Abstract
Background
Hypertension (HTN) is a risk factor for dementia and animal studies suggest that centrally active (cross the blood brain barrier) angiotensin converting enzyme (ACE) inhibitors may protect against dementia beyond HTN control.
Methods
Participants in the Cardiovascular Health Study cognition substudy (mean age 75 yrs) with treated HTN and no diagnosis of heart failure (n= 1054) were followed for a median of 6 years to determine whether cumulative exposure to ACE inhibitors (as a class and by central activity), compared to other antihypertensive agents, was associated with lower risk of incident dementia, cognitive decline (by the modified mini mental state exam, 3MSE), or incident disability in instrumental activities of daily living (IADL).
Results
Among 414 participants exposed to ACE inhibitors and 640 not, there were 158 cases of incident dementia. Compared to other anti-HTN drugs, there was no association between exposure to all ACE inhibitors and risk of dementia (HR 1.01, 95% CI 0.88–1.15), difference in 3MSE scores (−0.32 points/yr, p=0.15), or odds of IADL disability (OR (95% CI) 1.06 (0.99–1.14). Adjusted results were similar. However, centrally active ACE inhibitors were associated with 65% less decline in 3MSE scores per year of exposure (p= 0.01) and non-centrally active ACE inhibitors were associated with greater risk of incident dementia (adjusted HR 1.20 (1.00–1.43) per year of exposure) and greater odds of IADL disability (adjusted OR 1.16 (1.03–1.30) per year of exposure) compared to other anti-HTN drugs.
Conclusions
While ACE inhibitors as a class do not appear to be independently associated with dementia risk or cognitive decline in older hypertensive adults, there may be within class differences in regards to these outcomes. These results should be confirmed with an RCT of a centrally active ACE inhibitor in the prevention of cognitive decline and dementia.
INTRODUCTION
The prevalence of Alzheimer's disease in the US is projected to increase to approximately 9 to 13 million by 2050.1,2 Conservative estimates project that two new cases will be diagnosed every minute in the US by then, and that delaying the onset of dementia, even by one year, would have a significant public health impact, reducing the number of cases over 10 years by an estimated 210,000.2 Hypertension (HTN) is an important risk factor for the development of dementia, of both the vascular and Alzheimer types.3–5 Epidemiologic data from large cohort studies has typically shown an association between the use of antihypertensive drugs and lower risk of dementia.6–8 However, controlled trials of commonly used classes of antihypertensive drugs (calcium channel blockers, beta blockers, diuretics, and angiotensin-converting enzyme (ACE) inhibitors), have yielded mixed results with respect to their protective effect on the incidence of dementia 9–12 In addition, a Cochrane meta-analysis found that blood pressure reduction (by all drug classes combined) was not significantly associated with reduced risk of cognitive impairment or dementia.13 Thus, it raises the question whether a mechanism independent of (or in addition to) blood pressure lowering accounts for the variable protective effects on cognition that have been described.
Several lines of evidence support the hypothesis that ACE inhibitors may have benefits on cognition beyond blood pressure control. The brain is known to possess an intrinsic renin-angiotensin system (RAS) that is involved in memory and cognition.14 Although specific mechanisms are unclear, stimulation of the RAS is involved in the activation of inflammatory cytokines that may play a role in degenerative dementias.15,16 A study in hypertensive rats found that lifetime treatment with captopril (an ACE inhibitor that crosses the blood brain barrier), but not hydralazine, significantly attenuates the age-related impairment in learning and memory despite equal blood pressure control in the two groups.17 These results support the contention that the mechanism of preservation of learning and memory may not be primarily due to the blood pressure lowering effect of captopril.
Using a large, population-based cohort, we aimed to determine i] whether ACE inhibitors as a class, compared to other antihypertensive agents, confer lower risk of incident dementia, cognitive decline, or incident disability in instrumental activities of daily living (IADL) among older adults with hypertension and ii] whether there is a difference between ACE inhibitors that cross the blood brain barrier (centrally active) compared to those that do not (non-centrally active). We hypothesized that centrally active ACE inhibitors, but not non-centrally active ones, would be associated with lower risk of incident dementia, cognitive decline, and IADL disability compared to other antihypertensive drugs.
