Abstract
The modifier of deaf waddler (mdfw) and age-related hearing loss (Ahl) loci were both discovered as inbred strain polymorphisms that affect hearing loss in mice. Both loci map to the same position on chromosome (Chr) 10. The mdfw locus interacts epistatically with the deaf waddler (dfw) mutation on Chr 6, and the Ahl locus is a major contributor to AHL in several inbred strains. To investigate the possibility of allelism, we examined the correspondence of mdfw and Ahl phenotypes among 12 inbred mouse strains. The effects of strain-specific mdfw alleles on hearing loss were assessed in dfw2J/+ F1 hybrids produced from mating BALB-dfw2J/+ mice with mice from each of 12 inbred strains. F1 hybrids were then assessed for hearing by auditory-evoked brainstem response threshold analysis and classified as dfw2J/+ or +/+ by polymerase chain reaction typing. Heterozygosity for dfw2J accelerated hearing loss in F1 hybrids derived from all strains tested, except those produced with the B6.CAST +Ahl congenic strain. dfw2J/+ F1 hybrids derived from parental strains 129P1/ReJ, A/J, BUB/BnJ, C57BR/cdJ, DBA/2J, NOD/LtJ and SKH2/J exhibited a severe hearing loss by 12 weeks of age. Those derived from strains 129T2/SvEmsJ, C3H/HeJ, CBA/CaJ and NON/LtJ exhibited only a slight to intermediate hearing loss at that age. The hearing loss associated with these strain-specific mdfw alleles corresponds with previously determined Ahl allele effects, providing additional evidence that mdfw and Ahl are manifestations of the same gene. A functional relationship therefore may exist between the Ca2+ transporting activity of the dfw gene (Atp2b2) and AHL.
Keywords: Hearing loss, Mouse, Inbred strain, Age-related hearing loss, Noise-induced hearing loss
1. Introduction
Presbycusis (or age-related hearing loss, AHL) is a progressive, primarily sensorineural hearing loss that affects more than 40% of the human population over 65 years of age, which can lead to a diminished quality of life for the elderly (Gorlin et al., 1995; Morton, 1991). The genetic basis of presbycusis is poorly understood because of the extreme difficulty in studying such a late-onset disease in human populations. Mice offer a promising approach for the genetic study of human presbycusis because of their short life span and ease of experimental manipulation. In addition, mice provide a good model system to study human hearing because of the anatomical, functional and pathological similarities between human and mouse ears (Steel, 1991).
AHL has been extensively studied in C57BL/6J mice (Henry and Chole, 1980; Hunter and Willott, 1987; Mikaelian, 1979; Willott et al., 1984) and a major underlying gene (Ahl) has been mapped to chromosome (Chr) 10 (Johnson et al., 1997). More than 20 inbred strains of mice have been discovered with AHL (Zheng et al., 1999). The same Chr 10 locus contributes to AHL in at least 10 of these strains (Johnson et al., 2000); however, the Ahl gene has not yet been identified at the molecular level. Susceptibility to noise-induced hearing loss (NIHL) has been shown to be associated with the propensity to develop AHL (Li et al., 1993) and with the Ahl gene in particular (Erway et al., 1996). Thus, the Ahl gene appears to play a major role in determining both AHL and NIHL susceptibility. AHL and NIHL vulnerability, however, may not be coupled in all inbred strains (Yoshida et al., 2000).
The map position of Ahl on Chr 10 coincides with that of another inbred strain polymorphism associated with hearing loss, the modifier of deaf waddler (mdfw) locus. The mdfw locus has been shown to interact epistatically with the deaf waddler (dfw) mutation on Chr 6 (Noben-Trauth et al., 1997). Mice homozygous for the deaf waddler mutation (dfw/dfw) exhibit a congenital hearing loss with an associated head bobbing and unbalanced gait. Mice that are heterozygous for dfw and that are also homozygous for a BALB/c-derived, recessive mdfw allele exhibit profound hearing loss by 10 weeks of age but have normal behavior and gait. Mice that are heterozygous for dfw but that have at least one copy of a CAST/Ei-derived, dominant Mdfw allele retain good hearing until old age. The genotype of the mdfw locus, regardless of allelic composition, has no affect on phenotypes of dfw/dfw or +/+ mice. While mutations of dfw have been shown to be alterations of the ATPase, Ca2+ transporting, plasma membrane 2 gene, Atp2b2 (Street et al., 1998), the mdfw gene has not yet been identified at the molecular level.
The mdfw and Ahl loci were both discovered as inbred strain polymorphisms that affect hearing loss and both map to the same position on Chr 10. To investigate the possibility of allelism, we examined the correspondence of mdfw and Ahl phenotypes among 12 inbred mouse strains. For example, the mdfw alleles from BALB/c and C57BL/6 strains were shown to allow hearing loss to occur in dfw/+ mice; whereas, the dominant Mdfw allele from CAST/Ei prevents hearing loss (Noben-Trauth et al., 1997). Consistent with these results, both the BALB/c and C57BL/6 strains have a recessive Ahl allele conferring hearing loss susceptibility that is different from the dominant, AHL-resistant CAST/Ei allele. To extend this comparative analysis, we examined 12 additional mouse strains for the effects of their mdfw alleles on hearing loss in dfw/+ F1 hybrids. Here, we show that Ahl and mdfw hearing loss phenotypes do indeed correspond among all inbred strains tested, suggesting that these two independently discovered loci represent the same gene. In AHL-susceptible strains, hearing loss is greatly accelerated when mice are heterozygous for the dfw mutation, suggesting a functional relationship between the Ca2+ transporting activity of the dfw gene and AHL or NIHL.
