Abstract
Conditions of increased cognitive or emotional demand activate dopamine release in a regionally selective manner. Whereas the brief millisecond response of dopamine neurons to salient stimuli suggests that dopamine’s influence on behaviour may be limited to signalling certain cues, the prolonged availability of dopamine in regions such as the prefrontal cortex and nucleus accumbens is consistent with the well described role of dopamine in maintaining motivation states, associative learning and working memory. The behaviourally elicited terminal release of dopamine is generally attributed to increased excitatory drive on dopamine neurons. Our findings here, however, indicate that this increase may involve active removal of a tonic inhibitory control on dopamine neurons exerted by the lateral habenula (LHb). Inhibition of LHb in behaving animals transiently increased dopamine release in the prefrontal cortex, nucleus accumbens and dorsolateral striatum. The inhibitory influence was more pronounced in the nucleus accumbens and striatum than in the prefrontal cortex. This pattern of regional dopamine activation after LHb inhibition mimicked conditions of reward availability but not increased cognitive demand. Electrical or chemical stimulation of LHb produced minimal reduction of extracellular dopamine, suggesting that in an awake brain the inhibition associated with tonic LHb activity represents a near-maximal influence on dopamine neurotransmission. These data indicate that LHb may be critical for functional differences in dopamine neurons by preferentially modulating dopamine neurons that project to the nucleus accumbens over those neurons that primarily project to the prefrontal cortex.
Keywords: cognition, rat, reward, schizophrenia, striatum, substance abuse
Introduction
Dopamine neurotransmission is implicated in processing information related to reward, motivation, learning, control of movement, and cognition (Wise & Rompre, 1989; Goldman-Rakic, 1998; Kelley, 2004; Calabresi et al., 2007). While dopamine neurons in awake animals exhibit brief phasic responses to stimuli that generally last < 500 ms (Schultz et al., 1993), phasic increases in extracellular levels of dopamine in cortical and subcortical regions during conditions of increased cognitive or emotional demand remain elevated for minutes (Abercrombie et al., 1989; Phillips et al., 2004; Stefani & Moghaddam, 2006). A significant proportion of dopamine receptors are extrasynaptic (Smiley et al., 1994); therefore, the sustained levels of extracellular dopamine may have functional significance in modulating dopamine-dependent ‘maintained’ behaviours such as motivational states, sustained attention or working memory. The persistent extracellular levels of dopamine may also be critical for some forms of cellular plasticity (Sun et al., 2005). While it is generally accepted that behavioural activation involves a sustained increase in cortical and striatal dopamine, the pathways that mediate this increase remain controversial. Regions such as the prefrontal cortex (PFC), amygdala, pedunculopontine nucleus and hippocampus have been implicated in the regulation of dopamine in anaesthetized preparations (Floresco et al., 1998; Mitchell et al., 2000); however, stimulation of these regions in awake animals produces a small or no increase in dopamine release (Jackson et al., 2001). These differences suggest that other regions are involved in mediating sustained dopamine activation during performance of cognitive tasks or exposure to motivationally salient contexts.
Although a great deal of emphasis has been placed on increased excitatory drive of dopamine neurons during behavioural activation, an alternative mechanism may involve active removal of a tonic inhibitory control on dopamine neurons. A candidate region for this mode of regulation is the lateral habenular nucleus (LHb). The LHb is a subdivision of the habenular complex (HbCpl), an epithalamic structure situated on the surface of the mediodorsal thalamic nucleus. It has been implicated in functions traditionally associated with dopamine such as reward processing, stress, declarative memory and attention (Kumari et al., 1999; Lecourtier et al., 2004; Lecourtier & Kelly, 2005; Matsumoto & Hikosaka, 2007). It innervates inhibitory gamma-aminobutyric acid (GABA) neurons in dopamine neuron-containing regions of the ventral tegmental area (VTA) and the substantia nigra (Herkenham & Nauta, 1979; Araki et al., 1988). Electrical stimulation of LHb inhibits dopamine neurons (Christoph et al., 1986) via stimulation of GABA-A receptors (Ji & Shepard, 2007). In primates, LHb and dopamine neurons display opposing patterns of activity during reward-predicting and -nonpredicting stimuli (Matsumoto & Hikosaka, 2007).
Here we tested the hypothesis that LHb maintains tonic inhibitory control of dopamine neurons in the awake brain. Phasic suppression of LHb activity would then lead to a transient increase in dopamine release in cortical and striatal terminal areas, and to behavioural activation. Extracellular levels of dopamine were measured in three regions [PFC, nucleus accumbens (NAc) and lateral striatum] which are implicated in goal-directed movement, cognition and reward. Activity of LHb was transiently inhibited by localized application of an alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor antagonist (LY293558). These results were compared with chemical (AMPA) or electrical stimulation of LHb. Locomotor activity and stereotypy were measured and correlated with levels of dopamine.
Materials and methods
Subjects
Adult male Sprague–Dawley rats (Harlan, Somerville, New Jersey, USA), weighing 320–350 g at the time of surgery, were used in this study. Before surgery, animals were housed in pairs on a 12-h light–dark cycle (lights on at 07.00 h) for at least 1 week after their arrival in the animal facilities. Experiments were performed during the light phase on awake and freely moving animals. All experimental protocols were approved by the University of Pittsburgh Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical and microdialysis procedures
Surgical procedures were performed under isoflurane anaesthesia. A thermostatically controlled electric heating pad was used to maintain the body temperature at 37 °C. Rats were placed in the stereotaxic frame with the tooth bar 3.3 mm below the ear bars in order to correspond to the atlas of Paxinos & Watson (1998). Dental cement was used to secure the probes and electrodes to the skull and to two skull screws. Immediately after the termination of surgery, the dialysis probes were connected to a liquid swivel-balance arm assembly. After surgery, rats were placed in a home-cage-sized clear polycarbonate cage (44 × 22 × 42 cm) containing fresh bedding, and were allowed to recover for 24 h prior to the collection of the dialysis samples. Animals had ad libitum access to food and water during the recovery period but not during the experiment. Probes were perfused with a modified Ringer’s solution containing the following (in mm): NaCl, 145; KCl, 2.7; MgCl2 1.0; and CaCl2; 1.2. The flow rate was set at 1.3 µL⁄min during the recovery period and 2.0 µL⁄min during the collection of dialysis samples. Dialysis samples were collected every 20 min and injected immediately into an HPLC system with electrochemical detection for the analysis of dopamine, as previously described (Adams & Moghaddam, 1998).
