Abstract
Recent evidence suggests that the hypocretin–orexin system participates in the regulation of reinforcement processes. The current studies examined the extent to which hypocretin neurotransmission regulates behavioral and neurochemical responses to cocaine, and behavioral responses to food reinforcement. These studies used a combination of fixed ratio, discrete trials, progressive ratio and threshold self-administration procedures to assess whether the hypocretin 1 receptor antagonist, SB-334867, reduces cocaine self-administration in rats. Progressive ratio sucrose self-administration procedures were also used to assess the extent to which SB-334867 reduces responding to a natural reinforcer in food-restricted and food-sated rats. Additionally, these studies used microdialysis and in vivo voltammetry in rats to examine whether SB-334867 attenuates the effects of cocaine on dopamine signaling within the nucleus accumbens core. Furthermore, in vitro voltammetry was used to examine whether hypocretin knockout mice display attenuated dopamine responses to cocaine. Results indicate that when SB-334867 was administered peripherally or within the ventral tegmental area, it reduced the motivation to self-administer cocaine and attenuated cocaine-induced enhancement of dopamine signaling. SB-334867 also reduced the motivation to self-administer sucrose in food-sated but not food-restricted rats. Finally, hypocretin knockout mice displayed altered baseline dopamine signaling and reduced dopamine responses to cocaine. Combined, these studies suggest that hypocretin neurotransmission participates in reinforcement processes, likely through modulation of the mesolimbic dopamine system. Additionally, the current observations suggest that the hypocretin system may provide a target for pharmacotherapies to treat cocaine addiction.
Keywords: fast scan cyclic voltammetry, microdialysis, mouse, rat, reward, SB-334867
Introduction
The hypocretin–orexin (HCRT) system consists of the HCRT-1 and HCRT-2 peptides, which bind to two receptor subtypes (HCRT1 and HCRT2 receptors). Extensive evidence indicates that the HCRTs regulate arousal-related processes, including sleep–wake function and locomotor activity (Hagan et al., 1999; Bourgin et al., 2000; Piper et al., 2000; España et al., 2001, 2002). Recently, this peptide system has also been implicated in the regulation of reinforcement processes. For example, the HCRT1 receptor antagonist SB-334867 blocks conditioned place preference (CPP) for morphine and cue- and stress-induced reinstatement of ethanol- and cocaine-seeking (Boutrel et al., 2005; Harris et al., 2005; Lawrence et al., 2006; Richards et al., 2008; Aston-Jones et al., 2009; Smith et al., 2009). Additionally, SB-334867 blocks behavioral sensitization to cocaine and associated changes in synaptic plasticity (Borgland et al., 2006). Consistent with this, HCRT knockout (KO) mice show reduced morphine dependence and display a decreased dopamine (DA) response to morphine (Georgescu et al., 2003; Narita et al., 2006). This latter observation suggests the possibility that HCRT regulation of reinforcement processing may involve actions on mesolimbic DA systems. Indeed, HCRT neurons innervate the ventral tegmental area (VTA) as well as the nucleus accumbens (NAc), where HCRT receptors are found (Peyron et al., 1998; Marcus et al., 2001; Fadel & Deutch, 2002). Furthermore, HCRT induces burst firing and potentiates glutamate-mediated excitatory drive of DA neurons (Korotkova et al., 2003; Borgland et al., 2006). Finally, HCRT-1 infusions into the VTA elicit DA release in the NAc and induce CPP (Narita et al., 2006, 2007), although a lack of HCRT-1 effects in the NAc core has also been reported (Vittoz & Berridge, 2006; Vittoz et al., 2008).
Recent data demonstrate that SB-334867 alters cocaine self-administration, although the effect appears to depend on the schedule of reinforcement employed. For example, SB-334867 has been shown to reduce cocaine-reinforced responding under a progressive ratio (PR) schedule (Borgland et al., 2009) but does not affect the rate of cocaine consumption under a fixed ratio (FR) schedule (Aston-Jones et al., 2009). These data suggest that HCRT signaling exerts differential effects on the mechanisms that control the motivation to self-administer cocaine vs. those that regulate drug intake. To further examine the extent to which HCRT signaling regulates reinforcement processing, the effects of intraperitoneal (i.p.) and/or intra-VTA SB-334867 were tested across a battery of self-administration protocols that model varying aspects of reinforcement. Rats were tested under an FR, discrete trials (DT), PR or threshold self-administration procedure to assess whether SB-334867 reduces cocaine consumption and/or the motivation to self-administer cocaine. The effects of SB-334867 were also tested on sucrose self-administration in both food-sated and food-restricted rats, to assess whether the HCRT system modulates responding to a natural reinforcer across varying satiety states. Additionally, microdialysis and in vivo voltammetry were used to examine whether SB-334867 attenuates the effects of cocaine on DA signaling within the NAc core. Finally, in vitro voltammetry was used to examine whether HCRT KO mice display disrupted DA responses to cocaine.
Materials and methods
Animals
Male Sprague–Dawley rats (375–450 g, Charles River, Wilmington, MA, USA), or homozygous HCRT KO and wild-type (WT) mice (male or female), had ad libitum access to food and water, and were kept on a reverse 12:12-h light/dark cycle (lights on at 15:00 h), unless otherwise noted. HCRT KO and WT mice were created on a C57BL/6J-129/SvEV background and then backcrossed with C57BL/6J mice for more than 10 generations (Chemelli et al., 1999). Mice were genotyped using polymerase chain reaction with a neo primer, 5′-CCGCTATCAGGACATAGCGTTGGC-3′, or a genomic primer, 5′-GACGACGGCCTCAGACTTCTTGGG-3′, and a genomic primer, 3′-TCACCCCCTTGGGAT AGCCCTTCC-5′, common to KO and WT mice. All protocols and animal care procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996), and approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences.
Surgery
Rats used for self-administration experiments were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg), and implanted with an intravenous (i.v.) Silastic catheter (CamCaths, Cambridge, UK) placed into the right jugular vein. Rats received post-surgical antibiotic (Neosporin; Wellcome Medical, Quebec, Canada) and analgesic (5 mg/kg; ketoprofen; Webster Veterinary, Sterling, MA, USA), and recovered for 3 days prior to training. Self-administration rats that received injections of SB-334867 into the VTA were also implanted with guide cannulae (Plastics One, Roanoke, VA, USA) aimed 4 mm dorsal to the VTA (+5.3 mm posterior, ±2.0 mm lateral, −3.5 mm ventral; 8° from vertical).
Rats used for microdialysis experiments were anesthetized identically and then placed in a stereotaxic apparatus. Concentric microdialysis probes (membrane length, 2 mm; CMA/Microdialysis, Stockholm, Sweden) were implanted into the NAc core (−1.6 mm anterior, +1.6 mm lateral, −6.1 mm ventral) via a guide cannula (CMA/Microdialysis). Microdialysis rats that received injections of SB-334867 into the VTA were also implanted with guide cannulae aimed at the VTA (see above). Rats received post-surgical analgesic (Buprenex, 0.05 mg/kg; Rickitt and Colman, Richmond, VA, USA), and recovered for 48 h prior to testing. Microdialysis probes were inserted approximately 16 h prior to the beginning of sample collection.
Rats used for the voltammetry experiments were anesthetized with 1.5 g/kg i.p. urethane, and implanted with a jugular catheter. Rats were placed in a stereotaxic apparatus and implanted with a bipolar stimulating electrode affixed to a 26-gauge guide cannula (Plastics One) aimed at the VTA (+5.3 mm posterior, +1.0 mm lateral, −7.2 to −7.6 mm ventral). Stimulator leads were separated by 1.0 mm, and the cannula tip ended 2.0 mm dorsal to the leads. A carbon fiber microelectrode was implanted within the core of the NAc (−1.3 mm anterior, +1.3 mm lateral, −6.5 to −7.0 mm ventral), and a reference electrode was implanted in the contralateral cortex (−2.5 mm anterior, −2.5 mm lateral, −2.0 mm ventral).
Self-administration
Cocaine self-administration procedures have been described previously (Roberts & Goeders, 1989; McGregor et al., 1996; Roberts et al., 2002). Rats were individually housed and trained to self-administer cocaine on an FR schedule in which single lever responses resulted in single cocaine injections. Intravenous catheters were connected through a stainless steel spring to a counterbalanced swivel (Instech Laboratories, Plymouth Meeting, PA, USA). Lever responses resulted in delivery of 0.75 mg/kg cocaine (in saline; National Institute on Drug Abuse) over a 5-s period followed by a 20-s time-out period. FR training sessions were terminated after 20 injections. Once stable patterns of cocaine self-administration were reached (~2–4 days), rats were switched to other self-administration schedules for additional training and SB-334867 testing. All rats were tested during the dark/activity phase of the light/dark cycle.
The FR schedule is known to engender stable rates of cocaine-reinforced responding, and thus it is often the initial self-administration approach to investigate the effects of a pharmacological treatment (LeSage et al., 1999). In the current experiments, rats were given 3-h access to cocaine-associated levers, an access condition that does not significantly alter the rate of cocaine intake over multiple days of self-administration (Wee et al., 2007). On experimental days, rats were treated with i.p. vehicle [10% β-cyclodextrin +4% dimethylsulfoxide (DMSO) in distilled H2O] or 30 mg/kg SB-334867 (in vehicle; Tocris Bioscience, Ellisville, MO, USA) 30 min prior to the beginning of the FR session (09:30 h). This dose of SB-334867 has previously been used to successfully block reinstatement of cocaine-seeking (Boutrel et al., 2005) and CPP for morphine (Harris et al., 2005). Rats were randomly assigned to receive either vehicle or SB-334867 in the first experimental session, and then received the opposite treatment on the following testing day, with a minimum of 2 days between treatments.
