Abstract
Lapses in self-care are commonly cited as a major cause of poor outcomes in persons with heart failure (HF). Not surprisingly, self-care is assumed to be central to improving health outcomes in this patient population. Empirically, however, this assumption is not well supported, and mechanistically, relationships between self-care and outcomes in HF have not yet been described. In this review, it is proposed that effective self-care maintenance (adherence) and self-care management (symptom evaluation and management) practices are complementary to optimal medical management in delaying HF progression and improving health outcomes in this population. Potential mechanisms through which effective HF self-care practices are complementary to pharmacological therapy in improving outcomes include; a) facilitating partial blockade and partial deactivation of deleterious neurohomones, b) limiting inflammatory processes, c) decreasing the need for administration of detrimental pharmacological agents, and d) minimizing myocardial hibernation. As these mechanisms are hypothetical, research findings are required to establish their validity. Several strategic research questions are proposed.
Keywords: heart failure, self-care, adherence, symptom management, physiology
Introduction
Poor outcomes in heart failure (HF) are commonly attributed to insufficient self-care practices.1–3 Likewise, it is a commonly held view that effective self-care is central to improving health outcomes in the HF population, ostensibly because persons with HF are responsible for the majority of their own care.4 Currently, there is inadequate evidence to support the assumption that better HF self-care translates into better outcomes. Similarly, assumed mechanisms through which HF self-care may influence health outcomes remain untested.
The purpose of this review is to present hypothetical mechanisms through which self-care may influence health outcomes in persons with HF. In general, we propose that effective self-care practices are cardioprotective. Specifically, it is proposed that persons who are more engaged in HF self-care may have better health outcomes due to: a) achieving and maintaining effective neurohormonal blockade and partial deactivation, b) minimizing inflammation, c) avoiding the need for deleterious pharmacological therapy, and d) limiting myocardial hibernation. In this review, a contemporary definition of HF self-care is provided. Key elements of these four hypothetical mechanisms are then reviewed in brief. This review concludes with clinical practice and research implications of these potential mechanisms.
Heart Failure Self-Care: Contemporary Perspective
HF self-care is an active process involving critical behaviors that help maintain physiologic homeostasis and prevent acute exacerbations (self-care maintenance). HF self-care also involves decision-making in which patients engage and actions they take to effectively evaluate and manage symptoms when they occur (self-care management).5 (Figure 1)
Figure 1. Contemporary Model of Heart Failure Self-Care.
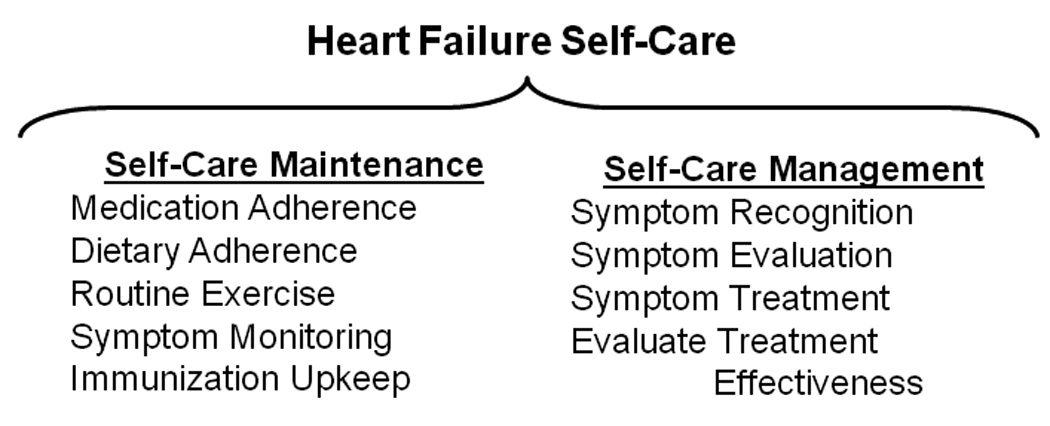
Heart Self-care maintenance in persons with heart failure includes routine daily practices, such as taking prescribed medications, maintaining fluid volume by limiting dietary sodium intake, and participating in health seeking behaviors like exercise. Self-care management in persons with heart failure includes symptom recognition and evaluation, engagement in strategies to ameliorate symptoms, and judgment of the effectiveness of self-directed treatment strategies. Source: Author
Heart Failure Self-Care Maintenance
Self-care maintenance behaviors reflect the degree to which a HF patient follows health actions set forth by and in agreement with a healthcare provider.6 This important aspect of HF self-care is commonly referred to as treatment adherence. Self-care maintenance behaviors include routine daily practices. HF patients who practice effective self-care maintenance are adherent to prescribed hormone blockers and diuretics, dietary sodium restriction, measurement of daily weight, and symptom surveillance.7 Further, patients who practice effective self-care maintenance exercise regularly, avoid potentially harmful medications like non-steroidal anti-inflammatory drugs, and participate in several other health-seeking behaviors like maintaining scheduled immunizations. In contrast, patients who practice poor self-care maintenance may go for periods of time without taking prescribed medications that help suppress deleterious hormones activated during HF. In addition, poor self-care maintenance can be manifested by non-adherence to daily treatment activities including dietary sodium restriction, and symptom surveillance.