Dialysis probes had an outer diameter of 330 µm and exposed tip of 1.0 mm for infusion of drugs into the LHb, thalamus (Thal) and dorsal hippocampus (DHipp). Probes used for measuring extracellular dopamine had an exposed tip of 3.0 mm for PFC and 2.0 mm for NAc and striatum. In most animals dopamine was measured from two regions simultaneously while LHb was inhibited or stimulated. Coordinates for probes were as follows (in mm): LHb [anteroposterior (AP), −3.8 from bregma; mediolateral (ML), 0.8; dorsoventral (DV), 4–5 from dura], Thal (AP, −2.2 from bregma; ML, 0.7; DV, 4.3–5.3 from dura), DHipp (AP, −3.3 from bregma; ML, 1.2; DV, 2.2–3.2 from dura), PFC (AP, +3.4 from bregma; ML, 0.8; DV, 2.8–5.8 from skull), NAc (AP, +1.7 from bregma; ML, 0.8; DV, 6.5–8.5 from skull) and striatum (AP, +1.7 from bregma; ML, 3.0; DV, 5–7 from skull). Coordinates for bipolar stainless steel electrodes used for electrical stimulation were as follows: LHb (AP, −3.8 from bregma; ML, 0.8; DV, 4.5 from dura), DHipp (AP, −3.8 from bregma; ML, 1.2; DV, 3.1 from dura) or Thal (AP, −2.2 from bregma; ML, 0.8; DV, 5.0 from dura). The probes used for detection of dopamine were lowered ipsilateral to LHb, Thal, or DHipp in one or two of the dopamine innervated regions (PFC, NAc and striatum). In the case of VTA stimulation, bipolar stainless steel electrodes were implanted in the VTA (AP, −5.3 from bregma; ML, 0.8; DV, −8.3). Regions and hemispheres were randomly chosen.
Drugs
Stock solutions of LY293558 (10 mm in distilled H20; gift from Eli Lilly, Indianapolis, IN, USA) and AMPA (10 mm in distilled H20; Sigma-RBI, St Louis, MO, USA) were prepared and kept frozen for a maximum of 1 month. Before use, aliquots of the stock solutions were diluted in Ringer’s solution to a final concentration of 100 µm.
Electrical stimulation parameters
Non-continuous burst stimuli were applied to both LHb and VTA. For LHb stimulation, 0.5-ms pulses were delivered at 20 Hz for 300 ms, with an interburst interval of 1 s and an amplitude of 60 µA for 20 min. These parameters were chosen in accordance with LHb neuronal patterns of activity as shown by Kim & Chang (2005). VTA neurons were stimulated with 1-ms pulses delivered at 100 Hz for 200 ms, with an interburst interval of 500 ms and amplitude of 60 µA for 20 min.
Behavioural measurements
Locomotor activity
Photobeams situated around the outside perimeter of the clear polycarbonate testing cage (Lafayette Instrument Company Inc., Lafayette, IN, USA) were used to assess spontaneous locomotion. Analysis of the beam-break data was performed with MotorMonitor software (v. 5.00; Hamilton-Kinder, LLC, Poway, CA, USA).
Stereotypy
The presence of stereotyped behaviours was rated for 30 s every 5 min, according to a scoring scale described previously (Adams & Moghaddam, 1998). Behaviours observed were as follows: grooming, sniffing (up or down), digging and rearing. A value of 1 was given for any specific behaviour that occurred during the 30 s of rating. Subsequently, ratings were pooled within 20-min periods to match each sample.
Histology
After the termination of each experiment, animals were anaesthetized with an intraperitoneal administration of chloral hydrate (400 mg⁄kg). They were perfused intracardially with 9% saline followed by 10% buffered formalin. Brains were removed and stored in 10% buffered formalin for at least 1 week. Serial sections (250 µm) of the fixed brains were stained with cresyl violet acetate and placements of microdialysis probes and tips of the stimulation electrodes were verified under a light microscope. Only rats with correct probe and electrode placements were included in the statistical analysis.
Statistical analysis
In all experiments, dopamine release was calculated for each dialysis sample as percentage ± SEM of baseline value (depicted as samples 1–3 in all figures).
Baseline was defined as the average of the three samples immediately before the application of any treatment. Dopamine levels were analysed with a two-way anova with region (PFC, NAc, striatum) as the between-subjects factor and sample (time) as the within-subject measure. Between-groups post hoc tests were performed using one-way anova with Tukey’s adjusted post hoc testing. Within-group post hoc tests were made using paired-sample t-tests (applying bi-directional hypothesis) with each post-treatment sample being compared to the mean of the three baseline samples. Simple correlations between dopamine release and measures of behavioural activity were performed using the average values for each sample. Lines were fitted for each dataset on the basis of an initial examination of the scatter plots, followed by use of the SigmaPlot regression tool (SigmaPlot version 8.0; SPSS, Chicago, IL, USA). The level for all statistical comparisons was P < 0.05. In the case of stereotypy score, significant effects were assessed using nonparametric statistical analysis (Friedman test).
