Abstract
Both IgG and secretory IgA Abs in mucosal secretions have been implicated in blocking the earliest events in HIV-1 transit across epithelial barriers, although the mechanisms by which this occurs remain largely unknown. In this study, we report the production and characterization of a human rIgA2 mAb that carries the V regions of IgG1 b12, a potent and broadly neutralizing anti-gp120 Ab which has been shown to protect macaques against vaginal simian/HIV challenge. Monomeric, dimeric, polymeric, and secretory IgA2 derivatives of b12 reacted with gp120 and neutralized CCR5- and CXCR4-tropic strains of HIV-1 in vitro. With respect to the protective effects of these Abs at mucosal surfaces, we demonstrated that IgG1 b12 and IgA2 b12 inhibited the transfer of cell-free HIV-1 from ME-180 cells, a human cervical epithelial cell line, as well as Caco-2 cells, a human colonic epithelial cell line, to human PBMCs. Inhibition of viral transfer was due to the ability of b12 to block both viral attachment to and uptake by epithelial cells. These data demonstrate that IgG and IgA MAbs directed against a highly conserved epitope on gp120 can interfere with the earliest steps in HIV-1 transmission across mucosal surfaces, and reveal a possible mechanism by which b12 protects the vaginal mucosal against viral challenge in vivo.
Transmission of HIV-1 occurs primarily through sexual contact and breastfeeding in which mucosal surfaces of the genital or gastrointestinal tracts, respectively, are exposed to HIV and/or HIV-infected cells (1, 2). For successful infection to occur, infectious virus must breach the mucosal barrier consisting of stratified squamous or columnar epithelial cells and gain access to target cells permissive for viral uptake and replication (3, 4). Once within the mucosa, HIV-1 establishes both local and systemic viral reservoirs with devastating immunological consequences, including depletion of CD4+ memory T cells and immunodeficiency (5, 6). Moreover, viral reservoirs are resistant to eradication by the cellular and humoral arms of the immune system and by antiviral therapies. The development of strategies to reduce sexual transmission of HIV-1 requires a better understanding of immunological factors that are capable of interrupting attachment, uptake, and transfer of HIV-1 by mucosal epithelial cells.
Evidence from macaque models of simian/HIV (SHIV)5 infection indicates that Abs directed against envelope spike glycoproteins (i.e., gp120 and gp41) are capable of conferring mucosal defense against HIV-1 (7–10). The most well-characterized anti-envelope Ab in this regard is IgG1 b12, one of only a handful of human mAbs known to be capable of neutralizing a broad range of primary HIV-1 isolates (11–13). IgG1 b12 recognizes a highly conserved epitope on gp120 involved in binding to CD4 (14). Macaques administered IgG1 b12 i.v. or topically (i.e., intravaginally) were protected against SHIV infection by the vaginal route (15, 16). The mechanism(s) by which IgG1 b12 inhibited viral infection of the vaginal mucosa was not determined in these experiments, but it is generally assumed that the Ab interferes with virus-host cell contact.
In humans and macaques, mucosal secretions contain a mixture of IgG and secretory IgA (SIgA) Abs (17). In secretions of the female genital tract, IgG and SIgA are found at roughly equal concentrations, whereas in the gastrointestinal tract, IgG concentrations are 30- to 100-fold lower than SIgA (1, 18 –20). SIgA is a polypeptide complex that consists of two (or more) IgA monomers joined at their C termini by a J chain that is covalently associated with secretory component (SC), a 70-kDa glycoprotein derived from the polymeric IgR during transcytosis across epithelial cells (21). Although both IgG and SIgA are capable of neutralizing viruses in mucosal secretions (22), the relative contribution of one Ab type over another in blocking sexual transmission of HIV-1 remains to be determined.
To better understand the mechanisms by which Abs against gp120 confer mucosal immunity to HIV, we have produced and characterized a recombinant human IgA2 mAb that carries the H and L chain variable domains of Ab b12. We demonstrate that monomeric and dimeric forms of recombinant human IgA2 b12 react with gp120 and neutralize HIV-1 infection of T cells. More importantly, we report that both IgG1 b12 and IgA2 b12 can prevent HIV-1 attachment, uptake, and transfer by cervical and intestinal epithelial cells. These data demonstrate that both IgG and IgA Abs against gp120 can interfere with the earliest steps in HIV-1 transmission across mucosal surfaces and reveal a possible mechanism by which IgG1 b12 protects the vaginal mucosa against viral challenge in vivo.
Materials and Methods
Construction of mammalian expression vector-encoding IgA2 b12
The plasmid pSM102 (see Fig. 1) encoding IgA2 b12 was constructed from pDR12 (12) by replacing the IgG1 constant domains with the IgA2 constant domains. The genomic DNA encoding human IgA2m (1) was amplified as a single 1.5-kb SacI-SalI DNA fragment by PCR using plasmid pcDNA3: cα2m (1) as template (23) and custom-designed primers 5′-GTCATCGTGAGCTCATCCCCGACCAGCCCCAAGGTG-3′ and 5′-TTTGACGTCGACTTTCCCAAGTGCTGAGACCCTGAGGAT-3′). The underlined sequences indicate the engineered restriction sites, and the bold sequences indicate the IgA2m1 coding region. The IgA2 constant domain was fused in-frame with the b12 VH coding sequence.
FIGURE 1.
Schematic of the IgA2 b12 expression vector. Plasmid pSM102 encodes the IgA1 b12 VH and VL (κ) chain, GS, as a selectable-amplifiable marker in mammalian cells, and β-lactamase for selection in Escherichia coli.
