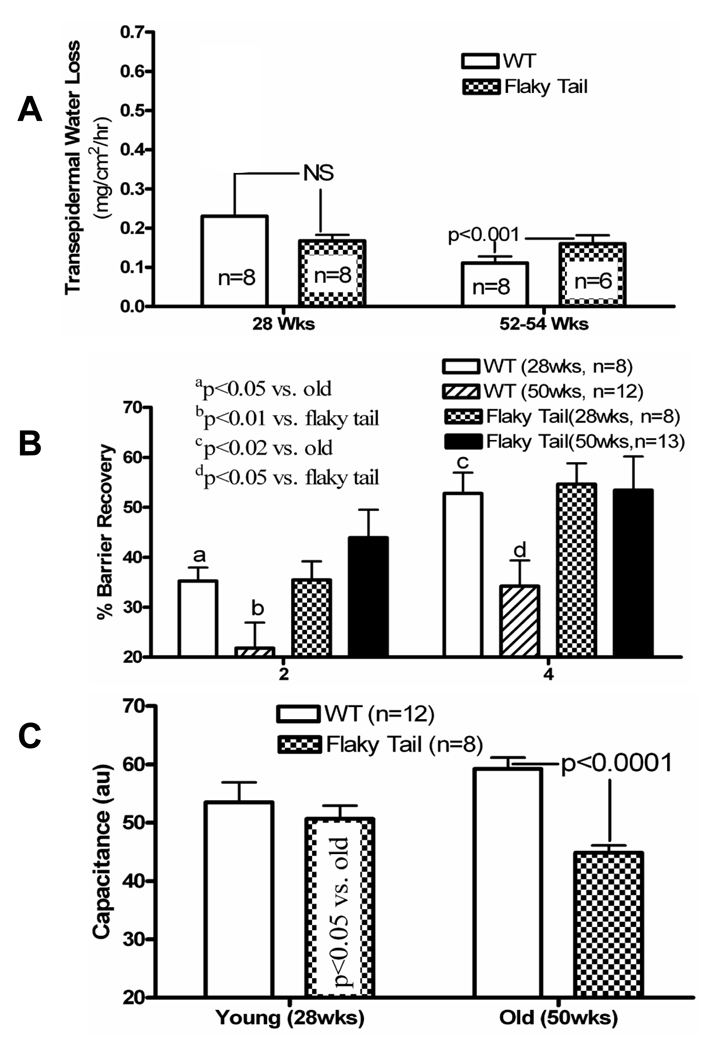
We next assessed epidermal functional parameters in ft/ft and wild-type (+/+) mice, using biophysical instrumentation. Permeability barrier homeostasis, assessed as the kinetics of transepidermal water loss (TEWL) levels after acute disruption 44, but basal barrier function declines with aging in +/+ aged (52–54 wk) mice, as previously described 44 (Fig. 1A). Though within the range of normal (≤ 2.5 mg/cm2/hr), basal TEWL levels increase significantly (by ≈20–30%) in 52–54 week old ft/ft mice compared to age-matched +/+ controls (Fig. 1A). As occurs in human AD [e.g., 30], the kinetics of barrier recovery after acute barrier abrogation accelerate in old ft/ft vs. +/+ mice (Fig. 1B). Such accelerated barrier recovery also occurs in other chronic inflammatory dermatoses, and is attributed to ongoing signaling of repair mechanisms, which are induced by barrier abrogation [e.g., 31, 32]. Finally, stratum corneum hydration, assessed as changes in electrical capacitance, does not differ in young ft/ft vs. +/+ mice (Fig. 1C), but stratum corneum hydration is significantly lower in young ft/ft mice, with a further decline in older ft/ft mice (Fig. 1C), again mirroring known changes in human AD 33, 45.
Figure 1. Abnormal Barrier Function and Stratum Corneum Hydration in Flaky Tail Mice.
A: Basal transepidermal water loss (TEWL) was assessed with an electrolytic analyzer as mg/cm2/hr, and expressed as (mean) ± SEM. B: Barrier disruption was achieved with repeated tape stripping until TEWL levels ≥ 3× normal; then % recovery from an initial ‘0’ value, was measured 2 and 4 hrs after stripping, and expressed as mean +/−SEM. C: Stratum corneum hydration was measured as electrical capacitance on shaved back skin in flaky tail and wild-type mice, and shown as changes in arbitrary units (AU) +/− SEM (n=8–13, as indicated).
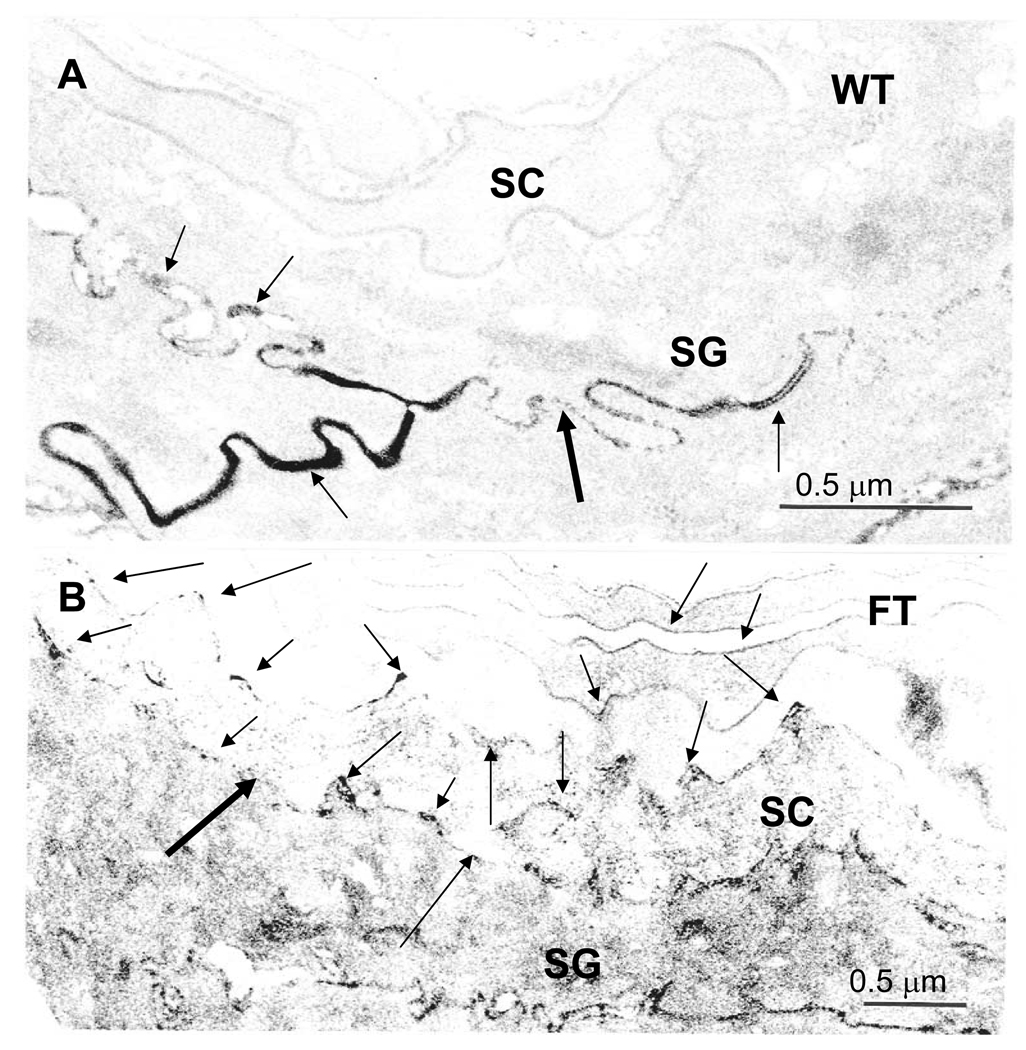
Since basal TEWL levels lie within the normal range in ft/ft mice, we next assessed barrier function in these mice under basal conditions by an alternate approach (i.e., ultrastructural visualization of tracer perfusion). The high resolution of this method also allowed us to determine simultaneously whether the barrier abnormality can be attributed to a defective corneocyte or to enhanced paracellular transport. In +/+ mice, the water-soluble, low molecular weight, electron-dense tracer, lanthanum nitrate, does not breach the stratum granulosum-stratum corneum interface (Fig. 2A). In contrast, tracer not only breaches this layer, but also extends several layers outward into the stratum corneum in ft/ft mice (Fig. 2B). Notably, despite the intracellular localization of flg, the tracer remains largely confined to the stratum corneum extracellular spaces in ft/ft mice (Fig. 2B, arrows). Together, these results demonstrate that flg-deficiency leads to alterations in basal barrier function via a defect in the stratum corneum extracellular matrix.