Heart Failure Self-Care Management
Self-care management refers to a patient’s ability to quickly recognize HF symptoms when they occur, engage in a management strategy to ameliorate symptoms, and judge the effectiveness of their self-directed treatment strategies.8 In the event of a sign of fluid excess or subtle congestive symptom, patients who practice effective self-care management quickly recognize the change and act to ameliorate symptoms by limiting fluid or sodium intake, or taking additional doses of low-dose diuretics as a treatment strategy. Persons who practice effective self-care management are able to judge the effectiveness of self-engaged treatments7 and determine if they must return to a state of surveillance or involve healthcare practitioners in the management of HF symptoms.9 On the contrary, patients who practice poor self-care management are not likely to quickly recognize signs and symptoms as manifestations of their chronic HF, and are not likely to take appropriate action when congestive symptoms occur. Further, patients who practice poor self-care management are unlikely to be able to judge the effectiveness of their self-engaged treatments.
Potential Mechanisms Through Which Self-Care Influences Health Outcomes
Heart Failure Self-Care and Cardioprotection
In persons with HF, cardioprotective strategies aim to delay the progression of cardiomyocyte death and untoward outcomes, such as advanced ventricular dysfunction or frequent episodes of clinical congestion that lead to re-hospitalization and death.10 In the broadest sense, effective self-care is complementary to optimal medical management in delaying HF progression and poor health outcomes and in this way is cardioprotective. Although four hypothetical mechanisms by which HF self-care may influence outcomes are presented individually, it is more likely that effective self-care practices influence multiple if not all four means of cardioprotection concurrently.
Potential Mechanism I: Neurohormonal Blockade and Partial Deactivation
According to a widely held theory of the natural progression of HF,11 hormones produced in response to HF cause hemodynamic congestion. Congestion may leave an indelible mark by overloading the heart and further impairing cardiac hemodynamics.12 Unrecognized and untreated congestion eventually leads to burdensome and disabling HF symptoms,13 progressive cardiocirculatory deterioration, and eventually death. Effective self-care maintenance and management may help interrupt and delay this progression of HF.
Predominantly in response to changes in arterial pressure and filling the sympathetic nervous system (SNS) mounts a rapid, robust and complex response that is largely mediated by norepinephrine (NE). The initial cardiovascular effects of SNS activation include positive chronotropism, dromotropism, inotropism and lusitropism, as well as venous and arteriolar vasoconstriction.14, 15 These cardiocirculatory adaptations initially help restore cardiac output and mean arterial pressure. Renin-angiotensin-aldosterone system (RAAS) activation, triggered by decreased renal pressure16 and SNS stimulation,17 is also initially compensatory in restoring mean arterial pressure. As an example, angiotensin II (ANG-II), a RAAS mediator, is a potent vasoconstrictor that also stimulates aldosterone-mediated water and sodium conservation,18 which in combination restores blood volume, cardiac output and mean arterial pressure. Chronic SNS and RAAS activation, however, lead to increased afterload, hemodynamic and clinical congestion,19 increased myocardial oxygen demand, intracellular calcium toxicity, as well as proliferative14 and pro-inflammatory effects.20
Effective self-care maintenance includes taking neurohormonal blockers, such as β-adrenergic blockers and angiotensin converting enzyme (ACE) inhibitors regularly as prescribed. The essential step of medication adherence helps minimize the cardiocirculatory burden of elevated levels of NE and ANG-II and has numerous and well established health outcome benefits. Maintaining optimal sodium and water balance also may minimize activation of certain neurohormonal systems. Although a source of unresolved controversy,21 adhering to dietary fluid and sodium restriction are thought to be effective in maintaining optimal sodium and water balance and minimizing the risk of congestive episodes. This assumption is based on evidence that lapses in dietary restriction increase the risk of HF readmission and mortality,22, 23 and the fundamental principle that neurohormonal activation limits the body’s ability to excrete ingested sodium and water.24
Effective self-care management is also essential to limiting the burden of neurohormones and partially deactivating neurohormonal systems. Engagement in symptom management is dependent on sensing and interpreting symptoms when they occur, which can be difficult for HF patients.25 Persons who are able to recognize HF symptoms early, however, are likely to engage in self-initiated treatment strategies that are relatively less aggressive and to avoid long delays in treatment-seeking that have been identified in this patient population.26 For example, persons with HF may be able to manage their symptoms by merely restricting fluid or sodium intake or to manage their congestion with comparatively low-dose diuretics. Decreased congestion, as a result of low-dose diuretics and limited sodium and water intake, helps optimize blood volume and cardiac hemodynamics and thereby deactivates several neurohomonal systems including SNS and RAAS. At the same time, such small, patient-managed adjustments decrease the risk of reactive increases in neurohormonal activation that can occur with greater changes in sodium intake27 and in response to high-dose diuretics.28 Further, managing fluid and sodium intake may improve congestion and the effectiveness of β-blocking agents.29 That is, β-blocking agents are thought to have greater cardioprotective effects in the absence of clinical congestion.30 Thus, patients who effectively manage episodes of congestion may benefit from improved neurohormonal blocker efficacy.