Establishment of stable CHO cell lines secreting monomeric and dimeric/polymeric forms of IgA b12
We used the selectable-amplifiable marker, glutamine synthetase (GS) encoded on plasmid pSM102 (see Fig. 1), to produce stable cell lines secreting high levels of IgA b12. The use of GS as a selectable-amplifiable marker has been described previously (12, 24). CHO-K1 were seeded into 6-well cell culture plates and maintained in glutamine-free MEM (GMEM; Mediatech) containing 5% FCS (HyClone) and methionine sulfoximine (MSX; Sigma-Aldrich) at a range of concentrations (20 –120 μM). Supernatants of pSM102-transfected cells were screened by ELISA for the presence of IgA Abs reactive with gp120 (see below). Cells from positive wells were collected by trypsinization and cloned three times by limiting dilution (25). A single clone secreting monomeric IgA2 b12, herein referred to as 100-02-C9, was chosen for further study based on a number of criteria, including levels of Ab production and stability in culture.
To produce cell lines secreting dimeric IgA2 b12, clone 100-02-C9 was transfected with plasmid pcDNA3Hygro::J chain encoding human J chain and selected for growth in GMEM-MSX medium containing hygromycin (400 μg/ml) (23). We identified transfectants secreting polymeric forms of IgA2 b12 by screening cell supernatants using a SC-binding ELISA (26). Two stable transfectants, J2 and J10, were cloned three times by limiting dilution and used for further studies.
Clone 100-02-C9 was maintained in GMEM supplemented with 5% FCS (Ultra Low Bovine IgG FBS; Invitrogen Life Technologies) and 80 μM MSX (Sigma-Aldrich). Clone J1 secreting was maintained in the same medium with the addition of hygromycin B (400 μg/ml). Large scale cell culture was performed using 10-layer CellStacks (Corning).
Immunoprecipitation and Western blot analysis of IgA2 b12
Supernatants from CHO cell lines (~3 ml) were mixed with polyclonal goat anti-human α-chain antiserum (Cappel/ICN) followed by protein G agarose beads (Pierce), which bind goat IgG but not human IgA (27). Protein G agarose beads (~40 μl) were collected by centrifugation and resuspended in 2× Laemmli sample buffer, boiled for 5 min, and then subjected to SDS-PAGE on 4 –18% gradient gel (Bio-Rad). The proteins were transferred to a polyvinylidene difluoride membrane (0.45-μm pore size; Bio-Rad) by electroelution and then probed with biotin-labeled goat anti-human IgA Fab (1/5000) (Tago Immunologicals) and avidin-HRP (Sigma-Aldrich). Membranes were developed using the ECL kit (Amersham Pharmacia) and exposed to Kodak X-OMAT film (Fisher-Scientific). When necessary, polyacrylamide gels were stained with Gel Code Blue (Pierce) to visualize proteins.
Purification of monomeric and dimeric forms of IgA2 b12
Abs were affinity purified from cell culture supernatants using ImmunoPure Immobilized Protein L (Pierce), which binds human κ L chains, and then were subjected to size exclusion chromatography by a Sephacryl S300 High Resolution column (GE Healthcare) to separate dimeric and monomeric forms of IgA2 b12. The column was calibrated using the following molecular mass standards: dextran 2000 kDa, ferritan 440 kDa, catalase 220 kDa, and RNase A 10 kDa. Peak fractions of IgAb2 were pooled and purity was checked by SDS-PAGE and concentrations of the IgA molecular forms were determined by ELISA and absorbance spectroscopy (28).
Expression of recombinant human rSC
rSC was produced by transfection of CHO-K1 cells with pCL-2, a plasmid encoding human rSC provided to us by J. Woof (University of Dundee Medical School, Dundee, Scotland; Refs. 29 –31). Transfectants were identified by hygromycin B (200 μg/ml; Roche). Stable clones secreting rSC were isolated by limiting dilution and assayed for expression of rSC by ELISA.
gp120 ELISA
HIV-1-specific ELISAs to detect IgA b12 were done by coating Nunc Maxisorp 96-well microtiter plates (Fisher-Scientific) with HIV-1IIIB lysate (Calypte Biomedical) or rHIV-1IIIB gp120 (ImmunoDiagnostics) using 100 ng of protein/well in PBS (pH 7.4). After overnight incubation, plates were blocked with PBS plus BSA (1% w/v) and Tween 20 (0.1%) before the addition of IgA b12 or IgA b12 cell culture supernatants. The plates were washed and then probed with peroxidase-conjugated, affinity-purified goat polyclonal Abs (0.5 μg/ml) specific for human IgA (Southern Biotechnology Associates) or human κ-chain (Sigma-Aldrich). Plates were developed using the one-component tetramethylbenzidine substrate (Kirkegaard & Perry) and read using a spectrophotometer (Molecular Devices). Total IgA levels were determined by sandwich ELISA using plates coated with goat anti-human IgA (Cappel) diluted 1/1000 into PBS and detected using biotin-labeled goat-anti-human, as done previously (18). Human IgA2 (Calbiochem) was used as a standard.
Ab-binding curves
Ninety-six-well microtiter plates (Corning) were coated with HIV-1MN gp120 (2 μg/ml in PBS (pH 7.4); Immunodiagnostics) overnight at 4°C. The plates were blocked with 4% nonfat dry milk for 30 min at room temperature and then incubated for 2 h at 37°C with serial dilutions of mAbs. The plates were developed by incubation with alkaline phosphatase-labeled goat anti-human κ L chain Abs and the alkaline phosphatase substrate kit (Pierce), then analyzed using a microtiter plate spectrophotometer set at 405 nm. The concentrations of mAbs that gave an OD of 1.0 at 405 nm were used for the avidity determination and competitive inhibition assays (see below).