METHODS
Participants and Study Design
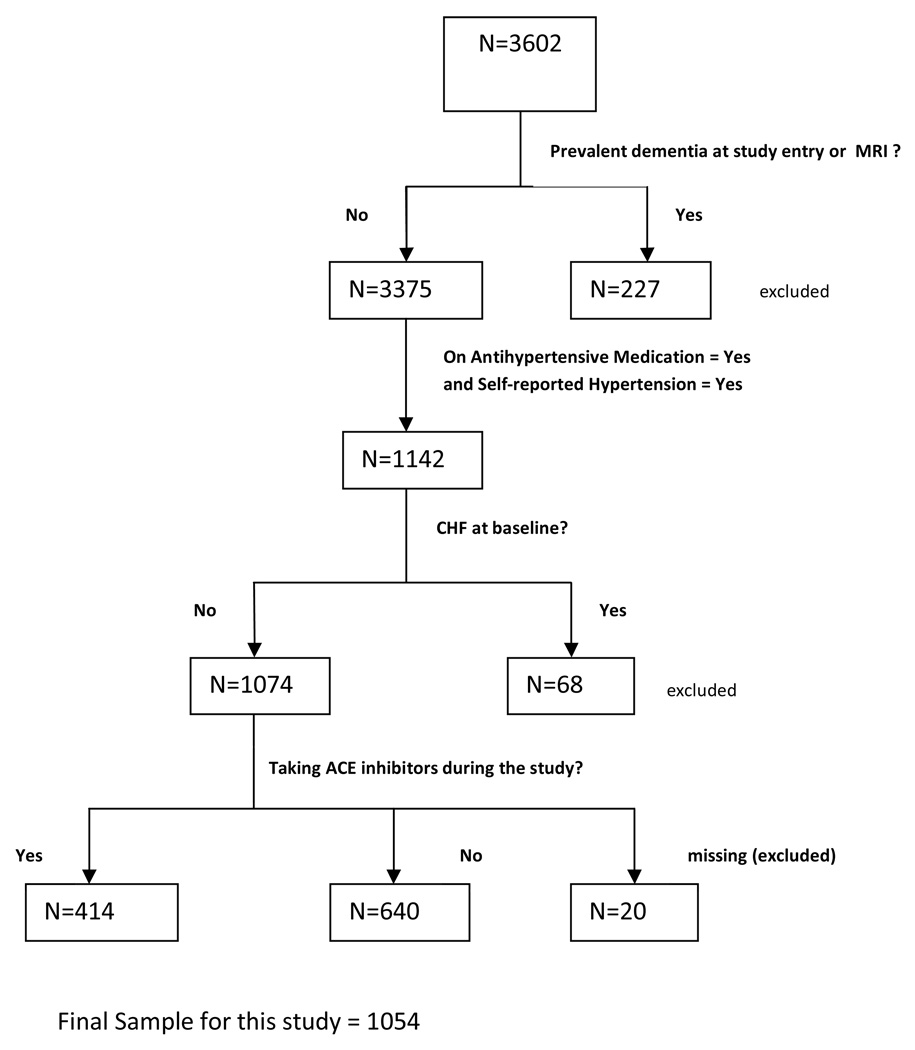
These analyses use longitudinal data from the Cardiovascular Health Study, a prospective multi-center, population-based cohort study of cardiovascular risk factors in 5,888 community-dwelling older adults. An ancillary study was conducted to evaluate the incidence and prevalence of dementia in a subset of the cohort (Cardiovascular Health Cognition sub-study, 3,602 participants) who had brain magnetic resonance imaging (MRI) between 1991 and 1994.18,19 Participants were evaluated at baseline with clinical assessments including physical, cognitive, and functional assessments as well as laboratory testing. Medication, function, cognitive, physical exam, and medical history data were updated at annual participant visits. For this analysis, participants determined to have prevalent dementia at the time of the MRI (defined as “baseline” for these analyses) were excluded. In order to limit confounding by indication, we also restricted our analyses to participants with treated HTN defined as self-reported HTN and taking an antihypertensive medication. Because ACE inhibitors are also commonly used for congestive heart failure treatment and because heart failure may impair cognition, we further excluded patients with heart failure at baseline. After applying these entry and exclusion criterion, 1054 participants formed our study population (Figure 1).
Figure 1. Sample Population.
MRI= brain magnetic resonance imaging; CHF= congestive heart failure
The year of the MRI served as the baseline visit. MRIs were obtained from 1991–1994. Follow-up continued through 1999 or until the participant received a diagnosis of dementia or was lost to follow-up, whichever came first.
Predictor/Exposure
The predictor of interest was cumulative exposure to ACE inhibitors. Participants brought in all medications used in the prior two weeks and each was recorded by study staff. ACE inhibitors were classified into two groups according to their ability to cross the blood brain barrier. These determinations were made based primarily on experiments in rats. The two most common means of measuring ability to cross the blood brain barrier were 1) analysis of ground up, tissue specific ACE activity after administration of ACE inhibitors orally or subcutaneously and 2) tissue specific imaging of a radio-labeled ACE inhibitor after administration of various ACE inhibitors (which compete for binding with the radio-labeled ACE inhibitor).After review of the literature and pharmaceutical package inserts, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as crossing the blood brain barrier, while benazepril, enalapril, moexapril, and quinapril were classified as not (Box). 20–28 If data on use of ACE inhibitors were missing for an examination, and the values before and after that examination agreed, the missing value was replaced with the value observed for those visits. If data for more than two visits were missing, no imputations of missing data were attempted. Only 1.3% of the values were imputed.
Box: Centrally and Non-Centrally Active ACE Inhibitors
| Centrally Active ACE Inhibitors |
Non-Centrally Active ACE Inhibitors |
|---|---|
| Captopril | Benazepril |
| Fosinopril | Enalapril |
| Lisinopril | Moexepril |
| Perindopril | Quinapril |
| Trandolapril | Ramipril |
| Zofenopril |
Outcomes
For the purposes of follow-up, the start of the study was defined for each participant as the year when he/she had the MRI and the end of the study was defined as the date of dementia diagnosis, his/her last evaluation in 1999, or loss to follow-up, whichever came first. Because HTN is a risk factor for both Alzheimer’s dementia as well as vascular dementia,3–5 the sum of which accounted for more than 96% of incident dementia cases in CHS,19 time to incident, all-cause dementia was the primary outcome. Participants at high risk for dementia based on pre-specified criteria18 and all minority participants underwent detailed evaluations. After clinical evaluation, neuropsychiatric testing, and an MRI scan, the diagnosis of dementia was adjudicated by a committee of neurologists and psychiatrists and has been extensively described elsewhere.18,19
Pre-specified secondary outcomes included change in cognition over time measured by the Modified Mini Mental State Exam (3MSE),29 and dependence in instrumental activities of daily living (IADL).30 Both of these outcomes, measured annually, are correlated with dementia and may pick up deficits in cognition before a diagnosis of dementia is made. The 3MSE is a 100 point test of global cognition similar to the Folstein Mini Mental State Exam with higher scores indicating better performance. Six IADL were measured (shopping, meal preparation, money management, telephone use, light housework, and heavy housework) and scored as independent (0) or dependent (1) and summed. For this analysis we dichotomized participants as completely independent or dependent in one or more IADL. We excluded participants with dependencies in IADL at baseline (N= 252).