Generalized neurohormonal activation, involving the secretion of additional hormones like arginine vasopressin (AVP) and endothelin 1 (ET-1), occurs late in the progression of HF.12 AVP is secreted in response to arterial underfilling,31 and causes vasoconstriction, water conservation, and enhanced catecholamine responsiveness, all which contribute to congestion. ET-1 is produced within endothelial cells in response to various stimuli including shear stress, hypoxia, and other circulating hormones such as ANG-II and AVP.32 ET-1 causes potent and sustained vasoconstriction,33 as well as positive chronotropism and inotropism in contractile cells.32 While pharmaceutical agents that block AVP and ET-1 may be prescribed in addition to standard therapy, the secretion of these hormones also can be minimized by suppression of SNS and RAAS and optimization of cardiac hemodynamics with effective self-care management practices. As an example, prompt self-management of congestion may improve cardiac hemodynamics enough to deactivate non-osmotic AVP secretion. In addition, partial SNS suppression by the prompt treatment of subtle HF symptoms and adherence to β-adrenergic blockers limits ET-1 secretion.34
Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) are secreted from cardiomyocytes in response to wall stretch.35 Secretion of both natriuretic peptides is also stimulated by several circulating substances including NE, ANG-II, AVP, ET-1 and numerous inflammatory cytokines.35 The natriuretic peptides have beneficial cardioprotective effects, including diuresis, natriuresis, cardiomyocyte relaxation and vasodilatation.35 These cardioprotective effects, however, are often opposed by concomitant increases in neurohormones, such as NE and ANG-II, with antagonistic cardiocirculatory effects.24, 27 Thus, the full cardioprotective benefits of the natriuretic peptides are only achieved when the effects of other neurohormonal systems (e.g. SNS & RAAS) are partially blocked pharmacologically. In this manner, persons who are adequately engaged in effective self-care maintenance practices are more likely to benefit from the action of endogenous ANP and BNP, as the effects of cardiostimulatory and vasoconstrictive hormones are suppressed. Benefits of another cardioprotective hormone, bradykinin, can also be enhanced by adherence to ACE inhibitors.36
In summary, (Figure 2) persons who engage in effective HF self-care practices are more likely to maintain fluid volume balance and avoid clinical congestion. They are able to partially block the detrimental neurohormones of HF, such as NE, ANG-II, AVP and ET-1, and at the same time partially deactivate these neurohormonal systems. Persons who are engaged in effective self-care practices are able to have a favorable balance between natriuretic peptides and disadvantageous hormones and to benefit from the augmented effect of other endogenous cardioprotective hormones like bradykinin. In addition, because they are more likely to manage episodes of congestion, persons with HF who are engaged in effective self-care are able to garner the benefit of enhanced pharmacological effectiveness.
Figure 2. Hypothetical Mechanisms Through Which Heart Failure Self-Care Influences Health Outcomes.
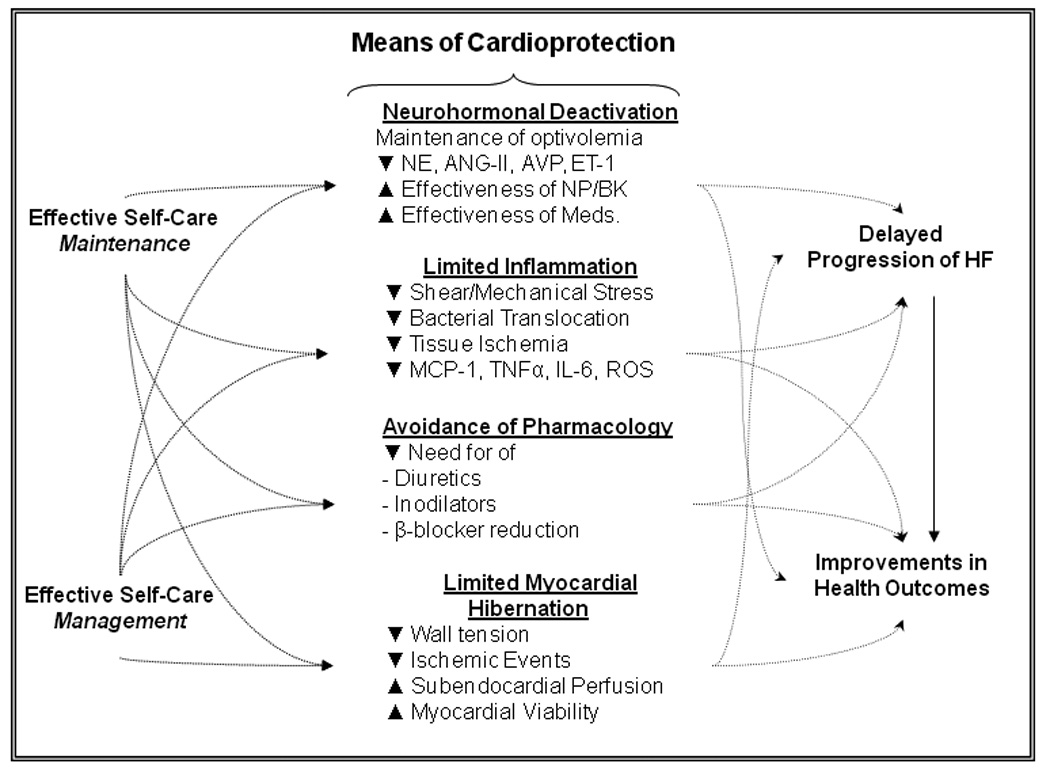
Effective self-care behaviors are cardioprotective, potentially delaying HF progression and improving outcomes in persons with HF. It is proposed that effective self-care practices are complementary to pharmacological therapies in improving outcomes by promoting neurohormonal blockade and deactivation, limiting inflammatory processes, decreasing the likelihood of detrimental changes to pharmacological management, and limiting the extent of myocardial hibernation. See text for details. Abbreviations: ANG-II = angiotensin II, AVP = arginine vasopressin, BK = bradykinin, ET-1 = endothelin, HF = heart failure, IL-6 = interleukin 6, Meds. = medications, MCP-1 = monocyte chemoattractant protein 1, NE = norepinephrine, NP = natriuretic peptides, ROS = reactive oxygen species, TNFα = tumor necrosis factor alpha. Source: Author
Potential Mechanism II: Minimizing Inflammation
According to the “cytokine hypothesis”,37 several inflammatory mediators are expressed in response to HF, and both modulate and contribute to HF pathogenesis. Pro-inflammatory cytokines are thought to promote ventricular remodeling by inducing ventricular hypertrophy, fibrosis and apoptosis.38 Several potential mechanisms through which inflammatory mediators are activated in HF have been proposed.