Ab avidity and affinity for gp120
The avidity (or functional affinity) of each form of b12 mAb was determined by disrupting mAb binding to the solid-phase Ag using increasing amounts of ammonium thiocyanate (NH4SCN; 0 – 4.0 M), a mild denaturing agent for 30 min after mAb binding to the solid phase, before adding the labeled conjugate Ab, as described (32, 33). The affinity of each form of b12 mAb was measured by competitive inhibition of Ab binding to the solid phase by preincubation of mAb (concentration yielding OD of 1.0) with serial 3-fold dilutions of soluble HIV-1MN gp120 Ag (0 –1 μg/ml) overnight at 4°C, as previously described (34 –36). The amounts of un-bound mAbs were measured as described above. For both the NH4SCN and competitive inhibition assays, the ODs in duplicate wells were averaged; the background with no Ag was subtracted. The molar concentrations yielding a 50% inhibition of OD with inhibitor or soluble Ag were determined as the affinity index (AI) and competitive inhibition index, respectively. The latter was used to estimate the dissociation constant (KD) using the formula KD = a0((A0/A0 − A) − 1) as described (34).
HIV-1 neutralization assays
HIV-1BaL (R5) and HIV-1IIIb (X4) were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program (NIH ARRRP; nos. 510 and 398, respectively). For conventional neutralization assays, viruses (50 50% tissue culture-infective dose (TCID50)/ml) were mixed with IgG1 or IgA2 b12 (monomeric, dimeric, and polymeric forms) and then added in duplicate to 96-well flat-bottom microtiter plates (Costar) and incubated for 30 min at 37°C. PBMCs from healthy adult donors seronegative for HIV-1 and hepatitis B and C were isolated from buffy coats by Ficoll-Hypaque gradient centrifugation. Before infection, PBMCs were activated by overnight incubation with PHA (5 μg/ml; Sigma-Aldrich) in RPMI 1640 with 10% heat-inactivated FBS (HyClone), 5% recombinant human IL-2 (Roche), and 50 μg/ml gentamicin (Invitrogen Life Technologies) at 37°C and 5% CO2. The cells were then washed and cultured for 3 days in RPMI 1640 with 10% FBS and 5% IL-2 before being added to the virus-Ab mixtures. A total of 2 × 105 cells in 100 μl were added to each well and incubated for 2 h at 37°C to permit viral infection. The PBMCs were washed three times to remove unbound virus, and 6 days later the cell supernatants were assayed for p24 levels by ELISA (Beckman Coulter).
Neutralization activity of the various forms of Ab b12 was confirmed using HIV-1 envelope-pseudotyped viruses, which are capable of single-round replication generated by cotransfection of 293T cells with the pNL4-3.luc.RE vector (N. Landau, NIH ARRRP) and the psVIIIex7 env-expressing vector, provided by J. Sodroski (Dana-Farber Cancer Institute) (37). The assays were conducted by seeding U87.CD4.CCR5 cells (NIH ARRRP) in totals of 1.5 × 104 cells/well in a 96-well flat-bottom tissue culture-treated plate (Corning). Seeded cells were contained in a 100-μl volume of medium (DMEM containing 10% FBS, 300 μg of G418/ml, glutamine, and penicillin-streptomycin) and incubated for 24 h at 37°C in 5% CO2. Separately, Ab and virus mixtures were made by adding 60 μl of serially diluted Ab in medium to an equal volume of medium containing 2 × 105 relative light units of pseudotyped virus and incubated for 1 h at 37°C. Following incubation, 100 μl of the Ab and virus mixtures were transferred to the U87 cells and incubated for 3 days at 37°C in 5% CO2. Cell lysates were generated by adding 60 μl of cell culture lysis reagent (Promega), incubated at room temperature for 5 min, pelleted by centrifugation at 1200 × g for 2 min. A total of 20 μl of lysate was transferred to opaque assay plates (Corning), luciferase reagent was added (Promega), and luciferase activity was measured on a luminometer (Orion; Berthold Detection Systems). The degree of virus neutralization by Ab was determined by measuring luciferase activity. The percent neutralization at a given Ab concentration was express as: ((luciferase activity in the absence of Ab − luciferase activity in the presence of a given Ab concentration)/luciferase activity in the absence of antibody) × 100. The following molecular masses were used to calculate the molar concentration of each Ab (Table I): IgG1 150 kDa; mIgA2 170 kDa; dIgA2 (with J chain) 360 kDa; SIgA2 b12 (with J chain and SC) 430 kDa. The molecular mass of pIgA2 b12, which is a mixture of monomer, dimer, and higher molecular mass molecules, was assumed to be 320 kDa, the dominant species expressed by clone J1 expressing pIgA2 b12 as determined by gel filtration chromatography.
Table I.
Neutralization of HIV-1-pseudotyped virus in luciferase reporter assays
| Virus |
||||||
|---|---|---|---|---|---|---|
| JR-FL | JR-CSF | HxB2 | ||||
| mAb (nM)a | IC90b | IC50c | IC90 | IC50 | IC90 | IC50 |
| IgG1 | 1.3 | 0.7 | 5.3 | 0.7 | 0.7 | <0.27 |
| mIgA2 | 4.1 | 0.6 | >58.8 | 2.4 | 0.6 | <0.24 |
| dIgA2 | 1.1 | 0.3 | 31.3 | 1.1 | 0.6 | <0.11 |
| pIgA2 | 1.3 | 0.3 | 25.0 | 0.6 | 0.3 | <0.11 |
| SIgA2 | 0.9 | 0.2 | 16.7 | 0.2 | 0.2 | <0.1 |
| mAb (μg/ml) | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 |
| IgG1 | 0.2 | 0.1 | 0.8 | 0.1 | 0.1 | <0.04 |
| mIgA2 | 0.7 | 0.1 | >10 | 0.4 | 0.1 | <0.04 |
| dIgA2 | 0.4 | 0.1 | 10.0 | 0.4 | 0.2 | <0.04 |
| pIgA2 | 0.4 | 0.1 | 9.1 | 0.2 | 0.1 | <0.04 |
| SIgA2 | 0.4 | 0.1 | 7.2 | 0.1 | 0.1 | <0.04 |
The following molecular masses were used to calculate the molar concentration of each Ab: IgG1 150 kDa; mIgA2 170 kDa; dIgA2 (with J chain) 360 kDa; SIgA2 b12 (with J chain and SC) 430 kDa. The molecular mass of pIgA2 b12, which is a mixture of monomer, dimer, and higher molecular mass molecules, was assumed to be 320 kDa, the dominant species expressed by clone 1 expressing pIgA2 b12 as determined by gel filtration chromatography.