The DT procedure used in these studies is an extension of an FR schedule that allows 24-h access to cocaine but limits the number of injections that an animal can receive each hour. Although this protocol involves low effort to obtain single cocaine injections, it does not allow the animal to titrate to preferred blood levels of cocaine. This degree of cocaine intake engenders a pattern of responding in which rats self-administer cocaine almost exclusively during the active/dark phase of the light/dark cycle (Fig. 1A and C) (Brebner et al., 1999; Roberts et al., 2002). Under this DT schedule, rats had the opportunity to self-administer cocaine during 10-min trials initiated at 20-min intervals (three trials per hour, 24 h/day). Trials began with the presentation of a cocaine-associated lever, and were terminated when the rat depressed the lever, or after a 10-min time-out period. Lever responses triggered delivery of 1.5 mg/kg cocaine. DT sessions continued for 2–3 days until stable patterns of self-administration were obtained. On experimental days, rats were treated with i.p. vehicle or 7.5, 15 or 30 mg/kg SB-334867 at 11:30 h. This time was chosen on the basis of previous observations (Brebner et al., 1999; Roberts et al., 2002), indicating that rats display high rates of cocaine intake between 10:00 h and 15:00 h (Fig. 1A and C). Rats were treated with vehicle and each dose of SB-334867 using a Latin-square design, with a minimum of 2 days between treatments.
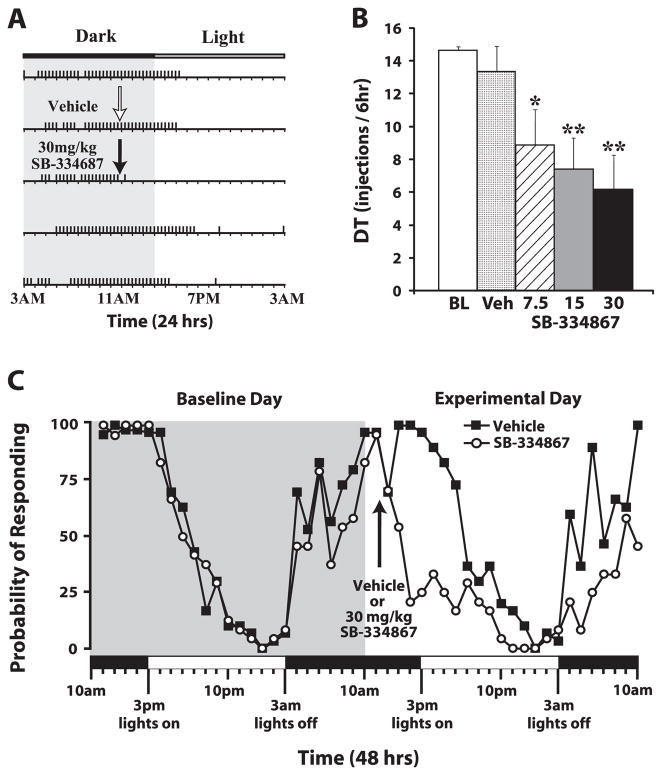
Fig. 1.
SB-334867 reduces cocaine self-administration on a discrete trials (DT) schedule. (A) A response pattern from an individual rat across 5 days of testing. Horizontal lines represent 24-h periods. Vertical tick marks represent trials in which a 1.5 mg/kg cocaine injection was taken. In this case, the rat received either an intraperitoneal (i.p.) vehicle (Veh) (white arrow) or 30 mg/kg SB-334867 injection (black arrow). (B) The mean number ± standard error of the mean of cocaine injections taken over the 6-h period following i.p. injections of vehicle (n = 8) or SB-334867 (7.5, 15 and 30 mg/kg; n = 8). (C) The mean probabilities of responding for cocaine over a 48-h period. The gray shaded area depicts the 24 h prior to receiving drug treatment, and thus reflects cocaine responding under baseline (BL) conditions. The unshaded area shows the 24-h period during which rats received either vehicle or 30 mg/kg SB-334867. The black arrow indicates the time of injection (11:30 h). Alternating bars on the x-axis denote the dark phase (black: 03:00 h to 15:00 h) and light phase (white: 15:00 h to 03:00 h). *P < 0.05 and **P < 0.01 relative to baseline.
The PR schedule is useful for assessing the reinforcing efficacy of cocaine. Rats were given access to a response lever at 10:00 h, and single cocaine injections were contingent upon an increasing number of responses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603 (Richardson & Roberts, 1996). When the required number of responses was made, a single 0.75 mg/kg cocaine injection was delivered. The self-administration session was terminated after 6 h, and the number of injections taken before a 1-h time period elapsed with no further injections taken was defined as the ‘breakpoint’.
For experiments involving i.p. injections, rats were treated with vehicle or 7.5, 15 or 30 mg/kg SB-334867 30 min prior to the beginning of the PR session (09:30 h). Rats were treated with vehicle and each dose of SB-334867 using a Latin-square design, with a minimum of 2 days between treatments.
For experiments involving intra-VTA infusions, rats were treated either unilaterally or bilaterally with 250 nL (100 nL/min) of vehicle or SB-334867 into the VTA 30 min prior to the beginning of the PR session (09:30 h). Briefly, 33-gauge infusion needles that extended 4.0 mm beyond the cannulae tips (Plastics One) were loaded with vehicle (DMSO) or 10 nmol SB-334867 (in vehicle) and then inserted into the guide cannulae. Similar doses of SB-334867 have previously been shown to reduce locomotor sensitization to cocaine (Borgland et al., 2006), CPP for morphine (Narita et al., 2006), and nicotine self-administration (Hollander et al., 2008). Furthermore, DMSO has been shown to be an acceptable vehicle for central injections, and does not result in apparent damage to neural tissue (Fig. S1) (Blevins et al., 2002). Infusions were performed using a microprocessor-controlled infusion pump (Harvard Apparatus, South Natick, MA, USA), and following completion of the infusion, needles were removed. Rats were randomly assigned to receive either vehicle or SB-334867 in the first experimental session, and then received the opposite treatment on the following testing day, with a minimum of 2 days between treatments.
To examine the effects of SB-334867 on responding to a natural reinforcer, food-sated and food-restricted rats were tested on a PR sucrose self-administration schedule. During the first 2 days of training on an FR schedule, all rats were food-restricted (food deprivation for 21 h per day). Rats had daily access to sucrose (45-mg sucrose pellets; Research Diets Inc., Brunswick, NJ, USA) during the 2-h session, and this was followed by free access to regular rat chow for 1 h. All rats acquired the task (> 50 pellets over 1 h) within the first 2 days of training, and were subsequently switched to a PR self-administration procedure for testing with SB-334867. At this point, rats in the food-sated group were switched to ad libitum access to rat chow in their home cage, with no further manipulations of their food intake other than the sucrose self-administration procedure. Rats in the food-restricted group continued on the food restriction protocol for the remainder of the study. These rats consumed 8–10 g of food (~45–50% of free access food consumption) during the 1-h feeding period, and their average weight was 90% (range, 86.5–94.5%) of ad libitum weight measured immediately prior to the beginning of food restriction. Rats in the food-sated group gained an average of 8% body weight over the course of the study.
The sucrose PR session was identical to that described above for cocaine self-administration, except that rats were tested for 2 h rather than 6 h. This length of testing was selected on the basis of pilot studies indicating that > 90% of food intake occurred within the first 2 h of a 6-h session. Once stable patterns of self-administration were reached on the PR schedule, rats were randomly assigned to receive either vehicle or 30 mg/kg SB-334867 (09:30 h) in the first experimental session, and then received the opposite treatment on the following testing day, with a minimum of 2 days between treatments.
A novel self-administration procedure has been developed that is designed to assess the reinforcing threshold of cocaine. It is an adaptation of a previous threshold protocol that used multiple days of testing (Zittel-Lazarini et al., 2007; Oleson & Roberts, 2009). In the current paradigm, rats were tested on an FR schedule across a descending series of cocaine doses within a single session. This within-session threshold procedure is useful for determining the dose of cocaine that is minimally effective in supporting self-administration behavior, and provides information concerning both consumption and maximal unit price (Pmax), a behavioral economic index of price (see Fig. 3). Rats were trained on an FR schedule, as described above, and then switched to the threshold procedure when stable responding rates were obtained. Under the threshold procedure, individual lever responses resulted in delivery of a dose that decreased every 10 min. The dose of cocaine was varied by manipulating the volume of drug delivery (duration of injection) while maintaining a constant drug concentration. Injection durations were reduced on a quarter-log scale as follows: 3162, 1780, 1000, 562, 316, 188, 100, 56, 31, 18 and 10 ms. The calculated unit dose equivalents (5 mg/mL × 1.6 mL/min × pump duration) were 422, 237, 133, 75, 42, 25, 13, 7.5, 4.1, 2.4 and 1.3 μg per infusion (see Fig. 3D); these are associated with doses of 1120, 630, 350, 200, 110, 60, 30, 20, 10, 5 and 3 μg/kg per infusion for a rat weighing 375 g. On experimental days, rats were treated with vehicle or 7.5, 15 or 30 mg/kg SB-334867 30 min prior to the beginning of the threshold session (09:30 h). Rats were treated with vehicle and each dose of SB-334867 using a Latin-square design, with a minimum of 2 days between treatments.