Covariates
Potential confounders of the relationship between ACE inhibitor use and cognition were considered. Demographics included age, sex, race (classified as white vs. other), education (classified as <high school, high school, and college or graduate school), and income (categorized as <$12,000/yr, $12,000–34,999/yr, or ≥$35,000/yr). Health related behaviors included smoking status (never, former, current), alcohol consumption per week (none, 1–7 drinks/week, and >7 drinks/week), and exercise (kcal per week expended). We also considered baseline comorbidities including diabetes (classified as none, impaired fasting glucose, and diabetes), coronary artery disease, history of stroke, renal insufficiency (using baseline serum creatinine as a surrogate measure), hyperlipidemia (using baseline serum low density lipoprotein, LDL, as a surrogate measure), inflammation (using C-reactive protein as a surrogate measure), and depression (measured with Center for Epidemiologic Studies Depression (CES-D) scale31 annually). Measures of dementia risk, including baseline 3MSE scores and apolipoprotein E (APOE) allele status (presence or absence of an e4 allele) were also obtained. Systolic blood pressure, incident stroke, and any other antihypertensive medication use were recorded at annual visits.
Statistical Analyses
Bivariate analyses were done to determine the associations between covariates of interest and exposure to ACE inhibitors as well as incident dementia. Continuous variables were examined with t-tests and categorical variables were examined using the chi-squared test. Covariates which were not associated (p>0.10) with either the predictor (exposure to ACE inhibitors) or the outcome (incident dementia) were not included in our multivariable models. Those variables included exercise and CRP. APOE-e4 was used not as a covariate but was considered as a potential effect modifier in the relationship between ACE inhibitors and incident dementia. There was no statistically significant interaction found, and thus the analyses were ultimately not stratified by APOE-e4 status.
Time dependent proportional hazards regression analyses were used to model the relationship of time-to-dementia and exposure to ACE inhibitors (total years on ACE inhibitors). Exposure to ACE inhibitors was recorded longitudinally. At each follow-up, exposure to ACE inhibitors was defined as total number of years on ACE inhibitors up to the year before the current follow-up (cumulative exposure, 1 year lag). For example an individual who survived (not demented) after 6 years and had ACE inhibitor use at baseline, years 3 and 4, but not at years 1, 2 and 5, would have a cumulative exposure to ACE inhibitors that equals 1 up to the beginning of year 3, equal to 2 up to the beginning of year 4, and equal to 3 up to the beginning of year 6. We adjusted for potential confounders (described above) in a stepwise fashion including baseline demographics; health-related behaviors; co-morbidities and labs; and baseline 3MSE score. We also controlled for time-dependent covariates including incident stroke, systolic BP, depression scores, and use of other hypertension drugs annually at each follow-up. We adjusted for the use of other hypertension drugs at each visit because we were interested in knowing what impact ACE inhibitors had on dementia risk (or other outcomes) independent of the use of other antihypertensive agents. Though we restricted our population to treated hypertensive patients and excluded those with heart failure at baseline, we further attempted to avoid confounding by indication for ACE inhibitors by censoring participants at the time a new heart failure diagnosis was made. Because dementia is insidious in its onset, exposures thought to be protective would need to be present well before the diagnosis. Thus, we incorporated a one year lag between the exposure and the outcome.32 Results are presented as hazards ratios (HR) and 95% confidence intervals (CI) providing the relative risk for an increase of one year of ACE inhibitor exposure with the reference group being participants who entered the study on a HTN drug, but were not yet exposed to ACE inhibitors. Proportional hazards assumptions for ACE inhibitor use were tested and met.33
Because we hypothesized, a priori, that ACE inhibitors which cross the blood brain barrier would have a different effect than those that do not, further analyses were conducted to compare the risk of dementia for centrally active and non-centrally active ACE inhibitors as cumulative, time-dependent predictors, again with those not yet exposed to ACE inhibitors as the reference group. The statistical models were fitted as those described above except that two predictor variables were now included: cumulative exposure to centrally active ACE inhibitors and cumulative exposure to non-centrally active ACE inhibitors. In sensitivity analyses, we also examined exposure to the subclasses of ACE inhibitors as time dependent covariates (yes/no each year, rather than cumulative exposure) to assess the effect of current exposure on cognitive performance.
The association between cumulative exposure to ACE inhibitors and change in 3MSE scores over time was modeled using mixed effects models. The predictor variable was the same as in time-dependent proportional hazards models and the same set of covariates was used to build the adjusted model with the exception of baseline 3MSE score. The effects of centrally active and non-centrally active ACE inhibitors were similarly modeled by including two predictors in the model. Autoregressive (1) structure was used to account for within-subject correlation of scores over time. The results are presented as the change in 3MSE score (points) associated with every one additional year of exposure to ACE inhibitors as compared to non-exposure. Sensitivity analyses as described above were also performed.
For the IADL disability outcome, annual IADL scores were re-coded as binary outcomes (0 vs 1 or more) as described previously. Only the patients whose IADL scores were 0 at baseline were included for this analysis. A generalized estimating equations (GEE) model with repeated binary response was fitted to assess the association between cumulative exposure to ACE inhibitors and incident IADL disability. The same set of covariates was used to fit the adjusted model as were used with the incident dementia outcome and a one year lag was again imposed. Autoregressive (1) structure was used to account for within subject correlation. Results are presented as odds ratios (OR) and 95% CI and represent the odds of being IADL disabled for every year of exposure to ACE inhibitors as compared to non-exposure. All statistical analyses were performed using SAS 9.1 (Cary, NC).