2. Materials and methods
2.1. Mice and genetic crosses
The dfw2J mutation arose in a congenic substrain of BALB/cByJ (Noben-Trauth et al., 1997) and this strain is hence referred to as CBy-dfw2J. F1 hybrids were produced from matings of CBy-dfw2J/+ heterozygotes with mice from each of the inbred strains to be tested for mdfw alleles. Half of the F1 hybrids from each mating are expected to be dfw2J/+ (test group) and half +/+ (control group). To alleviate husbandry difficulties encountered with diabetic mice, we used the resistant NOD.NON-H2nb1 congenic strain, rather than its diabetic NOD/LtJ progenitor, but for brevity have designated these mice as NOD/LtJ. The care of mice used in this study was approved by the Animal Care and Use Committee of The Jackson Laboratory. The Jackson Laboratory is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
2.2. dfw genotyping
To distinguish between dfw2J/+ and +/+ genotypes, DNA was extracted from tail tips of F1 hybrids and assayed by polymerase chain reaction (PCR) (Street et al., 1998). The first set of PCR primers amplifies a 108 bp product from dfw2J but not wild-type genomic DNA: forward CATCTGCTCAGACAAGACGA, reverse GCATTGATGGAGCTGGGATC. The second set of PCR primers amplifies a 162 bp product from wild-type but not dfw2J genomic DNA: forward CATCTGCTCAGACAAGACAG, reverse GGTGTAGGCGCTGTTGATGG. The PCR reactions for each primer pair were carried out in separate 10 μl total volumes, containing 20 ng genomic DNA, using previously described methods (Ward-Bailey et al., 1996), except that the annealing temperature was 60°C.
2.3. Auditory-evoked brainstem response (ABR) threshold measurements
The parental strains, F1 hybrids and backcross mice were tested for ABR thresholds with equipment from Intelligent Hearing Systems (IHS, Miami, FL, USA) using previously described methods and equipment (Zheng et al., 1999). Subdermal needle electrodes are inserted at the vertex and ventrolaterally to both ears of anesthetized mice. Specific auditory stimuli (broadband click and pure-tone pips of 8, 16 and 32 kHz) from high frequency transducers are delivered binaurally through plastic tubes to the ear canals. Evoked brainstem responses are amplified and averaged and their wave patterns displayed on a computer screen. Auditory thresholds are obtained for each stimulus by varying the sound pressure level to identify the lowest level at which an ABR pattern can be recognized.
3. Results
CBy-dfw2J/+ mice (which are mdfw/mdfw) were mated with mice from each of 12 inbred strains to produce F1 hybrids that are either dfw2J/+ or +/+ at the dfw locus and heterozygous at the mdfw locus. In all F1 hybrids, the unknown mdfw allele of the strain to be tested is in heterozygous combination with the recessive mdfw allele from the CBy strain. The dfw2J/+ and +/+ genotypes of the F1 progeny were distinguished by PCR analysis and their hearing was assessed by ABR threshold analysis.
Heterozygosity for dfw2J caused a severe hearing impairment (ABR thresholds >60 dB above normal) by 12 weeks of age in seven of the 12 tested F1 hybrids (Table 1), derived from parental strains 129P1/ReJ, A/J, BUB/BnJ, C57BR/cdJ, DBA/2J, NOD/LtJ and SKH2/ J. Heterozygosity for dfw2J had less of a detrimental effect on hearing in the other F1 hybrids (Table 2), derived from parental strains 129T2/SvEmsJ, C3H/ HeJ, CBA/CaJ, NON/LtJ (all with only a mild impairment, ABR thresholds 20–40 dB above normal) and B6.CAST +Ahl (no impairment). B6.CAST +Ahl is a congenic strain that was produced by successively backcrossing the CAST/Ei +Ahl allele (conferring resistance to AHL) onto the C57BL/6J inbred strain (Johnson et al., 1997). The CAST/Ei Mdfw allele (which protects against hearing loss in dfw/+ mice (Noben-Trauth et al., 1997)) is contained within the congenic segment of the B6.CAST +Ahl strain, because of the identical map positions of Ahl and mdfw on Chr 10.
Table 1.