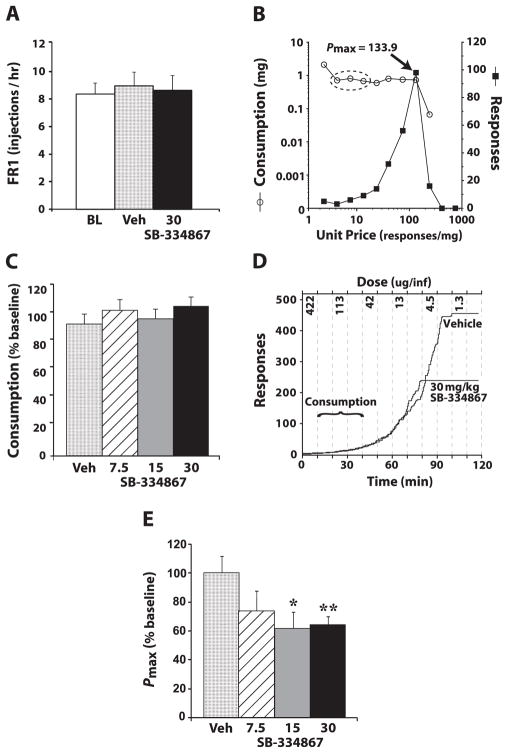
Fig. 3.
SB-334867 does not affect cocaine consumption but reduces responding as the unit price of cocaine is increased. (A) The mean number ± standard error of the mean of 0.75 mg/kg cocaine injections taken per hour following intraperitoneal (i.p.) injection of vehicle (Veh) (n = 6) or 30 mg/kg SB-334867 (n = 6) on a fixed ratio (FR) schedule. (B) An individual event record showing how the dependent measures of consumption (open circles) and price are extracted from these same data. Consumption is calculated by averaging cocaine intake across three bins (demarcated by dashed oval; see Materials and methods). Pmax is defined as the point at which maximal responding occurs on the price–response function (filled squares). (C) The mean ± standard error of the mean percentage baseline (BL) consumption of cocaine following i.p. injection of vehicle (n = 9) or SB-334867 (7.5, 15 or 30 mg/kg; n = 9) on the threshold schedule. (D) Event records from an individual rat that received an i.p. injection of vehicle or 30 mg/kg SB-334867. Dashed lines denote times in which cocaine doses were reduced (every 10 min; only every other dose is shown, for clarity). Note that the rate of responding increases as the dose of cocaine is lowered throughout the session. (E) Shown are the mean ± standard error of the mean Pmax values, expressed as a percentage of baseline, following i.p. injections of vehicle or SB-334867. *P < 0.05 and **P < 0.01 relative to vehicle.
Microdialysis
Microdialysis probes were perfused (0.8 μL/min) with sterile artificial cerebrospinal fluid (148 mM NaCl; 2.7 mM KCl; 1.2 mM CaCl2; 0.85 mM MgCl2; pH 7.4). Samples were collected every 20 min and analyzed for DA by high-performance liquid chromatography (HPLC) and electrochemical detection (BAS, West Lafayette, IN, USA). At least six baseline samples were collected, and this was followed by either an i.p. injection of vehicle or 30 mg/kg SB-334867, or an intra-VTA infusion of vehicle or 10 nmol SB-334867. Forty minutes after injections/infusions, animals received a single i.p. injection of 10 mg/kg cocaine. These studies used single i.p cocaine injections to better compare the current findings with those obtained from an extensive microdialysis literature using i.p. cocaine delivery as the standard administration route. Dialysate samples were subsequently collected every 20 min for 2 h. To account for the time required for analytes to exit sampling lines, data were shifted accordingly (20-min shift).
HPLC
The HPLC (Bianalytical Systems, Mt Vernon, IN, USA) apparatus consisted of a syringe pump, a glassy carbon working electrode, a reference electrode, and an electrochemical detector. A 2 × 50 mm (3-μm particle) reverse-phase column (Luna-Phenomenex, Torrance, CA, USA) was used to separate compounds. The applied potential was +650 mV as referenced to an Ag/AgCl electrode. The mobile phase [75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium salt, 100 μL/L triethylamine, 25 μM EDTA, 10% acetonitrile (v/v), pH 3.0] was pumped at a rate of 170 μL/min, with a detection limit for DA of 10 pM. DA quantification was achieved by comparing samples with DA standards of known concentration.
In vivo voltammetry
Voltammetry studies were conducted to provide a detailed examination of pharmacologically induced changes in DA release and uptake following intra-VTA SB-334867 treatment. Urethane-anesthetized rats were used to avoid potential interference from behavioral factors and to avoid the alterations in DA uptake kinetics that can occur when using other anesthetics (Greco & Garris, 2003). Importantly, the effects of DA uptake inhibitors, such as cocaine, are similar in both freely moving and urethane-anesthetized rats (Greco & Garris, 2003; Wightman et al., 2007). Following surgery, a stimulating electrode was lowered into the VTA, and the carbon fiber electrode was initially lowered into the caudate putamen (−1.3 anterior, +1.3 lateral, −4.5 ventral), until a 1-s, 60-Hz monophasic (2 ms; 120 μA) stimulation train elicited a robust DA signal. The caudate putamen displays higher levels of DA release and faster uptake (~4 μM) than the NAc core (~2.5 μM) (Kuczenski et al., 1991; Jones et al., 1995; Wu et al., 2001), and thus it is a useful region for maximizing recording conditions. Once stimulator and carbon fiber electrode locations achieved adequate levels of release in the caudate putamen, the carbon fiber electrode was lowered 2–2.5 mm further into the NAc core, which yields lower DA release levels and slower DA uptake. Once stable baselines were established in the NAc core, a 33-gauge infusion needle (Plastics One) containing vehicle (DMSO) or 10 nmol SB-334867 (in vehicle) was inserted into the guide cannula. Infusion needles extended 2.0 mm beyond the cannula tip to the same dorsal/ventral location as the stimulator leads. After a minimum of 10 min, a 250-nL infusion was made over 2.5 min (100 nL/min), using an infusion pump (Harvard Apparatus). Intravenous cocaine injections (1.5 mg/kg) were administered as an experimenter-delivered, 2-s, ~200-μL bolus, 40 min after infusions of vehicle or SB-334867.
Given the high temporal resolution obtained with voltammetry techniques, the current studies used i.v. cocaine injections to examine the rapid temporal profile of SB-334867 effects on baseline and cocaine-induced alterations in DA signaling. Cocaine was delivered 40 min following SB-334867, on the basis of preliminary data indicating that SB-334867 consistently reduced levels of stimulated DA release within this time frame (Fig. 6A). Electrically stimulated DA response parameters were acquired 30 s after cocaine injection and every 5 min thereafter.
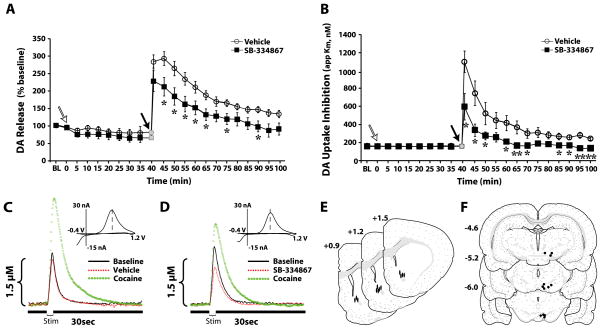
Fig. 6.
Intra-ventral tegmental area (VTA) SB-334867 disrupts phasic dopamine (DA) signaling and attenuates DA responses to cocaine. (A) The mean ± standard error of the mean of stimulated DA release expressed as a percentage of baseline (BL). (B) The mean ± standard error of the mean of DA uptake inhibition (apparent affinity, Km) following intra-VTA infusions of vehicle (n = 6) or 10 nmol of SB-334867 (n = 6). Gray symbols shown at time 0 represent a non-collection time period during which 1.5 mg/kg cocaine was injected intravenously over a 2-s period. (C and D) Representative concentration–time plots and cyclic voltammograms (insets) of DA responses from rats that received pretreatment infusions of (C) vehicle or (D) 10 nmol of SB-334867 into the VTA. Stim represents the time of electrical stimulation. (Insets in C and D) Cyclic voltammograms depict two current peaks, one at 600 mV (positive deflection) for DA oxidation, and one at −200 mV (negative deflection) for reduction of DA-O-quinone. The position of the peaks identifies the substance oxidized as DA. (E and F) Schematic depictions of (E) carbon fiber electrode locations within the nucleus accumbens core (filled ovals) and (F) infusion needle locations in the VTA (filled circles). Distance from bregma is shown beside each coronal section (Swanson, 1998). *P < 0.05 and **P < 0.01 relative to vehicle.
In vitro voltammetry
Mice were killed by decapitation, and their brains were rapidly removed and prepared as previously described (John & Jones, 2007). Coronal slices (400 μm) of the striatum were maintained at room temperature in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid, consisting of 126 mM NaCl, 25 mM NaHCO3, 11 mM D-glucose, 2.5 mM KCl, 2.4 mM CaCl2, 1.2 mm MgCl2, 1.2 mm NaH2PO4, and 0.4 mm L-ascorbic acid (pH adjusted to 7.4). A carbon fiber microelectrode was positioned 75 μm below the surface of the slice in the NAc core. DA release was evoked every 5 min by a 4-ms, one-pulse stimulation (monophasic, 120 μA) from a bipolar stimulating electrode (Plastics One) placed 100–200 μm from the carbon fiber microelectrode. Drugs were applied by superfusion (1 mL/min), using cumulative increases in concentration (Jones et al., 1995; John & Jones, 2007).