Concentrations of Ab required to neutralize 90% of the indicated virus, presented in nanomoles or micrograms per milliliter.
Concentrations of Ab required to neutralize 50% of the indicated virus, presented in nanomoles or micrograms per milliliter.
Epithelial cell transfer of HIV-1 to PBMCs
ME180 (HTB-33; American Type Culture Collection (ATCC)) and Caco-2 (HTB-37; ATCC) cells were cultured in Eagle’s MEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies). For the HIV-1 transfer assays, ME180 and Caco-2 epithelial cells were plated in 96-well flat-bottom microtiter plates at 1 × 105 cells/ml (200 μl/well) 2 days before infection. HIV-1BaL or HIV-1IIIb (50 TCID50/ml) were incubated in the absence or presence of IgG1 or IgA2 b12, or relevant isotype control Abs for 30 min at 37°C. The virus-Ab mixtures were then applied to confluent epithelial cell monolayers and incubated for 2 h at 37°C. The monolayers were washed three times with medium to remove any unbound virus and overlaid with activated PBMCs (2 × 105/well in 200 μl). Cell-free supernatants were harvested 6 days after coculture and assessed for p24 levels by ELISA. For studies aimed at determining at which step(s) b12 inhibits viral transfer from epithelial cells to PBMCs, mAbs were added 1) concurrently with HIV-1BaL or HIV-1IIIb (50 TCID50/ml; designated +/−), 2) after epithelial cells had been washed to remove free virus (designated −/+), virus-Ab complexes, or free Ab; 3) or at both steps (designated +/+).
Inhibition of HIV-1BaL attachment and internalization by ME180 cells
We used a protocol adapted from Bobardt et al. (38) to measure HIV-1 attachment and uptake by human cervical ME180 cells. Briefly, ME180 cells grown to confluence in 96-well flat-bottom plates were exposed in duplicate to HIV-1BaL (20 ng p24/well) in the absence or presence of IgG1 or IgA2 b12, or relevant isotype control Abs. Cell monolayers were washed twice to remove free virus and virus Ab complexes, and then treated with lysis solution (Beckman Coulter). Levels of p24 Ag were measured in cell lysates by ELISA. The p24 Ag levels were considered representative of the total amount of cell associated virus (i.e., virus on the cell surface, as well as virus that had bee internalized). To determine only the amount of virus internalized by ME180 cells, the monolayers were washed two times to remove free, unbound virus, and then treated with 0.05% trypsin-EDTA (Invitrogen Life Technologies) for 8 min at 37°C to proteolytically remove surface-bound virus (39 – 41). Preliminary dose-response curves revealed that 0.05% trypsin resulted in the maximal removal of virus from the cell surfaces: higher concentrations of trypsin did not reduce HIV-1 levels further. After treatment with trypsin, the cells were treated with lysis solution and lysates were tested for p24 Ag, as described above.
Results
Production of monomeric and dimeric forms of recombinant human IgA2 b12
Ab IgG1 b12 was originally engineered from a Fab by cloning the b12 VH and VL genes into a mammalian combinatorial expression vector (12). Using this original vector, we switched the isotype of b12 by replacing the IgG1 constant domains with a PCR fragment encoding the constant domains of human IgA2(m1) (23), resulting in the plasmid pSM102 (Fig. 1). Plasmid pSM102 was then transfected into CHO cells, and several cell lines were established which secreted mIgA2 b12 into cell supernatants. One cell line in particular, 100-02-C9, was chosen for further study because of its stability and robust production of Ab. Clone 100-02-C9 was adapted to protein/serum-free medium and mIgA2 b12 was purified from cell supernatants by protein L affinity chromatography. Analysis of purified mIgA2 b12 by nonreducing SDS-PAGE revealed a protein species of ~150 kDa, which corresponds to the expected molecular mass of mIgA2 b12 (Fig. 2A).
FIGURE 2.
Expression and purification of monomeric and dimeric forms of IgA2 b12. A, Nonreducing SDS-PAGE analysis of monomeric, dimeric, and polymeric forms of IgA2 b12, as compared with IgG1 b12. Ab preparations were size-fractionated by nonreducing SDS-PAGE and stained with Coomassie blue. Monomeric IgA2 b12 migrated with a molecular mass of ~150 kDa (filled arrowhead), whereas higher molecular mass forms (>220 kDa; open arrowheads) were present in the dimeric and polymeric IgA b12 preparations. A protein band of ~50 kDa was present in all IgA b12 preparation (*), and likely corresponds to κ L chain dimers that dissociate from intact Ig (52). IgG1 b12 migrated with a molecular mass of ~160 kDa. B, Separation of dimeric and monomeric forms of IgA2 b12 by FPLC gel filtration. As described in Materials and Methods, Abs were eluted from by isocratic elution on HiPrep 16/60 Sephacryl S-300 High Resolution column at a flow rate of 0.5 ml/min. Peaks containing dimeric IgA2 b12 (50.02 peak) and monomeric IgA2 b12 (61.52 peak) were determined by ELISA and SDS-PAGE. The following molecular mass standards were used to calibrate the column: dextran 2000 kDa, ferritan 440 kDa, catalase 220 kDa, and RNase A 10 kDa. C, Anti-J chain Western blot analysis. Monomeric, dimeric, and polymeric forms of IgA2 b12, as well as IgG1 b12, were subjected to nonreducing SDS-PAGE, transferred to nitrocellulose, and probed with an anti-human J chain Ab. The minor band observed in the IgG1 b12 lane is probably due to spill over from the adjacent pIgA2 b12 sample.