Laminar shear stress exerts an anti-inflammatory effect in healthy humans.39 In contrast, when the endothelium is disrupted by hemodynamic stress, leukocyte and platelet adhesion and expression of pro-inflammatory cytokines like monocyte chemoattractant protein-1 (MCP-1) is triggered.40 In addition, direct hemodynamic stretch triggers myocardial production of the pro-inflammatory cytokines tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6).41 TNFα is thought to cause profound contractile dysfunction and induction of apoptosis.42 Chronic elevation of IL-6 leads to ventricular hypertrophy43 and negative inotropism.44 It also is hypothesized that IL-6 activation in HF inhibits the expression of cardioprotective peptides like ANP.43 Cytokines have been implicated in the induction of reactive oxygen species, which contribute to the progression of HF by decreasing contractile force and inducing apoptosis.45 Accordingly, actions that ameliorate hemodynamic stress and mechanical stretch are likely to limit the expression and secretion of inflammatory mediators.
Maintaining volume homeostasis and optimal cardiac hemodynamics decreases the likelihood of mechanical stress and stretch. In patients with HF, mechanical stress and stretch are controlled through the early and effective management of episodes of congestion. That is, HF patients who quickly recognize and treat HF symptoms are likely to ameliorate episodes of congestion that contribute to mechanical stress and stretch and subsequent expression of pro-inflammatory mediators. Adherence to neurohormonal blockers may also limit the impact of inflammatory mediators in persons with HF. For example, there is evidence that β-blocking agents decrease levels of TNFα and IL-6 in patients with cardiomyopathy.46 In addition, improvement in ejection fraction and functional capacity after initiation of β-blocking agents in patients with HF is highly correlated with a concomitant decrease in plasma IL-6.47
Infection also may lead to inflammation in patients with HF. Seemingly simple health maintenance behaviors such as hand washing, preventative dental health, and maintenance of scheduled immunizations have the potential to limit inflammation and the associated detrimental cardiac effects. As an example, poor dental health is linked to development of cardiovascular disease and mortality.48 Further, the risk of hospitalization for HF is increased during influenza season,49 a risk that can be minimized by taking influenza vaccine.50 As an additional source of infection, bacterial endotoxins translocate from the gut to the blood stream during episodes of congestion, a possible cause of elevated levels of inflammatory markers in patients with HF.51, 52 Symptom monitoring and management of episodes of congestion appear to be critical. That is, it is conceivable that patients who are more vigilant in monitoring for subtle congestive signs and symptoms and who engage in effective management strategies are more likely to minimize bacterial translocation that occurs during episodes of congestion.
Tissue ischemia can also induce pro-inflammatory cytokine production. Intracellular stress signals respond to hypoxia and subsequent tissue injury, inducing expression of IL-6,41 TNFα, and MCP-1.40 One way of improving oxygen delivery is routine exercise. Although the source of ongoing debate, there is evidence that exercise increases anaerobic threshold,53 and increases peak oxygen uptake in persons with HF,54 and increases coronary flow reserve in persons with coronary artery disease.55 Thus exercise may be one way in which persons with HF can optimize tissue oxygen delivery and minimize ischemia. Adhering to β-blockers also aids in reducing myocardial ischemia, as these agents decrease myocardial oxygen demand.56 Moreover, managing episodes of congestion is likely to decrease wall tension that decreases subendocardial perfusion and limits optimal cardiac hemodynamics. In fact, the majority of subendocardial perfusion occurs during diastole. If end-diastolic volumes and pressures are high then wall tension is also high, limiting blood flow to the myocardial muscle.57 Patients who are able to recognize early symptoms of congestion and engage in effective management strategies are more likely to optimize diastolic filling pressures and limit the potential ischemic insult from such congestive episodes.