Results
LHb inhibition increased spontaneous activity and dopamine release in the PFC, NAc and striatum
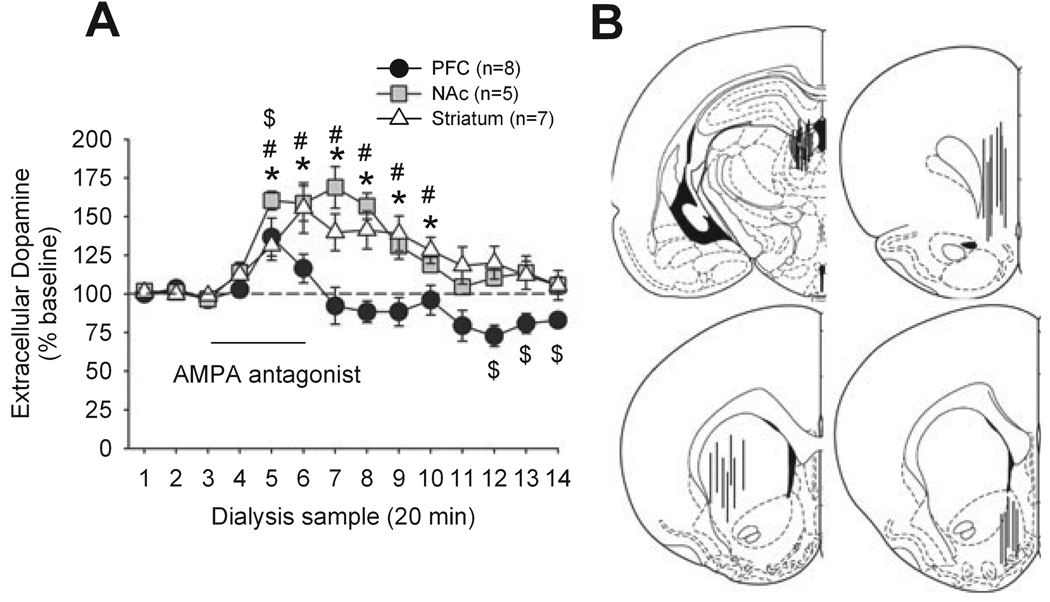
After three stable baseline samples were collected in the PFC, NAc or striatum, the activity of LHb was inhibited by the AMPA receptor antagonist LY293558 (Fig. 1). This treatment increased dopamine release in all three regions. However, the increase in PFC was shorter in magnitude and duration. A two-way anova with region as factor and sample as repeated measure indicated a significant effect of region (F2,16 = 5.50, P < 0.05), a significant effect of sample (F15,240 = 15.9, P < 0.0001) and a significant region × sample interaction (F30,240 = 2.93, P < 0.0001). Analysis of each region with a one-way anova with sample as the repeated measure indicated a significant effect of sample: PFC (F15,90 = 4.61, P < 0.0001), NAc (F16,50 = 12.96, P < 0.0001) and striatum (F13,91 = 7.98, P < 0.0001). Between-regions post hoc tests indicated a significant difference between PFC and NAc (P < 0.05), a significant difference between PFC and striatum (P < 0.05) and no difference between NAc and striatum (P > 0.05).
FIG. 1.
(A) Effect of application of the AMPA receptor antagonist LY293558 to the LHb via a microdialysis probe on the release of dopamine in the PFC, NAc and dorsal striatum. $P < 0.05 (PFC) *P < 0.05 (NAc), #P < 0.05 (striatum) compared to baseline. (B) Representations of probe placements in LHb (top left), PFC (top right), striatum (bottom left) and NAc (bottom right; plates are modified from Paxinos & Watson, 1998).
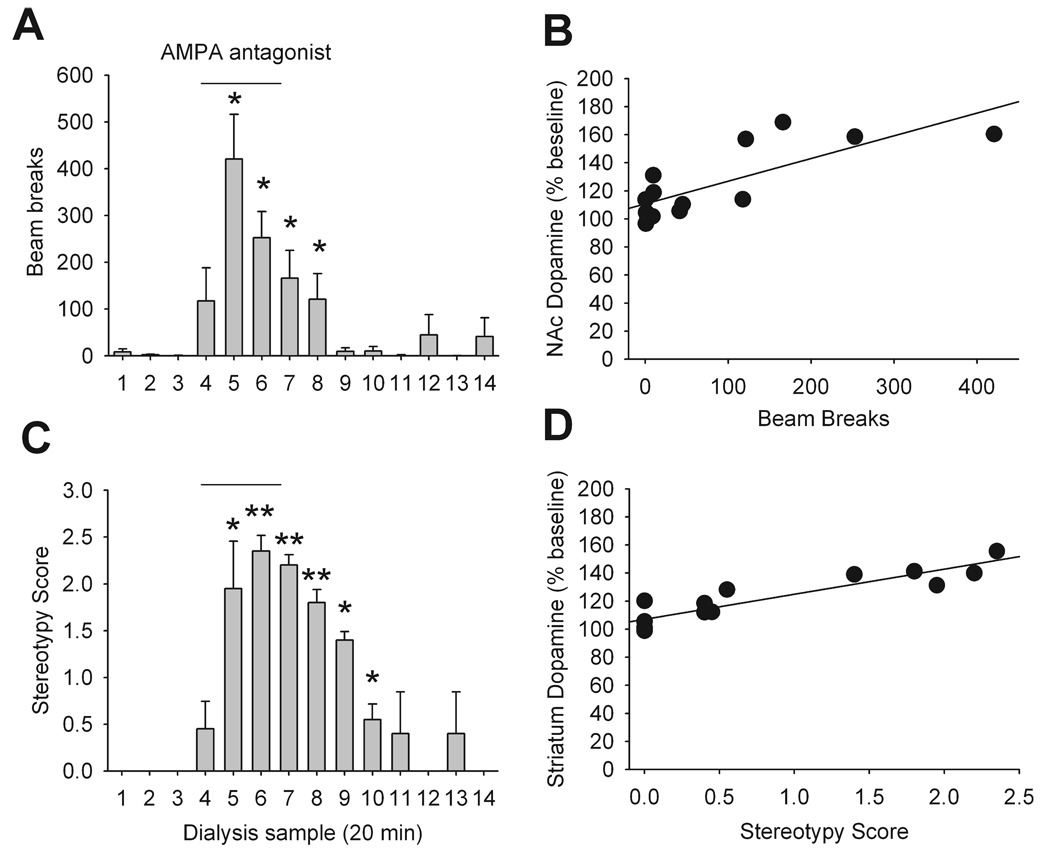
During the microdialysis studies, spontaneous locomotion and behavioural stereotypy were assessed. As demonstrated in Fig. 2, inhibition of LHb resulted in phasic increases in locomotor activity (F13,52 = 8.48, P < 0.0001) and expression of behavioural stereotypy (P < 0.0001). Increased dopamine neurotransmission in the NAc and striatum have been associated with increased locomotion and stereotypy, respectively (Kelly et al., 1975). We found that locomotor activity was positively correlated with increased dopamine release in NAc and stereotypy score positively correlated with increased dopamine release in striatum (Fig. 2).
