Abstract
Recent rodent studies suggest that gonadal hormones influence extinction of conditioned fear. Here we investigated sex differences in, and the influence of estradiol and progesterone on, fear extinction in healthy humans. Men and women underwent a two-day paradigm in which fear conditioning and extinction learning took place on day 1 and extinction recall was tested on day 2. Visual cues were used as the conditioned stimuli and a mild electric shock was used as the unconditioned stimulus. Skin conductance was recorded throughout the experiment and used to measure conditioned responses (CRs). Blood samples were obtained from all women to measure estradiol and progesterone levels. We found that higher estradiol during extinction learning enhanced subsequent extinction recall but had no effects on fear acquisition or extinction learning itself. Sex differences were only observed during acquisition, with men exhibiting significantly higher CRs. After dividing women into low- and high-estradiol groups, men showed comparable extinction recall to high-estradiol women, and both of these groups showed higher extinction recall than low-estradiol women. Therefore, sex differences in extinction memory emerged only after taking into account women's estradiol levels. Lower estradiol may impair extinction consolidation in women. These findings could have practical applications in the treatment of anxiety disorders through cognitive and behavioral therapies.
Keywords: estrogen, progesterone, sex difference, fear, menstrual cycle, learning and memory
Failure to extinguish conditioned fear may play an important role in the pathogenesis of anxiety disorders (Davis et al., 2006; Milad et al., 2008; Milad and Rauch, 2007; Milad et al., 2006b; Orr et al., 2000; Rauch et al., 2006). Extinction forms the theoretical basis of treatment by exposure (Rothbaum and Davis, 2003). A substantial number of studies conducted in rodents and more recently in humans indicate that brain regions associated with the acquisition and consolidation of conditioned fear extinction are implicated in anxiety disorders. These areas include the amygdala, ventromedial prefrontal cortex (vmPFC), and hippocampus (for review, see Quirk and Mueller, 2008). These brain regions are sexually dimorphic and contain a high density of gonadal hormone receptors (Giedd et al., 1996; Goldstein et al., 2001; Ostlund et al., 2003). This may explain why measures of fear and arousal are associated with changes in hormonal levels over the menstrual cycle (Cahill, 2003; Goldstein et al., 2005; Gupta et al., 2001; Jasnow et al., 2005) and sex differences in arousal (Goldstein et al., 2010). Such findings also hold promise for clarifying important sex differences in anxiety disorders (Kinrys and Wygant, 2005; Pigott, 2003).
We recently showed that female rats undergoing extinction learning during the proestrous phase of the estrus cycle (when estradiol and progesterone levels are elevated) exhibited better extinction memory during subsequent extinction recall (i.e., retention) testing (Milad et al., 2009a). Moreover, exogenously administered estradiol and progesterone facilitated extinction recall, whereas estradiol and progesterone receptor antagonists impaired it (Milad et al., 2009a). A recent study found that estradiol administration into the hippocampus in female rats facilitated fear extinction (Chang et al., 2009). Collectively, these data suggest that gonadal hormones influence the consolidation of extinction memory.
In a study of healthy humans, we showed that phase of the menstrual cycle influences consolidation of fear extinction (Milad et al., 2006a), but gonadal hormones were not measured. The present goal was to explore the associations of estradiol and progesterone with physiological aspects of acquisition and extinction of conditioned fear in healthy women at different phases of their menstrual cycles, and to compare their responses to those of healthy men. Estradiol and progesterone levels were measured from blood samples obtained prior to the initiation of a previously described two-day fear conditioning and extinction experiment (Milad et al., 2005; Milad et al., 2007; Rauch et al., 2005). On Day 1, participants underwent fear conditioning in virtual context A and extinction learning in virtual context B. On Day 2, extinction recall (context B) was tested using skin conductance response (SCR) as a measure of fear (Milad et al., 2005; Rauch et al., 2005). Based on our recent findings in female rats and the literature reviewed above, we hypothesized that estradiol and progesterone would facilitate the extinction of conditioned fear and/or the consolidation thereof, such that women with higher levels of these hormones would display lower conditioned responses (CRs) during subsequent extinction recall. Further, these hormonal differences would, in part, account for sex differences in conditioned fear extinction.