Data acquisition
The electrode potential was linearly scanned from −0.4 to 1.2 V and back to −0.4 V vs. Ag/AgCl. Cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms, using a scan rate of 400 V/s, by means of a voltammeter/amperometer (Chem-Clamp; Dagan Corporation, Minneapolis, MN, USA). The magnitude of electrically evoked DA release and transporter-mediated uptake kinetics, including maximal uptake rate (Vmax) and apparent affinity of endogenous DA (Km), were monitored. Extracellular concentrations of DA were assessed by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with electrode calibrations of known concentrations of DA (1–10 μM). DA overflow curves were fitted to a Michaelis–Menten-based kinetic model (Wu et al., 2001), using Labview software (National Instruments, Austin, TX, USA). Changes in uptake and release were obtained by setting baseline levels of Km (prior to any drug treatment) to 0.16 μM and establishing a baseline Vmax individually for each subject. Following cocaine injection/application, Vmax was held constant for the remainder of the experiment, and thus cocaine-induced changes in uptake were attributable to changes in Km.
Histology
Rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), and 0.5% Chicago Blue Dye (Sigma-Aldrich Inc., St Louis, MO, USA) was microinjected (250 nL) into the VTA. Rats were perfused transcardially with 60 mL of 0.9% saline followed by 60 mL of 10% formalin (pH 7.0; Sigma-Aldrich Inc.), and brains were removed and cryoprotected in 30% sucrose solution (in 0.01 M phosphate buffer, pH 7.4). Sections were sliced (40 μm) and Nissl-stained using neutral red dye to identify the carbon fiber microelectrode (Fig. 6E) and microdialysis probe (Fig. 5C) locations within the NAc core. To verify microinfusion needle locations within the VTA, sections were Nissl-stained or processed immunohistochemically for tyrosine hydroxylase, a marker of DA neurons (Figs 2D, 5D and 6F; Fig. S1).
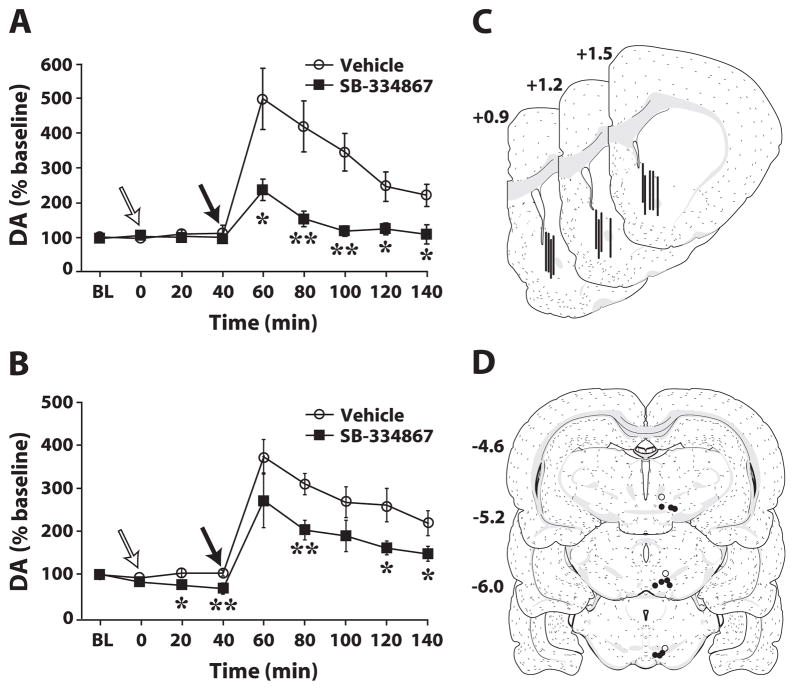
Fig. 5.
Peripheral and intra-ventral tegmental area (VTA) SB-334867 attenuates cocaine-induced elevations in tonic dopamine (DA) in the nucleus accumbens (NAc) core. Shown are the mean level ± standard error of the mean of DA within the NAc core following (A) intraperitoneal (i.p.) injection of vehicle (n = 6) or 30 mg/kg SB-334867 (n = 6), or (B) intra-VTA injection of vehicle (n = 6) or 10 nmol of SB-334867 (n = 6). White arrows indicate the time of vehicle or SB-334867 injection, and black arrows indicate the time of 10 mg/kg i.p. cocaine injection. (C) Schematic depictions of representative microdialysis probe placements (vertical bars) in the NAc core from both i.p. and intra-VTA experiments. (D) Shown are schematic depictions of effective (filled circles) and ineffective (open circles) infusion needle locations in the VTA. Distance from bregma is shown beside each coronal section (Swanson, 1998). *P < 0.05 and **P < 0.01 relative to vehicle. BL, baseline.
Fig. 2.
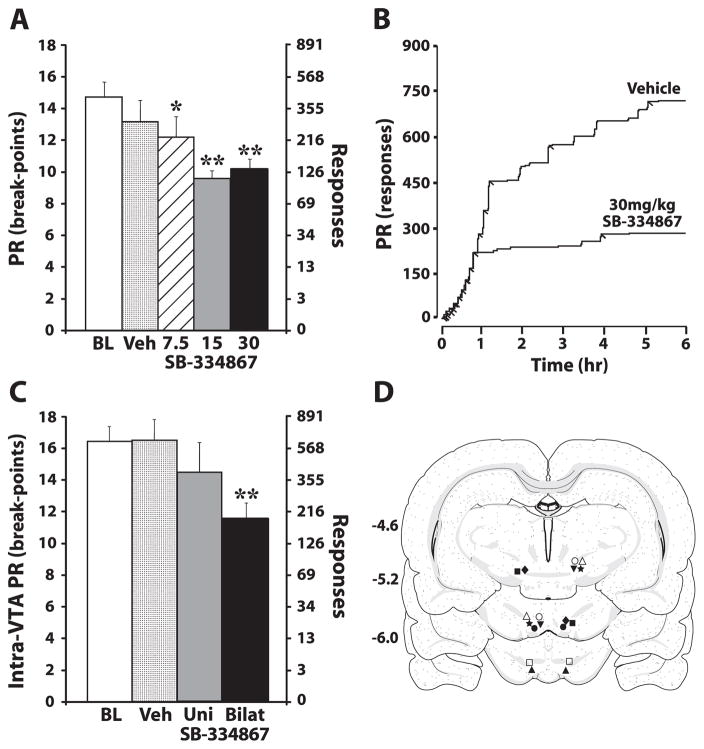
Peripheral and intra-ventral tegmental area (VTA) SB-334867 reduces cocaine self-administration on a progressive ratio (PR) schedule. (A) The mean number ± standard error of the mean of 0.75 mg/kg cocaine breakpoints following intraperitoneal (i.p.) injection of vehicle (Veh) (n = 6) or SB-334867 (7.5, 15 and 30 mg/kg; n = 6). (B) Event records from an individual rat that received an i.p. injection of vehicle or 30 mg/kg SB-334867. Cocaine injections are indicated by diagonal tick marks. (C) The mean number ± standard error of the mean of cocaine breakpoints following unilateral (Uni) or bilateral (Bilat) intra-VTA infusions of vehicle (unilateral, n = 5; bilateral, n = 6) or 10 nmol of SB-334867 (unilateral, n = 5; bilateral, n = 6). (D) Schematic depictions of effective (filled matched symbols) and ineffective (open matched symbols) bilateral infusion locations in the VTA from individual rats. Distance from bregma is shown beside each coronal section (Swanson, 1998). *P < 0.05 and **P < 0.01 relative to baseline (BL).
The carbon fiber portion of the microelectrode is too small (5 μm in diameter and 100 μm in length) to produce damage that is detectable with a light microscope. Nevertheless, the track produced by the glass capillary, which occurs approximately 1 mm dorsal to the carbon fiber, can be readily visualized, and thus it is possible to precisely locate the rostrocaudal and mediolateral location of the electrode. The dorso-ventral position of the electrode can be determined on the basis of the known final stereotaxic coordinates to which the electrode was lowered (−6.5 to −7.0 mm ventral), from the relative location of the tract produced by the glass capillary, and from voltammetric data collected during the experiment. Specifically, it is known that the caudate putamen exhibits greater DA release and faster uptake rates than the NAc core, which is located immediately ventral to the caudate putamen (Jones et al., 1995; Wu et al., 2001). Likewise, the NAc core displays greater DA release and faster uptake rates than the NAc shell, which is located medial and ventral to the NAc core (Jones et al., 1996; España & Jones, 2008). Thus, on the basis of the unique profile of DA dynamics in each of these striatal structures, it is possible to distinguish recordings of the NAc core vs. the caudate putamen or the NAc shell. Consequently, all voltammetry recordings were initiated in the caudate putamen, and DA dynamics were compared with final recordings made in the NAc core. Therefore, by combining the visual verification of electrode locations obtained using standard histology techniques, the known coordinates to which the electrode was lowered into the brain, and the unique profile of DA dynamics from the final recording location, it was possible to accurately reconstruct the dorsoventral position of the carbon fiber electrode.