FIG. 2.
Behavioural response to the administration of the AMPA antagonist LY293558 in LHb. (A) Assessment of locomotor activity in the five rats whose NAc dopamine release was measured during LY293558 administration in LHb. Each point (beam breaks, mean ± SEM) corresponds to a 20-min sample collection. *P < 0.05 vs. baseline. (B) There was a positive correlation between locomotor activity and NAc dopamine release (r = 0.78, P < 0.05) and PFC dopamine release (r = 0.6, P < 0.05; data not shown). (C) Assessment of stereotypy score in the seven rats whose striatum dopamine release was measured during LY293558 administration in LHb. Each point (score, mean ± SEM) corresponds to a 20-minute sample collection. *P < 0.05, **P < 0.01 vs. baseline. (D) There was a positive correlation between stereotypy score and striatum dopamine release (r = 0.58, P < 0.05) and no correlation with PFC dopamine release (r = 0.2, P > 0.05; data not shown).
Similar patterns of dopamine release in NAc, but not PFC, were evoked by VTA stimulation
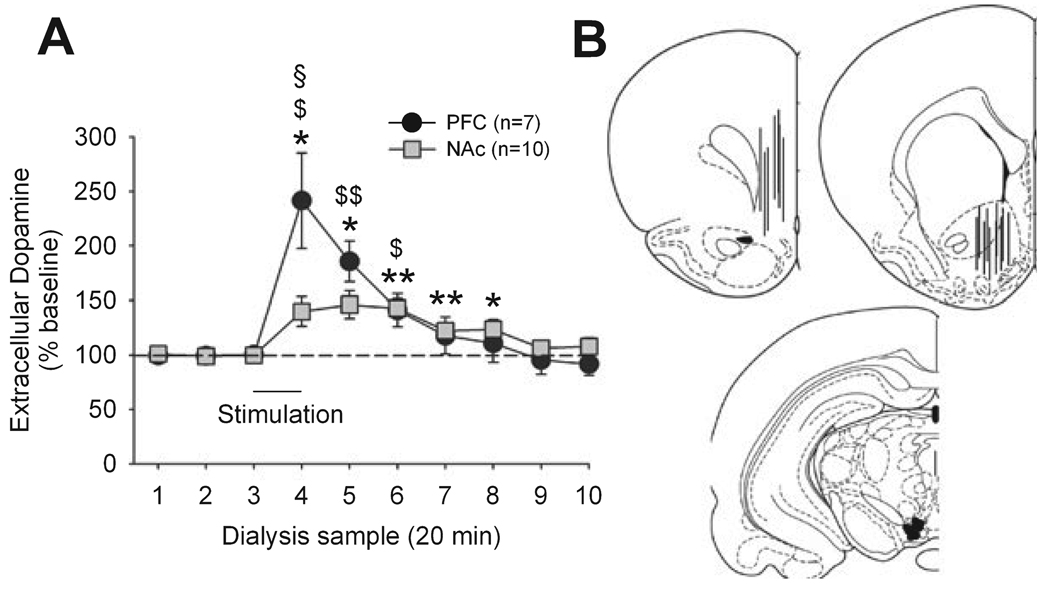
Given the significantly smaller effect of LHb inhibition on PFC dopamine release compared to the other regions, we asked whether generalized activation of dopamine neurons produces a similar pattern of activation in dopamine release in these regions. This question was addressed by electrically stimulating dopamine cell bodies in the VTA while simultaneously measuring dopamine efflux in the PFC and NAc (Fig. 3). Dopamine neurons in the VTA project to both PFC and NAc. As expected, stimulation of VTA significantly increased dopamine release in both regions (PFC: F7,42 = 9.58, P < 0.01; NAc: F7,63 = 7.87, P < 0.01). In contrast to LHb inhibition, however, VTA stimulation produced a larger increase in PFC dopamine than in NAc dopamine release. The increase in NAc was nearly identical in magnitude to that observed after LHb inhibition (Fig. 1) whereas PFC levels increased nearly twice as much.
FIG. 3.
(A) Impact of VTA electric stimulation on dopamine release in NAc and PFC. *P < 0.05, **P < 0.01 (NAc vs. baseline), $P < 0.05, $$P < 0.01 (PFC vs. baseline), §P < 0.05 (PFC vs. NAc). $$P < 0.01 (B) Representations of probe placements in PFC (top left) and NAc (top right) and electrodes placements in VTA (bottom; plates are modified from Paxinos & Watson, 1998).