RESULTS
The average age of participants at baseline was 75 years. Approximately 64% were women and 76% were white. Those who had ever been exposed to ACE inhibitors during the study period (n = 414) were significantly more likely to be male, have higher income, drink more than 7 drinks/week, have higher creatinine and LDL values, have higher systolic blood pressure, and were more than twice as likely to have diabetes at baseline. (Table 1) Of the 414 participants who were exposed to ACE inhibitors, 224 took only centrally active ACE inhibitors, 138 took only non-centrally active ACE inhibitors, and 45 took both (at different times) over the course of the study. There were no significant differences in characteristics of participants who took centrally active versus non-centrally active ACE inhibitors except that those taking centrally active ACE inhibitors had slightly higher baseline 3MSE scores (93.2 and 91.7 points respectively, p = 0.02). Over a median follow-up of 6 years (interquartile range 5–6 years), the mean (SD) exposure to ACE inhibitors was 3.24 (1.9) years and specifically to centrally active, 3.06 (1.83) years and non-centrally active, 2.70 (1.78) years. Approximately 38% of ACE inhibitor users were continuous users throughout the study period, with no difference in the length of continuous use between those on centrally versus non-centrally active ACE inhibitors.
Table 1.
Baseline Characteristics of CHS Participants by Exposure to ACE Inhibitors N=1054, followed for 5255 person-years
| Ever ACE inhibitor |
Never ACE inhibitor |
P Value | |
|---|---|---|---|
| Mean(SD) or % | Mean(SD) or % | ||
| N | 414 | 640 | |
| Age | 74.5 (4.7) | 75.0 (5.0) | 0.10 |
| Female | 57.5 | 67.8 | <0.001 |
| White race | 76.6 | 76.1 | 0.86 |
| Education | 0.57 | ||
| <High School | 25.9 | 27.2 | |
| =High School | 31.6 | 28.6 | |
| >High School | 42.5 | 44.2 | |
| Income (per year) | 0.002 | ||
| <$12,000 | 19.2 | 29.4 | |
| $12,000–35,999 | 57.2 | 50.3 | |
| ≥$35,000 | 23.5 | 20.3 | |
| Smoking History | 0.74 | ||
| Never | 47.1 | 49.4 | |
| Former | 45.6 | 43.2 | |
| Current | 7.4 | 7.3 | |
| Alcohol use | 0.13 | ||
| none | 54.0 | 57.9 | |
| 1–7 drinks/wk | 33.9 | 33.7 | |
| >7 drinks/wk | 12.1 | 8.5 | |
| 3MSE score | 92.5 (5.7) | 92.0 (6.5) | 0.06 |
| CES-D Score | 5.3 (4.9) | 5.0 (4.7) | 0.28 |
| Creatinine (mg/dL) | 1.1 (0.3) | 1.0 (0.3) | 0.01 |
| C-Reactive Protein (mg/L) | 5.7 (9.0) | 5.8 (9.7) | 0.88 |
| LDL (mg/dL) | 129.3 (35.1) | 125.8 (32.5) | 0.10 |
| ≥1 APOE-e4 allele | 22.6 | 25.2 | 0.35 |
| Diabetes | <.001 | ||
| None | 69.4 | 80.1 | |
| Impaired Fasting Glucose | 8.0 | 8.3 | |
| Diabetes | 22.6 | 11.6 | |
| Coronary Artery Disease | 17.4 | 19.4 | 0.42 |
| Systolic Blood Pressure | 146.1 (22.8) | 138.9 (19.5) | <.001 |
| History of Stroke | 5.1 | 5.0 | 0.96 |
| Asprin use | 35.8 | 37.7 | 0.53 |
ACE = angiotensin converting enzyme; 3MSE = Modified Mini Mental State Exam; CES-D = Center for Epidemiologic Studies- Depression Scale; LDL = serum low density lipoprotein, APOE-e4 = apolipoprotein E- epsilon 4 allele
Incident Dementia
There were 158 incident cases of dementia (101 Alzheimer’s, 21 Vascular, 30 mixed Alzheimer’s and Vascular, 6 other causes). Of the 158 incident cases, 111 occurred in particpants never exposed to ACE inhibitors (followed for 3599 person-years) and 47 occurred in participants who had been exposed to ACE inhibitors (followed for 1656 person-years once exposed to ACE inhibitors). Among hypertensive older adults on drug therapy, we found no difference in the risk of dementia for users of ACE inhibitors (as a class) compared with other antihypertensive drug users (adjusted HR 1.01, 95% CI 0.87–1.18). However, when examined separately by blood brain barrier crossing status, exposure to ACE inhibitors that did not cross the blood brain barrier was associated with a greater risk of dementia by 20% per year of exposure compared to non-ACE inhibitor users (adjusted HR 1.20, 95% CI 1.00–1.43). Given an average of approximately three years of exposure, this finding translates into a HR of 1.73 over three years. Table 2 shows the results of the stepwise model building. There was very little change in hazard ratios regardless of the covariates added. The results of sensitivity analyses, in which exposures to the subclasses of ACE inhibitors were examined as time dependent covariates (measuring current exposure rather than cumulative exposure), were qualitatively similar to the primary analyses; however, the results were not statistically significant in multi-variable adjusted models (HR (95% CI) for ACE inhibitors not crossing the blood brain barrier 1.10 (0.61–1.99), and 1.03 (0.61–1.73) for ACE inhibitors crossing the blood brain barrier).
Table 2.