Figure 2. Abnormal Paracellular Permeability Barrier to a Subcutaneous, Water-Soluble Tracer in Flaky Tail Mice.
Explants of back skin from sex-matched, 12 wk old, wild-type (WT) and flaky tail (FT) mice (n = 3 each) were immersed dermis-side-downward on a solution containing 4% colloidal lanthanum nitrate (pH 7.5) for 30 min to 2 hr, followed by fixation in Karnovsky’s solution (see Methods). Colloidal lanthanum travels outward through the extracellular spaces, but does not reach the stratum corneum (SC) in WT skin (A, arrows). In contrast, in FT skin, lanthanum tracer extends into the lower SC, primarily via the extracellular spaces (B, arrows), suggesting impaired ‘inside-to-outside’ barrier function. SG = stratum granulosum; A+B, osmium tetroxide post-fixation; Mag bars = 0.5 µm.
Enhanced Uptake of Epicutaneously-Applied, Water-Soluble Tracers via the Extracellular Route
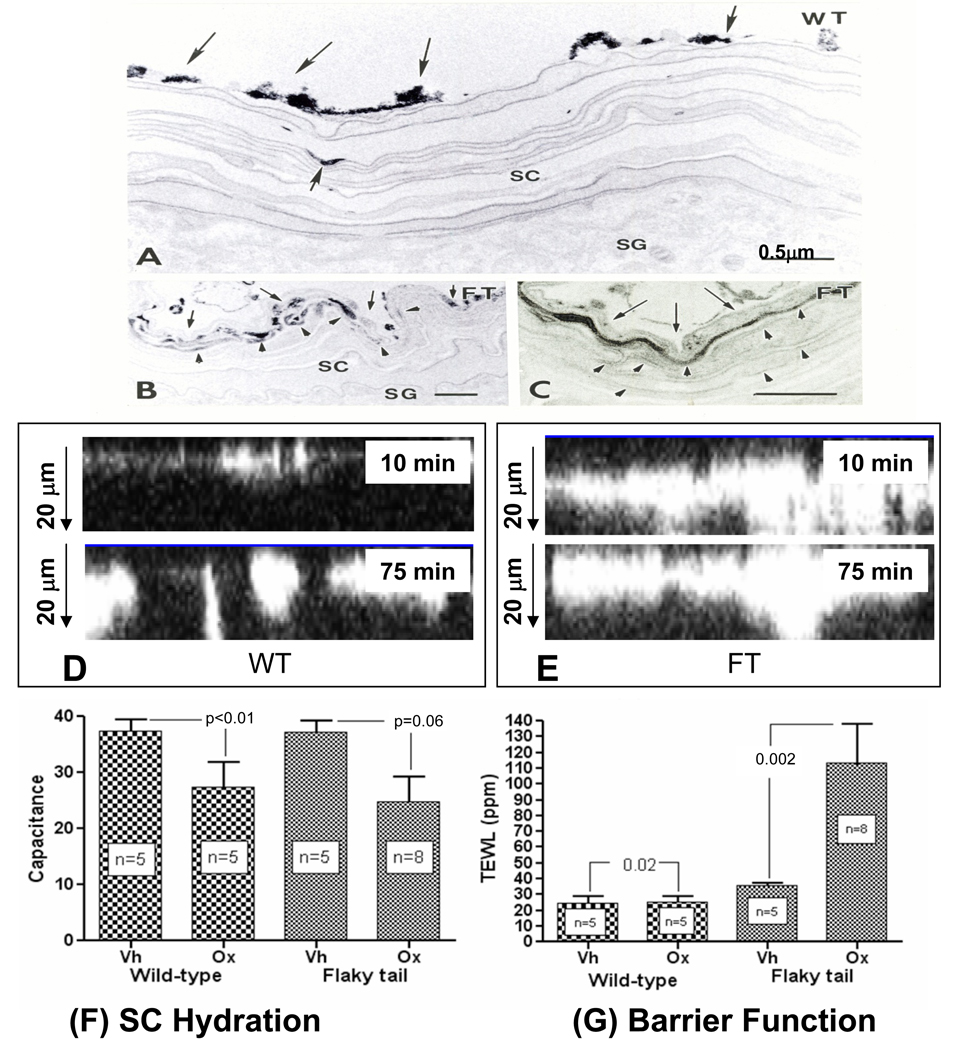
Normal stratum corneum does not allow entry of water-soluble xenobiotes 46. Therefore, we next determined whether epicutaneously-applied, water-soluble tracers enter the stratum corneum of flg-deficient mice, and by which route. Lanthanum nitrate tracer was applied topically to the flanks of ft/ft and +/+ mice for 30 min to two hrs, while under general anesthesia. As shown previously 46, lanthanum fails to breach the stratum corneum of +/+ mice (Fig. 3A). In contrast, tracer permeates 3–5 layers deep into the stratum corneum of ft/ft mice by 2 hrs (Fig. 3B&C). Notably, the tracer localizes solely within the extracellular spaces, indicating again that penetration occurs via the paracellular route (Fig. 3C, arrows). In parallel studies, we visualized the permeation of the low-molecular weight, water-soluble fluorophore, calcium green, by dual photon, confocal microscopy. Differences in the extent of penetration can be seen as early as 10 min after application of tracer to ft/ft skin, with further penetration evident by 75 min in ft/ft mice (Fig. 3D&E). In contrast, very little of the fluorophore can be detected beneath the outer stratum corneum of +/+ mice at 75 min. Together, these results demonstrate that the stratum corneum of flg-deficient mice is more permeable to water-soluble tracers, and that penetration of an electron-dense, water-soluble tracer occurs via the same paracellular pathway that is utilized by water itself, exiting the skin.