To produce dimeric/polymeric forms of IgA2 b12, clone 100-02-C9 was supertransfected with plasmid pcDNA3Hygro::J chain, encoding human J chain (23). We identified several stable cell lines, notably J2 (“clone 1”) and J10 (“clone 2”), which secreted high molecular mass (i.e., dimeric and/or polymeric) forms of IgA2 b12. Total IgA2 b12 present in the supernatants of J2 cell cultures, which contain a mixture of monomers, dimers, and higher molecular mass polymers, were purified by protein L affinity column and referred to simply as “polymeric” IgA2 b12 (pIgA2 b12). Dimeric forms of IgA2 b12 were purified from higher molecular forms IgA2 b12 by size-exclusion chromatography (Fig. 2B). As expected, dimeric IgA2 migrated with an apparent molecular mass >220 kDa (Fig. 2A). Additional minor bands of slightly higher and lower molecular masses (e.g., 200 –250 kDa) were also evident by SDS-PAGE, and likely represent dIgA2 b12 with varying degrees of glycosylation (42). Western blot analysis confirmed that J chain was associated with dIgA2 b12, but not mIgA2 b12 or IgG1 (Fig. 2C). Additionally, IgA2 b12 derived from clones J2 and J10 (but not from 100-02-C9) were capable of binding to human SC in a SC solid phase-binding assay (Fig. 3), further demonstrating that J chain was properly incorporated into dimeric/polymeric forms of IgA2 b12. It should be underscored that the use of the term polymeric IgA2 b12 in this manuscript refers to a mixture of monomers, dimmers, and higher molecular mass polymeric forms of Ab.
FIGURE 3.
SC associates with polymeric IgA2 b12. Microtiter plates were coated with polyclonal goat anti-human IgA Abs, and then overlaid with human secretory IgA (positive control), monomeric IgA2 b12 (negative control), or dimeric IgA2 b12 from CHO cell clones J2 or J10. The plates were then probed with human SC and then developed using anti-human SC Abs. Each data point represents the average of two microtiter wells tested in parallel.
Reactivity of IgA b12 with gp120
We used ELISA to confirm that the different forms of IgA2 b12 retained their ability to bind gp120. The use of anti-κ chain-specific secondary Abs in our ELISAs enabled us to directly compare the binding profiles of different Ab isotypes (i.e., IgA and IgG). As shown in Fig. 4A, all three forms of IgA2 b12 (monomeric, dimeric, and polymeric) reacted with gp120MN in a dose-dependent manner with binding profiles similar to that obtained with IgG1 b12. Identical results were obtained when ELISAs were performed using gp120IIIB and gp120JR-FL (data not shown).
FIGURE 4.
Reactivity, avidity, and affinity of IgA2 b12 for gp120. The relative reactivity, avidity, and affinity of different forms of purified IgA2 b12 were compared with IgG1 b12. A, ELISA analysis examining the reactivity IgA2 and IgG1 b12 with gp120MN. Anti-human κ chain Abs were used as secondary reagents to allow direct comparison of reactivity of different b12 isotypes with gp120. B, Functional avidity of IgA2 and IgG1 b12 for gp120MN as determined by ammonium thiocyanate dissociation (see Materials and Methods). The AI (inset) is defined as the amount of ammonium thiocyanate (M) required to elute 50% of the Ab from gp120. C, The relative affinities of IgA2 and IgG1 b12 for gp120MN were determined indirectly by competitive inhibition. Abs were mixed with increasing concentrations of soluble gp120MN before being applied to ELISA plates coated with the same Ag. The amount of soluble gp120 required to reduce Ab binding by 50% was defined as the competitive inhibition index (inset, left). The approximate KD (inset, right) was determined as described in Materials and Methods.
To assess the relative avidity (i.e., functional affinity) of different forms of IgA2 b12 for gp120, we determined the amount of ammonium thiocyanate required to elute 50% mAb bound to gp120 (32, 33). From this, we calculated the AI for each mAb preparation, as described in Materials and Methods. All three forms of IgA b12 eluted from the plate at roughly equivalent amounts of ammonium thiocyanate (0.2– 0.6 M), thereby yielding similar AIs (Fig. 4B). Although slightly higher levels of ammonium thiocyanate were required to IgG1 b12, this difference was not significant. The relative affinities of each form of IgA b12 as estimated using a competitive inhibition assay were also similar (Fig. 4C). From these experiments, we conclude that monomeric and dimeric forms of IgA2 b12 recognize gp120 at levels comparable to IgG1 b12.