F1 strain hybrids in which dfw2J heterozygosity caused a severe hearing impairment by 12 weeks of age
| Unique parent of F1 hybrid | Genotype | Age (weeks) | Sex | ABR thresholds |
|||
|---|---|---|---|---|---|---|---|
| click | 8 kHz | 16 kHz | 32 kHz | ||||
| Normal hearing mice (Zheng et al., 1999) | <55 | <40 | <35 | <60 | |||
| 129P1/ReJ | +/+ | 4 | m | 50 | 45 | 35 | 60 |
| 129P1/ReJ | +/+ | 4 | m | 40 | 35 | 20 | 45 |
| 129P1/ReJ | +/+ | 4 | m | 40 | 25 | 15 | 40 |
| 129P1/ReJ | +/+ | 4 | m | 40 | 30 | 15 | 50 |
| 129P1/ReJ | dfw/+ | 4 | f | 80 | 55 | 45 | 80 |
| 129P1/ReJ | dfw/+ | 4 | m | 85 | 70 | 50 | 95 |
| 129P1/ReJ | dfw/+ | 4 | m | 85 | 65 | 50 | 90 |
| 129P1/ReJ | dfw/+ | 4 | m | 55 | 50 | 30 | 75 |
| 129P1/ReJ | +/+ | 6 | m | 35 | 35 | 10 | 45 |
| 129P1/ReJ | +/+ | 6 | m | 35 | 35 | 15 | 45 |
| 129P1/ReJ | +/+ | 6 | m | 35 | 30 | 10 | 50 |
| 129P1/ReJ | dfw/+ | 6 | f | 95 | 75 | 55 | 95 |
| 129P1/ReJ | dfw/+ | 6 | f | 119 | 104 | 99 | 119 |
| 129P1/ReJ | dfw/+ | 6 | m | 90 | 85 | 90 | 105 |
| 129P1/ReJ | dfw/+ | 6 | m | 85 | 80 | 90 | 105 |
| 129P1/ReJ | +/+ | 9 | m | 40 | 35 | 15 | 45 |
| 129P1/ReJ | +/+ | 9 | m | 40 | 35 | 20 | 45 |
| 129P1/ReJ | +/+ | 9 | m | 40 | 40 | 15 | 60 |
| 129P1/ReJ | dfw/+ | 9 | f | 115 | 95 | 90 | 119 |
| 129P1/ReJ | dfw/+ | 9 | f | 119 | 104 | 99 | 119 |
| 129P1/ReJ | dfw/+ | 9 | m | 100 | 85 | 95 | 115 |
| 129P1/ReJ | dfw/+ | 9 | m | 90 | 85 | 90 | 110 |
| 129P1/ReJ | +/+ | 12 | f | 40 | 45 | 15 | 60 |
| 129P1/ReJ | +/+ | 12 | m | 40 | 30 | 15 | 45 |
| 129P1/ReJ | +/+ | 12 | m | 40 | 40 | 20 | 60 |
| 129P1/ReJ | +/+ | 12 | f | 40 | 35 | 15 | 60 |
| 129P1/ReJ | +/+ | 12 | f | 40 | 35 | 15 | 60 |
| 129P1/ReJ | +/+ | 12 | m | 40 | 35 | 15 | 55 |
| 129P1/ReJ | dfw/+ | 12 | f | 119 | 104 | 99 | 119 |
| 129P1/ReJ | dfw/+ | 12 | f | 119 | 104 | 99 | 119 |
| 129P1/ReJ | dfw/+ | 12 | m | 110 | 95 | 90 | 119 |
| 129P1/ReJ | dfw/+ | 12 | m | 100 | 100 | 95 | 119 |
| A/J | +/+ | 12 | f | 35 | 20 | 15 | 50 |
| A/J | +/+ | 12 | f | 35 | 25 | 15 | 45 |
| A/J | +/+ | 12 | m | 35 | 25 | 15 | 45 |
| A/J | dfw/+ | 12 | f | 119 | 95 | 95 | 119 |
| A/J | dfw/+ | 12 | f | 119 | 104 | 99 | 119 |
| BUB/BnJ | +/+ | 12 | f | 35 | 25 | 20 | 45 |
| BUB/BnJ | +/+ | 12 | f | 45 | 30 | 20 | 50 |
| BUB/BnJ | +/+ | 12 | m | 40 | 25 | 20 | 45 |
| BUB/BnJ | +/+ | 12 | m | 35 | 20 | 15 | 35 |
| BUB/BnJ | +/+ | 12 | m | 40 | 25 | 25 | 40 |
| BUB/BnJ | dfw/+ | 12 | m | 115 | 95 | 85 | 119 |
| BUB/BnJ | dfw/+ | 12 | f | 115 | 104 | 90 | 119 |
| BUB/BnJ | dfw/+ | 12 | f | 110 | 100 | 90 | 119 |
| BUB/BnJ | dfw/+ | 12 | f | 110 | 95 | 90 | 110 |
| C57BR/cdJ | +/+ | 12 | f | 35 | 25 | 10 | 40 |
| C57BR/cdJ | +/+ | 12 | f | 35 | 20 | 10 | 35 |
| C57BR/cdJ | +/+ | 12 | f | 35 | 25 | 15 | 40 |
| C57BR/cdJ | +/+ | 12 | f | 35 | 20 | 10 | 35 |
| C57BR/cdJ | +/+ | 12 | m | 35 | 25 | 15 | 40 |
| C57BR/cdJ | dfw/+ | 12 | f | 119 | 100 | 95 | 119 |
| C57BR/cdJ | dfw/+ | 12 | m | 110 | 90 | 95 | 119 |
| C57BR/cdJ | dfw/+ | 12 | m | 119 | 104 | 99 | 119 |