In summary, through effective self-care maintenance and management behaviors, persons with HF may limit active inflammatory processes and cytokine elevations that are linked to poor outcomes in HF. Potential mechanisms include the reduction of infectious processes, like influenza or dental caries, and the prevention or management of congestion that may foster gut bacterial translocation or myocardial mechanical stress and ischemia in persons with HF.
Potential Mechanism III: Avoiding the Need for Deleterious Pharmacology
When the management of HF symptoms is refractory to self-care, neurohormonal activation and inflammation severely impair cardiac hemodynamics and induce further myocardial injury,19 often necessitating emergency department and/or hospital admission. Managing acute decompensated HF (also referred to as acute HF syndrome58) solely under the hemodynamic paradigm requires high-dose diuretics, inodilators (agents with both positive inotropic and vasodilator effects) and the withdrawal of β-blockers. Effective self-care maintenance and management may prevent acute decompensated HF and the need for complex and aggressive management that is potentially deleterious.
Once effective neurohormonal blockade is achieved, there is strong advocacy for the management of HF with minimal diuretic dosing or without diuretics.28 This is due in part to a growing body of evidence that the use of diuretics is associated with poor outcomes in persons with HF.59–62 Potential reasons for hazards associated with diuretics include resultant electrolyte disturbances, excessive neurohormonal activation, hypovolemia and renal impairment.28 Routine use of inodilator agents in the treatment of persons with acute or chronic HF may also result in poor outcomes.63–66 Inodilators, like certain exogenous catecholamines and phosphodiesterase inhibitors, induce tachycardia, ischemia, hypotension and necrosis, and increase ectopy.63 Although they may transiently improve function, the risks of this class of drugs appear to outweigh their long-term benefits. In addition, during hospitalization for acute episodes of congestion, β-blocking agents are often reduced or temporarily discontinued. Reduction of β-blocker dose or cessation, however, is associated with an increased risk of mortality.67
Strict adherence to neurohormonal blockers, fluid and sodium intake reduction/restriction and preventative health measures are key aspects of HF self-care maintenance that may help prevent the evolution of acute decompensated HF. Symptom monitoring is a particularly important aspect of self-care maintenance that facilitates early recognition and actions that effectively ameliorate symptoms when they occur. Persons who practice effective symptom monitoring may recognize early satiety or fatigue or clothes feeling tight as evidence of congestion requiring treatment. On the contrary, if subtle or even hallmark signs and symptoms are missed or not considered symptoms of HF, congestive episodes are likely to progress to acute decompensated HF where self-initiated treatments may be less effective.
Self-care management of HF symptoms also prevents acute decompensated HF and the need for aggressive management. HF patients experience daily fluctuations in HF symptoms,68 making it difficult to interpret symptom severity and even the presence of symptoms with an insidious onset.69 Persons who are able to quickly recognize worsening HF symptoms, however, are more likely to engage in timely and effective self-care management strategies that ameliorate symptoms by means that are much less aggressive than high-dose diuretic or inodilator therapy. Further, HF patients who are able to judge the effectiveness of their self-initiated treatment strategies are more likely to take appropriate action in follow-up of episodes of congestion. Namely, persons engaged in effective self-care management are able to assess the need for continued or alternative symptom treatment, engagement of healthcare providers in their symptom management, and/or continued symptom surveillance.
Potential Mechanism IV: Myocardial Hibernation
Myocardial hibernation is the phenomenon of chronic and reversible ventricular dysfunction due to inadequate coronary perfusion.70 Unlike complete coronary artery occlusion, coronary hypoperfusion that causes myocardial hibernation is not severe enough to induce necrosis or apoptosis. Rather, ischemic insults reduce inotropic force of segments of the ventricle.70 The pathogenesis of hibernating myocardium is largely unclear. Structural alterations, including contractile protein depletion and fibrosis and metabolic derangements, as well as decreased coronary flow reserve71 and resting myocardial perfusion72 have been previously cited as potential contributors to myocardial hibernation.
Hibernating myocardium is problematic for patients with HF, as these regions of the ventricle are prone to repeated episodes of ischemia and may progress to necrosis or apoptosis.71 Repeated ischemic insults can be due to progressive stenosis or wall tension that limits subendocardial blood flow. Most importantly, hibernating myocardium represents viable myocardial tissue, the function of which may be improved by ameliorating the mismatch between coronary flow and function. Coronary revascularization has certain utility in improving the function of hibernating myocardium. Maintaining blood volume homeostasis by adhering to prescribed dietary restrictions and taking HF medications as prescribed may reduce the frequency and duration of episodes of increased wall tension and ischemia. Rapid self-management of congestive symptoms also has implications in improving myocardial viability. That is, patients who practice effective self-care management are more likely to avoid prolonged episodes of increased transmural pressure and myocardial ischemia that trigger the progression of compensated myocardium into hibernation, or from hibernating myocardium to necrosis/apoptosis.
One mechanism through which β-adrenergic blockers are thought to improve myocardial function in HF is through improvements in the balance of myocardial oxygen supply and demand. In support of this hypothesis, patients with known hibernating myocardium have a greater increase in ejection fraction after the addition of a β-blocking agent than HF patients without hibernating myocardium.73 It is thought that this improvement in contractile function may be due, at least in part, to improved function of areas of the myocardium that were hibernating. Accordingly, prescription of β-blockers was proposed as a complementary or alternative therapy to revascularization in patients with hibernating myocardium.73 Adherence to β-blockers may well improve viability of hibernating myocardium. Moreover, routine exercise may also have important implications, particularly if the increased coronary flow reserve found in persons with coronary artery disease who exercise55 also exists is persons with non-ischemic HF.