Figure 3. Epicutaneous Tracer Penetrates Flaky Tail Stratum Corneum via the Extracellular Spaces.
A–C: Flanks of flaky tail (FT) and wild-type (WT) mice (n = 3 each) were immersed in 4% lanthanum nitrate solution for 30 min to 2 hrs, followed by aldehyde fixation. After 2 hrs, little or no tracer entered the stratum corneum (SC) of WT mice (A, single small arrow). In contrast, abundant tracer reaches ≥4 layers into the SC in FT mice (B, arrowheads), which on higher magnification [C] can be seen to localize to extracellular domains (C, arrowheads). D+E: Calcium green was applied to freshly-obtained explants from ft/ft and wt mice (n = 3 each), and penetration was assessed by dual-photon confocal microscopy, along the ‘z’ axis after 10 and 75 min. Note much deeper ‘outside-to-inside’ penetration of calcium green in ft/ft mice at both time points. Flaky tail (FT) mice, repeatedly-challenged with low-dose Ox, display a more severe barrier abnormality (G), but no further decline in stratum corneum (SC) hydration in comparison to wild-type (WT) mice (F). A–C: Osmium tetroxide post-fixation; mag bar = 0.5 µm.
Structural Basis for Altered Permeability in Flaky Tail Mice
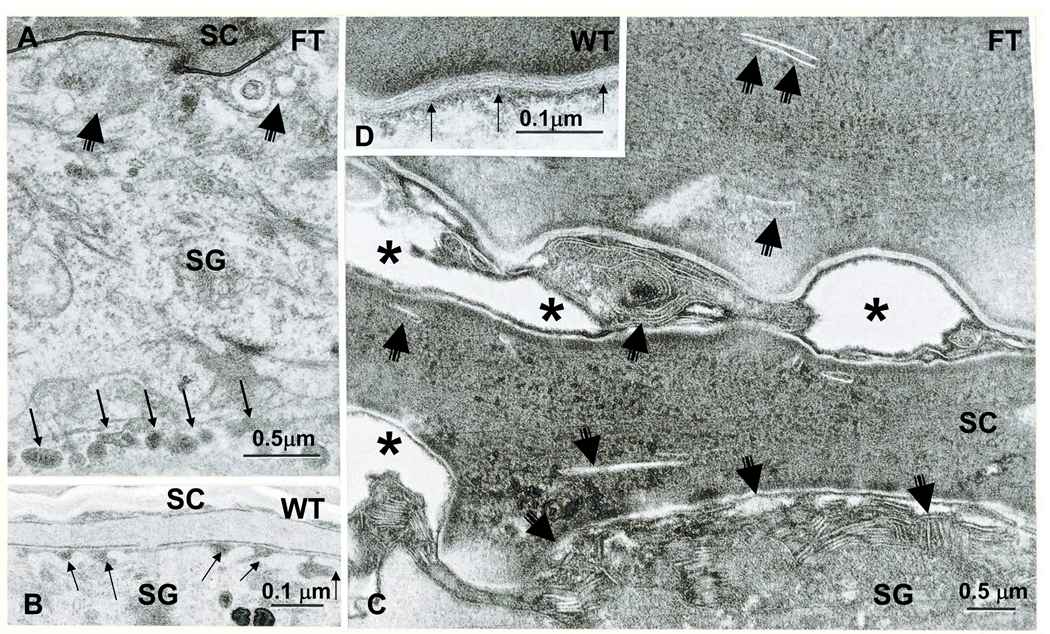
We next assessed the structural basis for the extracellular barrier abnormality in ft/ft mice. By standard transmission electron microscopy, ft/ft mice display not only the previouslyreported paucity of F-type keratohyalin granules in the stratum granulosum [cited in 19], but also a previously-undescribed abnormality in lamellar body secretion (Fig. 4). Lamellar body formation appears normal (neither lamellar body density nor organelle contents differ in ft/ft vs. +/+ mice). Yet, substantial numbers of lamellar bodies appear to be retained within terminally-differentiating keratinocytes in ft/ft mice (Fig. 4A vs. B). These unsecreted organelles then become entombed within the cytosol of nascent corneocytes, instead of being secreted in toto into the extracellular matrix, as occurs in +/+ mice (Fig. 4C vs. D). Partial entombment (failed secretion) of lamellar bodies could also be demonstrated by an alternate method: identification of the fate of a lamellar body content marker (acid lipase) by ultrastructural cytochemistry 32, 40. In +/+ epidermis, enzyme activity localizes both to lamellar body contents (suppl Fig. 2A), and within the stratum corneum, solely to the extracellular domains (suppl Fig. 2B). Flaky tail mice reveal a similar localization of enzyme activity to lamellar bodies, but evidence of reduced secretion; i.e., lower amounts of initially-secreted enzyme at the stratum granulosum-stratum corneum interface, as well as sparse (reduced) enzyme product in the stratum corneum interstices (suppl Fig. 2C, arrows). In contrast to +/+ mice, the cytosol of many ft/ft corneocytes contains abundant enzyme activity, with enzyme activity localizing near retained lamellar arrays (suppl Fig. 2D), further evidence for entombment of lamellar body contents.
Figure 4. Abnormalities in the Lamellar Body Secretory System in Young Flaky Tail Mice.
Partial failure of lamellar body exocytosis is evident in flaky tail (FT) epidermis (A, C). Note lamellar bodies lined up in peripheral cytosol (A, multiple thin arrows); decreased secreted material at stratum granulosum (SG)-stratum corneum (SC) interface (A&C, short, fat arrows); decreased numbers of lamellar bilayers (C): delayed maturation of lamellar bilayers (C); and entombed lamellar body contents within the corneocyte cytosol (C, short, thin arrows). B&D: Normal lamellar body secretion (B, arrows) and extracellular lamellar bilayers in wild-type (WT) epidermis (D, thin arrows) A&B, osmium tetroxide post-fixation; C&D, ruthenium tetroxide post-fixation. Mag bars = A&C, 0.5 µm; B&D, 0.1 µm.
We next assessed the consequences of impaired lamellar body secretion and enhanced paracellular transport in ft/ft mice. As a result of impaired lamellar body secretion, the quantities of extracellular lamellar bilayers correspondingly decline in the stratum corneum of ft/ft mice (Fig. 4C vs. D). Moreover, the extracellular processing of newly-secreted lamellar body contents into ‘mature’ lamellar bilayers appears to be impaired or delayed in ft/ft mice (Fig. 4C vs. D). Together, these results suggest that either profilaggrin accumulation or flg deficiency impedes lamellar body secretion, leading to an abnormal stratum corneum extracellular matrix in ft/ft mice.