LHb stimulation inhibited dopamine release in NAc, but not in PFC or striatum
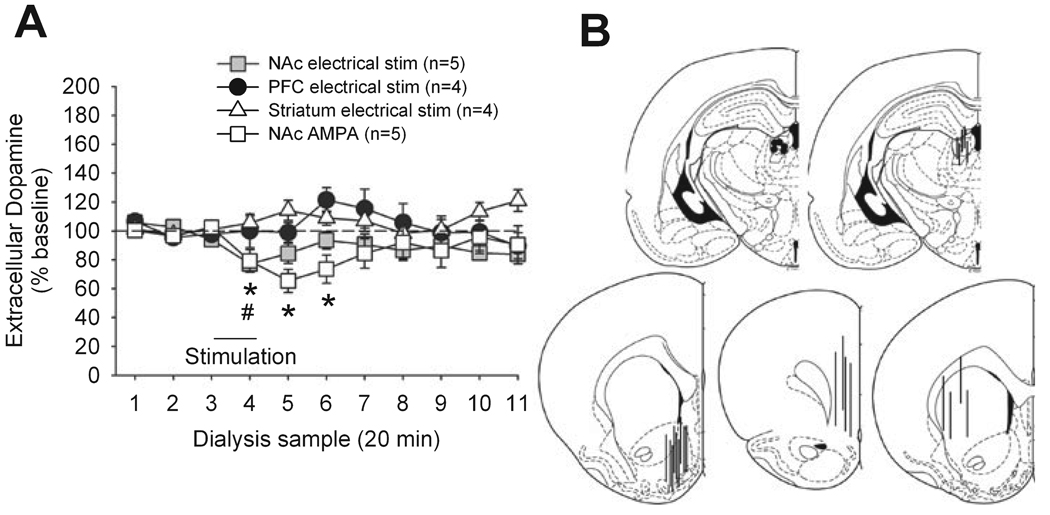
The LHb was electrically stimulated while dopamine was measured in NAc, PFC and striatum. This treatment produced a significant but small decrease in NAc dopamine release (F10,40 = 3.15,P < 0.05) but had no effect on PFC and striatal dopamine (Fig. 4). There were no changes in locomotion or stereotypy associated with this treatment (data not shown). Electrophysiological studies have shown that electrical stimulation of LHb profoundly reduces the firing of dopamine neurons (Christoph et al., 1986; Ji & Shepard, 2007) whereas we only observed a partial (or no) effect on dopamine release here. To confirm that electrical stimulation was sufficient to stimulate LHb neurons, we also examined the effect of robust chemical stimulation by AMPA (100 µm perfused for 15 min through the probe) on NAc dopamine levels (Fig. 4). This treatment also produced a small but significant decrease in NAc dopamine release (F10,40 = 3.13, P < 0.05) and did not produce any measurable behavioural effects (data not shown).
FIG. 4.
Impact of AMPA-mediated or electrical stimulation of the LHb on dopamine release in the NAc and PFC. (A) Results (dopamine release, mean ± SEM) are shown as percentages of baseline value (samples 1–3). Each sample represents a 20-min period. In both cases stimulation started immediately after the collection of sample 3 and lasted 20 min. A significant effect was only observed in the NAc; *P < 0.05 (AMPA), #P < 0.05 (electric) vs. baseline. (B) Representations of electrode or probe placement in the LHb (top) and probe placement in the NAc, PFC, and striatum (bottom).
Manipulating near-LHb structures failed to mimic its effects on DA release
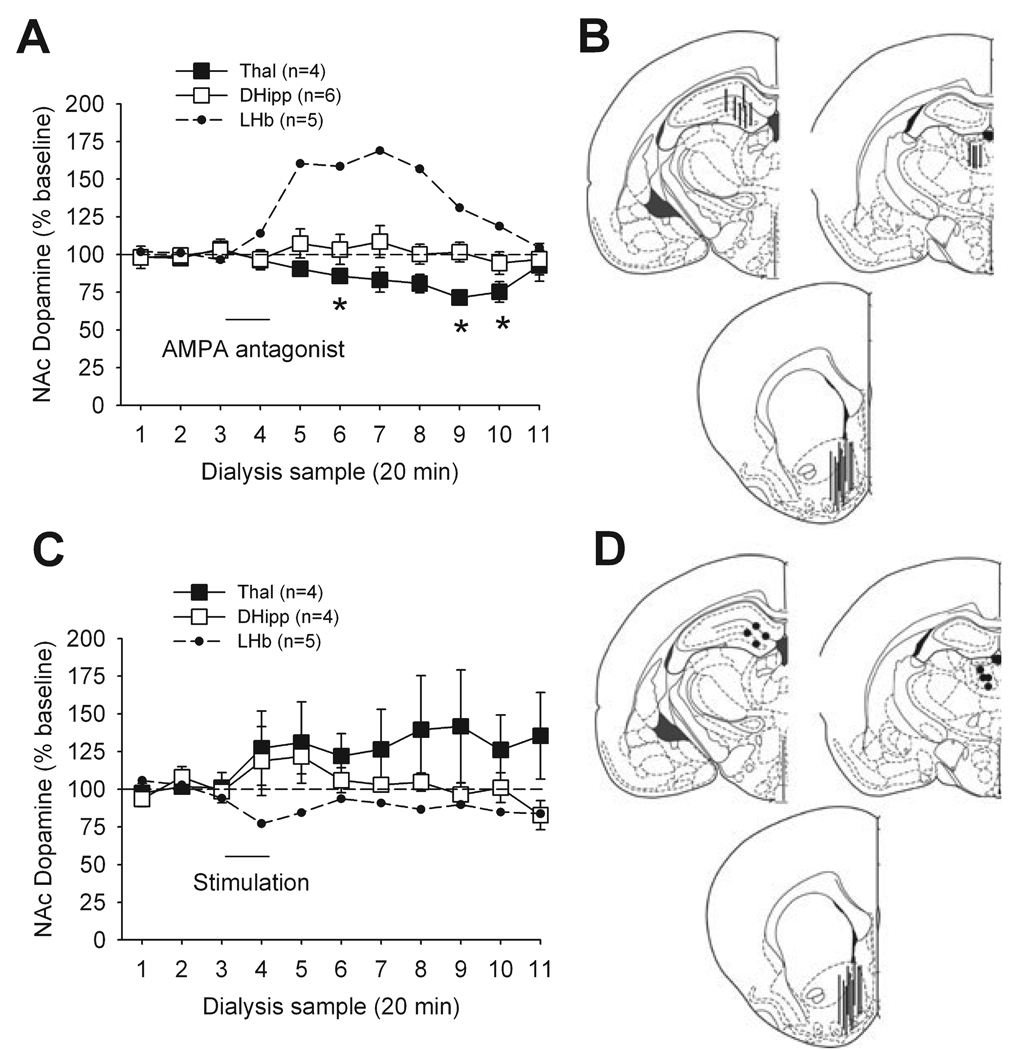
To ensure that our observations did not result from drug or current spread to structures near the LHb, we determined the effect of both electrical stimulation and AMPA antagonist application to DHipp and Thal, while dopamine was measured in NAc (Fig. 5). Application of the AMPA antagonist to the Thal significantly decreased the release of dopamine in NAc (F10,30 = 3.31, P < 0.05) but with a delayed onset and prolonged duration of action that appeared dissimilar to the effect of LHb activation. The same treatment in the DHipp had no significant effect on these measures. Electrical stimulation of either region did not have a significant effect on NAc dopamine. These treatments did not produce behavioural activation (data not shown).