Methods and Materials
Participants
Fifty-four participants (36 women, 18 men) ages 18-30 were recruited from the local community via advertisement. Women were divided by hormonal levels into two groups of 18 (see below). All participants were right-handed, without endocrinologic, neurologic, or other medical conditions, and without Axis I mental disorders, including substance dependence or abuse, as determined by the Structured Clinical Interview for DSM-IV (First et al., 2002). No participant was using psychoactive or other potentially confounding drugs or medications. All female participants had regular menstrual cycles by history and had not been using oral contraceptives or hormone replacement for at least three months. After a complete description of the procedures, written informed consent was obtained from all participants in accordance with the requirements of the Partners Healthcare Human Research Committee.
Women were studied during the early follicular phase (days 3-5 of the cycle, n = 6), late follicular phase (days 10-12, n = 10), early luteal phase (days 18-20, n = 11), or late luteal phase (days 25-27, n = 11). Women were divided into low-estradiol group (LE) and high-estradiol (HE) groups, and separately into low-progesterone (LP) and high-progesterone (HP) groups based on median splits regardless of menstrual phase. Sixty one percent of women in the LE group were part of the LP group, and 66% of women in the HE group were part of the HP group.
Conditioning and Extinction Procedure
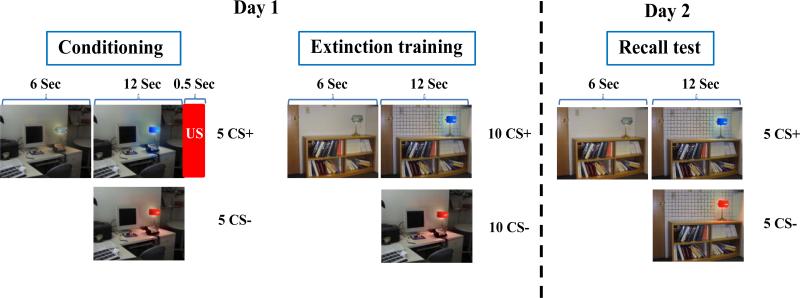
The fear conditioning and extinction procedures were identical to those previously described (Milad et al., 2006a; Milad et al., 2005). Digital photographs of two different rooms constituted the visual contexts. Each context contained a lamp that was shown first in the off position (no color) and then switched on to one of two different colors (blue or red), which constituted the conditioned stimuli (CSs) (see figure 1). The selection of the CS+ color (followed by shock) and CS- color (no shock) and the contexts were pseudorandom and counterbalanced across participants. Contexts and CSs were displayed on a computer monitor three feet in front of participants who were seated upright in a chair. The unconditioned stimulus (US) was a 500 ms electric shock previously selected by the participant to be “highly annoying but not painful” delivered through electrodes attached to the second and third fingers of the right hand (Milad et al., 2005; Orr et al., 2000). The shock electrodes remained attached during each session of the experiment, but the US was administered only during the Conditioning session.
Figure 1.
Fear conditioning and extinction protocol. CS+: conditioned stimulus, CS-: conditioned stimulus never paired with shock (unconditioned stimulus, US). Adapted from Milad et al., 2005.
The experimental protocol was conducted over two days. On Day 1, the Habituation session consisted of eight trials in which the to-be CS+ and to-be CS- (4 of each) were presented in a counterbalanced manner within both the to-be conditioning context and the to-be extinction context. The Conditioning session consisted of five CS+ and five CS- trials, presented within the conditioning context. The US occurred immediately following each CS+ offset (100% reinforcement). The Extinction session was divided into two sub-sessions: early and late, which were separated by an approximate one-minute rest period. Each sub-session consisted of five CS+ and five CS- trials, all presented within the extinction context. On Day 2, the Recall session was identical to an Extinction sub-session given on Day 1. The US was never presented on day 2. When questioned after completion of the study, all subjects were explicitly aware of the CS-US contingency.