| C57BR/cdJ | dfw/+ | 12 | m | 115 | 90 | 80 | 115 |
| C57BR/cdJ | dfw/+ | 12 | m | 119 | 104 | 99 | 119 |
| DBA/2J | +/+ | 12 | f | 35 | 20 | 10 | 30 |
| DBA/2J | +/+ | 12 | m | 35 | 20 | 15 | 35 |
| DBA/2J | +/+ | 12 | m | 35 | 15 | 10 | 35 |
| DBA/2J | dfw/+ | 12 | m | 119 | 95 | 85 | 115 |
| DBA/2J | dfw/+ | 12 | m | 115 | 95 | 80 | 110 |
| DBA/2J | dfw/+ | 12 | f | 119 | 104 | 95 | 119 |
| DBA/2J | dfw/+ | 12 | f | 119 | 104 | 99 | 119 |
| NOD/LtJ | +/+ | 12 | f | 35 | 25 | 10 | 45 |
| NOD/LtJ | +/+ | 12 | f | 40 | 45 | 25 | 55 |
| NOD/LtJ | +/+ | 12 | f | 40 | 30 | 15 | 40 |
| NOD/LtJ | +/+ | 12 | m | 35 | 30 | 15 | 45 |
| NOD/LtJ | +/+ | 12 | f | 35 | 25 | 15 | 40 |
| NOD/LtJ | dfw/+ | 12 | f | 119 | 104 | 99 | 119 |
| NOD/LtJ | dfw/+ | 12 | f | 119 | 100 | 95 | 119 |
| NOD/LtJ | dfw/+ | 12 | m | 115 | 100 | 95 | 115 |
All F1 hybrids were produced from mating CBy-dfw2J/+ mice with mice from each of the strains listed below. All ABR thresholds above normal values are shown in boldface.
Table 2.
F1 strain hybrids in which dfw2J heterozygosity caused only a slight to intermediate hearing loss at 12 weeks of age
| Unique parent of F1 hybrid | Genotype | Age (weeks) | Sex | ABR thresholds |
|||
|---|---|---|---|---|---|---|---|
| click | 8 kHz | 16 kHz | 32 kHz | ||||
| B6.CAST +Ahl | +/+ | 12 | f | 35 | 25 | 15 | 40 |
| B6.CAST +Ahl | +/+ | 12 | f | 40 | 25 | 15 | 35 |
| B6.CAST +Ahl | +/+ | 12 | f | 40 | 20 | 10 | 35 |
| B6.CAST +Ahl | +/+ | 12 | f | 35 | 20 | 15 | 35 |
| B6.CAST +Ahl | +/+ | 12 | m | 35 | 20 | 15 | 35 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 45 | 35 | 20 | 50 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 40 | 25 | 20 | 50 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 45 | 35 | 25 | 50 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 40 | 30 | 15 | 35 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 40 | 35 | 20 | 40 |
| B6.CAST +Ahl | dfw/+ | 12 | f | 40 | 30 | 15 | 35 |
| B6.CAST +Ahl | +/+ | 58 | m | 45 | 45 | 20 | 55 |
| B6.CAST +Ahl | +/+ | 58 | m | 40 | 40 | 15 | 45 |
| B6.CAST +Ahl | +/+ | 58 | f | 40 | 35 | 15 | 40 |
| B6.CAST +Ahl | +/+ | 58 | f | 40 | 30 | 20 | 45 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 40 | 45 | 20 | 40 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 40 | 45 | 15 | 45 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 40 | 45 | 20 | 45 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 45 | 45 | 20 | 50 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 40 | 35 | 20 | 40 |
| B6.CAST +Ahl | dfw/+ | 58 | f | 40 | 30 | 15 | 40 |
| 129T2/SvEmsJ | +/+ | 12 | f | 45 | 45 | 20 | 60 |
| 129T2/SvEmsJ | +/+ | 12 | f | 45 | 40 | 20 | 55 |
| 129T2/SvEmsJ | +/+ | 12 | m | 40 | 30 | 15 | 45 |
| 129T2/SvEmsJ | dfw/+ | 12 | f | 40 | 35 | 35 | 55 |
| 129T2/SvEmsJ | dfw/+ | 12 | m | 40 | 30 | 45 | 55 |
| 129T2/SvEmsJ | dfw/+ | 12 | m | 40 | 25 | 40 | 50 |
| 129T2/SvEmsJ | dfw/+ | 12 | m | 40 | 30 | 35 | 55 |