FIG. 5.
Impact of inhibition and stimulation of regions adjacent to LHb on dopamine release in NAc. (A) Administration of the AMPA antagonist LY293558 in the thalamus (Thal, ■) and dorsal hippocampus (DHipp, □) on dopamine release. Results (dopamine release, mean ± SEM) are shown as percentages of baseline value (samples 1–3). Each sample represents a 20-min period. LY293558 administration started immediately after the collection of sample 3 and lasted 20 min (DHipp) or 15 min (Thal). LHb results (small dots; see Fig. 1) are shown for comparison. *P < 0.05 (Thal, vs. baseline). (B) Representations of probe placements in DHipp (top left), Thal (top right) and NAc (bottom). (C) Impact of Thal and DHipp electrical stimulation on dopamine release in NAc. Results (dopamine release, mean ± SEM) are shown as percentages of baseline value (samples 1–3). LHb results (small dots) are shown for comparison. (D) Representations of electrode placements in DHipp (top left) and Thal (top right) and probe placements in NAc (bottom).
Discussion
Our data demonstrate that in an awake state the LHb maintains an inhibitory control over dopamine neurotransmission in cortical and striatal regions. Reducing this inhibition transiently increases dopamine release in a manner that resembles increases seen during salient conditions such as reward availability or increased cognitive demand. The inhibitory influence was most robust toward regulation of dopamine in NAc and striatum. Stimulation of LHb produced minimal reduction of extracellular dopamine, suggesting that tonic activity of LHb exerts a near-maximal effect on dopamine neurotransmission.
Preferential influence of LHb on dopamine release in the NAc compared to PFC
This is the first study to address the role of LHb on regulating dopamine transmission in different terminal regions of behaving animals. Inactivation of LHb by local application of an AMPA⁄kainate antagonist transiently suppressed postsynaptic fast-acting excitatory influence. This approach, unlike electric lesions or tetrodotoxin administration used to deactivate LHb in previous studies (Lisoprawski et al., 1980), spares fibers of passage which run along the stria medullaris, the HbCpl afferent pathway, and bypass the latter (Herkenham & Nauta, 1979). Inactivation of LHb inhibition by an AMPA receptor antagonist increased dopamine release in both NAc and striatum by ~60% over baseline. This increase was sustained for over an hour after the cessation of LHb inhibition and correlated with a general increase in spontaneous behavioural activity. The magnitude and duration of dopamine release in the NAc correlated with behavioural activation and was similar to that reported when animals are engaged in effort-related processes and reward-seeking behaviour (Salamone et al., 1994; Westerink et al., 1994; Bassareo & Di Chiara, 1997; Stefani & Moghaddam, 2006). For example, in food-restricted animals that are placed in a maze for 20–40 min for a reward-retrieval task, NAc extracellular dopamine is increased by 50–75% over baseline and remains elevated for over an hour after the animal has been removed from the maze (Stefani & Moghaddam, 2006). This pattern of activation suggests that the presence of reward or motivationally salient events increases NAc dopamine release by inhibiting LHb neuronal activity. This mechanism is consistent with recent electrophysiological studies in rhesus monkey showing that the activation of dopamine neurons in response to reward is accompanied by inhibition of LHb neurons (Matsumoto & Hikosaka, 2007).