Serological Measurements
All subjects underwent the experimental protocol in the morning and were instructed not to eat after midnight prior to participation. Blood samples were drawn on Days 1 and 2 20-30 minutes prior to the experiment. Estradiol levels were assessed using an RIA kit (Roche Diagnostics, Indianapolis, IN) with a sensitivity of 18.4 pmol/L and an intra-assay coefficient of variance (CV) of 1.6-5.7%. Progesterone levels were determined using an RIA kit (Roche Diagnostics, Indianapolis, IN) with a sensitivity of 0.095 nmol/L, and an intra-assay CV of 1.5 – 2.7%.
Psychometric Measures
On a day of psychiatric assessment prior to the experiment, each subject completed the Beck Anxiety and Beck Depression Inventories, Anxiety Sensitivity Index (ASI), STAI Trait (T) assessment, and NEO Five Factor Inventory (NEO-FFI). After undergoing conditioning and extinction on Day 1 of the experiment, subjects completed the STAI State (S) assessment.
Psychophysiological Measures
In each trial, the context image was presented for 18 seconds: six seconds alone (light off) followed by 12 seconds in combination with the CS+ or CS- (light on). The mean inter-trial interval was 16 seconds and the range 12-21 seconds. The SCR was calculated during each CS by subtracting the mean skin conductance level (SCL) during the two seconds immediately preceding context onset from the highest SCL recorded during the 12-second CS presentation. To reduce heteroscedasticity, each SCR was square-root transformed prior to analysis (for a negative SCR, the square root of the absolute value was calculated and given a negative sign). Differential responses were calculated by subtracting the SCRs to the CS- from those of the CS+, and these values were used to conduct all statistical analyses. Skin conductance level was measured during the five seconds preceding the onset of each habituation session trial and then averaged across all eight trials to yield baseline SCL. Unconditioned response (UCR) levels were calculated by subtracting the average SCR during the one second immediately following the shock (before the SCR starts to rise) from the maximum SCR during the five seconds after the shock.
To quantify the amount of extinction memory expressed during the recall test on Day 2, we calculated an “extinction retention index”, which takes into account the level of fear acquired during conditioning by each subject when measuring the recall of extinction. Each subject's average differential SCR during the first two trials of the Extinction Recall session was divided by their largest differential SCR during the Conditioning session and then multiplied by 100, yielding a percentage of maximal conditioned responding. This was subtracted from 100% to yield an extinction retention index. We chose the highest SCR during the Conditioning session as a reference to assess the extinction performance on Day 2. This contrasts with using the last few conditioning trials as is commonly done in animal studies (Quirk et al., 2000) because human CRs typically decline towards the end of conditioning. Unless specified, all data are presented as means ± standard error of the mean (S.E.). Analysis of variance (ANOVA) with repeated measures was used to analyze the data across experimental sessions, and analysis of covariance (ANCOVA) was used to adjust for potentially confounding variables such as baseline SCL, shock intensity, UCR, and anxiety sensitivity index when comparing men versus women. The statistical software was SPSS (Version 17.0, SPSS Inc., Chicago, IL 2008); the Tukey Honestly Significant Difference method was used in making post hoc comparisons. Student t-test was used when appropriate.
Results
Demographics, Personality Measures, and Baseline Physiological Measures
Men and women did not differ in age or years of education. Men exhibited higher mean baseline SCL, higher mean unconditioned SCR to the US, and higher mean shock level selection. Men scored lower on the NEO-FFI Agreeableness survey than women (for full statistics, see supplemental table 1).
Comparisons between the low-estradiol (LE, n = 18, mean 57.5 ± 5.7 pg/ml; median split 112 pg/ml) and high-estradiol (HE, n = 18, mean 256.7 ± 34.4 pg/ml) female groups revealed no differences in age, years of education, nor ethnicity. The HE group showed lower baseline SCL and scored lower on the NEO-FFI Extroversion survey than the LE group (supplemental table 2). Comparisons between the low-progesterone (LP, n = 18, mean 0.58 ± 0.04 pg/ml; median split 0.9 pg/ml) and high-progesterone (HP, n = 18, mean 8.3 ± 1.6 pg/ml) female groups revealed no differences in any demographic, physiological, or personality measure (supplemental table 3).