Risk of Incident Dementia per year of exposure to ACE inhibitors (as a class) and by blood brain barrier crossing status of the ACE inhibitor. The reference group is users of other antihypertensive drugs in both analyses.
| All ACE inhibitors | Centrally Active ACE inhibitors |
Non-Centrally Active ACE inhibitors |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value |
| Unadjusted | 1.01 | 0.88–1.15 | 0.90 | 0.89 | 0.74–1.08 | 0.25 | 1.18 | 1.00–1.39 | 0.05 |
| + demographics, health | 1.05 | 0.91–1.21 | 0.52 | 0.91 | 0.73–1.13 | 0.41 | 1.23 | 1.03–1.46 | 0.02 |
| behaviors, comorbidities/labsa | |||||||||
| above + time dependent systolic | 1.05 | 0.91–1.22 | 0.50 | 0.92 | 0.74–1.14 | 0.43 | 1.23 | 1.03–1.47 | 0.02 |
| BP and stroke | |||||||||
| All above + other HTN med | 1.01 | 0.87–1.18 | 0.86 | 0.88 | 0.70–1.09 | 0.24 | 1.20 | 1.00–1.43 | 0.05 |
age, sex, race, education, income, smoking, alcohol use, diabetes, coronary artery disease, serum creatinine, serum low density lipoprotein, 3MSE score, and time dependent depression scores
Change in 3MSE Scores
We measured 3MSE scores over time as a measure of cognitive decline that might be more sensitive to change than an incident dementia diagnosis. The adjusted mean change in 3MSE scores was −0.45 points per year for participants taking antihypertensive drugs other than ACE inhibitors. When examined as a class, for every year of exposure to ACE inhibitors compared with other antihypertensive drugs, there was no significant difference in decline in 3MSE scores (−0.28 points/year, p=0.09). However, when examined by blood brain barrier crossing status, use of ACE inhibitors that cross the blood brain barrier compared to other antihypertensive drugs was associated with 65% less decline per year of exposure (−0.16 points/year, p=0.01). (Table 3) The results of the sensitivity analyses using time dependent ACE inhibitor exposure were qualitatively similar, but not statistically significant in multivariable adjusted models.
Table 3.
Change in 3MSE (points per year of exposure to ACE inhibitors) a
| Unadjusted | Adjustedb | |||||
|---|---|---|---|---|---|---|
| 3MSE | SE | P Valuec | 3MSE | SE | P Valuec | |
| Other Antihypertensive Drugs (reference) | −0.49 | 0.07 | −0.45 | 0.06 | ||
| ACE inhibitors (all) | −0.32 | 0.11 | 0.15 | −0.28 | 0.09 | 0.09 |
| Centrally Active ACE inhibitors | −0.22 | 0.13 | 0.06 | −0.16 | 0.11 | 0.01 |
| Non-Centrally Active ACE inhibitors | −0.52 | 0.17 | 0.83 | −0.53 | 0.15 | 0.60 |
In separate models we examined all ACE inhibitors versus reference and centrally versus non-centrally active versus reference. The reference group was the same in both models.
Adjusted for age, sex, race, education, income, smoking, alcohol use, diabetes, coronary artery disease, serum creatinine, serum low density lipoprotein at baseline as well as depression scores, systolic blood pressure, stroke, and other hypertension drug use annually.
The P values listed reflect a comparison with the reference group.
IADL Disability
For every year of exposure to ACE inhibitors as a class, compared to other antihypertensive drugs, we found a greater odds (OR 1.10, 95% CI 1.02–1.20, p=0.02) of IADL disability. However, when examined by ability to cross the blood brain barrier, it is exposure to non-centrally active ACE inhibitors that is associated with the greater risk. For every year of exposure to those ACE inhibitors, there is 1.16 greater odds of being dependent in at least one IADL (95% CI 1.03–1.30, p=0.01) (Table 4).
Table 4.
Odds of IADL Disability per Year of Exposure to ACE inhibitors a
| Unadjusted | Adjusted b | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Other Antihypertensive Drugs (reference) | 1.00 | - | - | 1.00 | - | - |
| ACE inhibitors (all) | 1.06 | 0.99–1.14 | 0.09 | 1.10 | 1.02–1.20 | 0.02 |
| Centrally Active ACE inhibitors | 1.03 | 0.95–1.13 | 0.44 | 1.07 | 0.97–1.18 | 0.16 |
| Non-Centrally Active ACE inhibitors | 1.11 | 1.00–1.23 | 0.05 | 1.16 | 1.03–1.30 | 0.01 |
In separate models we examined all ACE inhibitors versus reference and centrally versus non-centrally active versus reference. The reference group was the same in both models.
Adjusted for age, sex, race, education, income, smoking, alcohol use, diabetes, coronary artery disease, serum creatinine, serum low density lipoprotein, and Modified Mini Mental State Exam score at baseline as well as depression score, systolic blood pressure, stroke, and other hypertension drug use annually.
DISCUSSION
In a large, well characterized cohort of treated, hypertensive older adults followed for a median of six years, duration of exposure to ACE inhibitors as a class versus other antihypertensive classes was not associated with a reduction in risk of dementia. However, when examined by central activity, exposure to ACE inhibitors that do not cross the blood brain barrier was associated with a 73% greater risk of incident dementia and a 56% greater risk of incident IADL disability over three years of exposure compared to other antihypertensive agents. In contrast, exposure to ACE inhibitors that do cross the blood brain barrier was associated with a 65% reduction in cognitive decline per year of exposure as measured by the 3MSE. And, qualitatively, the direction of results for all outcomes was in favor of ACE inhibitors that cross the blood brain barrier.