Filaggrin Deficiency Alters Thresholds for Irritant and Acute Allergic Contact Dermatitis
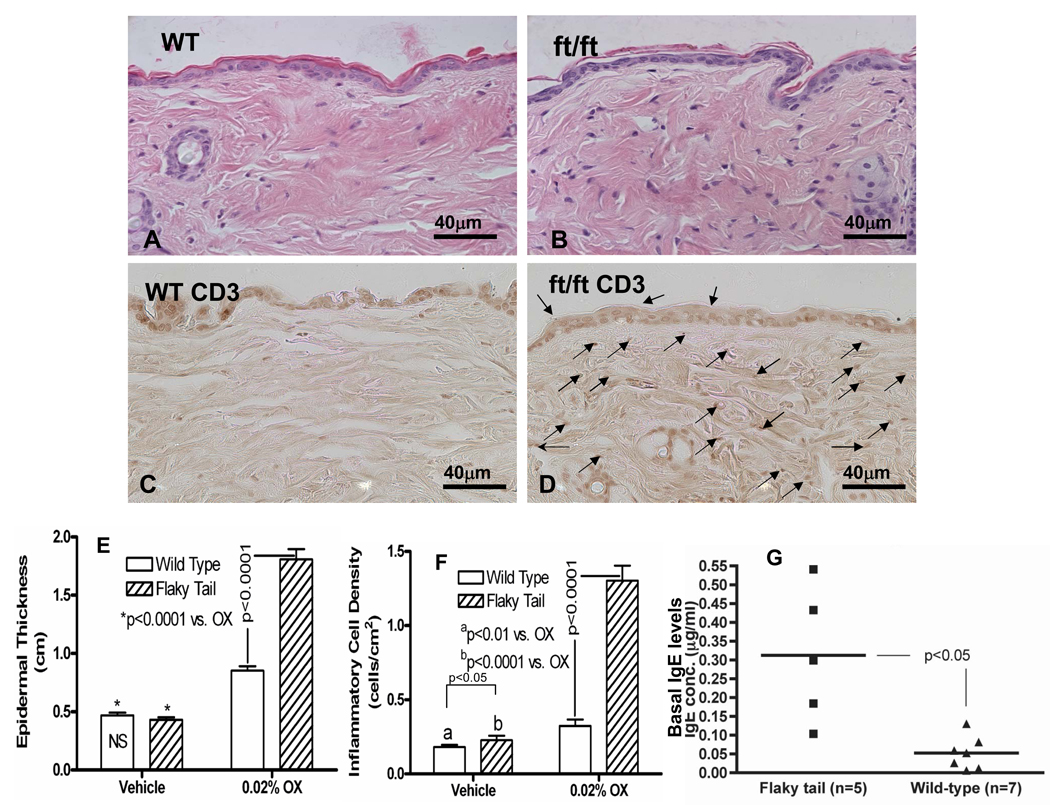
We next assessed whether the barrier abnormality in ft/ft mutant mice results in an increased propensity to develop cutaneous inflammation. Flaky tail skin displays low-grade inflammation under basal conditions, indicated by the presence of increased numbers of inflammatory cells under basal conditions (Figs. 5B, E&F; histologic images that correspond to these quantitative data are shown in supplementary Fig. 3C vs. A). Moreover, most, but not all ft/ft mice display elevated serum IgE levels, even under basal conditions (Fig. 5G). Together, these results suggest that ft/ft mice display low-grade inflammation, even under basal conditions.
Figure 5. Reduced Threshold for Development of AD-Like Inflammation in Response to Repeated Ox Challenges in Flaky Tail Mice.
Flaky tail (FT) and wild-type (WT) mice were sensitized to Ox, and then repeatedly challenged with a subthreshold concentration of Ox (0.02%) every other day to a shaved area of back skin for a total of 10 challenges. Under basal conditions, FT mice display low-grade inflammation (F, see also suppl. Fig. 3C vs. A). FT skin displayed much more prominent erythema, scaling and excoriations than does WT mouse skin (see text), a change that is associated with a modest inflammatory infiltrate (B vs. A & suppl. Fig. 3C), that was enriched in CD3+ dermal lymphocytes (D vs. C). Quantitative changes in epidermal hyperplasia and inflammation are shown in E&F. G: Serum IgE levels are significantly elevated in most flaky tail mice, even under basal conditions. Mag bars = 40 µm.
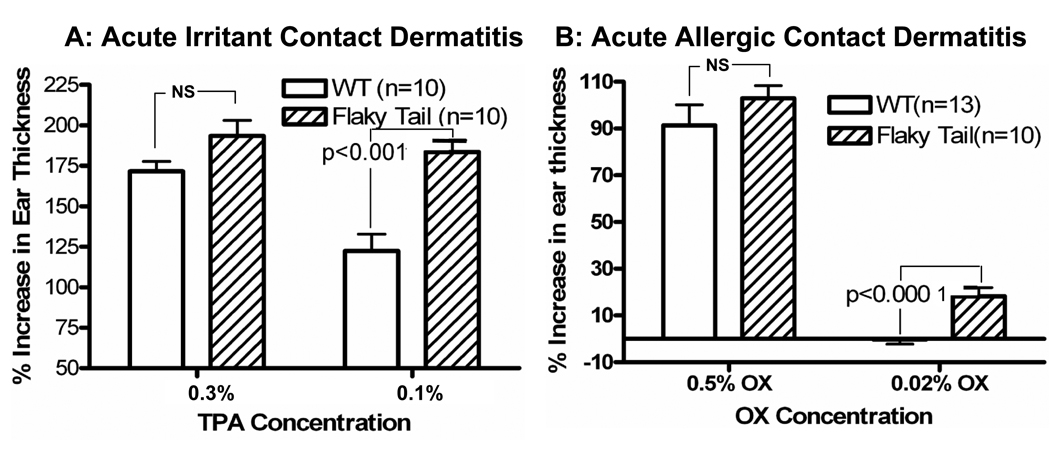
The phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA), elicits irritant contact dermatitis when applied topically to mouse ears, with the severity of the inflammatory response correlating with an increase in ear thickness two (2) hours after topical applications 34, 47. TPA was first applied at a standard concentration of 0.3% to the ears of ft/ft and +/+ mice. Although inflammation appeared greater in ft/ft mice, the differences in ear thickness in ft/ft vs. +/+ mice do not achieve statistical significance (Fig. 6A). However, at a lower dose of TPA (0.1%) that provokes only marginal inflammation in +/+ mice, ft/ft mice display a marked inflammatory response, with a significant increase in ear thickness (Fig. 5A;p <0.001; parallel histologic differences are shown in suppl Fig. 4). Together, these results demonstrate that ft/ft mice display an enhanced propensity to develop irritant contact dermatitis.