For immunohistochemical processing of tyrosine hydroxylase, sections were rinsed with 0.01 M phosphate-buffered saline (PBS) and then incubated for 20 min in a quench solution containing 0.75% hydrogen peroxide, rinsed again, and transferred for 30 min to a blocking solution containing 10% normal goat serum and 0.3% Triton X-100 in 0.01 M PBS. Sections were then rinsed, and incubated for 16 h at room temperature with rabbit anti-rat tyrosine hydroxylase antibody (1: 20 000; Millipore, Billerica, MA, USA) diluted in 0.01 M PBS. After incubation, tissue was rinsed with 0.01 M PBS, and incubated with biotinylated goat anti-rabbit antibody (1: 500; ABC kit; Vector Laboratories, Burlingame, CA, USA) for 90 min. Tissue was then rinsed with 0.01 M PBS, exposed for 90 min to an avidin–biotin peroxidase complex (ABC complex; Vector Laboratories), rinsed with 0.01 M PBS, and then stained with Vectastain Blue (Vector Laboratories) to yield a blue–gray precipitate. Sections were mounted on microscope slides (Fisher Scientific, Itasca, IL, USA), air-dried overnight, taken through a graded series of ethanol (50–100%), cleared for 24 h (D-limonene; VWR, West Chester, PA, USA), and coverslipped using DPX mounting medium (BDH Laboratory Supplies, Garden City, NY, USA). Photomicrographs were acquired with an Axiocam HRc digital camera (Carl Zeiss, Inc., Thornwood, NY, USA) connected to a Zeiss Axioplan microscope, adjusted for brightness and contrast using Adobe Photoshop CS4 software, and then labeled using Adobe Illustrator CS4 software.
Data analysis and statistics
Cocaine self-administration
Self-administration data for the FR studies were expressed as the rate of cocaine injections taken per hour. For the DT studies, data were expressed as the total number of injections taken during the 6 h following vehicle or SB-334867 treatment. For all PR studies, data were expressed as breakpoints (total number of injections or sucrose pellets taken) and total number of lever responses made during the session. No statistical differences were observed between unilateral and bilateral intra-VTA data for the baseline and vehicle conditions, thus these data were pooled for figure presentation (Fig. 2C) but not for statistical analyses. For the FR, DT and cocaine and sucrose PR self-administration studies, vehicle and SB-334867 data were compared with the previous 3 days of baseline responding. For the threshold studies, data were expressed as consumption and Pmax, and reported as percentage change from the preceding 3 days. Consumption was derived by averaging cocaine intake across three time bins (Fig. 3B). The first 10-min bin was excluded because of the confounding effects of drug ‘loading’, and the latter bins were excluded because of the confounding effects of price. In this manner, the mean consumption value derived from this data represents the preferred cocaine level to which the animal is titrating, similar to that encountered during an FR session (Fig. 3A). Unit price values were calculated by dividing the response requirement (one response) by the unit dose (dose of cocaine, which was reduced every 10 min). This produced the following unit-price values: 2.4, 4.2, 7.5, 13.4, 23.7, 39.9, 75.0, 133.9, 241.9, 416.7, and 750 (Fig. 3B, x-axis). Pmax was defined as the unit price at which maximal responding occurs, and was derived by assessing the apex of the price–response function (Fig. 3B, filled squares). It should be noted that Pmax coincides with the point at which cocaine consumption (Fig. 3B, open circles) changes from being maintained (inelastic demand) to not being maintained (elastic demand).
The effects of SB-334867 on FR and sucrose PR self-administration were assessed using one-way repeated-measures ANOVAs (baseline, vehicle, and 30 mg/kg SB-334867). Comparisons of baseline responding between the food-sated and food-restricted sucrose PR self-administration experiments were conducted using t-tests. The effects obtained from the DT and cocaine PR (i.p. SB-334867) studies were assessed using one-way repeated-measures ANOVAs (baseline, vehicle, and 7.5, 15 and 30 mg/kg SB-334867). The effects from the cocaine PR (intra-VTA SB-334867) studies were assessed using separate one-way repeated-measures ANOVAs for each of the unilateral and bilateral experiments (baseline, vehicle, and 10 nmol of SB-334867). Finally, effects from the threshold studies were assessed using one-way repeated-measures ANOVAs (vehicle, and 7.5, 15 and 30 mg/kg SB-334867). When statistical significance was obtained, simple effect analyses were conducted using individual one-way ANOVAs to examine pairwise comparisons of SB-334867 groups with vehicle or baseline control groups.
Microdialysis
For both the i.p. and intra-VTA microdialysis studies, data were calculated as the percentage change from baseline, with baseline (100%) being defined as the average of three samples that occurred prior to the injection of drug or vehicle. The effects of SB-334867 on cocaine-induced increases in extracellular DA within the NAc core were assessed using a two-way mixed design ANOVA, with drug (vehicle vs. 30 mg/kg SB-334867) as the between-subjects variable and time as the repeated-measures variable. Simple effect analyses were conducted to compare extracellular DA levels between groups using one-way ANOVAs.
In vivo voltammetry
Stimulated DA release was calculated as the percentage change from baseline, with baseline (100%) being defined as the average of three samples that occurred prior to the injection of cocaine. Changes in maximal uptake rate following SB-334867 were expressed as Vmax, and changes in uptake inhibition following cocaine were expressed as Km. DA release and uptake measures were derived from a Michaelis–Menten-based model (Wu et al., 2001). The effects of SB-334867 on Vmax were assessed using a t-test comparing Vmax prior to intra-VTA SB-334867 injection and Vmax 40 min later, immediately prior to cocaine injection (baseline vs. pre-cocaine). The effects of SB-334867 on DA release and cocaine-induced uptake inhibition (Km) were assessed using a two-way mixed design ANOVA, with drug (vehicle vs. SB-334867) as the between-subjects variable, and time as the repeated-measures variable. Where appropriate, simple effect analyses were conducted using one-way ANOVAs.
In vitro voltammetry
Baseline levels of DA release and Vmax, as well as cocaine-induced uptake inhibition (Km), were derived as described above. The effects of genotype (wild type vs. HCRT KO) on baseline levels of stimulated DA release and uptake were assessed using a t-test. The effects of genotype on cocaine-induced uptake inhibition were assessed using a two-way mixed design ANOVA, with genotype (wild type vs. HCRT KO) as the between-subjects variable, and drug concentration as the repeated-measures variable. Simple effect analyses were conducted using one-way ANOVAs. All statistical analyses were conducted using spss (SPSS Inc., Chicago, IL, USA).
Results
DT cocaine self-administration
To examine whether SB-334867 alters responding for 1.5 mg/kg cocaine under a DT schedule, animals were treated with i.p. vehicle (n = 8) or one of three doses of SB-334867 (7.5, 15 or 30 mg/kg; n = 8) during a time in which rats show a high probability of responding for cocaine (11:30 h). Responding on the DT schedule was characterized by high rates of cocaine intake during the activity/dark phase of the light/dark cycle (Fig. 1A and C). Relative to baseline, vehicle did not reduce the total number of cocaine injections taken. In contrast, there was a significant reduction in cocaine intake following SB-334867 treatment (F4,28 = 5.9, P < 0.001). Analyses over the 6 h following injection showed that all doses of SB-334867 significantly reduced responding for cocaine (Fig. 1B).
PR cocaine self-administration
Intraperitoneal experiments
To examine the extent to which SB-334867 reduces the reinforcing efficacy of 0.75 mg/kg cocaine, rats were treated with i.p. vehicle (n = 6) or SB-334867 (7.5, 15 or 30 mg/kg; n = 6) 30 min prior to the beginning of the PR session (09:30 h). As shown in Fig. 2A and B, under baseline conditions, rats reached an average breakpoint (number of cocaine injections taken) of 14.8 ± 0.9, which was associated with 539.5 ± 97.7 lever responses. Vehicle injections did not significantly alter breakpoints (13.2 ± 1.2) or the total number of lever responses (401.2 ± 124.4). Similar to what was found previously with 10 mg/kg i.p. SB-334867 (Borgland et al., 2009), SB-334867 produced a significant overall reduction in breakpoints (F4,20 = 6.0, P < 0.005) and the number of lever responses (F4,20 = 3.8, P < 0.05). Subsequent analyses demonstrated that all doses of SB-334867 significantly reduced breakpoints (Fig. 2A; 7.5 mg/kg, 12.2 ± 1.5; 15 mg/kg, 9.8 ± 0.8; 30 mg/kg, 10.3 ± 0.6). Additionally, the 15 mg/kg (131.2 ± 31.9) and 30 mg/kg (159.2 ± 26.9) doses also significantly reduced the total number of lever responses.
Analysis of cumulative records from individual rats tested under the PR schedule indicated that SB-334867 reduced the total number of lever responses without altering the initial pattern of cocaine intake. Rats treated with SB-334867 showed initial response rates identical to those of vehicle-treated rats, but completed fewer responses before reaching their final breakpoint (Fig. 2B).
Intra-VTA experiments
To assess whether SB-334867 reduces the reinforcing efficacy of 0.75 mg/kg cocaine via actions in the VTA, rats were treated with either unilateral intra-VTA vehicle (n = 5) or SB-334867 (10 nmol; n = 5), or bilateral intra-VTA vehicle (n = 6) or SB-334867 (10 nmol; n = 6), 30 min prior to the beginning of the PR session (09:30 h). Under baseline conditions, rats reached an average breakpoint of 16.3 ± 0.8, which was associated with 857.2 ± 138.6 lever responses (Fig. 2C). Unilateral and bilateral injections of vehicle into the VTA (250 nL) did not significantly alter breakpoint number (unilateral, 16.4 ± 1.9; bilateral, 16.5 ± 1.2) or the total number of lever responses (unilateral, 809.0 ± 250.2; bilateral, 764.0 ± 222.5). As shown in Fig. 2C, as compared with baseline, unilateral injections of SB-334867 did not significantly alter breakpoints (14.5 ± 1.8) or the number of lever responses (496.5 ± 180.4), although a trend for decreases in each of these measures was observed. In contrast, bilateral injections of SB-334867 produced a significant reduction in breakpoints (11.5 ± 1.0, F2,10 = 12.8, P < 0.01) and the number of lever responses (169.2 ± 50.9, F2,10 = 4.8, P < 0.05). Similar to what was found with the i.p. SB-334867 studies, cumulative records from individual rats indicated that bilateral intra-VTA injections of SB-334867 reduced the total number of lever responses without altering the initial pattern of cocaine intake. Figure 2D shows a schematic representation of bilateral infusion needle locations within the VTA. Infusions placed outside the VTA (n = 3) had no significant effect on self-administration (breakpoints, 15.3 ± 0.9; lever responses, 565.3 ± 165.1).