| 129T2/SvEmsJ | +/+ | 29 | f | 45 | 45 | 25 | 55 |
| 129T2/SvEmsJ | +/+ | 29 | f | 45 | 40 | 20 | 55 |
| 129T2/SvEmsJ | +/+ | 29 | m | 40 | 40 | 20 | 50 |
| 129T2/SvEmsJ | dfw/+ | 29 | f | 45 | 35 | 50 | 60 |
| 129T2/SvEmsJ | dfw/+ | 29 | m | 50 | 35 | 60 | 60 |
| 129T2/SvEmsJ | dfw/+ | 29 | m | 45 | 35 | 70 | 60 |
| 129T2/SvEmsJ | dfw/+ | 29 | m | 45 | 35 | 60 | 60 |
| CBA/CaJ | +/+ | 12 | f | 35 | 20 | 15 | 30 |
| CBA/CaJ | +/+ | 12 | f | 35 | 20 | 10 | 30 |
| CBA/CaJ | +/+ | 12 | f | 35 | 20 | 10 | 30 |
| CBA/CaJ | +/+ | 12 | m | 30 | 15 | 10 | 30 |
| CBA/CaJ | +/+ | 12 | m | 35 | 15 | 10 | 30 |
| CBA/CaJ | +/+ | 12 | m | 35 | 20 | 10 | 30 |
| CBA/CaJ | dfw/+ | 12 | f | 65 | 50 | 60 | 65 |
| CBA/CaJ | dfw/+ | 12 | f | 45 | 25 | 45 | 45 |
| CBA/CaJ | dfw/+ | 12 | f | 50 | 30 | 55 | 65 |
| CBA/CaJ | dfw/+ | 12 | f | 45 | 25 | 45 | 40 |
| CBA/CaJ | dfw/+ | 12 | f | 50 | 25 | 55 | 45 |
| CBA/CaJ | dfw/+ | 12 | f | 35 | 20 | 50 | 40 |
| CBA/CaJ | +/+ | 28 | f | 40 | 25 | 10 | 35 |
| CBA/CaJ | +/+ | 28 | f | 30 | 20 | 10 | 30 |
| CBA/CaJ | +/+ | 28 | m | 40 | 35 | 20 | 35 |
| CBA/CaJ | +/+ | 28 | m | 35 | 25 | 15 | 35 |
| CBA/CaJ | +/+ | 28 | m | 35 | 25 | 15 | 35 |
| CBA/CaJ | +/+ | 28 | f | 35 | 30 | 15 | 35 |
| CBA/CaJ | dfw/+ | 28 | f | 85 | 70 | 65 | 90 |
| CBA/CaJ | dfw/+ | 28 | f | 90 | 75 | 70 | 95 |
| CBA/CaJ | dfw/+ | 28 | f | 100 | 85 | 85 | 105 |
| CBA/CaJ | dfw/+ | 28 | f | 105 | 85 | 90 | 105 |
| NON/LtJ | +/+ | 12 | f | 30 | 20 | 10 | 35 |
| NON/LtJ | +/+ | 12 | m | 40 | 25 | 20 | 40 |
| NON/LtJ | +/+ | 12 | f | 35 | 20 | 15 | 35 |
| NON/LtJ | +/+ | 12 | f | 35 | 25 | 10 | 40 |
| NON/LtJ | +/+ | 12 | m | 35 | 20 | 10 | 35 |
| NON/LtJ | dfw/+ | 12 | f | 85 | 65 | 50 | 90 |
| NON/LtJ | dfw/+ | 12 | f | 75 | 55 | 55 | 80 |
| NON/LtJ | dfw/+ | 12 | f | 80 | 70 | 50 | 80 |
| NON/LtJ | dfw/+ | 12 | m | 80 | 75 | 55 | 90 |
| NON/LtJ | dfw/+ | 12 | m | 90 | 75 | 55 | 85 |
| NON/LtJ | dfw/+ | 12 | m | 80 | 65 | 55 | 75 |
| NON/LtJ | +/+ | 24 | f | 35 | 25 | 15 | 35 |
| NON/LtJ | +/+ | 24 | f | 35 | 30 | 15 | 45 |
| NON/LtJ | +/+ | 24 | f | 35 | 25 | 10 | 35 |
| NON/LtJ | +/+ | 24 | f | 35 | 25 | 10 | 45 |
| NON/LtJ | +/+ | 24 | f | 30 | 25 | 10 | 35 |
| NON/LtJ | dfw/+ | 24 | m | 85 | 50 | 80 | 90 |
| NON/LtJ | dfw/+ | 24 | m | 95 | 70 | 70 | 95 |
| NON/LtJ | dfw/+ | 24 | m | 119 | 100 | 90 | 119 |
| C3H/HeJ | +/+ | 12 | f | 35 | 26 | 15 | 40 |
| C3H/HeJ | +/+ | 12 | f | 35 | 30 | 20 | 40 |
| C3H/HeJ | +/+ | 12 | f | 30 | 30 | 15 | 35 |
| C3H/HeJ | dfw/+ | 12 | f | 65 | 55 | 50 | 80 |
| C3H/HeJ | dfw/+ | 12 | f | 55 | 25 | 50 | 55 |
| C3H/HeJ | dfw/+ | 12 | m | 65 | 30 | 45 | 55 |
| C3H/HeJ | dfw/+ | 12 | m | 65 | 55 | 50 | 80 |
| C3H/HeJ | dfw/+ | 12 | m | 45 | 25 | 40 | 45 |
| C3H/HeJ | dfw/+ | 12 | m | 65 | 60 | 45 | 75 |
| C3H/HeJ | +/+ | 23 | f | 45 | 35 | 15 | 35 |
| C3H/HeJ | +/+ | 23 | f | 45 | 35 | 20 | 35 |
| C3H/HeJ | +/+ | 23 | m | 40 | 30 | 20 | 40 |
| C3H/HeJ | dfw/+ | 23 | f | 85 | 80 | 75 | 90 |
| C3H/HeJ | dfw/+ | 23 | f | 80 | 70 | 75 | 90 |