Figure 6. Altered Sensitivity to Irritant/Hapten-Induced Allergic Contact Dermatitis in Flaky Tail Mice.
In young, wild-type mice, TPA induces irritant contact dermatitis, while Ox induces allergic contact dermatitis following a single, full-strength challenge dose 38. While higher concentrations produced comparable changes in young wide-type (WT) and flaky tail mice, lower doses of either TPA (A) or Ox (B) induce inflammation only in flaky tail mice. Changes in ear thickness correlate with severity of inflammation (see suppl Fig. 4).
We next assessed whether ft/ft mice display an altered threshold to the development of acute allergic contact dermatitis. For these studies, we utilized a single challenge dose (2%) of the universal hapten, oxazolone (Ox), followed one week later by topical application to the ear of either a single challenge dose at a concentration (0.5%) which produces a prominent dermatitis after 24–48 hrs 47, 48, or a much lower hapten concentration (0.02%), which does not produce visible inflammation in +/+ mice. As with the topical irritant (c.f., Fig. 5A), dermatitis appears more exaggerated in ft/ft mice, after challenges with the standard (0.5%) hapten dose, but again the results do not achieve statistical significance (Fig. 5B). At the lower challenge dose of Ox (0.02%), dermatitis does not develop in +/+ mice, while in contrast, substantial inflammation is evident in similarly-challenged ft/ft mice (Fig. 5B; p<0.0001 vs. +/+; parallel histologic differences are shown in suppl Fig. 4). These results show that ft/ft mice display a reduced threshold for the development of hapten-induced, acute allergic contact dermatitis.
Filaggrin Deficiency Predisposes To the Development of an AD-Like Dermatosis in Mice
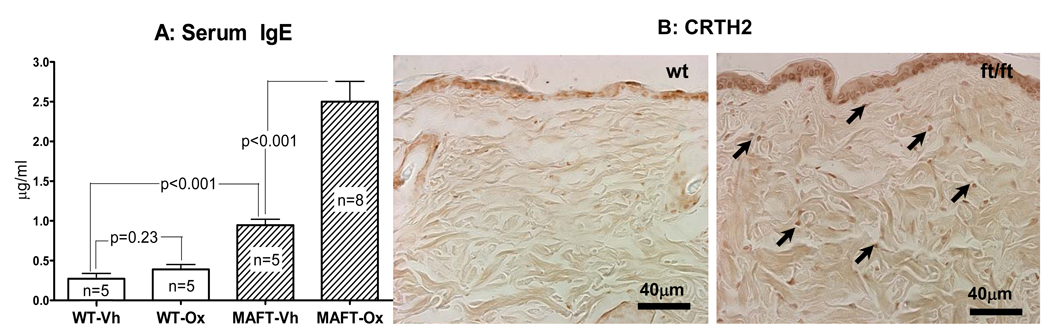
We previously showed that Ox sensitization, followed by repeated (10×) hapten challenges over 20–21 days produces a dermatosis with certain structural, biochemical, and immunologic features of human AD 49. In the next set of studies, we used reduced challenge doses of Ox (10 challenges with 0.02% vs. the standard 0.5% concentration) in ft/ft and +/+ mice. Whereas +/+ mice display minimal signs of inflammation, ft/ft mice reveal erythema, hyperkeratosis, epidermal hyperplasia, and histologically-prominent inflammation at the lower hapten concentration (Figs. 6E&F; suppl Fig. 3). Moreover, while virtually no CD3+ lymphocytes are detected in +/+ mouse skin, a moderately-high density of CD3+ cells can be seen in ft/ft mice repeatedly challenged with low-dose Ox (Fig. 6D). Flaky tail mice also display numerous prostaglandin D2 receptor (CRTH2)-positive cells, consistent with a th2-dominant immunophenotype, as well as much higher serum IGE levels than do comparably-treated +/+ mice (Figs. 7A&B). Finally, ft/ft mice, repeatedly challenged with low-dose Ox, display reduced immunostaining for mBD3 (mouse homologue of hBD2 [suppl Fig. 5D]), but staining for cathelicidin antimicrobial peptide (CAMP = mouse homologue of LL37) instead appears to increase (suppl Fig. 5C). Together, these results indicate that flg deficiency predisposes to development of a hapten-stimulated AD-like dermatosis in mice.
Figure 7. Flaky Tail Mice Demonstrate Increased Th2 Response to Repeated Low-Dose Ox Challenges.
Serum was collected from young flaky tail (FT) and wild-type (WT) mice before and after Ox sensitization, and either ten low-dose Ox (0.02%) or vehicle treatments, followed by assessment of IgE levels by ELISA. While no difference from baseline was seen between vehicle and Ox-treated WT mice, a large increase was observed in FT mice (B). Likewise, low-dose Ox-challenged FT mice demonstrate increased staining for CRTH2-bearing cells (A, arrows) in Ox-treated, FT mice. Mag bars = 40 µm.
More Severe Barrier Abnormality in Filaggrin-Deficient Mice with AD
In normal mice, repeated challenges with 0.5% Ox result in a progressively more-severe barrier abnormality 49, consistent with a proposed ‘outside-inside-outside’ pathogenic cycle in AD 1, 2. We next assessed whether repeated, sub-threshold (0.02%) doses of Ox would provoke functional abnormalities in ft/ft mice that occur only at higher doses in +/+ mice. While stratum corneum hydration does not decline in such Ox-challenged mice (Fig. 3F), ft/ft mice develop a severe permeability barrier abnormality at sub-threshold doses of hapten (Fig. 3G), evidenced by an acceleration of TEWL levels to levels produced by full-challenge hapten doses in +/+ or normal mice (c.f., 49). These results show that development of an AD-like dermatosis in flg-deficient mice is paralleled by a further exacerbation of the permeability barrier abnormality.
DISCUSSION
Human atopic dermatitis (AD) is increasingly viewed as a primary barrier disorder, based upon recent data from multiple populations that have demonstrated an association with mutations in FLG, the gene encoding the stratum corneum structural protein, filaggrin (FLG). Yet, while these molecular genetic studies have shifted views of AD pathogenesis towards a new ‘outside-to-inside’ pathogenic paradigm 1, 2, they leave unanswered mechanistic questions of how FLG-deficiency leads not only to a barrier abnormality, but also to an inflammatory phenotype in AD. Because such questions are difficult to address in the complex, multifactorial environment of human disease, we addressed these issues in an animal model of flg deficiency, the flaky tail (ft/ft) mouse. Recently, these mice were shown to carry a frameshift mutation with one base pair deletion (5303delA) in exon3 of flg 24, which mimics two common, distal mutations in human AD (i.e., R2447X in repeat 7 and 1033del4 in repeat 10) 6, 28.