Fear Conditioning and Extinction
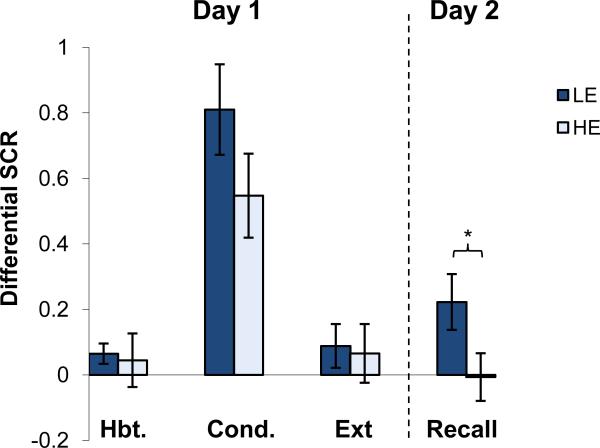
Group differences in differential responses (CS+ minus CS-) during Habituation, Conditioning, Extinction Learning, and Recall comparing women with high estradiol (HE) and women with low estradiol (LE) are shown in Figure 2. Repeated-measures ANOVA with two factors (experimental Session × Group) revealed significant main effects of Session (F(1,34) = 21.1, p < 0.001) and Group (F(1,34) = 4.18, p < 0.05) and no significant interaction (F(3,102) = 0.98, p = 0.40). Post-hoc analysis showed that the two groups did not differ significantly during Habituation, Conditioning, or Extinction Learning on day 1 (p values > 0.05). During Recall, the HE group exhibited significantly lower CRs relative to the LE group (p < 0.05 see figure 2). A further examination of the relationship between estradiol levels and extinction retention in women, operationalized as correlating estradiol levels on day 1 and extinction retention index, revealed a significant positive correlation (r = 0.39, p = 0.03).
Figure 2.
Conditioned responses (SCRs) of high and low estradiol (HE and LE respectively) females across each session of the experiment. Habituation shows the mean of five trials, Conditioning displays the mean max response, Extinction shows the mean of the last two trials of each subject, Recall shows the mean of the first two trials. All data are shown based on differential response (CS+ minus CS-). Significant differences (p < .05) are indicated by *.
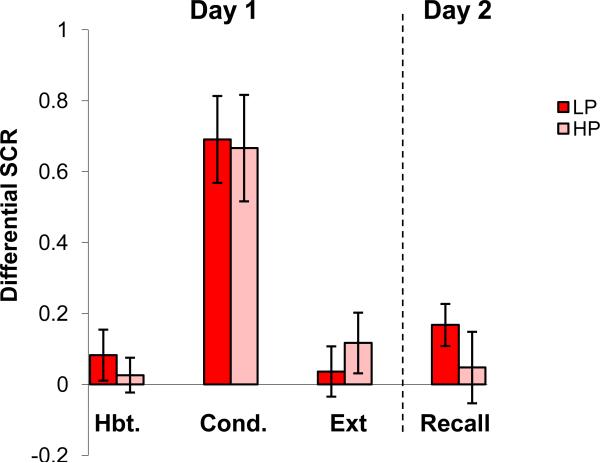
Group differences during each experimental session (CS+ minus CS-) in high progesterone (HP) versus low progesterone (LP) women are shown in Figure 3. ANOVA revealed a significant main effect of Session (F(1,34) = 20.8, p < 0.001), but not of Group (F(1,34) = .19, p = 0.67), or interaction (F(3,102) = 0.40, p = 0.84), indicating that the LP and HP groups did not differ at any experimental session on day 1 or day 2.
Figure 3.
Conditioned responses (SCRs) of high (HP) and low progesterone (LP) women across each session of the experiment. See Figure 2 footnote for details. No significant differences were found.