| C3H/HeJ | dfw/+ | 23 | m | 80 | 60 | 50 | 105 |
| C3H/HeJ | dfw/+ | 23 | m | 70 | 60 | 65 | 90 |
| C3H/HeJ | dfw/+ | 23 | m | 90 | 85 | 90 | 115 |
| C3H/HeJ | dfw/+ | 23 | f | 110 | 90 | 95 | 119 |
| C3H/HeJ | +/+ | 34 | f | 40 | 25 | 20 | 45 |
| C3H/HeJ | +/+ | 34 | f | 40 | 30 | 25 | 45 |
| C3H/HeJ | +/+ | 34 | f | 40 | 30 | 20 | 45 |
| C3H/HeJ | dfw/+ | 34 | f | 119 | 100 | 95 | 119 |
| C3H/HeJ | dfw/+ | 34 | m | 115 | 100 | 99 | 119 |
| C3H/HeJ | dfw/+ | 34 | m | 115 | 95 | 95 | 115 |
| C3H/HeJ | dfw/+ | 34 | m | 110 | 100 | 95 | 115 |
| C3H/HeJ | dfw/+ | 34 | m | 110 | 90 | 85 | 110 |
| C3H/HeJ | dfw/+ | 34 | m | 115 | 100 | 85 | 115 |
Hearing remained normal in dfw2J/+ hybrids with B6.CAST +Ahl. All F1 hybrids were produced from mating CBy-dfw2J/+ mice with mice from each of the strains listed below. ABR thresholds above normal values are shown in boldface.
Some of the F1 hybrids were tested at multiple ages to determine hearing loss onset times and progression. The dfw2J/+ F1 hybrids derived from the 129P1/ReJ strain exhibited a mild hearing impairment at 4 weeks of age that progressed to severe impairment by 9 weeks of age (Table 1). The dfw2J/+ F1 hybrids derived from the B6.CAST +Ahl strain retained normal hearing levels even at 58 weeks of age (Table 2). The mild hearing impairment observed at 12 weeks of age in F1 hybrids derived from strains 129T2/SvEmsJ, CBA/CaJ, NON/LtJ and C3H/HeJ progressed to intermediate impairment by 23–29 weeks of age and then to severe impairment by 34 weeks of age (in C3H/HeJ-derived dfw2J/+ mice).
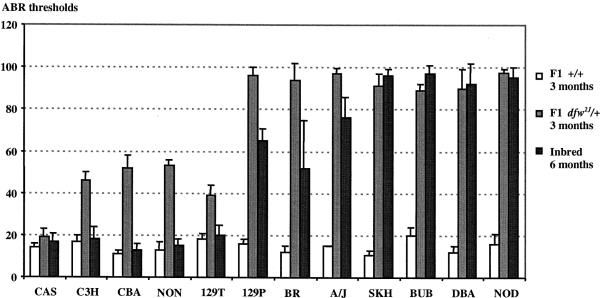
The hearing loss associated with dfw heterozygosity in each F1 strain hybrid was then compared with the AHL associated with its parental inbred strain. The ABR thresholds of dfw2J/+ F1 hybrids at 12 weeks of age, which indicate the effect of the mdfw allele, correspond with the ABR thresholds of the parental inbred strain at 6 months of age, which indicate the level of AHL associated with that strain (Fig. 1). The Chr 10 Ahl locus has been shown by backcross linkage analysis to be a major contributor to the AHL exhibited by these same strains (Johnson et al., 2000).
Fig. 1.
Comparison of mdfw and Ahl effects on hearing loss in 12 inbred mouse strains. The mdfw effects are shown as the average ABR thresholds of 3-month-old dfw2J/+ F1 hybrids (Tables 1 and 2), produced by mating mice from each inbred strain with CBy-dfw2J/+ mice. ABR thresholds of 3-month-old +/+ F1 hybrids are shown as controls for normal hearing. The Ahl effects are shown as the average ABR thresholds of 6-month-old mice from each of the inbred strains (Zheng et al., 1999 and unpublished data; at least five mice tested per strain). For clarity, only threshold responses to the 16 kHz pure-tone stimulus are shown. Strain abbreviations are CAS=B6.CAST +Ahl, C3H=C3H/HeJ, CBA=CBA/CaJ, NON=NON/LtJ, 129T=129T2/SvEmsJ, 129P=129P1/ReJ, BR=C57BR/cdJ, SKH=SKH2/J, BUB=BUB/BnJ, DBA=DBA/2J and NOD=NOD/LtJ.