The effect of LHb inhibition on PFC dopamine release was smaller in magnitude and duration than the increase seen in the NAc and striatum. This was surprising because extracellular dopamine in PFC generally increases far more than striatal or NAc dopamine in response to salient events such as stress and increased cognitive load (Abercrombie et al., 1989; Inglis & Moghaddam, 1999; Phillips et al., 2004; Stefani & Moghaddam, 2006). Given that an indiscriminate excitation of dopamine neurons in the VTA has been reported in response to LHb inhibition (Christoph et al., 1986; Ji & Shepard, 2007), we questioned whether a general activation of VTA neurons will preferentially increase dopamine release in NAc compared to PFC. Electrical stimulation of the VTA, however, mimicked the same magnitude of increase in NAc dopamine as that seen after LHb inhibition but produced a far larger increase in PFC. Thus it appears that LHb preferentially inhibits the activity of dopamine neurons that project to NAc. It is interesting that the increase in PFC dopamine release after LHb inhibition mimicked the small and short-lasting activation of dopamine in this region during reward retrieval (Stefani & Moghaddam, 2006) as opposed to the robust activation during cognitive tasks such as working memory or set-shifting (Phillips et al., 2004; Rossetti & Carboni, 2005; Stefani & Moghaddam, 2006; van der Meulen et al., 2007). Furthermore, cognitive impairments immediately observed in rats after habenula lesions generally involve classic basal ganglia deficits such as increased impulsivity as opposed to decreased accuracy (Lecourtier & Kelly, 2005). Thus, based on comparing the magnitude and duration of dopamine activation in NAc and PFC during LHb inhibition with that reported during different behavioural contexts, it may be hypothesized that LHb preferentially regulates the activity of those dopamine neurons that encode for processes related to reward seeking, and which primarily project to NAc, but not those neurons that subserve executive functions, and which primarily project to PFC. Anatomical studies indirectly support this notion; axons from the LHb predominantly innervate the posterior part of the VTA whereas VTA projections to the PFC primarily originate from the anterior portion of this structure (Swanson, 1982). It is noteworthy that the main inhibitory drive onto LHb neurons arises from the globus pallidus (Araki et al., 1984), which has been strongly implicated in reward-related behaviours (Tindell et al., 2006). Clearly, further anatomical characterization and electrophysiological studies using antidromic stimulation are necessary to establish the validity of this mechanism.
LHb primarily has a permissive role on dopamine release
LHb sends glutamatergic axons to the VTA and substantia nigra, which predominantly synapse on GABA interneurons (Bunney & Aghajanian, 1976; Herkenham & Nauta, 1979; Geisler & Zahm, 2005) and therefore indirectly inhibit dopamine neurons. Accordingly, previous electrophysiological studies have reported that LHb stimulation profoundly inhibits dopamine neurons in the rat (Christoph et al., 1986; Ji & Shepard, 2007) and monkey (Matsumoto & Hikosaka, 2007). Here we only observed a small decrease or no effect on dopamine release in NAc, striatum or PFC. Studies by Christoph et al. (1986) and Ji & Shepard (2007) were performed in anaesthetized rats and reported profound inhibition in 85–97% of dopamine neurons. It is difficult to compare these studies with ours because the activity of LHb neurons may be down-regulated in their preparation given that anaesthesia inhibits the activity of cortical neurons which provide a major excitatory input onto LHb neurons (Greatrex & Phillipson, 1982). In awake animals where a greater number of LHb neurons may be spontaneously active, LHb stimulation may be far less effective in stimulating the already depolarized GABAergic neurons in dopamine cell body regions. A recent primate study, however, showed that in awake animals electrical stimulation of LHb does inhibits dopamine neurons (Matsumoto & Hikosaka, 2007). The result of this study differed from the data reported in anaesthetized rats in that the magnitude of inhibition was smaller and varied greatly among neurons. In over half of the recorded dopamine neurons, LHb stimulation reduced their baseline activity by < 50%. Furthermore ~20% of dopamine neurons were not inhibited by LHb stimulation. Considering that single-unit recordings may be biased toward neurons with robust spontaneous activity, the portion of neurons unresponsive to LHb stimulation may have been greater across the entire population. This smaller level of inhibition together with reduced activation of regulatory mechanisms that usually inhibit dopamine release, such as reduced occupancy of presynaptic D2 autoreceptors, would explain the small, or lack of, effect of LHb stimulation on extracellular dopamine release. Thus while overstimulation of LHb in awake animals may inhibit some dopamine neurons, the impact of this effect in reducing dopamine efflux in terminal regions is small and limited to NAc. Overall, the modulatory role of LHb on extracellular dopamine in an awake brain appears to be one in which inhibition of the LHb produces increases in dopamine release that are much larger than decreases following LHb activation.
Specificity of the present results
A problem with local infusion or electrical stimulation studies is that the applied compound or current may mediate an effect by diffusing to surrounding structures. This is especially a concern with the present work because LHb is surrounded by other structures that have been reported to influence dopamine cell activity. To address this issue we repeated the stimulation and inhibition experiments in two additional regions adjacent to the LHb. These regions included DHipp and thalamus, where stimulation under some circumstances has been shown to enhance NAc or dorsal striatal dopamine release (Kilpatrick et al., 1986; Strecker & Moneta, 1994; Churchill et al., 1996; Peleg-Raibstein & Feldon, 2006). Our studies showed that DHipp and thalamus inhibition or stimulation did not mimick the impact of LHb inhibition or stimulation on dopamine release. Thus, whereas these regions may influence dopamine-related functions, the data presented here were not contaminated by diffusion of compounds or current from LHb to surrounding structures.
Implications for brain disorders
Basic and clinical findings suggest that the function of the habenular complex, in particular the LHb, may be compromised in psychiatric disorders, in particular schizophrenia and addiction (Sandyk, 1992; Caputo et al., 1998; Ellison, 2002; Lecourtier et al., 2005; Heldt & Ressler, 2006). Both of these disorders have traditionally been associated with abnormal dopamine neurotransmission (Carlsson, 1978; Wise & Rompre, 1989). In the case of addictive disorders, dopamine projections to NAc are thought to be critical for maintaining behaviours that mediate drug-taking (Berridge & Robinson, 1998; Kelley, 1999). The recent electrophysiological findings that the activity of dopamine neurons which signal reward coincides with LHb inhibition (Matsumoto & Hikosaka, 2007), together with the present results that LHb inhibition preferentially increase NAc dopamine release, are consistent with a role for LHb pathology in mediating abnormal reward-seeking behaviour. In schizophrenia, although primary pathology in the dopamine system has not been found in post-mortem studies, imaging studies in individuals with schizophrenia support the idea that limbic striatal, and not cortical, dopamine is hyperactive (Abi-Dargham et al., 2000). Compromised LHb function is entirely consistent with this mechanism because it will abnormally augment dopaminergic tone in dorsal and ventral striatum in the absence of pathology within dopamine neurons.