FR cocaine self-administration
To examine the extent to which SB-334867 alters responding for unrestricted access to 0.75 mg/kg cocaine, animals were treated with i.p. vehicle (n = 6) or SB-334867 (30 mg/kg, n = 6) 30 min prior to the beginning of an FR session (09:30 h). Similar to a previous finding (Aston-Jones et al., 2009), no differences in rates of drug responding were observed between groups. Under baseline conditions, the rate of cocaine intake was 8.3 ± 0.8 injections/h, and neither vehicle (8.9 ± 1.0 injections/h) nor 30 mg/kg SB-334867 (8.6 ± 1.0 injections/h) had any effect on this rate (Fig. 3A).
Threshold cocaine self-administration
To further assess the extent to which SB-334867 alters the motivation to take cocaine, rats were treated with vehicle (n = 9) or SB-334867 (7.5, 15 or 30 mg/kg, n = 9) 30 min prior to the beginning of the threshold session (09:30 h). The threshold procedure used in the current experiments provides information on both consumption and price within a single self-administration session (Fig. 3B–E). Across the 2-h session, doses of cocaine were reduced every 10 min. During the initial portion of the session, when individual lever responses resulted in delivery of relatively high doses of cocaine (1.12–0.2 mg/kg), rats were able to titrate to a preferred blood level of cocaine with relatively little effort. Similar to what was observed in the FR experiments (Fig. 3A), in the threshold procedure SB-334867 had no effect on cocaine consumption (Fig. 3B–D). In contrast, as the dose of cocaine was gradually lowered (0.06–0.0003 mg/kg cocaine), rats were required to respond more frequently to maintain blood levels of cocaine, thereby increasing the unit price of cocaine. This is illustrated in Fig. 3B, which shows an event record from an individual rat that reached a Pmax of 133.9. This latter portion of the threshold session is akin to a PR schedule, in which rats must respond increasingly greater numbers of times to obtain the same amount of cocaine (Fig. 3B and D). Under baseline conditions, rats reached an average Pmax (unit price that maintains self-administration) of 284.9 ± 39.2. Relative to baseline, vehicle injections did not significantly alter Pmax. In contrast, SB-334867 produced a significant overall reduction in Pmax (F3,32 = 2.9, P < 0.05). Further analyses indicated that both the 15 and 30 mg/kg doses of SB-334867 significantly reduced Pmax (Fig. 3E).
PR sucrose self-administration
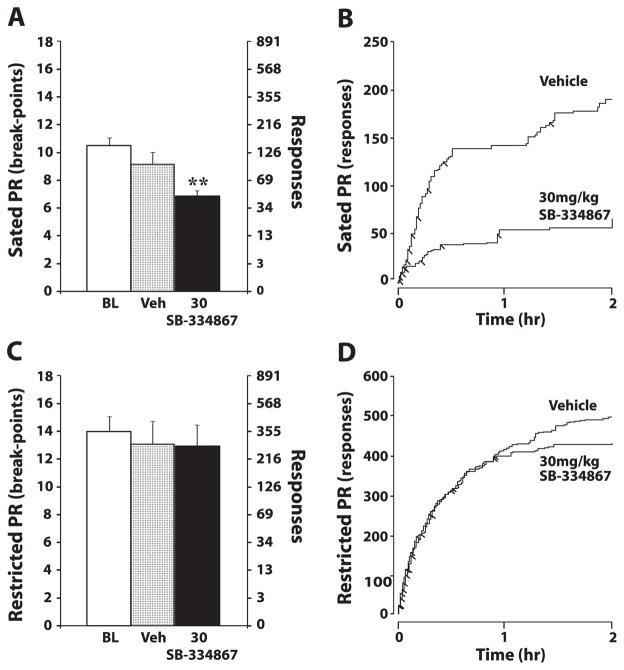
To examine whether SB-334867 alters responding for a natural reinforcer across varying satiety states, food-sated and food-restricted rats were treated with i.p. vehicle (food-sated, n = 7; food-restricted, n = 8) or 30 mg/kg SB-334867 (food-sated, n = 7; food-restricted, n = 8) 30 min prior to the beginning of the PR sucrose self-administration session (09:30 h). Similar to previous findings (Salamone et al., 1991; Aberman & Salamone, 1999; Thorpe et al., 2005; Barbano et al., 2009), differences in motivational drive observed between food-sated and food-restricted rats resulted in significant differences in baseline responding (breakpoints, t13 = 3.1, P < 0.01; lever responses, t13 = 2.9, P < 0.05). Under baseline conditions, food-sated rats reached an average breakpoint (number of pellets taken) of 10.5 ± 0.3, which was associated with 182.2 ± 16.4 lever responses. Conversely, food-restricted rats reached an average breakpoint of 14.0 ± 1.0, which was associated with 427.9 ± 89.2 lever responses. Figure 4 shows that, relative to baseline, vehicle injections did not significantly alter breakpoints or the number of lever responses in either food-sated rats (9.1 ± 0.7; 126.1 ± 22.9) or food-restricted rats (13.0 ± 1.4; 378.6 ± 103.9). By comparison, SB-334867 produced a robust reduction in breakpoints (6.7 ± 0.5, F2,12 = 9.7, P < 0.01) and the number of lever responses (60.4 ± 11.4, F2,10 = 13.5, P < 0.01) in food-sated rats. However, in food-restricted rats, SB-334867 had no effect on breakpoints (12.9 ± 1.3), and produced only a modest, non-significant decrease in the number of lever responses (349.8 ± 95.9).
Fig. 4.
SB-334867 reduces progressive ratio (PR) sucrose self-administration in food-sated but not food-restricted rats. (A) The mean ± standard error of the mean breakpoints (number of sugar pellets taken), following intraperitoneal (i.p.) injections of vehicle (n = 7) or SB-334867 (30 mg/kg, n = 7) in food-sated rats. (B) Cumulative PR records from a food-sated rat that received an i.p. injection of vehicle or 30 mg/kg SB-334867. Sucrose pellet deliveries are indicated by diagonal tick marks. (C) Shown are the mean ± standard error of the mean breakpoints following i.p. injections of vehicle (n = 8) or SB-334867 (30 mg/kg; n = 8) in food-restricted rats. (D) Cumulative PR records from a food-restricted rat that received an i.p. injection of vehicle or 30 mg/kg SB-334867. **P < 0.01.
Analysis of cumulative records from individual rats indicated that in food-sated rats, SB-334867 reduced the total number of lever responses for sucrose relatively early in the session. Thus, although these animals showed initial response rates similar to those of vehicle-treated rats (first three or four sucrose pellets), they quickly reduced their response rates and completed fewer responses overall (Fig. 4B). SB-334867 had little effect on the pattern of lever responses in food-restricted rats (Fig. 4D).
Microdialysis
Intraperitoneal experiments
The effects of i.p. SB-334867 on tonic DA signaling in the NAc core were assessed using microdialysis. Rats were pretreated with either vehicle (n = 6) or SB-334867 (n = 6) 40 min prior to receiving an i.p. injection of 10 mg/kg cocaine. Neither vehicle nor SB-334867 had a significant effect on baseline DA levels, although a modest trend for decreased DA was observed following SB-334867 injection (Fig. 5A). Following cocaine injection, vehicle-treated rats displayed typical increases in DA. In contrast, SB-334867 (30 mg/kg) produced a marked reduction in cocaine-induced increases in DA (treatment, F1,10 = 23.5, P < 0.001; time, F10,100 = 16.2, P < 0.001; treatment × time, F10,100 = 6.8, P < 0.001).
Intra-VTA experiments
To assess whether SB-334867 attenuates cocaine-induced increases in DA via actions in the VTA, vehicle (n = 6) or SB-334867 (10 nmol; n = 6) was microinjected into the VTA 40 min prior to an i.p. injection of 10 mg/kg cocaine. As shown in Fig. 5B, intra-VTA SB-334867 significantly reduced baseline DA levels and significantly attenuated the effects of cocaine (treatment, F1,11 = 11.4, P < 0.01; time, F10,100 = 38.7, P < 0.001; treatment × time, F10,110 = 2.4, P < 0.05). Figure 5C shows a schematic representation of a subset of microdialysis probe locations in the NAc core for the i.p. and intra-VTA studies. Figure 5D shows a schematic representation of infusion needle locations within the VTA. Infusions placed outside the VTA (n = 3) did not attenuate cocaine-induced changes in DA levels.