The finding that the association of ACE inhibitors with cognition depends on whether the drug crosses the blood brain barrier has also been reported by Ohrui et al34 who found no difference in the incidence of Alzheimer’s disease (n= 90 cases) in Japanese patients on various types of antihypertensive drugs, but did find a significantly lower risk of AD in a subgroup analysis of ACE inhibitors that cross the blood brain barrier (captopril and perindopril) vs. those that do not (enalapril, imidapril). There were no data on cognitive scores or functional status reported. Improvement in cognitive function, independent of blood pressure control, has also been shown with the angiotensin II receptor blocker (ARB), losartan, which crosses the blood brain barrier.35 However, in the SCOPE trial, participants receiving candesartan-based antihypertensive therapy were no less likely to develop dementia than participants receiving other classes of antihypertensives.12 In secondary analyses of the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), there was not a significant reduction in the incidence of all cause dementia among patients assigned to perindopril (centrally active ACE inhibitor), but there was a reduced risk of cognitive decline (defined by decline in MMSE score).11 The CHS population in this study differs from that of PROGRESS in that all participants in PROGRESS had a cerebrovascular accident prior to entry into the study.
There is biologic plausibility for why centrally active ACE inhibitors may benefit cognition. For example, in addition to the anti-inflammatory actions of ACE inhibitors as a mechanism for reduced cognitive decline discussed in the introduction, several other mechanisms are also plausible. First, there are increased concentrations of ACE, angiotensin II, and AT1 receptors in the cerebral cortex of AD patients36,37 and angiotensin II has been shown to inhibit acetylcholine release in rats and humans.38,39 Thus, blocking ACE activity with a centrally active ACE inhibitor could decrease angiotensin II levels, potentially reducing the inhibitory action on acetylcholine release and thereby increase acetylcholine concentration. This mechanism may relate more to acute effects of central ACE inhibition on cognitive testing (such as 3MSE) than to long term dementia risk. There is also microvascular pathology and decreased cerebral blood flow in AD.40 Angiotensin II is a vasoconstrictor and an increase in perivascular staining for ACE and angiotensin II has been shown in AD patients.36 Thus, centrally active ACE inhibitors may improve cerebral blood flow. On the other hand, a recent study showed that ACE may be important in converting amyloid--β 1–42 into Aβ1–40 and that ACE inhibitors block this process and increase Aβ1–42 deposition in the brain of mice.41 These results suggest that ACE inhibitors that cross the blood brain barrier might increase AD risk. The net effects of these potentially opposing mechanisms should be determined in a randomized trial.
In this study, exposure to ACE inhibitors that do not cross the blood brain barrier was associated with greater risk of incident dementia and IADL disability (which are primarily cognitive tasks) when compared to other classes of antihypertensive drugs. While there is biologic plausibility for the reduction in cognitive decline seen among patients taking centrally active ACE inhibitors, why would non-centrally active ACE inhibitors be associated with a greater risk of dementia and IADL disability? While it is possible that non-centrally active ACE inhibitors are harmful, it is more likely that they are simply less helpful in prevention of dementia and IADL disability than other antihypertensive drug classes combined. ACE inhibitors may be less effective in reducing the risk of selected blood pressure related complications than other blood pressure lowering drugs, and, perhaps most pertinently to cognition, the risk of stroke remains higher than when other classes of antihypertensive agents are used.42,43 These previous studies support the possibility that the effects of different agents on cognition might differ. Similarly, our results support the hypothesis that the centrally active ACE inhibitors are associated with lower risk of cognitive decline via mechanisms other than blood pressure control. A difference in the brain mechanisms of centrally and non-centrally active ACE inhibitors may account for some of the variable trial results with respect to ACE inhibitors and cognition. The results of the sensitivity analyses using time dependent, current exposure to ACE inhibitor subclasses were consistent with the directions of associations in our primary analyses, but were not statistically significant, and may be interpreted as providing insight more relevant to the association of acute exposures than to those of chronic or cumulative exposures. When interpreted in the context of our primary results, these additional analyses provide support for the contention that cumulative (or chronic) exposure to ACE inhibitor subclasses may be more strongly associated with risk of dementia and cognitive decline than is acute exposure.
While this study is based on a large, well characterized cohort with extensive cognitive follow-up and sub-clinical disease markers, there are several methodological limitations to highlight. As with all pharmacoepidemiology studies, it is impossible to entirely rule out confounding by indication. However, we significantly limited the effect of this type of confounding by methods of restriction and adjustment.44 We restricted our analyses to treated hypertensive patients, thus everyone had an indication for an ACE inhibitor. Furthermore, we excluded people with the other main indication for ACE inhibitors, congestive heart failure, and censored people if they developed CHF during the study. While hypertensive patients with diabetes are more likely to receive ACE inhibitors, we did control for diabetes in the analyses. Since diabetes is associated with cognitive decline, an increased proportion of diabetics among ACE inhibitor users would not explain the reduced risk of cognitive decline seen with ACE inhibitors that cross the blood brain barrier. In addition, it is unlikely that physicians consider whether ACE inhibitors cross the blood brain barrier when selecting which ACE inhibitor to prescribe and so it is unlikely that confounding by indication could explain the results. In addition to confounding by indication, there could also be residual confounding from other factors. While CHS has a rich set of clinical measures and biomarkers, many of which we controlled for, there is always the possibility of residual confounding.