Acknowledgement
This work was supported by the US National Institute of Mental Health.
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- DHipp
dorsal hippocampus
- GABA
gamma-aminobutyric acid
- HbCpl
habenular complex
- LHb
lateral habenular nucleus
- PFC
prefrontal cortex
- NAc
nucleus accumbens
- Thal
thalamus
- VTA
ventral tegmental area
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MS. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles L, Weiss R, Cooper T, Mann J, Van Heertum R, Gorman J, M L. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl Acad. Sci. USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, McGeer PL, Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988;441:319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Araki M, McGeer PL, McGeer EG. Retrograde HRP tracing combined with a pharmacohistochemical method for GABA transaminase for the identification of presumptive GABAergic projections to the habenula. Brain Res. 1984;304:271–277. doi: 10.1016/0006-8993(84)90330-5. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res. 1976;117:423–435. doi: 10.1016/0006-8993(76)90751-4. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Caputo A, Ghiringhelli L, Dieci M, Giobbio GM, Tenconi F, Ferrari L, Gimosti E, Prato K, Vita A. Epithalamus calcifications in schizophrenia. Eur Arch. Psychiatry Clin. Neurosci. 1998;248:272–276. doi: 10.1007/s004060050049. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Antipsychotic drugs, neurotransmitters, and schizophrenia. Am. J. Psychiatry. 1978;135:164–173. doi: 10.1176/ajp.135.2.165. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J. Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Duffy P, Kalivas PW. The mediodorsal nucleus of the thalamus in rats – II. Behavioral and neurochemical effects of GABA agonists. Neuroscience. 1996;70:103–112. doi: 10.1016/0306-4522(95)00352-j. [DOI] [PubMed] [Google Scholar]
- Ellison G. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur. Neuropsychopharmacol. 2002;12:287–297. doi: 10.1016/s0924-977x(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Floresco S, Yang C, Phillips A, Blaha C. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anesthetized rat. Eur. J. Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J. Comp. Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. The cortical dopamine system: Role in memory and cognition. Adv. Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Greatrex RM, Phillipson OT. Demonstration of synaptic input from prefrontal cortex to the habenula in the rat. Brain Res. 1982;238:192–197. doi: 10.1016/0006-8993(82)90782-x. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Lesions of the habenula produce stress- and dopamine-dependent alterations in prepulse inhibition and locomotion. Brain Res. 2006;1074:229–239. doi: 10.1016/j.brainres.2005.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J. Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost A, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J. Neurochem. 2001;78:920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA (A) receptor-mediated mechanism. J. Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology. 1999;27:198–213. [Google Scholar]
- Kelley A. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Pycock CJ, Riches I, Phillipson OT. Thalamic control of dopaminergic functions in the caudate-putamen of the rat – III. The effects of lesions in the parafascicular-intralaminar nuclei on D2 dopamine receptors and high affinity dopamine uptake. Neuroscience. 1986;19:991–1005. doi: 10.1016/0306-4522(86)90311-8. [DOI] [PubMed] [Google Scholar]
- Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J. Comp. Neurol. 2005;483:236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Mulligan OF, Checkley SA, Gray NS, Hemsley DR, Thornton JC, Corr PJ, Toone BK, Gray JA. Effects of d-amphetamine and haloperidol on latent inhibition in healthy male volunteers. J. Psychopharmacol. 1999;13:398–405. doi: 10.1177/026988119901300411. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. Bilateral lesions of the habenula induce attentional disturbances in rats. Neuropsychopharmacology. 2005;30:484–496. doi: 10.1038/sj.npp.1300595. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur. J. Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Saboureau M, Kelly CD, Pévet P, Kelly PH. Impaired cognitive performance in rats after complete epithalamus lesions, but not after pinealectomy alone. Behav. Brain Res. 2005;161:276–285. doi: 10.1016/j.bbr.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Joosten RN, de Bruin JP, Feenstra MG. Dopamine and noradrenaline efflux in the medial prefrontal cortex during serial reversals and extinction of instrumental goal-directed behavior. Cereb. Cortex. 2007;17:1444–1453. doi: 10.1093/cercor/bhl057. [DOI] [PubMed] [Google Scholar]
- Mitchell SN, Yee BK, Feldon J, Gray JA, Rawlins JN. Activation of the retrohippocampal region in the rat causes dopamine release in the nucleus accumbens: disruption by fornix section. Eur. J. Pharmacol. 2000;407:131–138. doi: 10.1016/s0014-2999(00)00741-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J. Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J. Neurosci. 2005;25:2322–2329. doi: 10.1523/JNEUROSCI.3038-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Sandyk R. Pineal and habenula calcification in schizophrenia. Int. J. Neurosci. 1992;67:19–30. doi: 10.3109/00207459208994773. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: Predominant and extrasynaptic localization in dendritic spines. Neurobiology. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani M, Moghaddam B. Rule learning and reward contingency are asociated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucelus accumbens, and dorsal striatum. J. Neurosci. 2006;26:8810–8818. doi: 10.1523/JNEUROSCI.1656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker RE, Moneta ME. Electrical stimulation of the kindled hippocampus briefly increases extracellular dopamine in the nucleus accumbens. Neurosci. Lett. 1994;176:173–177. doi: 10.1016/0304-3940(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. The projection of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J. Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Teismann A, de Vries JB. Increase in dopamine release from the nucleus accumbens in response to feeding: a model to study interactions between drugs and naturally activated dopaminergic neurons in the rat brain. Arch. Pharmacol. 1994;349:230–235. doi: 10.1007/BF00169288. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu. Rev. Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]