Neutralization of HIV-1 by IgA2 b12
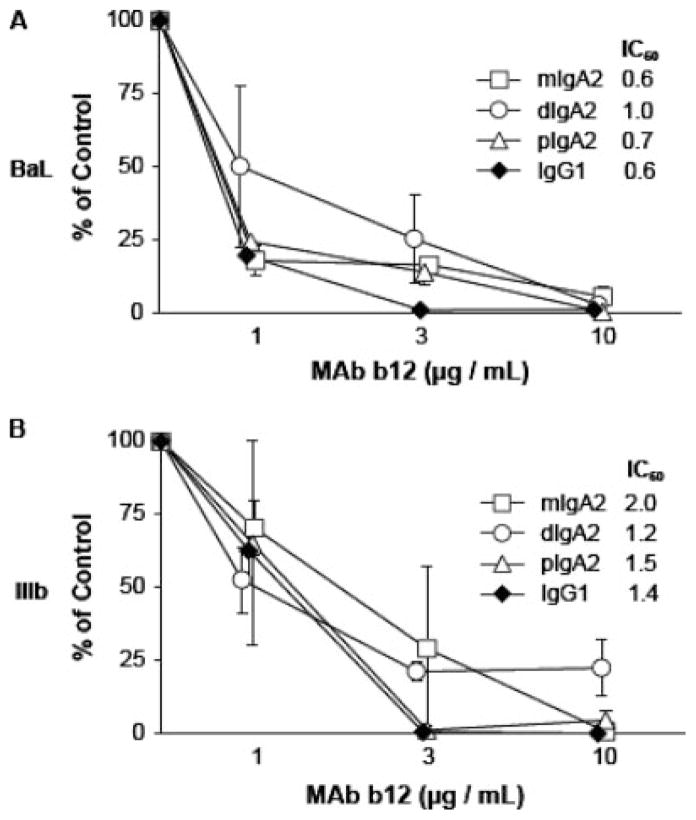
We next examined the ability of different forms of IgA2 b12 to inhibit CCR5-tropic and CXCR4-tropic strains of HIV-1 from infecting PBMCs. Monomeric, dimeric, and polymeric forms of IgA2 b12 demonstrated a dose-dependent inhibition of HIVBaL (CCR5-tropic) and HIVIIIB (CXCR4-tropic) infection of PBMCs similar to that obtained with IgG1 b12 (Fig. 5). No significant differences in the IC50 were observed among the different Abs tested in this assay. We also performed pseudovirus infection assays, which are considerably more sensitive than conventional T cell infection assays (37). Three pseudoviruses were tested: HIV-1JR-FL and HIV-1JR-CSF, two CCR5-tropic virus isolates, and HIV-1HxB2, a CXCR4-tropic virus which is virtually the same as HIVIIIB. These studies revealed subtle differences in neutralization activities among the different derivatives of b12 (Table I). These differences were most apparent when mAbs were examined as a function of their molar concentrations used in the neutralization assays, and when we compared IC90 values, rather than the less stringent IC50 values. For example, monomeric IgA2 b12 was notably less effective than other forms of b12 at neutralizing HIV-1JR-FL and HIV-1JR-CSF, whereas it was no different at neutralizing HxB2 (Table I). In fact, all forms of IgA2 b12 were rather poor at neutralizing HIV-1JR-CSF for reasons that are not immediately apparent. In contrast, monomeric, dimeric, and polymeric forms of IgA2 b12 were slightly more effective than IgG1 at neutralizing HIV-1HxB2.
FIGURE 5.
Neutralization of HIV-1 infection of PBMCs by IgA2 b12. Monomeric, dimeric, and polymeric (i.e., a mixture of monomers, dimers, and higher molecular mass polymers) IgA2 b12, as well as IgG1 b12, at indicated concentrations were incubated with (A) HIV-1BaL or (B) HIV-1IIIb for 30 min before being mixed with activated PBMCs. Levels of p24 in PBMC culture supernatants were determined 6 days later by ELISA, as described in Materials and Methods. Viral p24 in the control wells were consistently >14 ng/ml, confirming that the control cells were productively infected with HIV. The mAb concentration (micrograms per milliliter) necessary to inhibit 50% of viral infection, referred to as the IC50, is indicated in the inset.
We were also successful in producing a small amount of purified dimeric IgA2 b12 complexed with human SC (A. Hessell and D. Burton, manuscript in preparation) that enabled us to examine the capacity of SIgA2 b12 to neutralize HIV-1. As shown in Table I, the IC50 and IC90 for SIgA2 b12 were slightly lower than those obtained with dIgA2 b12, suggesting that the addition of SC may marginally enhance the capacity of IgA b12 to block HIV-1 infection of T cells.
Inhibition of HIV-1 transfer from epithelial cells to PBMCs
Although cervical epithelial cells are not considered permissive for HIV-1 infection per se, they may facilitate virus transfer to other cell types within the mucosa (2, 41). To determine whether Ab b12, which is directed against a highly conserved epitope within the CD4-binding site of gp120, can interfere with this step in viral transmission, we incubated HIV-1BaL and HIV-1IIIb with IgG1 b12, or IgA2 b12, and then applied the mixtures to monolayers of ME180 cells, a human-derived cervical epithelial cell line, or Caco-2 cells, a human-derived intestinal epithelial cell line. HIV-1 “transfer” from epithelial cells to CD4+, chemokine receptor+ cells was assessed by the addition of activated PBMCs to the epithelial cell monolayers. p24 levels were measured in the supernatants of the cocultures 6 days later (see Materials and Methods). We found that IgG1 b12, as well as monomeric and dimeric forms of IgA2 b12, inhibited HIV-1 infection of PBMCs in both the cervical and colonic epithelial cell transfer assay (Fig. 6). To discern whether the mAbs were interfering with viral attachment to and/or uptake by the epithelial cell monolayers (“step 1”), or inhibiting viral transfer from epithelial cells to PBMCs (“step 2”), the transfer assay was modified such that Ab b12 was added concurrently with HIV-1BaL and HIV-1IIIb (as done above), or added after virus had been allowed to attach to, and be internalized by, epithelial cells. As shown in Fig. 7, the addition of IgG1 b12 or dimeric IgA2 b12 at either step 1 (+/−), step 2 (−/+), or both (+/+) prevented HIV-1 infection of PBMCs, indicating in fact that b12 can block viral attachment of epithelial cells, as well as epithelial-PBMC transfer. Similar concentrations of Ab b12 were required to inhibit step 1 or step 2 of the epithelial transfer assay. It should be noted that at much higher doses of challenge virus (~4000 TCID50/ml), Ab applied before viral attachment to epithelial cells was more efficient at blocking infection of PBMCs than was Ab added after epithelial attachment/uptake (data not shown). Thus, b12 can act at either step in the epithelial transfer assay, but appears more efficient when added before viral epithelial exposure.