4. Discussion
The relationships illustrated in Fig. 1 clearly demonstrate the phenotypic correspondence of strain-specific mdfw and Ahl alleles. Hearing loss progression is greatly accelerated in mice that are heterozygous for the dfw2J mutation in AHL-susceptible strains, such as 129P1/ReJ, A/J, BUB/BnJ, C57BR/cdJ, DBA/2J, NOD/LtJ and SKH2/J (Table 1) and the previously described BALB/c and C57BL/6J strains (Noben-Trauth et al., 1997). This effect is much reduced in AHL-resistant strains, such as 129T2/SvEmsJ, C3H/HeJ, CBA/CaJ and NON/LtJ (Table 2) and absent in the congenic strain B6.CAST +Ahl (Table 2) and its parental donor strain CAST/Ei (Noben-Trauth et al., 1997). These results suggest there may be three allelic forms of mdfw: the allele conferring susceptibility to hearing loss (present in 129P1/ReJ, A/J, BALB/c, BUB/BnJ, C57BL/6J, C57BR/cdJ, DBA/2J, NOD/LtJ and SKH2/J) being recessive to alleles conferring partial resistance (present in 129T2/SvEmsJ, C3H/HeJ, CBA/CaJ and NON/LtJ) and complete resistance (present in CAST/Ei).
If Ahl and mdfw are allelic, as these results support, then a functional relationship may exist between the dfw gene (Atp2b2) and AHL or NIHL. The Atp2b2 gene product functions as a Ca2+ pump in hair cells (Street et al., 1998) and acoustic over-stimulation has been shown to increase cytoplasmic Ca2+ concentrations in outer hair cells (Fridberger et al., 1998). Thus, consequences related to an elevation of intracellular Ca2+ provide a possible link to the cumulative loss of outer hair cells characteristic of AHL and NIHL.
The accelerated hearing loss in dfw2J/+ hybrids potentially can be used as an early predictor of strains that are likely to exhibit late-onset AHL. For example, dfw2J/+ F1 hybrids with CBA/CaJ exhibit a slight but significant hearing loss at 3 months of age (Fig. 1). Although we did not detect AHL at 6 months of age in inbred CBA/CaJ mice, AHL has been reported in this strain at a much older age (17–25 months; Parham et al., 1999). Because hearing loss onset is much earlier in dfw2J/+ mice than in +/+ mice of a given strain, experiments to study AHL pathogenesis could be greatly shortened by using dfw2J/+ mice. NIHL experiments might also benefit by using dfw2J/+ mice. Mice that are dfw2J/+ are likely to exhibit an increased vulnerability to NIHL and thus provide a more sensitive model for NIHL studies. Our results predict that NIHL susceptibility and resistance will follow the strain relationships shown in Fig. 1. To produce dfw2J/+ mice for such studies, F1 hybrids can be produced as herein described, by crossing the strain of interest with CBy-dfw2J/+ mice. The resulting F1 hybrids will be genetically identical except at the dfw locus. One half of the F1 hybrids are expected to have the +/+ genotype, and these make ideal controls for the dfw2J/+ mice. As previously described, these two genotypes are easily distinguished by PCR typing.
The hearing loss in the 129P1/ReJ strain was much greater than in the 129T2/SvEmsJ strain, despite the similarity of their strain nomenclature. At 6 weeks of age, dfw2J/+ F1 hybrids derived from the 129P1/ReJ strain already exhibited a severe hearing impairment (Table 1), whereas dfw2J/+ F1 hybrids derived from the 129T2/SvEmsJ strain exhibited only a slight hearing impairment at 29 weeks of age (Table 2). At 6 months of age, 129P1/ReJ inbred strain mice exhibited AHL (average ABR thresholds >60 dB), whereas 129T2/SvEmsJ inbred strain mice retained normal hearing at that age (Fig. 1). The differences in hearing between these two strains is not that surprising considering the extensive genetic variability that has been documented among 129 substrains, a result of both deliberate and accidental outcrossing (Simpson et al., 1997). Although hearing loss before 3 months of age has been reported for three of the 129-related substrains (129P3/J, 129P1/ReJ and 129X1/SvJ; Zheng et al., 1999), this is not the case for all 129 substrains, as shown by our results for the 129T2/SvEmsJ strain. In this regard, the reported resistance of the 129S6/SvEvTac strain to NIHL (Yoshida et al., 2000) is less surprising than originally thought, because this 129 substrain (like 129T2/SvEmsJ) may be resistant to AHL.
The waltzer deafness mutation (v) maps to the same Chr 10 position as Ahl and mdfw and, therefore, may be an allele of the same gene. The cochlear pathology (hair cell loss and spiral ganglion cell degeneration) of v mutants (Deol, 1956) is similar to, but more severe than, that associated with Ahl and mdfw hearing loss. The v mutation, however, may be more amenable to a positional cloning approach for gene identification (Bryda et al., 1997) than are the Ahl and mdfw strain polymorphisms. Once the v gene is identified, it could be tested as a candidate for Ahl and mdfw. The region of mouse Chr 10 containing Ahl, mdfw and v has homologies with human Chr 10q21–q22, where the recessive, nonsyndromic deafness gene DFNB12 and the Usher syndrome type ID gene (USH1D) have been mapped (Chaib et al., 1996; Wayne et al., 1996). The gene or genes identified at the v locus in the mouse and the DFNB12/USH1D locus in humans may play important roles in AHL and NIHL susceptibility, as well as in the more severe deafness traits by which they were originally discovered.