When women were divided into LE and HE groups, mean progesterone levels significantly differed (LE 1.6 + 0.63 vs. HE 7.2 + 1.8, t(35) = 2.95 p = 0.005). Mean estradiol levels in the LP and HP groups, however, were not statistically different (LP 131.3 ± 35.5 vs. HP 182.8 ± 38.2, t(35) = 1.07, p = 0.29). Two-factor ANCOVA for LE versus HE with progesterone level as a covariate yielded a comparably significant group main effect (p <0.05) to that noted above, suggesting that progesterone was not influencing fear extinction.
However, as mentioned previously, 61% of women in the LE group were also part of the LP group, and 66% of women in the HE group were part of the HP group. Thus, while our data suggest no progesterone effects on extinction recall, we did not obtain sufficient differential variance between the two groups to fully examine the effects of progesterone independent of estradiol. In addition, we conducted analyses to examine the impact of estradiol either opposed or unopposed by progesterone, operationalized as an E:P ratio. Here we again conducted a median split and a correlation analysis, based on the E:P ratio, that revealed no significant effects (data not shown).
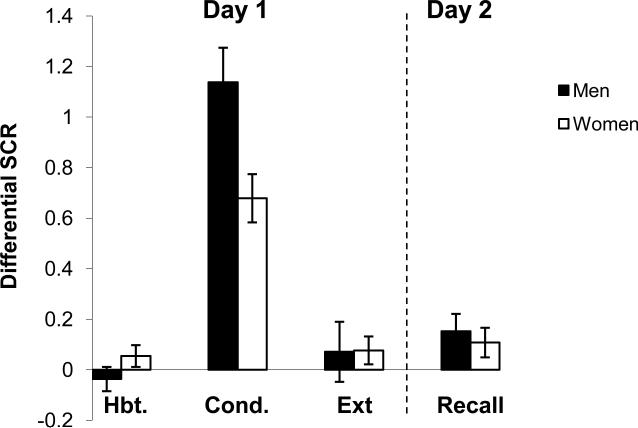
Women were pooled to assess sex differences in differential responses (CS+ minus CS-) during each experimental session (see Figure 4). Repeated-measures ANOVA (Session × Sex) revealed a significant main effect of Session (F(1,52) = 6.1, p < 0.05) and significant interaction (F(3,156) = 4.6, p < 0.01). Post-hoc analysis revealed that men exhibited significantly higher conditioned responses during the Conditioning phase relative to women (p<0.05).
Figure 4.
Conditioned responses (SCRs) of men and all women across each session of the experiment. See Figure 2 footnote for details.
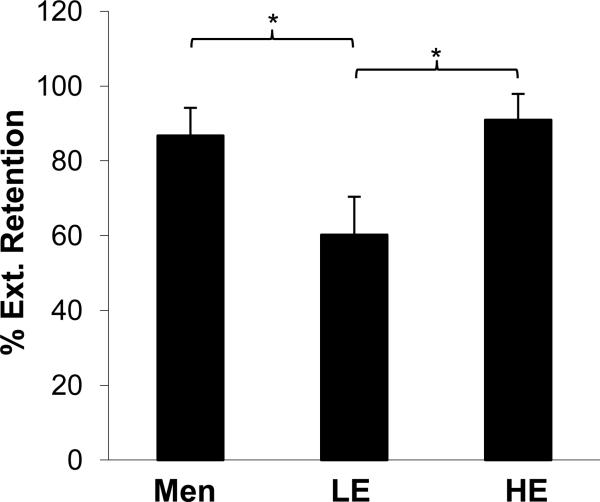
Regarding extinction retention index, Student's t-test revealed no significant difference between men and women in extinction retention (85.9% ± 7.7% and 74.6% ± 7.1% respectively, t = -1.01, p = 0.32). However, as shown in Figure 5, mean extinction retention indices for male, HE female, and LE females were significantly different (F(2,48) = 3.5, p < 0.04). Post-hoc analysis revealed that men and HE women did not differ (p = 0.69), whereas both of these groups showed significantly higher extinction retention (i.e. less fear) than LE women (p < 0.02 for HE vs. LE women, and p = 0.04 for men vs. LE women).