Treatment related imbalances in loss to follow-up among patients becoming cognitively impaired has also been cited as a potential source of bias in clinical trials measuring cognition as an outcome.45 There were not significant differences in loss to follow-up among categories of ACE inhibitor use in this study. The classification of centrally and non-centrally active ACE inhibitors was made predominantly based on basic science animal data because human data was generally lacking and due to variable methods of measurement, we were unable to provide units of measure for degrees of centrally activity. And while a compound’s ability to cross the blood brain barrier largely depends on its size, charge, and lipophilicity, the integrity of the blood brain barrier and the dose of the medication could influence its central activity. However, if there was misclassification (i.e., drugs classified as not centrally active did actually cross the blood brain barrier), we would expect this to bias the results towards the null. Another potential source of misclassification bias of the exposure is that we do not know what the exposure to ACE inhibitors was prior to baseline. However, this likely affects few of our participants since ACE inhibitor use didn't gain momentum until the late 1980s (only 10% of antihypertensive users were taking ACE inhibitors in 1988).46 We could not account for timing of ACE inhibitor exposure. For example, our models treated three years of exposure to ACE inhibitors the same, regardless of whether it occurred early or late in the study. We do note, however, that approximately 40% of ACE inhibitor users were continuous users throughout the study period and that the median duration of use did not differ between users of centrally and non-centrally active ACE inhibitors. There were few cases of dementia that were not attributable to Alzheimer’s disease, thus we could not separate our analyses by dementia type. Further studies are needed to determine if the effects of ACE inhibitors on cognition and dementia risk is the same for the most common dementia subtypes.
The potential public health impact of these findings is significant given the burden of cognitive impairment-related disability, the high prevalence of HTN, and the potential impact that the choice of agents to manage HTN may have beyond blood pressure control and cardiovascular disease risk. Based on the results of this study, compared to other antihypertensive drug classes, the use of non-centrally active ACE inhibitors (independent of use of other antihypertensive drugs) is associated with greater risk of IADL disability by approximately 56% and a 73% greater risk of dementia after three years of exposure. Conversely, use of centrally active ACE inhibitors was associated with 65% slower cognitive decline on a global measure of cognition. While these results come from an observational study and should be confirmed with a randomized, controlled trial of the use of a centrally active ACE inhibitor in the prevention of cognitive decline and dementia, it would appear that there may be within the class differences in the association of ACE inhibitors and cognition.
Acknowledgements
Sources of funding and support:
Dr. Sink is supported by the Hartford Geriatrics Health Outcomes Research Scholars, WFU Pepper Center (P30 AG21332), and the Kulynych Center for Research in Cognition. The Cardiovascular Health Study was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke, grant AG15928 from the National Institute on Aging, and grant 5R01HL074745-04.
Footnotes
| Conception and design |
Acquisition of data |
analysis and interpretation of data |
drafting of manuscript |
Critical revision of the manuscript |
Obtaining funding |
|
|---|---|---|---|---|---|---|
| Kaycee M. Sink, MD, MAS | X | X | X | X | X | |
| Xiaoyan Leng, PhD | X | X | X | X | ||
| Jeff Williamson, MD, MHS | X | X | X | |||
| Stephen B. Kritchevsky, PhD | X | X | X | |||
| Kristine Yaffe, MD | X | X | X | |||
| Bruce Psaty, MD, PhD | X | X | X | X | X | |
| Lewis Kuller, MD | X | X | X | X | X | |
| Sevil Yasar, MD, PhD | X | X | X | |||
| Hal Atkinson, MD | X | X | X | |||
| Mike Robbins, PhD | X | X | X | |||
| David Goff, MD, PhD | X | X | X |
Potential Conflicts of Interest:
Dr. Goff has a research grant from Merck. None of the other authors have any potential conflicts of interest to report.
A full list of principal CHS investigators and institutions can be found at http://www.chsnhlbi.org/pi.htm.
Authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study sponsors had no role in the study design, data analyses, interpretation of the data, or manuscript preparation.
Findings from this manuscript were presented in part at the 2007 annual meeting of the American Geriatrics Society (Seattle, WA, May 5, 2007).
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoog I. The relationship between blood pressure and dementia: a review. Biomed Pharmacother. 1997;51(9):367–375. doi: 10.1016/s0753-3322(97)89428-0. [DOI] [PubMed] [Google Scholar]
- 4.Hanon O, Seux ML, Lenoir H, Rigaud AS, Forette F. Hypertension and dementia. Curr Cardiol Rep. 2003;5(6):435–440. doi: 10.1007/s11886-003-0104-2. [DOI] [PubMed] [Google Scholar]
- 5.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 6.Guo Z, Fratiglioni L, Zhu L, Fastbom J, Winblad B, Viitanen M. Occurrence and progression of dementia in a community population aged 75 years and older: relationship of antihypertensive medication use. Arch Neurol. 1999;56(8):991–996. doi: 10.1001/archneur.56.8.991. [DOI] [PubMed] [Google Scholar]