In our examination of ft/ft mice, we first confirm that flg deficiency alone suffices to induce a barrier abnormality and low-grade inflammation under basal conditions, as shown recently for ichthyosis vulgaris 50. We also provide subcellular pathogenic insights, showing that despite its intracellular localization, flg deficiency results in enhanced paracellular permeability to both intradermally and epicutaneously applied water-soluble tracers, which do not penetrate +/+ stratum corneum. Movement of these low-molecular weight, water-soluble tracers through the stratum corneum interstices likely reflects the pathway for hapten ingress in AD, since similarly-sized, water-soluble antigens, such as nickel, more frequently induce allergic contact dermatitis in humans with AD 51. However, since our ft/ft mice were not on a homogenous C578BL6 background, but also express a hair phenotype (matted), we cannot eliminate the possibility that some of our observations could have been influenced by the concurrent matted mutation. Nevertheless, the recent work by Fallon, et al 24 shows enhanced antigen ingress in mice with the same flg mutation, but no matted in their background. Thus, flg deficiency alone likely explains the barrier abnormality that we demonstrate here in flaky tail mice.
Localizing the barrier defect in FLG deficiency to the extracellular matrix is an important first step in understanding AD pathogenesis. Yet, a key question remains unanswered: how can a defect in an intracellular protein, such as FLG, yield a more-porous extracellular pathway? FLG has two putative functions: 1) mediation of stratum corneum hydration through humidity-dependent proteolysis of FLG into its constituent amino acids and their deiminated products (e.g., histidine → trans-urocanic acid)18, 52; and 2) keratin intermediate filament compaction within the corneocyte cytosol 19, 53. Examination of either of these roles does not immediately suggest a mechanism by which FLG deficiency could lead to increased extracellular permeability. Potential clues are provided by other inherited defects of intracellular corneocyte proteins that also provoke increased paracellular permeability, but by divergent mechanisms 54. In one example, in K1/10-deficient epidermolytic hyperkeratosis, dominant-negative pairing of the mutant protein disrupts the cytoskeleton, impairing lamellar body secretion 32. In a second, alternate mechanism, both loricrin keratoderma and transglutaminase 1-deficient lamellar ichthyosis yield an abnormal corneocyte scaffold that disrupts the supramolecular organization of the extracellular lamellar bilayers 36, 37. A third mechanism, mutations in the V1 subdomain of K1 that produce palmar plantar keratoderma, provokes detachment of keratin filaments from the cornified envelope 55. This defect could link an abnormally-collapsed corneocyte to an extracellular defect, yet frozen sections of flg-deficient stratum corneum display corneocytes of normal shape and dimensions (Elias, unpublished observations). Our ultrastructural studies on ft/ft mice show a partial failure of lamellar body secretion, as shown previously for non-genotyped human AD 56. Likewise, our preliminary studies in genotyped ichthyosis vulgaris +/− AD patients show a similar blockade in lamellar body secretion 50. Thus, analogous to K1/10-deficient epidermolytic hyperkeratosis 32, unprocessed profilaggrin might impede lamellar body secretion (op. cit.) (suppl. Fig. 2). This mechanism likely also pertains to human AD associated with distal FLG mutations, in which residual profilaggrin expression is seen, but processed FLG is reduced 28. Yet, it is not clear how this mechanism might apply to those cases of AD caused by more proximal FLG mutations, which instead decrease both profilaggrin and FLG. A fourth mechanism, which could apply to both categories of FLG mutations, could be linked to decreased generation of acidic metabolites of FLG 1, 2 suppl. Fig. 6). Deficiency in these polycarboxylic acids could result in a net increase in the pH of the stratum corneum, which in turn could activate neutral-pH-dependent kallikreins, with a variety of negative, downstream consequences for the barrier 1,2. Such a pH-dependent mechanism could also lead to deactivation of two key ceramide-generating enzymes, acidic sphingomyelinase and β-glucocerebrosidase (suppl. Fig. 6)57. This mechanism may not, however, be operative in ft/ft mice, because the surface pH of these mice remains normal, perhaps due to compensatory upregulation of alternate, endogenous acidifying mechanisms [rev. in 58], such as solute carrier family 9 (sodium-hydrogen exchanger, 1 (slc9a) (old symbol NHE1) 59 and/or secretory phospholipase A2 60. Nonetheless, surface pH measurements do not always reflect pH alterations within specific microdomains of the stratum corneum 59. We did not specifically examine such potential, compensatory mechanisms in this study.
While our studies demonstrate the increased movement of topically-applied, water soluble compounds through the stratum corneum in ft/ft mice, the movement of water from inside to outside through the stratum corneum (TEWL) is not markedly altered in young ft/ft mice, becoming only slightly elevated in older ft/ft mice in comparison to older +/+ mice. The development of a barrier abnormality with aging of ft/ft mice could reflect the added stress from a steeper water gradient across the SC of older animals reflecting the observed decrease in SC hydration (Fig. 1C). Alternatively or additionally, it could reflect age-related differences in permeability barrier homeostasis 44, 61. While the explanation for the discordance in TEWL rates vs. xenobiote penetration is not clear, it could reflect differences in the thresholds for the barriers to water loss vs. xenobiote ingress/egress, respectively. Pertinently, to increase TEWL levels by tape stripping, multiple layers of the stratum corneum must be removed 62. Thus, there appears to be substantial redundancy, with only a relatively small amount of stratum corneum required to maintain a normal TEWL. Conversely, the structural basis for inhibiting water movement from inside to outside (i.e., TEWL) could differ from the structural basis for inhibiting the egress or ingress of somewhat larger, water-soluble compounds. Since there are several possible mechanisms that could explain how FLG deficiency leads to a marked abnormality in xenobiote permeation, without altering water loss, additional studies will be required to distinguish among these alternatives.
Having established that FLG deficiency results in an extracellular barrier defect, we next addressed a question of key functional significance; namely, does this barrier defect suffice to alter thresholds to the development of inflammation from epicutaneously-applied irritants and haptens? While FLG mutations are associated with the inflammatory disease, AD (op. cit.), the relationship between FLG deficiency and inflammation is obscured by the association of the same FLG mutations with the supposedly noninflammatory disorder of cornification, ichthyosis vulgaris (IV). Additional stressors to the barrier could be required to provoke human AD 1, 2, because human skin displays an inherently more competent barrier 63–65 (see below). Nevertheless, the presumption that IV is non-inflammatory should now be re-examined, in light of our observations, and those of Fallon, et al. 24, which show that flg deficiency alone results in epidermal hyperplasia, low-grade inflammation, and elevated IgE levels in otherwise normal mice.