Implications for Clinical Practice and Research
Teaching and fostering effective self-care practices in persons with HF is assumed to be evidence-based. To date, however, there are limited data to support this claim. As described, there are several possible mechanisms through which patients with HF may be able to impact their own health through effective self-care practices. Helping patients with HF learn how to master the maintenance of their health and management of HF symptoms remains central to multidisciplinary HF disease management programs. Improvements in self-care practices may be complementary to optimal pharmacological treatment of HF, and may be the link to improving patient outcomes that has been missing for decades in this population.
As the majority of research regarding HF self-care has aimed to identify factors that influence HF self-care ability, there are several implicit programs of research suggested. Specifically, the link between HF self-care behavior (both self-care maintenance and self-care management) and physiological surrogates and clinical outcomes has yet to be made. Accordingly, there are five general research questions associated with this aspect of HF self-care research (Table 1). First, is there an association between level of engagement in self-care and indices of neurohormonal activation in persons with HF? Second, is there an association between level of engagement in self-care and indices of inflammation? Third, is there a link between effective self-care and the decreased need for acute pharmacological agents with controversial or deleterious effects? Fourth, is there an association between HF self-care and myocardial viability? Finally, is there a direct association between self-care and health outcomes, like HF readmission and mortality?
Table 1.
Research Priorities. In order to begin validating the hypothetical mechanism described herein, several research questions are proposed.
| Research Questions |
|---|
|
Neurohormonal blockade and deactivation: 1) Are higher levels of engagement in self-care behaviors associated with lower plasma levels of NE, ANG-II, AVP, ET-1, ANP or BNP? |
|
Inflammation: 1) Are higher levels of engagement in self-care behaviors associated with lower plasma levels of TNFα, IL-6, CRP, or decreased incidence of systemic infection? |
|
Need for aggressive and disadvantageous pharmacological therapy: 1) Are higher levels of engagement in self-care behaviors associated with decreased high-dose diuretic or inodilator use, or less neurohormonal blocker discontinuation? |
|
Myocardial hibernation: 1) Do patients who are more engaged in self-care behaviors have less hibernating myocardium, or have greater improvements in EF after the initiation of β-blockade? |
Abbreviations: β-blockade = beta adrenergic blockade, ANP = atrial natriuretic peptide, ANG-II = angiotensin II, AVP = arginine vasopressin, BNP = B-type natriuretic peptide, CRP = C-reactive protein, EF = ejection fraction, ET-1 = endothelin 1, IL-6 = interleukin 6, TNFα = tumor necrosis factor alpha.
Source: Author
Conclusion
It is a commonly held view that self-care is a major contributor to optimal HF management. This manuscript explored several hypothetical mechanisms through which HF self care may influence outcomes. In general, HF self-care is thought to be cardioprotective, and complementary to optimal medical management in the improvement of health outcomes. Specifically, it is thought that effective HF self-care practices facilitate partial neurohormonal blockade and deactivation, limit inflammatory processes, minimize the need for disadvantageous pharmacological therapy, and minimize myocardial hibernation. These hypothetical mechanisms need to be validated by the results of ongoing and future clinical research.
Acknowledgments
Funding acknowledgement: The project described was supported by award number F31NR010299 from the National Institute of Nursing Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher S. Lee, Doctoral Candidate & Lecturer, University of Pennsylvania School of Nursing, Philadelphia, PA.
Nancy C. Tkacs, Associate Professor, University of Pennsylvania School of Nursing, Philadelphia, PA.
Barbara Riegel, Professor, University of Pennsylvania School of Nursing, Philadelphia, PA.