FIGURE 6.
Inhibition of HIV-1 transfer from epithelial cells to PBMC target cells by b12 mAbs. Cell-free HIV-1BaL or HIV-1IIIb preparations were incubated with IgG1 or IgA2 b12, and then applied to confluent monolayers of ME180 cells (left, A and B) or Caco-2 cells (right, C and D). Transfer was assessed by the addition of activated PBMCs and measuring p24 levels in culture supernatants by ELISA. The IC50 (micrograms per milliliter) is indicated in the inset table. Isotype-matched control Abs of irrelevant specificities showed no inhibition of HIV-1 transfer (data not shown).
FIGURE 7.
b12 mAbs block inhibition of HIV-1 transfer from epithelial cells to PBMCs. Cell-free HIV-1BaL or HIV-1IIIb preparations applied to confluent monolayers of ME180 cells (left, A and B) or Caco-2 cells (right, C and D) were incubated with IgG1 or IgA2 b12: 1) before epithelial exposure (+/−); 2) after epithelial exposure and washing but before addition of PBMCs (−/+), or both times (+/+), and then transfer was assessed by the addition of activated PBMCs and measuring p24 levels in culture supernatant by ELISA.
Inhibition of HIV-1 attachment to and uptake by cervical epithelial cells
In primary intestinal epithelial cells, it was reported that HIV-1 is internalized by endocytosis before being transferred to CD4+, co-receptor+ cells permissive for viral replication (4). From our experiments, it was unclear whether Ab b12 in step 1 was interfering with virus attachment to epithelial cells, and/or blocking virus uptake (i.e., endocytosis) into epithelial cells. To differentiate between attachment and uptake, we modified our transfer assays to include a trypsin digestion step which allowed us to proteolytically inactivate virus bound to cell surfaces, but not affect viruses that had been endocytosed (39 – 41). Both IgG1 b12 and IgA2 b12 demonstrated a dose-dependent reduction in the total amount of HIV-1BaL associated with ME180 cells (Fig. 8). In contrast, control IgG1 or IgA2 Abs had no effect on virus-epithelial interactions. Treatment of HIV-1-exposed ME180 cells with trypsin revealed that ~50% of the total cell-associated virus was resistant to digestion, suggesting that a fraction of HIV-1 was located intracellularly. The amount of HIV-1 recovered from trypsin-treated ME180 cells was reduced when HIV-1BaL was treated with IgG1 b12or IgA2 b12, inferring that b12 is capable of inhibiting virus uptake, as well as virus attachment to cervical epithelial cells.
FIGURE 8.
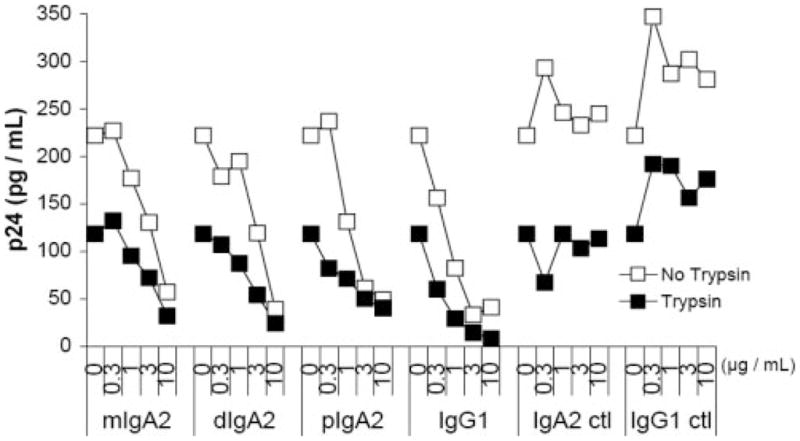
Inhibition of HIV-1 BaL internalization in ME180 cells by b12 mAbs. Increasing concentrations of IgG1 or IgA2 b12 were mixed with HIV-1BaL before application to ME180 cells. After 1 h at 37°C, the cells were washed to remove unbound virus and then lysed. Levels of p24 Ag in cell lysates were considered representative of the total amount of virus bound specifically to cell surfaces, plus the amount of virus that had been internalized (□). Alternatively, ME180 cells were treated with trypsin-EDTA (0.05%) for 8 min before lysis to effectively remove virus bound to the cell surfaces. Levels of p24 Ag in cell lysates from trypsin-treated cells were considered representative of the only amount virus internalized (■).
Discussion
Understanding the molecular mechanisms by which IgG and IgA Abs against gp120 interfere with HIV-1 transmission across mucosal surfaces has important implications for the design of effective vaccines and topical antivirals. In this study, we have successfully produced and purified recombinant monomeric and dimeric human monoclonal IgA2 Abs carrying the V regions of gp120-specific Ab b12 and characterized their functional activity in vitro. Human J chain was properly associated with dimeric IgA2 b12 as evidenced by Western blot analysis and by the ability of dimeric IgA2 b12 to bind human SC in a solid phase-binding assay. Although dimeric/polymeric forms of IgA can occur in the absence of J chain, these complexes cannot bind SC (43). rIgA2 b12 recognized gp120 by ELISA and neutralized HIV-1 infection of both activated PBMC and in a T cell line at levels comparable to IgG1 b12, indicating that both monomeric and dimeric forms of IgA2 b12 are fully functional. Finally, we have demonstrated for the first time that both IgG1 and IgA2 b12 are capable of preventing HIV-1 attachment to and uptake by colonic and cervical epithelial cells, revealing a possible mechanism by which Ab b12 protects mucosal compartments from HIV-1 infection in vivo.