Voltammetry
In vivo experiments
The effects of intra-VTA SB-334867 on phasic DA signaling were examined using in vivo voltammetry. Rats received SB-334867 (n = 6) or vehicle (n = 6) directly into the VTA 40 min prior to receiving a single i.v. injection of 1.5 mg/kg cocaine. As shown in Fig. 6, prior to pharmacological manipulations, electrically evoked DA responses were stable, and remained at baseline levels following vehicle infusions (250 nL) into the VTA. SB-334867 (10 nmol) alone had no effect on Vmax or Km. In contrast, within 10 min of administration, SB-334867 produced a modest, yet significant, reduction in stimulated DA release (64–74% of control; F10,50 = 4.9, P < 0.001; Fig. 6A and D).
SB-334867 also attenuated the effects of i.v. cocaine on stimulated DA release (treatment, F1,9 = 6.1, P < 0.05; time, F23,207 = 37.8, P < 0.001; treatment × time, F23,207 = 8.7, P < 0.001). Following vehicle pretreatment, 1.5 mg/kg i.v. cocaine increased DA levels to 294 ± 20% within 5 min of injection (Fig. 6A and C). In contrast, following SB-334867, cocaine increased levels to only 236 ± 36% during this time frame. This effect of SB-334867 was first observed at the 30-s time point, but was not statistically significant until 5 min after cocaine injection (Fig. 6A and D).
A similar effect of SB-334867 was also observed on cocaine-induced inhibition of DA uptake (Km; treatment, F1,9 = 17.6, P < 0.005; time, F23,207 = 26.9, P < 0.001; treatment × time, F23,207 = 4.5, P < 0.001). Prior to cocaine administration, neither vehicle nor SB-334867 infusions into the VTA had any effect on DA uptake (Fig. 6B). Although i.v. cocaine inhibited DA uptake in animals pretreated with either vehicle or SB-334867, the magnitude of inhibition was significantly attenuated in animals treated with SB-334867 (vehicle, 670.7 ± 85.2%; SB-334867, 372.2 ± 88.5%; Fig. 6B–D). Figure 6E and F shows schematic representations of carbon fiber microelectrode tip locations in the NAc core and infusion needle locations in the VTA, respectively.
In vitro experiments
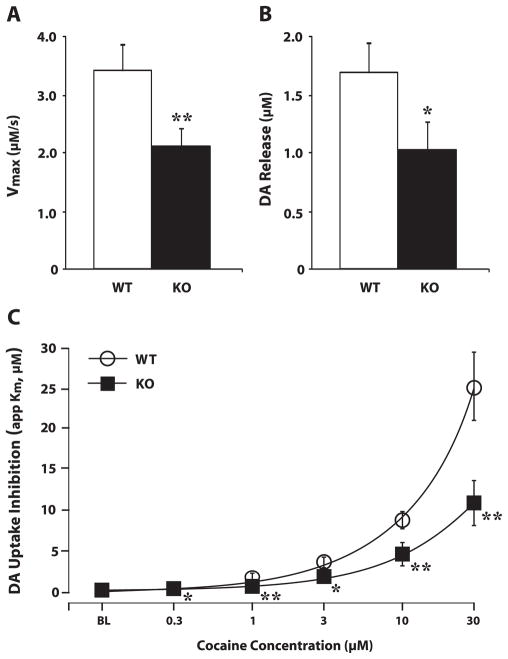
To further assess whether the HCRT system influences baseline and cocaine-induced phasic DA signaling, in vitro voltammetry was conducted in brain slices containing the NAc core of WT (n = 7) and HCRT KO (n = 7) mice. Under baseline conditions, HCRT KO mice displayed reduced stimulated DA release (t12 = 1.9, P < 0.05) and slower DA uptake (Vmax; t12 = 3.5, P < 0.01) relative to WT mice (Fig. 7A and B). As shown in Fig. 7C, cocaine increased DA uptake inhibition (Km) in both WT and HCRT KO mice. However, the effects of cocaine were attenuated in HCRT KO mice (genotype, F1,11 = 14.4, P < 0.01; concentration, F5,55 = 46.9, P < 0.001; genotype × concentration, F5,55 = 7.4, P < 0.001).
Fig. 7.
Hypocretin (HCRT) knockout (KO) mice display disrupted dopamine (DA) signaling and attenuated DA responses to cocaine. (A and B) The mean ± standard error of the mean of stimulated (A) DA release and (B) maximal uptake rate (Vmax) in wild-type (WT) (n = 7) and HCRT KO (n = 7) mouse slices containing the nucleus accumbens (NAc) core. (C) Shown are the mean ± standard error of the mean DA uptake inhibition (apparent affinity, Km) in WT (n = 7) and HCRT KO (n = 7) mouse slices across cumulative doses of cocaine. *P < 0.05 and **P < 0.01 relative to vehicle.
Discussion
The current observations indicate that blockade of HCRT signaling reduces the motivation to self-administer cocaine under conditions that require effortful responding or in which access to cocaine is limited. Additionally, disruptions in HCRT signaling reduce baseline and cocaine-induced DA responses within the NAc core. Importantly, the effects of SB-334867 were similar when it was administered systemically or into the VTA, suggesting that HCRT participates in reinforcement processes and that these actions involve modulation of the mesolimbic DA system. Finally, the current studies also demonstrate that SB-334867 reduces sucrose intake in food-sated but not food-restricted rats.
Cocaine self-administration
In the current studies, rats were tested across a battery of self-administration schedules to model multiple aspects of reinforcement processing. Under the FR schedule, which is useful for assessing changes in cocaine consumption, rats had unrestricted access to cocaine during daily 3-h sessions, and thus were able to titrate cocaine intake to preferred blood levels with little effort. Similar to a previous finding (Aston-Jones et al., 2009), SB-334867 had no effect on cocaine intake under this schedule, suggesting that HCRT may not be a critical factor in supporting cocaine self-administration when conditions allow for nearly effortless access to cocaine.
In contrast to what was found in the FR studies, SB-334867 reduced cocaine intake when access was restricted using a DT procedure. The DT schedule used here prevents rats from maximizing their blood levels of cocaine by restricting cocaine access to three trials per hour, and engenders a characteristic pattern of intake in which animals are disinclined to self-administer cocaine during the light phase (Roberts et al., 2002). The complex interaction between dose and availability occurring during the current DT paradigm renders this schedule more vulnerable to pharmacological and physiological influences than schedules with less restricted access to cocaine. Thus, under these conditions, SB-334867 reduced cocaine intake in a manner similar to other drugs that selectively reduce intake under DT conditions, but not under an FR schedule (Brebner et al., 2000; Smith et al., 2004).
The PR schedule is useful for assessing changes in the motivational influences of drugs. During the early portions of the PR session, single cocaine injections are obtained with relatively low effort. In contrast, as the lever response requirement is increased, rats must exert progressively greater effort to obtain the same cocaine injection. Under these conditions, both i.p. and bilateral intra-VTA SB-334867 injections reduced breakpoints without altering the initial pattern of behavior. These effects are similar to recent findings showing that a 10 mg/kg i.p. dose of SB-334867 reduces PR responding for cocaine (Borgland et al., 2009).
The threshold self-administration procedure used here provides information on both consumption and price within a single session. Lever responses during the early portion of the session resulted in high doses of cocaine, and consequently rats could readily titrate to preferred blood levels, similar to an FR schedule. It is not surprising, therefore, that SB-334867 had no effect on cocaine intake under these conditions. In contrast, as the dose of cocaine was lowered throughout the session, rats were required to respond more frequently to maintain blood levels of cocaine. This is akin to a PR schedule in which increasingly greater numbers of responses are required to obtain the same amount of drug. At this higher cocaine price, SB-334867 decreased responding earlier in the session, at threshold doses that were sufficient to maintain self-administration in vehicle-treated rats (Pmax).
Combined, these results indicate that HCRT neurotransmission is necessary to support cocaine self-administration under conditions with high-effort requirements or in which titrating to preferred blood levels of cocaine is restricted. Furthermore, given that intra-VTA SB-334867 and i.p. SB-334867 reduced cocaine intake to a similar extent, it is likely that HCRT influences cocaine reinforcement, via actions within the VTA.
Dopamine signaling
The attenuation of cocaine self-administration may be attributable to reduced DA signaling within the NAc core. Microdialysis experiments show that intra-VTA, and to a certain extent peripheral SB-334867, reduced tonic DA levels in the NAc core prior to cocaine administration. Furthermore, both i.p. and intra-VTA SB-334867 significantly reduced DA responses to cocaine. These observations are consistent with previous work demonstrating that HCRT-1 increases DA levels in the NAc (Narita et al., 2006), although other studies did not find HCRT-1 effects in the NAc core (Vittoz & Berridge, 2006).
In vivo voltammetry studies indicated similar alterations in DA signaling to those observed with microdialysis. In these studies, intra-VTA SB-334867 significantly reduced electrically evoked DA release in the NAc core prior to cocaine administration. Additionally, SB-334867 attenuated the effects of cocaine on both DA release and uptake inhibition. In vitro voltammetry experiments also demonstrated that HCRT KO mice had reduced DA release and slower uptake under baseline conditions, and attenuated cocaine-induced DA uptake inhibition. Combined, the in vivo and in vitro voltammetry observations suggest that HCRT reduces DA responses to cocaine, in part, by attenuating cocaine-induced DA uptake inhibition.