Figure 5.
Percent extinction retention for men, women with high estradiol (HE) levels, and women with low estradiol (LE) levels. Significant differences (p < .05) are indicated by *.
Discussion
We examined the influence of natural fluctuations of gonadal hormones on the acquisition, extinction learning, and extinction recall of conditioned fear in women. Estradiol levels did not affect the acquisition or extinction of conditioned fear but facilitated extinction memory recall. These results suggest a possible role of estradiol in the consolidation of extinction memories. With regard to sex differences, when gonadal hormones were not considered, sex differences were observed during the acquisition of conditioned fear, with men showing elevated CRs relative to women, replicating our previous report (Milad et al., 2006a). When taking the women's estradiol levels into consideration, however, sex differences were observed during the extinction retention test. Men and women with high estradiol levels exhibited significantly more extinction memory than women with low estradiol levels.
Several studies previously examined sex differences in fear conditioning in humans (van der Molen et al., 1988; Zorawski et al., 2005). However, the influence of estradiol and on fear extinction and extinction memory has not been addressed. Our findings here suggest that estradiol appears to significantly facilitate extinction recall in women. Such a role for estradiol in extinction retention has recently received support from the rodent literature. In an auditory fear conditioning paradigm, we recently showed that administration of estradiol to female rats significantly enhanced extinction recall whereas injection of estradiol receptor antagonists impaired it (Milad et al., 2009a). Chang et al., (2009) have shown that injections of estradiol into the hippocampus facilitate extinction of contextual fear. Moreover, estradiol has anxiolytic effects in rodents (Lund et al., 2005; Walf and Frye, 2005) and appears to facilitate memory formation in several behavioral paradigms (Leuner et al., 2004), including extinction of passive avoidance (Rivas-Arancibia and Vazquez-Pereyra, 1994) and conditioned-taste aversion (Yuan and Chambers, 1999). Previously, we assessed the influence of menstrual phase on extinction recall by focusing on two phases of the menstrual cycle: early follicular, where estradiol and progesterone levels are low, and late follicular, where estradiol levels are high and progesterone relatively low (Milad et al., 2006a). However, hormonal levels were not directly measured in that study, and therefore estimates relied solely on the reports of the participants regarding their menstrual phase in contrast to the study reported here.
Unlike estradiol, progesterone did not appear to have a significant effect on fear extinction. This is interesting given that we have recently shown that progesterone does appear to have a significant role in facilitating recall of fear extinction in female rats (Milad et al., 2009a). Moreover, it has also been shown that progesterone administration to rodents facilitates cognitive performance in a variety of behavioral tasks such as object recognition (Frye and Walf, 2008). Allopregnanolone, a metabolite of progesterone, has anxiolytic effects that are mediated through brain regions involved in fear extinction such as the amygdala and the vmPFC (Engin and Treit, 2007). The substantial overlap of estradiol and progesterone in our sample did not allow us to fully examine the effects of progesterone independent of estradiol. Thus, it remains possible that progesterone may have an effect on fear extinction consolidation in women directly or perhaps by interacting with estradiol. Additional studies are needed to further examine the role of progesterone in fear extinction in women.
One study of healthy human subjects reported no sex differences in fear conditioning (Zorawski et al., 2005), whereas another reported elevated CRs in women relative to men (Guimaraes et al., 1991). The apparent discrepancy between the results of the above studies and our data presented herein may be due, in part, to at least two factors. First, these studies did not control for menstrual cycle phase in their sample. Second, these studies included an unidentified number of women on birth control (who by definition would not be cycling), with other women who were cycling, thus introducing potential confounds with regard to investigating sex effects. However, men showed elevated conditioned responses during the acquisition phase relative to women, which was consistent with our previous results (Milad et al., 2006a), and with finding increased freezing in male relative to female rodents in cued and contextual fear conditioning (Aguilar et al., 2003; Gupta et al., 2001; Wiltgen et al., 2001). Our findings are also consistent with a recent functional MRI study that demonstrated increased brain activity in stress response circuitry in men compared with women during ovulation (when estradiol is high relative to progesterone) in contrast to men compared with women during the early follicular period (during which estradiol and progesterone are low) (Goldstein et al., 2010).