In order to ascertain whether FLG deficiency alone leads to increased susceptibility to inflammatory dermatoses, we employed previously described methods for eliciting irritant and acute allergic contact dermatitis 38, 47, as well as an AD-like mouse model 49, in ft/ft vs. +/+ mice. Our results show that FLG deficiency alone lowers thresholds to inflammation following epicutaneous applications of either irritants or haptens. Most importantly, we demonstrate development of an AD-like phenotype following applications of reduced concentrations of haptens, which do not produce th2-dominant inflammation in +/+ mice. The AD-like phenotype included increased CRTH2-positive cells, decreased immunostaining for mBD3 (the murine homologue of hBD2), and elevated serum IgE levels. Similarly, antigen-challenged mice, with the same flg mutation as ft/ft mice, also display multiple features of th2 inflammation 24. Interestingly, these flg-deficient mice reportedly did not develop significantly higher IgE levels24, likely reflecting a less severe phenotype due to differences in bioavailability of our hapten vs. the complete antigen employed by Fallon, et al. 24. Finally, the apparent reduction in mBD3 expression could reflect downstream down-regulation by th2 cytokines 16.
Notably, inflammation can be induced in flg-deficient mice, without a requirement for additional, acquired stressors, such as reduced ambient humidity, high pH surfactants, and/or increased psychological stress, conditions that are known to both further compromise barrier function, and to precipitate AD in humans 1–3. This leaves unanswered, however, the question of why many IV patients, including some with double-allele FLG mutations, do not display a concomitant AD phenotype 25, 26. Two alternative or perhaps coincident explanations could explain this apparent paradox. First, it is possible that all patients with FLG mutations have the potential to develop AD, based upon their inherited barrier defect alone, but repeated epicutaneous deposition of allergens, sufficient to provoke TH2-dominant inflammation, might not occur in some IV patients. Second, in mice, unlike humans, development of inflammation might not require the superimposition of additional acquired stressors, because as noted above, mouse skin displays reduced barrier competence in comparison to human skin 63–65. This inherent difference could also explain why hapten-induced, acute allergic contact dermatitis converts to an AD-like phenotype following repeated challenges in mice, a transition that is not known to occur in humans.
In summary, we show here that FLG deficiency, as occurs in many cases of human AD, suffices to provoke a barrier abnormality, which in turn allows enhanced permeation of water-soluble tracers via the paracellular route. The paracellular defect can be attributed to abnormal extracellular lamellar bilayers, resulting from compromised lamellar body secretion. These structural abnormalities allow enhanced irritant/hapten ingress, resulting in reduced inflammatory thresholds, providing a mechanistic link between FLG deficiency and development of AD. Finally, these studies substantiate and explain the new, ‘outside-to-inside’ paradigm of AD pathogenesis.
KEY MESSAGES
Filaggrin deficiency alone provokes a permeability barrier abnormality (no further acquired stressors are necessary).
The barrier abnormality localizes to the extracellular spaces of the stratum corneum, where if facilitates bidirectional, paracellular percutaneous transport of water-soluble xenobiotes.
The paracellular abnormality can be ascribed to impaired secretion of epidermal lamellar bodies.
The barrier abnormality correlates with reduced inflammatory thresholds to topical irritants and haptens.
Repeated, low dose hapten applications that fail to elicit inflammation in wild-type mice, provoke inflammation with certain th2 features in filaggrin-deficient mice, worsening the barrier abnormality, thereby completing an ‘outside-inside-(back to) outside’ pathogenic cycle in murine AD.
Short Summary of Clinical Implications
FLG deficiency (in human AD) provokes a paracellular barrier abnormality, caused by abnormal extracellular lamellar bilayers, resulting from compromised lamellar body secretion. These structural abnormalities reduce inflammatory thresholds to irritants/haptens, providing a mechanistic link between FLG deficiency and development of AD, explaining the new, ‘outside-to-inside’ paradigm of AD pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Ms. Joan Wakefield for superb editorial assistance. These studies were supported by NIH grants AR019098, AG028492, AI059311, and the Medical Research Service, Department of Veterans Affairs.
Abbreviations
- AD
atopic dermatitis
- BD
βdefensin
- CAMP
cathelicidin antimicrobial peptide
- flg (mouse) or FLG (human)
filaggrin
- ft/ft
flaky tail
- Krt
keratin
- Ox
oxazolone
- TEWL
transepidermal water loss
- TM
targeted mutant
- TPA
12-O-tetradecanoylphorbol-13-acetate
- +/+
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias PM, Steinhoff M. "Outside-to-inside" (and now back to "outside") pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene-environment interactions. J Allergy Clin Immunol. 2006;118:3–21. doi: 10.1016/j.jaci.2006.04.042. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 4.Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WH, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682–689. doi: 10.1038/jid.2008.280. [DOI] [PubMed] [Google Scholar]
- 5.Boguniewicz M, Leung DY. 10. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–S480. doi: 10.1016/j.jaci.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 7.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006;126:1200–1202. doi: 10.1038/sj.jid.5700365. [DOI] [PubMed] [Google Scholar]
- 9.Weidinger S, Rodriguez E, Stahl C, Wagenpfeil S, Klopp N, Illig T, et al. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol. 2007;127:724–726. doi: 10.1038/sj.jid.5700630. [DOI] [PubMed] [Google Scholar]
- 10.Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–1284. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- 11.Brown SJ, Sandilands A, Zhao Y, Liao H, Relton CL, Meggitt SJ, et al. Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol. 2008;128:1591–1594. doi: 10.1038/sj.jid.5701206. [DOI] [PubMed] [Google Scholar]
- 12.Jung T, Stingl G. Atopic dermatitis: therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008;122:1074–1081. doi: 10.1016/j.jaci.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006;38:399–400. doi: 10.1038/ng0406-399. [DOI] [PubMed] [Google Scholar]
- 14.Scharschmidt TC, Segre JA. Modeling atopic dermatitis with increasingly complex mouse models. J Invest Dermatol. 2008;128:1061–1064. doi: 10.1038/sj.jid.5701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 16.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 17.Elias P, Schmuth M. Abnormal skin barrier in the etio-pathogenesis of atopic dermatitis. Curr Allergy Asthma Rep. 2009 doi: 10.1007/s11882-009-0037-y. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol. 1986;115:84–92. doi: 10.1016/0012-1606(86)90230-7. [DOI] [PubMed] [Google Scholar]
- 19.Presland R, Rothnagal J, Lawrence O. Profilaggrin and the fused S100 family of calcium-binding proteins. In: Elias PM, Feingold K, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 111–140. [Google Scholar]
- 20.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 21.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 22.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic Review Series: Skin Lipids.Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandilands A, Terron-Kwiatkowski A, Hull PR, O'Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 26.Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 27.Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–1081. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 28.Gruber R, Terron-Kwiatkowski A, Sandilands A, Utermann G, Fritsch P, Janecke A, et al. Proximal versus distal filaggrin mutations in ichthyosis vulgaris. J Invest Dermatol. 2007;127:S87. [Google Scholar]