References
- 1.Lainscak M, Cleland JG, Lenzen MJ, Keber I, Goode K, Follath F, et al. Nonpharmacologic measures and drug compliance in patients with heart failure: data from the EuroHeart Failure Survey. Am J Cardiol. 2007;99(6B):31D–37D. doi: 10.1016/j.amjcard.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Hauptman PJ. Medication adherence in heart failure. Heart Fail Rev. 2008;13(1):99–106. doi: 10.1007/s10741-007-9020-7. [DOI] [PubMed] [Google Scholar]
- 3.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80(5):437–441. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockwell JM, Riegel B. Predictors of self-care in persons with heart failure. Heart Lung. 2001;30(1):18–25. doi: 10.1067/mhl.2001.112503. [DOI] [PubMed] [Google Scholar]
- 5.Riegel B, Carlson B, Glaser D. Development and testing of a clinical tool measuring self-management of heart failure. Heart Lung. 2000;29(1):4–15. doi: 10.1016/s0147-9563(00)90033-5. [DOI] [PubMed] [Google Scholar]
- 6.Cleemput I, Kesteloot K. Economic implications of non-compliance in health care. Lancet. 2002;359(9324):2129–2130. doi: 10.1016/S0140-6736(02)09114-6. [DOI] [PubMed] [Google Scholar]
- 7.Riegel B, Dickson VV. A situation-specific theory of heart failure self-care. J Cardiovasc Nurs. 2008;23(3):190–196. doi: 10.1097/01.JCN.0000305091.35259.85. [DOI] [PubMed] [Google Scholar]
- 8.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10(4):350–360. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Jaarsma T, Abu-Saad HH, Dracup K, Halfens R. Self-care behaviour of patients with heart failure. Scand J Caring Sci. 2000;14(2):112–119. [PubMed] [Google Scholar]
- 10.Gaze DC. The role of existing and novel cardiac biomarkers for cardioprotection. Curr Opin Investig Drugs. 2007;8(9):711–717. [PubMed] [Google Scholar]
- 11.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20(1):248–254. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 12.Davila DF, Nunez TJ, Odreman R, de Davila CA. Mechanisms of neurohormonal activation in chronic congestive heart failure: pathophysiology and therapeutic implications. Int J Cardiol. 2005;101(3):343–346. doi: 10.1016/j.ijcard.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Shin DD, Thomas TO, Brandimarte F, Fonarow GC, Abraham WT. Congestion is an important diagnostic and therapeutic target in heart failure. Rev Cardiovasc Med. 2006;7 Suppl 1:S12–S24. [PubMed] [Google Scholar]
- 14.Katz AM. Heart failure: a hemodynamic disorder complicated by maladaptive proliferative responses. J Cell Mol Med. 2003;7(1):1–10. doi: 10.1111/j.1582-4934.2003.tb00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CS, Tkacs NC. Current Concepts of Neurohormonal Activation in Heart Failure: Mediators and Mechanisms. AACN Adv Crit Care. 2008;19(4):364–385. doi: 10.1097/01.AACN.0000340718.93742.c4. [DOI] [PubMed] [Google Scholar]
- 16.Schweda F, Segerer F, Castrop H, Schnermann J, Kurtz A. Blood pressure-dependent inhibition of Renin secretion requires A1 adenosine receptors. Hypertension. 2005;46(4):780–786. doi: 10.1161/01.HYP.0000183963.07801.65. [DOI] [PubMed] [Google Scholar]
- 17.Persson PB. Renin: origin, secretion and synthesis. J Physiol. 2003;552(Pt 3):667–671. doi: 10.1113/jphysiol.2003.049890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension. 2004;44(6):913–918. doi: 10.1161/01.HYP.0000146483.78994.56. [DOI] [PubMed] [Google Scholar]
- 19.Chen HH, Schrier RW. Pathophysiology of volume overload in acute heart failure syndromes. Am J Med. 2006;119(12) Suppl 1:S11–S16. doi: 10.1016/j.amjmed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 21.Lennie TA. Nutrition self-care in heart failure: state of the science. J Cardiovasc Nurs. 2008;23(3):197–204. doi: 10.1097/01.JCN.0000317426.14092.60. [DOI] [PubMed] [Google Scholar]
- 22.Bennett SJ, Huster GA, Baker SL, Milgrom LB, Kirchgassner A, Birt J, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168–174. [PubMed] [Google Scholar]
- 23.Tsuyuki RT, McKelvie RS, Arnold JM, Avezum A, Jr, Barretto AC, Carvalho AC, et al. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med. 2001;161(19):2337–2342. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- 24.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 25.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29(2):74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 26.Evangelista LS, Dracup K, Doering LV. Treatment-seeking delays in heart failure patients. J Heart Lung Transplant. 2000;19(10):932–938. doi: 10.1016/s1053-2498(00)00186-8. [DOI] [PubMed] [Google Scholar]
- 27.Alvelos M, Ferreira A, Bettencourt P, Serrao P, Pestana M, Cerqueira-Gomes M, et al. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur J Heart Fail. 2004;6(5):593–599. doi: 10.1016/j.ejheart.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Patel J, Smith M, Heywood JT. Optimal use of diuretics in patients with heart failure. Curr Treat Options Cardiovasc Med. 2007;9(4):332–342. doi: 10.1007/s11936-007-0028-z. [DOI] [PubMed] [Google Scholar]
- 29.Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(4):384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 30.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 31.Holmes CL, Landry DW, Granton JT. Science Review: Vasopressin and the cardiovascular system part 2 - clinical physiology. Crit Care. 2004;8(1):15–23. doi: 10.1186/cc2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16(8):1081–1098. doi: 10.1097/00004872-199816080-00001. [DOI] [PubMed] [Google Scholar]
- 33.Attina T, Camidge R, Newby DE, Webb DJ. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart. 2005;91(6):825–831. doi: 10.1136/hrt.2004.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nessler J, Nessler B, Kitlinski M, Gackowski A, Piwowarska W, Stepniewski M. Concentration of BNP, endothelin 1, pro-inflammatory cytokines (TNF-alpha, IL-6) and exercise capacity in patients with heart failure treated with carvedilol. Kardiol Pol. 2008;66(2):144–151. [PubMed] [Google Scholar]
- 35.Ruskoaho H. Cardiac hormones as diagnostic tools in heart failure. Endocr Rev. 2003;24(3):341–356. doi: 10.1210/er.2003-0006. [DOI] [PubMed] [Google Scholar]
- 36.Sharma JN. The kallikrein-kinin system: from mediator of inflammation to modulator of cardioprotection. Inflammopharmacology. 2005;12(5–6):591–596. doi: 10.1163/156856005774382760. [DOI] [PubMed] [Google Scholar]