The effects of disrupted HCRT neurotransmission on DA signaling and cocaine effects are likely due to decreased DA neuron activity in the VTA, through direct effects on either DA neurons or GABA interneurons. This hypothesis is strengthened by work indicating that HCRT neurotransmission regulates excitatory synaptic transmission in DA neurons (Borgland et al., 2006), and potentiates glutamatergic synaptic transmission in brain slices from animals with a history of cocaine self-administration (Borgland et al., 2009). Furthermore, recent observations indicate that, in addition to blocking DA reuptake, cocaine also increases the incidence of DA release events in the NAc shell (Aragona et al., 2008) and stimulates glutamate release in the VTA of cocaine-experienced rats (Wise et al., 2008). Thus, it is possible that reduced effects of cocaine in HCRT KO mice or rats treated with SB-334867 could be related to attenuation of cocaine enhancement of DA signaling at the VTA. Additionally, it is possible that reduced DA neuronal activity in HCRT KO mice, or following SB-334867 treatment, could result in changes in DA terminals in the NAc (e.g. via phosphorylation or glycosylation of the DA transporter), leading to altered transporter function that ultimately reduces cocaine’s effectiveness (Li et al., 2004; Foster et al., 2008; Mortensen et al., 2008).
The microdialysis and voltammetry observations presented here indicate that both acute and chronic disruption in HCRT neurotransmission result in altered DA signaling and reduced DA responses to cocaine. These data concur with previous studies indicating that SB-334867 reduces CPP for morphine and attenuates morphine withdrawal, and that HCRT KO mice display decreased DA responses to morphine in the NAc (Georgescu et al., 2003; Narita et al., 2006). When combined with the current cocaine self-administration findings, these observations provide substantial support for the hypothesis that HCRT neurotransmission is necessary for normal DA signaling and for cocaine self-administration behavior, and that these effects may involve actions within the VTA.
Sucrose self-administration
To examine the effects of SB-334867 on responding for a natural reward under varying satiety conditions, PR sucrose self-administration in food-sated and food-restricted rats was performed. In these studies, SB-334867 significantly reduced breakpoints in food-sated rats, but had no effect in food-restricted rats. Previous studies indicate that an animal’s satiety state can dictate their motivation to work for food and their sensitivity to self-administration manipulations that reduce responding [e.g. increasing ratio requirements (Salamone et al., 1991; Aberman & Salamone, 1999; Thorpe et al., 2005; Barbano et al., 2009)]. In the current studies, food-sated rats showed significantly lower motivation to work for sucrose than did food-restricted rats, and these animals were more sensitive to the effects of SB-334867. Although the current observations may appear to conflict with previous observations (Thorpe et al., 2005; Lawrence et al., 2006; Hollander et al., 2008; Nair et al., 2008; Borgland et al., 2009), this is the first study to test the effects of SB-334867 across different satiety states, making direct comparisons with previous work difficult.
It is possible that the differential effects of SB-334867 observed between food-sated and food-restricted rats could be related to variations in the responsivity of DA systems between these two groups of rats. Indeed, previous reports have indicated that food-restricted rats display a variety of alterations in DA function, including increased tyrosine hydroxylase activity (Pan et al., 2006), enhanced DA uptake (Zhen et al., 2006), and reduced basal levels of DA in the NAc (Pothos et al., 1995). Furthermore, food-restricted rats also display enhanced NAc DA responses to food (Wilson et al., 1995), cocaine and amphetamine (Pothos et al., 1995; Rouge-Pont et al., 1995; Cadoni et al., 2003). Thus, the same adaptations in DA signaling that render food-restricted rats more sensitive to the rewarding properties of food or psychostimulants may underlie their insensitivity to SB-334867 effects on the motivation to work for sucrose.
The differential effects of SB-334867 on food-sated, but not food-restricted, rats suggest that disrupted HCRT neurotransmission may not affect food intake when animals are motivated by nutritional factors. This is consistent with a recent report indicating that in food-restricted rats, SB-334867 reduces high-fat food intake, while regular food consumption is preserved (Borgland et al., 2009). Given that an ideal pharmacotherapy for cocaine addiction would not only reduce the motivation to take cocaine, but also preserve the ability of the animal to meet nutritional requirements, the current findings suggest that the HCRT system may be a promising target for intervention.
HCRT and arousal
Extensive evidence indicates that HCRT regulates arousal-related processes, including sleep–wake function and locomotor activity (Hagan et al., 1999; Bourgin et al., 2000; Piper et al., 2000; España et al., 2001, 2002). Consequently, it is possible that SB-334867 reduces self-administration via generalized disruptions in arousal rather than through actions on reinforcement mechanisms. However, in the FR studies and in the early portions of the PR and threshold experiments, conditions in which maintaining preferred blood levels of cocaine requires relatively low effort, SB-334867 had no effect on the rate of cocaine responding. Under these conditions, SB-334867-treated rats showed normal levels of responding for cocaine, suggesting that rats were not sedated and were entirely capable of responding on levers. Furthermore, in food-restricted rats, SB-334867 did not reduce sucrose self-administration, indicating that highly motivated rats also had the capacity to respond on levers. Taken together, the results showing that SB-334867 decreases cocaine intake on the DT, PR and threshold schedules, but does not affect the responding under an FR or sucrose-reinforced PR schedule in food-restricted rats, suggest that the pharmacological effects of SB-334867 cannot be readily explained by generalized SB-334867 effects on sedation or motor activity. This interpretation is consistent with previous reports indicating that SB-334867 does not elicit sleep and does not alter responding for other natural rewards (Lawrence et al., 2006; Deng et al., 2007; Rasmussen et al., 2007; Hollander et al., 2008; Dugovic et al., 2009).
Conclusions
The current studies demonstrate that blockade of HCRT receptors reduces cocaine intake across a variety of self-administration protocols and reduces sucrose intake in food-sated but not food-restricted rats. Disrupted HCRT signaling also reduces baseline and cocaine-induced DA responses within the NAc core. These studies suggest that HCRT is critically involved in cocaine self-administration behavior through actions on the VTA–mesolimbic DA system.
Acknowledgments
We would like to thank L.N. Thomas, J.R. Melchior (M.S.) and J.K. Konstantopoulos for their expert technical assistance. These studies were supported by K01 DA025279 (R.A. España), R01 DA021325 (S.R. Jones), P50 DA06634 (D.C.S. Roberts and S.R. Jones), R01 DA14030 (D.C.S. Roberts), and F31DA024525 (E.B. Oleson).
Abbreviations
- CPP
conditioned place preference
- DA
dopamine
- DMSO
dimethylsulfoxide
- DT
discrete trials
- FR
fixed ratio
- HCRT
hypocretin-orexin
- HPLC
high-performance liquid chromatography
- i.p
intraperitoneal
- i.v
intravenous
- Km
apparent affinity
- KO
knockout
- NAc
nucleus accumbens
- PBS
phosphate-buffered saline
- Pmax
maximal unit price
- PR
progressive ratio
- Vmax
maximal uptake rate
- VTA
ventral tegmental area
- WT
wild-type
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Fig. S1. Photomicrograph of intra-VTA infusion site.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology (Berl) 2009;203:475–487. doi: 10.1007/s00213-008-1390-6. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav. 2002;71:277–282. doi: 10.1016/s0091-3057(01)00659-1. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DC. The GABA(B) agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–1804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology (Berl) 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di CG. Selective psycho-stimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- España RA, Jones SR. A neurochemical perspective on dopamine interactions with psychostimulants. In: David H, editor. The Nucleus Accumbens: Neurotransmitters & Related Behaviours. Research Signpost; Kerala: 2008. pp. 329–368. [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin–dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco PG, Garris PA. In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol. 2003;479:117–125. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Kilts CD, Wightman RM. Comparison of dopamine uptake in the basolateral amygdaloid nucleus, caudate-putamen, and nucleus accumbens of the rat. J Neurochem. 1995;64:2581–2589. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Odell SJ, Marshall JF, Wightman RM. Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse. 1996;23:224–231. doi: 10.1002/(SICI)1098-2396(199607)23:3<224::AID-SYN12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine – relationship between locomotor and stereotypy response profiles and caudate and accumbens-dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Stafford D, Glowa JR. Preclinical research on cocaine self-administration: environmental determinants and their interaction with pharmacological treatment. Neurosci Biobehav Rev. 1999;23:717–741. doi: 10.1016/s0149-7634(99)00015-9. [DOI] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McGregor A, Baker G, Roberts DC. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 1996;53:5–9. doi: 10.1016/0091-3057(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Larsen MB, Prasad BM, Amara SG. Genetic complementation screen identifies a mitogen-activated protein kinase phosphatase, MKP3, as a regulator of dopamine transporter trafficking. Mol Biol Cell. 2008;19:2818–2829. doi: 10.1091/mbc.E07-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur J Neurosci. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Berman Y, Haberny S, Meller E, Carr KD. Synthesis, protein levels, activity, and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur J Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Hsu MA, Noone S, Johnson BG, Thompson LK, Hemrick-Luecke SK. The orexin-1 antagonist SB-334867 blocks antipsychotic treatment emergent catalepsy: implications for the treatment of extrapyramidal symptoms. Schizophr Bull. 2007;33:1291–1297. doi: 10.1093/schbul/sbm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Goeders NE. Drug self-administration: experimental methods and determinants. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Neuromethods. Humana Press; Clifton, N.J: 1989. pp. 349–398. [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le MM, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DC. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berl) 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps:Structure of the Rat Brain. Elsevier Science Publishers B.V.; Amsterdam: 1998. [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin/orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC. Dopaminergic correlates of motivated behavior: importance of drive. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Zhen J, Reith ME, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]
- Zittel-Lazarini A, Cador M, Ahmed SH. A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology (Berl) 2007;192:337–346. doi: 10.1007/s00213-007-0724-0. [DOI] [PubMed] [Google Scholar]