- 29.Sundberg J. The matted (ma) mutation, chromosome 3.Handbook of mouse mutations with skin and hair abnormalities. Animal models and biomedical tools. 1994:345–349. [Google Scholar]
- 30.Seidenari S, Giusti G. Objective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm Venereol. 1995;75:429–433. doi: 10.2340/0001555575429433. [DOI] [PubMed] [Google Scholar]
- 31.Demerjian M, Crumrine DA, Milstone LM, Williams ML, Elias PM. Barrier dysfunction and pathogenesis of neutral lipid storage disease with ichthyosis (Chanarin-Dorfman syndrome) J Invest Dermatol. 2006;126:2032–2038. doi: 10.1038/sj.jid.5700332. [DOI] [PubMed] [Google Scholar]
- 32.Schmuth M, Yosipovitch G, Williams ML, Weber F, Hintner H, Ortiz-Urda S, et al. Pathogenesis of the permeability barrier abnormality in epidermolytic hyperkeratosis. J Invest Dermatol. 2001;117:837–847. doi: 10.1046/j.0022-202x.2001.01471.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol. 2003;139:1417–1422. doi: 10.1001/archderm.139.11.1417. [DOI] [PubMed] [Google Scholar]
- 34.Man MQ, Barish GD, Schmuth M, Crumrine D, Barak Y, Chang S, et al. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J Invest Dermatol. 2008;128:370–377. doi: 10.1038/sj.jid.5701026. [DOI] [PubMed] [Google Scholar]
- 35.Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, et al. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis) J Invest Dermatol. 2004;122:909–922. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- 37.Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, et al. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11:248–256. doi: 10.1034/j.1600-0625.2001.110308.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheu MY, Fowler AJ, Kao J, Schmuth M, Schoonjans K, Auwerx J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- 39.Demerjian M, Man MQ, Choi EH, Brown BE, Crumrine D, Chang S, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15:154–160. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 40.Menon GK, Ghadially R, Williams ML, Elias PM. Lamellar bodies as delivery systems of hydrolytic enzymes: implications for normal and abnormal desquamation. Br J Dermatol. 1992;126:337–345. doi: 10.1111/j.1365-2133.1992.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 41.Rassner UA, Crumrine DA, Nau P, Elias PM. Microwave incubation improves lipolytic enzyme preservation for ultrastructural cytochemistry. Histochem J. 1997;29:387–392. doi: 10.1023/a:1026438917856. [DOI] [PubMed] [Google Scholar]
- 42.Hochuli E, Kupfer E, Maurer R, Meister W, Mercadal Y, Schmidt K. Lipstatin an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini.II.Chemistry and structure elucidation. J Antibiot (Tokyo) 1987;40:1086–1091. doi: 10.7164/antibiotics.40.1086. [DOI] [PubMed] [Google Scholar]
- 43.Hou SY, Mitra AK, White SH, Menon GK, Ghadially R, Elias PM. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991;96:215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- 44.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest. 1995;95:2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fluhr JW, Kuss O, Diepgen T, Lazzerini S, Pelosi A, Gloor M, et al. Testing for irritation with a multifactorial approach: comparison of eight non-invasive measuring techniques on five different irritation types. Br J Dermatol. 2001;145:696–703. doi: 10.1046/j.1365-2133.2001.04431.x. [DOI] [PubMed] [Google Scholar]
- 46.Menon GK, Elias PM. Morphologic basis for a pore-pathway in mammalian stratum corneum. Skin Pharmacol. 1997;10:235–246. doi: 10.1159/000211511. [DOI] [PubMed] [Google Scholar]
- 47.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheu HM, Lee JY, Kuo KW, Tsai JC. Permeability barrier abnormality of hairless mouse epidermis after topical corticosteroid: characterization of stratum corneum lipids by ruthenium tetroxide staining and high-performance thin-layer chromatography. J Dermatol. 1998;25:281–289. doi: 10.1111/j.1346-8138.1998.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 49.Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmuth M, Crumrine D, Presland RB, Fleckman P, Fritsch PO, Elias PM. Basis for the epidermal functional abnormalities in granular layer-absent (AGL) vs-present (PGL) ichthyosis vulgaris. J Invest Dermatol. 2005;124:A72. [Google Scholar]
- 51.Novak N, Baurecht H, Schafer T, Rodriguez E, Wagenpfeil S, Klopp N, et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol. 2008;128:1430–1435. doi: 10.1038/sj.jid.5701190. [DOI] [PubMed] [Google Scholar]
- 52.Rawlings AV, Scott IR, Harding CR, Bowser PA. Stratum corneum moisturization at the molecular level. J Invest Dermatol. 1994;103:731–741. doi: 10.1111/1523-1747.ep12398620. [DOI] [PubMed] [Google Scholar]
- 53.Dale BA, Presland RB, Lewis SP, Underwood RA, Fleckman P. Transient expression of epidermal filaggrin in cultured cells causes collapse of intermediate filament networks with alteration of cell shape and nuclear integrity. J Invest Dermatol. 1997;108:179–187. doi: 10.1111/1523-1747.ep12334205. [DOI] [PubMed] [Google Scholar]
- 54.Schmuth M, Gruber R, PM E, Williams M. Ichthyosis update: towards a function-driven model of pathogenesis of the disorders of cornification and the role of corneocyte proteins in these disorders. Adv Dermatol. 2007;23:231–256. doi: 10.1016/j.yadr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Candi E, Tarcsa E, Digiovanna JJ, Compton JG, Elias PM, Marekov LN, et al. A highly conserved lysine residue on the head domain of type II keratins is essential for the attachment of keratin intermediate filaments to the cornified cell envelope through isopeptide crosslinking by transglutaminases. Proc Natl Acad Sci U S A. 1998;95:2067–2072. doi: 10.1073/pnas.95.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fartasch M, Bassukas ID, Diepgen TL. Disturbed extruding mechanism of lamellar bodies in dry non-eczematous skin of atopics. Br J Dermatol. 1992;127:221–227. doi: 10.1111/j.1365-2133.1992.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 57.Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 58.Elias PM. Physiological lipids for barrier repair in dermatology. In: Draelos Z, editor. Cosmeceuticals. 2005. [Google Scholar]
- 59.Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002;277:47399–47406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- 60.Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 61.Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847–2856. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]
- 62.Gunathilake R, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, et al. pH-Regulated Mechanisms Account for Pigment-Type Differences in Epidermal Barrier Function. J Invest Dermatol. 2009 doi: 10.1038/jid.2008.442. Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bond JR, Barry BW. Hairless mouse skin is limited as a model for assessing the effects of penetration enhancers in human skin. J Invest Dermatol. 1988;90:810–813. doi: 10.1111/1523-1747.ep12462031. [DOI] [PubMed] [Google Scholar]
- 64.Bond JR, Barry BW. Limitations of hairless mouse skin as a model for in vitro permeation studies through human skin: hydration damage. J Invest Dermatol. 1988;90:486–489. doi: 10.1111/1523-1747.ep12460958. [DOI] [PubMed] [Google Scholar]
- 65.Hinz RS, Hodson CD, Lorence CR, Guy RH. In vitro percutaneous penetration: evaluation of the utility of hairless mouse skin. J Invest Dermatol. 1989;93:87–91. doi: 10.1111/1523-1747.ep12277361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.