- 37.Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2(3):243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 38.Gullestad L, Aukrust P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol. 2005;95(11A):17C–23C. doi: 10.1016/j.amjcard.2005.03.008. discussion 38C–40C. [DOI] [PubMed] [Google Scholar]
- 39.World CJ, Garin G, Berk B. Vascular shear stress and activation of inflammatory genes. Curr Atheroscler Rep. 2006;8(3):240–244. doi: 10.1007/s11883-006-0079-8. [DOI] [PubMed] [Google Scholar]
- 40.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med. 2005;37(2):74–85. doi: 10.1080/07853890510007232. [DOI] [PubMed] [Google Scholar]
- 41.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94(12):1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 42.von Haehling S, Jankowska EA, Anker SD. Tumour necrosis factor-alpha and the failing heart--pathophysiology and therapeutic implications. Basic Res Cardiol. 2004;99(1):18–28. doi: 10.1007/s00395-003-0433-8. [DOI] [PubMed] [Google Scholar]
- 43.Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45(2):183–193. doi: 10.1536/jhj.45.183. [DOI] [PubMed] [Google Scholar]
- 44.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91(11):988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 45.Lopez Farre A, Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38(6):1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 46.Cinquegrana G, D'Aniello L, Landi M, Spinelli L, Grande G, De Prisco F, et al. Effects of different degrees of sympathetic antagonism on cytokine network in patients with ischemic dilated cardiomyopathy. J Card Fail. 2005;11(3):213–219. doi: 10.1016/j.cardfail.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Mayer B, Holmer SR, Hengstenberg C, Lieb W, Pfeifer M, Schunkert H. Functional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levels. Int J Cardiol. 2005;103(2):182–186. doi: 10.1016/j.ijcard.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 48.Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C. Poor oral health is associated with coronary heart disease and elevated systemic inflammatory and haemostatic factors. J Clin Periodontol. 2004;31(1):25–29. doi: 10.1111/j.0303-6979.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 49.Sandoval C, Walter SD, Krueger P, Smieja M, Smith A, Yusuf S, et al. Risk of hospitalization during influenza season among a cohort of patients with congestive heart failure. Epidemiol Infect. 2007;135(4):574–582. doi: 10.1017/S095026880600714X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 51.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 52.Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5(5):609–614. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 53.Wielenga RP, Huisveld IA, Bol E, Dunselman PH, Erdman RA, Baselier MR, et al. Results of the Chronic Heart Failure and Graded Exercise study (CHANGE) Safety and effects of physical training in chronic heart failure. Eur Heart J. 1999;20(12):872–879. doi: 10.1053/euhj.1999.1485. [DOI] [PubMed] [Google Scholar]
- 54.McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144(1):23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 55.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342(7):454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 56.O'Rourke ST. Antianginal actions of beta-adrenoceptor antagonists. Am J Pharm Educ. 2007;71(5):95. doi: 10.5688/aj710595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz AM. Physiology of the Heart. Forth ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 58.Fonarow GC. Acute decompensated heart failure: challenges and opportunities. Rev Cardiovasc Med. 2007;8 Suppl 5:S3–S12. [PubMed] [Google Scholar]
- 59.Domanski M, Tian X, Haigney M, Pitt B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J Card Fail. 2006;12(5):327–332. doi: 10.1016/j.cardfail.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27(12):1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97(12):1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 62.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bayram M, De Luca L, Massie MB, Gheorghiade M. Reassessment of dobutamine, dopamine, and milrinone in the management of acute heart failure syndromes. Am J Cardiol. 2005;96(6A):47G–58G. doi: 10.1016/j.amjcard.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 64.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. Jama. 2002;287(12):1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 65.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41(6):997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 66.O'Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr, McNulty SE, Grossman SH, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138(1 Pt 1):78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 67.Metra M, Torp-Pedersen C, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, et al. Should beta-blocker therapy be reduced or withdrawn after an episode of decompensated heart failure? Results from COMET. Eur J Heart Fail. 2007;9(9):901–909. doi: 10.1016/j.ejheart.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 68.Webel AR, Frazier SK, Lennie T, Moser DK. Daily variability in dyspnea, edema and body weight in heart failure patients. Eur J Cardiovasc Nurs. 2006 doi: 10.1016/j.ejcnurse.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Jurgens CY, Fain JA, Riegel B. Psychometric Testing of the Heart Failure Somatic Awareness Scale. J Cardiovasc Nurs. 2006;21(2):95–102. doi: 10.1097/00005082-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med. 1998;339(3):173–181. doi: 10.1056/NEJM199807163390307. [DOI] [PubMed] [Google Scholar]
- 71.Bhatia G, Sosin M, Leahy JF, Connolly DL, Davis RC, Lip GY. Hibernating myocardium in heart failure. Expert Rev Cardiovasc Ther. 2005;3(1):111–122. doi: 10.1586/14779072.3.1.111. [DOI] [PubMed] [Google Scholar]
- 72.Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, et al. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112(21):3289–3296. doi: 10.1161/CIRCULATIONAHA.105.549170. [DOI] [PubMed] [Google Scholar]
- 73.Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362(9377):14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]