- 7.in't Veld BA, Ruitenberg A, Hofman A, Stricker BH, Breteler MM. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol Aging. 2001;22(3):407–412. doi: 10.1016/s0197-4580(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 8.Khachaturian AS, Zandi PP, Lyketsos CG, et al. Antihypertensive Medication Use and Incident Alzheimer Disease: The Cache County Study. Archives of Neurology. 2006;63(5):686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 9.Amenta F, Mignini F, Rabbia F, Tomassoni D, Veglio F. Protective effect of anti-hypertensive treatment on cognitive function in essential hypertension: analysis of published clinical data. J Neurol Sci. 2002:203–204. 147–151. doi: 10.1016/s0022-510x(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 10.Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162(18):2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 11.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163(9):1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 12.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness B, Todd S, Passmore P, Bullock R. The effects of blood pressure lowering on development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease. Cochrane Database Syst Rev. 2006 Feb;:CD004034. doi: 10.1002/14651858.CD004034.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Wright JW, Reichert JR, Davis CJ, Harding JW. Neural plasticity and the brain renin-angiotensin system. Neurosci Biobehav Rev. 2002;26(5):529–552. doi: 10.1016/s0149-7634(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 15.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 17.Wyss JM, Kadish I, van Groen T. Age-related decline in spatial learning and memory: attenuation by captopril. Clin Exp Hypertens. 2003;25(7):455–474. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 19.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 20.Tan J, Wang JM, Leenen FH. Inhibition of brain angiotensin-converting enzyme by peripheral administration of trandolapril versus lisinopril in Wistar rats. Am J Hypertens. 2005;18(2 Pt 1):158–164. doi: 10.1016/j.amjhyper.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Cushman DW, Wang FL, Fung WC, Harvey CM, DeForrest JM. Differentiation of angiotensin-converting enzyme (ACE) inhibitors by their selective inhibition of ACE in physiologically important target organs. Am J Hypertens. 1989;2(4):294–306. doi: 10.1093/ajh/2.4.294. [DOI] [PubMed] [Google Scholar]
- 22.Jouquey S, Mathieu MN, Hamon G, Chevillard C. Effect of chronic treatment with trandolapril or enalapril on brain ACE activity in spontaneously hypertensive rats. Neuropharmacology. 1995;34(12):1689–1692. doi: 10.1016/0028-3908(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins TA, Mendelsohn FA, Chai SY. Angiotensin-converting enzyme modulates dopamine turnover in the striatum. J Neurochem. 1997;68(3):1304–1311. doi: 10.1046/j.1471-4159.1997.68031304.x. [DOI] [PubMed] [Google Scholar]
- 24.Gohlke P, Scholkens B, Henning R, Urbach H, Unger T. Inhibition of converting enzyme in brain tissue and cerebrospinal fluid of rats following chronic oral treatment with the converting enzyme inhibitors ramipril and Hoe 288. J Cardiovasc Pharmacol. 1989;14 Suppl 4:S32–S36. [PubMed] [Google Scholar]
- 25.Chrysant SG, Chrysant GS. Pharmacological and clinical profile of moexipril: a concise review. J Clin Pharmacol. 2004;44(8):827–836. doi: 10.1177/0091270004267194. [DOI] [PubMed] [Google Scholar]
- 26.Chai SY, Perich R, Jackson B, Mendelsohn FA, Johnston CI. Acute and chronic effects of angiotensin-converting enzyme inhibitors on tissue angiotensin-converting enzyme. Clin Exp Pharmacol Physiol Suppl. 1992;19:7–12. doi: 10.1111/j.1440-1681.1992.tb02803.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CI, Fabris B, Yamada H, et al. Comparative studies of tissue inhibition by angiotensin converting enzyme inhibitors. J Hypertens Suppl. 1989;7(5):S11–S16. [PubMed] [Google Scholar]
- 28.Johnston CI, Mendelsohn FA, Cubela RB, Jackson B, Kohzuki M, Fabris B. Inhibition of angiotensin converting enzyme (ACE) in plasma and tissues: studies ex vivo after administration of ACE inhibitors. J Hypertens Suppl. 1988;6(3):S17–S22. [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 32.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62(7):1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 33.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer: 1997. [Google Scholar]
- 34.Ohrui T, Matsui T, Yamaya M, et al. Angiotensin-Converting Enzyme Inhibitors and Incidence of Alzheimer's Disease in Japan. Journal of the American Geriatrics Society. 2004;52(4):649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x. [DOI] [PubMed] [Google Scholar]
- 35.Tedesco MA, Ratti G, Di Salvo G, Natale F. Does the angiotensin II receptor antagonist losartan improve cognitive function? Drugs Aging. 2002;19(10):723–732. doi: 10.2165/00002512-200219100-00001. [DOI] [PubMed] [Google Scholar]
- 36.Savaskan E, Hock C, Olivieri G, et al. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer's dementia. Neurobiol Aging. 2001;22(4):541–546. doi: 10.1016/s0197-4580(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 37.Barnes NM, Cheng CH, Costall B, Naylor RJ, Williams TJ, Wischik CM. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer's disease. Eur J Pharmacol. 1991;200(2–3):289–292. doi: 10.1016/0014-2999(91)90584-d. [DOI] [PubMed] [Google Scholar]
- 38.Barnes JM, Barnes NM, Costall B, et al. Angiotensin II inhibits acetylcholine release from human temporal cortex: implications for cognition. Brain Res. 1990;507(2):341–343. doi: 10.1016/0006-8993(90)90294-l. [DOI] [PubMed] [Google Scholar]
- 39.Barnes JM, Barnes NM, Costall B, Horovitz ZP, Naylor RJ. Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res. 1989;491(1):136–143. doi: 10.1016/0006-8993(89)90095-4. [DOI] [PubMed] [Google Scholar]
- 40.Ruitenberg A, den HT, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57(6):789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 41.Zou K, Yamaguchi H, Akatsu H, et al. Angiotensin-converting enzyme converts amyloid beta-protein 1–42 (Abeta(1–42)) to Abeta(1–40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27(32):8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 43.Psaty BM, Lumley T, Furberg CD, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289(19):2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 44.Psaty BM, Koepsell TD, Lin D, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47(6):749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 45.Di BM, Pahor M, Franse LV, et al. Dementia and disability outcomes in large hypertension trials: lessons learned from the systolic hypertension in the elderly program (SHEP) trial. Am J Epidemiol. 2001;153(1):72–78. doi: 10.1093/aje/153.1.72. [DOI] [PubMed] [Google Scholar]
- 46.Barker WH, Mullooly JP, Linton KL. Trends in hypertension prevalence, treatment, and control: in a well-defined older population. Hypertension. 1998;31(1 Pt 2):552–559. doi: 10.1161/01.hyp.31.1.552. [DOI] [PubMed] [Google Scholar]