In conclusion, the results presented here provide evidence that the two independently discovered loci Ahl and mdfw are different manifestations of the same gene. In AHL-susceptible strains, hearing loss is greatly accelerated in mice that are heterozygous for the dfw2J mutation. Hearing assessment of dfw2J/+ F1 strain hybrids, therefore, potentially can be used to predict the susceptibility of the parental strains to AHL or NIHL. The equivalency of mdfw and Ahl further suggests that a functional relationship may exist between the Ca2+ transporting activity of the dfw gene (Atp2b2) and AHL or NIHL.
Acknowledgements
We thank Dr. Patsy Nishina, Dr. Greg Cox and Dr. Konrad Noben-Trauth for their careful review of this manuscript and Ken Bosom and Heping Yu for their expert technical assistance. We also thank Dr. Lawrence Erway for his help in establishing the ABR testing system at The Jackson Laboratory and Val Street for providing primer sequences to distinguish dfw genotypes. This work is supported by contract DC62108 from the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders (NIDCD). Institutional shared services are supported by National Cancer Institute Support grant CA34196.
References
- Bryda EC, Ling H, Flaherty L. A high-resolution genetic map around waltzer on mouse chromosome 10 and identification of a new allele of waltzer. Mamm. Genome. 1997;8:1–4. doi: 10.1007/s003359900336. [DOI] [PubMed] [Google Scholar]
- Chaib H, Place C, Salem N, Dode C, Chardenoux S, Weissen-bach J, el Zir E, Loiselet J, Petit C. Mapping of DFNB12, a gene for a non-syndromal autosomal recessive deafness, to chromosome 10q21–22. Hum. Mol. Genet. 1996;5:1061–1064. doi: 10.1093/hmg/5.7.1061. [DOI] [PubMed] [Google Scholar]
- Deol MS. The anatomy and development of the mutants pirouette, shaker-1 and waltzer in the mouse. Proc. R. Soc. Lond. B: Biol. Sci. 1956;145:206–213. doi: 10.1098/rspb.1956.0028. [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau YW, Davis RR, Krieg EF. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear. Res. 1996;93:181–187. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc. Natl. Acad. Sci. USA. 1998;95:7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Toriello HV, Cohen MM. Hereditary Hearing Loss and it's Syndromes. Vol. 28. Oxford University Press; Oxford, NY: 1995. [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss on the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear. Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear. Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Erway LC. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics. 2000;70:171–180. doi: 10.1006/geno.2000.6377. [DOI] [PubMed] [Google Scholar]
- Li HS, Hultcrantz M, Borg E. Influence of age on noise-induced permanent threshold shifts in CBA/Ca and C57BL/6J mice. Audiology. 1993;32:195–204. doi: 10.3109/00206099309072935. [DOI] [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/bl6 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscopy. 1979;34:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Morton NE. Genetic epidemiology of hearing loss. Ann. N. Y. Acad. Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR, Nishina PM. mdfw: a deafness susceptibility locus that interacts with deaf waddler (dfw) Genomics. 1997;44:266–272. doi: 10.1006/geno.1997.4869. [DOI] [PubMed] [Google Scholar]
- Parham K, Sun XM, Kim DO. Distortion product otoa-coustic emissions in the CBA/J mouse model of presbycusis. Hear. Res. 1999;134:29–38. doi: 10.1016/s0378-5955(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Steel KP. Similarities between mice and humans with hereditary deafness. Ann. N. Y. Acad. Sci. 1991;630:68–79. doi: 10.1111/j.1749-6632.1991.tb19576.x. [DOI] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat. Genet. 1998;19:390–394. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- Ward-Bailey PF, Johnson KR, Handel MA, Harris BS, Davisson MT. A new mouse mutation causing male sterility and histoincompatibility. Mamm. Genome. 1996;7:793–797. doi: 10.1007/s003359900239. [DOI] [PubMed] [Google Scholar]
- Wayne S, Der Kaloustian VM, Schloss M, Polomeno R, Scott DA, Hejtmancik JF, Sheffield VC, Smith RJ. Localization of the Usher syndrome type ID gene (Ush1D) to chromosome 10. Hum. Mol. Genet. 1996;5:1689–1692. doi: 10.1093/hmg/5.10.1689. [DOI] [PubMed] [Google Scholar]
- Willott JF, Kulig J, Satterfield T. The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment. Hear. Res. 1984;16:161–167. doi: 10.1016/0378-5955(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Hequembourg SJ, Atencio CA, Rosowski JJ, Liberman MC. Acoustic injury in mice: 129/SvEv is exceptionally resistant to noise-induced hearing loss. Hear. Res. 2000;141:97–106. doi: 10.1016/s0378-5955(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]