In the present study, similar to the stress response in our previous work (Goldstein et al., 2010), men consistently displayed elevated expression of CRs during fear conditioning relative to both HE and LE groups in women (data not shown). Thus, these data indicate that sex differences during fear acquisition may be mediated by other mechanisms, including other hormonal systems that may or may not interact with estrogen and progesterone. For example, it has been previously shown that cortisol levels are associated with facilitated memory recall during acute stress in women (Andreano et al., 2008). Pre-conditioning injections of cortisol reduced conditioned fear in men while it had the opposite effect in women (Merz et al., 2010; Stark et al., 2006), thus suggesting that sex differences during fear learning may be mediated via the impact of cortisol (Andreano and Cahill, 2009). Another possibility for the observed sex differences during fear conditioning may be due to influences of gonadal hormones during early development on brain regions mediating fear conditioning (Dalla and Shors, 2009).
The level of extinction memory in men was comparable to that in women with high estradiol, whereas women with low estradiol showed significantly lower extinction memory during the recall test relative to men. This finding is counterintuitive given that the levels of estradiol in men would be most comparable to those found in low-estradiol women, and therefore one would have expected comparable extinction between these two groups. We speculate that the observed differences may be due to the elevated levels of testosterone in men. It has been shown that testosterone injections into male rats facilitated learning of inhibitory avoidance, reduced anxiety levels, and facilitated extinction of conditioned fear in mice (Edinger and Frye, 2007; Frye et al., 2008). Testosterone may have direct effects on facilitating extinction in men and may have indirect effects through its aromatization into estrogen. The role of testosterone in men and women on extinction memory consolidation should be further examined given that there are few studies investigating this issue.
It is worth noting that we observed a significant group difference in the extroversion trait between the HE and LE groups and significant group difference in the agreeableness factor between men and women. In a previous study, we found that only extroversion was positively correlated with fear extinction (Rauch et al., 2005). Yet, in the present sample, extroversion was higher in women with lower extinction memory (the lowe-stradiol group) and was lower in the women that showed enhanced extinction memory (the high estradiol group). Thus, these differences are unlikely to have influenced the between-group differences we observed in fear extinction. In addition, we observed a marginal difference between men and women in the ASI measure (supplemental table 1). This difference does not appear to influence group psychophysiological differences as such differences remained significant when co-varying for the ASI values.
Findings in the study reported here may have clinical implications given that several epidemiological studies have reported elevated incidence of anxiety disorders in women relative to men (Kinrys and Wygant, 2005; Pigott, 2003). Other studies have demonstrated sex differences in brain activity in processing emotional stimuli (Andreano and Cahill, 2009; Cahill, 2003; Goldstein et al., 2010; Protopopescu et al., 2005). Importantly, women diagnosed with premenstrual dysphoric disorder (PMDD) show increased anxiety and depression symptoms that are recurrent during every cycle, and that have recently been associated with genetic variation of the estrogen receptor alpha (Huo et al., 2007). Moreover, women diagnosed with PMDD exhibited increased amygdala and decreased vmPFC activation in the context of a behavioral inhibition paradigm (Protopopescu et al., 2008). Results of the present study suggest that there may be an association between natural variation of gonadal hormones, estradiol in particular, and the brain circuitry involved in fear inhibition. Collectively, therefore, it appears that low levels of gonadal hormones may be related to impaired fear extinction and impaired processing of emotional stimuli in women with various psychiatric disorders. Future studies of the neural circuitry of fear extinction, such as those conducted with healthy humans and patients with anxiety disorders such as PTSD (Bremner et al., 2005; Milad et al., 2009b), should consider obtaining information regarding gonadal hormone levels and examining their associations with activity in brain regions involved in fear inhibition. Such studies could potentially lead to novel ways to enhance the efficacy of extinction-based therapies for anxiety disorders. For example, concentrating exposure therapy at a particular phase of the menstrual cycle in women and/or administration of estradiol in conjunction with exposure therapy may strengthen extinction consolidation.
Supplementary Material
Acknowledgments
The work was supported by a grant from the National Institute of Mental Health (K01MH080346) to M.R.M. The project described was also supported by Grant 1UL1 RR025758-01, Harvard Clinical and Translational Science Center from the National Center for Research Resources, and ORWH-NIMH P50 MH082679 (JMG, P.I.) for JMG's time. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
List of Abbreviations
- CR
Conditioned Response
- vmPFC
ventromedial Prefrontal Cortex
- SCR
Skin Condunctance Response
- LE
Low Estrogen
- HE
High Estrogen
- LP
Low Progesterone
- HP
High Progesterone
- CS
Conditioned Stimulus
- US
Unconditioned Stimulus
- ASI
Anxiety Sensitivity Index
- SCL
Skin Conductance Level
- UCR
Unconditioned Response
- PTSD
Post-traumatic Stress Disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
Dr. Scott Rauch has received honoraria and/or consultation fees from Neurogen, Sepracor, Novartis and Medtronic. He has also participated in research funded by Medtronic, Cyberonics, Cephalon and Northstar. The remaining authors have no conflicts of interest to report.
Reference List
- Aguilar R, Gil L, Gray JA, Driscoll P, Flint J, Dawson GR, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Tobena A. Fearfulness and sex in F2 Roman rats: males display more fear though both sexes share the same fearfulness traits. Physiol Behav. 2003;78:723–732. doi: 10.1016/s0031-9384(03)00043-x. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009 doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behav Pharmacol. 2007;18:461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam Gibbon, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33:1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr., Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Hellewell J, Hensman R, Wang M, Deakin JF. Characterization of a psychophysiological model of classical fear conditioning in healthy volunteers: influence of gender, instruction, personality and placebo. Psychopharmacology (Berl) 1991;104:231–236. doi: 10.1007/BF02244184. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, Weinberger DR, Rubinow DR. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62:925–933. doi: 10.1016/j.biopsych.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2005 doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Kinrys G, Wygant LE. [Anxiety disorders in women: does gender matter to treatment?]. Rev Bras Psiquiatr. 2005;27(Suppl 2):S43–S50. doi: 10.1590/s1516-44462005000600003. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology. 2010;35:33–46. doi: 10.1016/j.psyneuen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006a;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009a doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AB, Shin LM, Lasko NB, Handwerger K, Orr SP, Rauch SL. Neurolbiological basis for failure to recall extinction memory in Posttraumatic Stress Disorder. Biol.Psychiatry . 2009b doi: 10.1016/j.biopsych.2009.06.026. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A Role for the Human Dorsal Anterior Cingulate Cortex in the Expression of Learned Fear. Biol.Psychiatry . 2007 doi: 10.1016/j.biopsych.2007.04.032. Ref Type: In Press. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Ann N Y Acad Sci. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006b doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, Silbersweig D, Stern E. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci U S A. 2005;102:16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108:87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Milad MR, Orr SP, Quinn BT, Fischl B, Pitman RK. Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport. 2005;16:1909–1912. doi: 10.1097/01.wnr.0000186599.66243.50. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rivas-Arancibia S, Vazquez-Pereyra F. Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life Sci. 1994;54:L363–L367. doi: 10.1016/0024-3205(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32:1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- van der Molen GM, Merckelbach H, van den Hout MA. The possible relation of the menstrual cycle to susceptibility to fear acquisition. J Behav Ther Exp Psychiatry. 1988;19:127–133. doi: 10.1016/0005-7916(88)90026-2. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behav Neurosci. 2001;115:26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm Behav. 1999;36:1–16. doi: 10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cogn Affect Behav Neurosci. 2005;5:191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.