Although two studies have demonstrated the ability of IgG1 b12 to protect macaques against mucosal SHIV challenge, the mechanism by which Ab b12 neutralized HIV-1 in vivo was not determined (15, 16). Results from our current study indicate that Ab b12 is capable of interfering with the earliest steps in mucosal transmission of HIV-1; attachment to and uptake by epithelial cells. These data are somewhat surprising because b12 recognizes an epitope overlapping the CD4-binding site on gp120, and the interaction of HIV-1 with epithelial cells is independent of CD4 (2, 4). Indeed, neither Caco2 nor ME180 epithelial cells express CD4 as detected by immunofluorescence or flow cytometry (E. N. Janoff and J. Palaia, unpublished data). Therefore, it is likely that Ab b12 interferes with viral attachment by steric hindrance or altering virus-host cell interactions through other means (44). Several groups have proposed that galactosyl ceramide serves as the primary receptor for HIV-1 on intestinal epithelial cells (45, 46). Galactosyl ceramide is a glycosphingolipid that protrudes only a few nanometers from the plasma membrane, so occupancy of IgG or IgA b12 on the surface of gp120 would be expected to interfere with virus-membrane contact. Although the receptor used by HIV-1 on cervical epithelial cells is currently unknown, the virus-epithelial cell interaction most certainly involves gp120.
Two other broadly neutralizing anti-HIV-1 human Abs, 2F5 and 2G12, have been class switched from IgG1 to IgM and IgA1 (47). 2G12 binds a conserved cluster of oligomannose side chains on gp120, and 2F5 targets a membrane proximal region of gp41. The neutralization activities of 2F5 and 2G12 were unchanged by isotype switching, except for 2G12 IgM, which inhibited HIV-1 infection of PBMCs ~28 times more efficiently than corresponding IgG (47). Whereas we converted Ab b12 to a human subclass IgA2, Wolbank et al. (47) converted 2G12 and 2F5 to subclass IgA1. IgA1 and IgA2 (of which there are two allotypic variants, m1 and m2) are found at roughly equal concentrations in mucosal secretions of the female genital tract and the large intestine (48). Although slight structural differences exist between IgA1 and IgA2 (e.g., IgA1 has an extended hinge region as compared with IgA2), there are currently no known functional differences between the two subclasses (17). In another report, Liu et al. (49) isotype switched the human mAb F425B4e8 from an IgG2 to other IgG subclasses (i.e., IgG1,3,4), as well as IgA1. mAb F425B4e8 recognizes an epitope at the base of the V3 loop of gp120 (50). Overall, the isotype variants of F425B4e8 performed similarly to each other with respect to recognition of gp120 and HIV-1 neutralization activity in vitro.
The feasibility of producing large amounts of IgA2 b12 in cell culture may have a number of practical applications. Most notable is the possibility of including IgA2 b12 in a topical microbicide aimed at reducing HIV-1 transmission across the mucosal surfaces of the vagina and ectocervix. In macaque models, IgG1 b12 applied vaginally in PBS or in a hydroxymethyl cellulose gel protected animals against a vaginal SHIV challenge, although protection was short-lived and required relatively high concentrations of Ab (16). We postulate that IgA2 b12, when complexed with SC, may provide better passive protection than that afforded by IgG1 b12 because SIgA is protease resistant and has a propensity to anchor in the mucus layer overlying epithelial cell surfaces (21). In fact, studies are underway to investigate the potential of SIgA2 b12 as a topical microbicide in the macaque model. Another application of IgA2 b12 is as a standard in ELISAs designed to detect anti-HIV-1 IgA Abs in human secretions. Accurately measuring anti-viral IgA titers in mucosal secretions is inherently difficult, and has been plagued, in part, by variation in ELISAs from laboratory to laboratory (20). Providing IgA2 b12 as a standard to public health and research laboratories would better enable the results of ELISAs from different laboratories around the world to be compared.
Determining the role of IgG and SIgA in conferring immunity to HIV-1 on mucosal surfaces has implications for HIV vaccine design and delivery. SIgA Abs are induced almost exclusively following mucosal vaccination, and are not elicited at any appreciable levels by systemic (e.g., i.m.) immunizations (1, 51). Whereas mice are considered to have a “common” mucosal immune system (i.e., immunization at one mucosal site stimulates a SIgA response at both local and both distant local mucosal responses), in humans, the shared mucosal immune system is more restricted. For example, oral immunization stimulates Abs in the proximal small intestine, but not in the distal large intestine or female genital tract (18). However, Abs in the female genital tract can be induced following vaginal or more practically intranasal vaccination. Delivery of mucosal vaccines also poses significant challenges, including the need for better adjuvants and formulations capable of withstanding the harsh environment of the respiratory and gastrointestinal tracts. The availability of fully functional IgG1 and IgA2 Abs that have identical V regions directed against a conserved neutralizing epitope on gp120 will enable us to define experimentally in animal models the “division of labor” that certainly exists between these two different Ab isotypes in mucosal tissues.
Acknowledgments
We thank Paul W. Parren (The Scripps Research Institute, La Jolla, CA), Stephanie Farrant (Children’s Hospital, Boston, MA), Crystal Piper (Wadsworth Center, Albany, NY), and Claudine Fasching (Minneapolis Veterans Affairs Medical Center, Minneapolis, MN) for technical assistance. We also acknowledge Finn-Eirik Johansen (University of Oslo, Oslo, Norway) for assistance with the SC-binding assay.
Footnotes
This work was supported by National Institutes of Health Grants AI33292 and AI55332 (to D.R.B.), Grants HD-41361, AI-48796, P30 AI-054907 (to E.N.J.), and Grant AI034757 (to M.R.N.). E.N.J. received additional support from the Veterans Affairs Research Service and the Colorado Center for AIDS Research. N.J.M. was supported by Mentored Research Scientist Award DK059295.
Abbreviations used in this paper: SHIV, simian/HIV; SIgA, secretory IgA; SC, secretory component; GS, glutamine synthetase; GMEM, glutamine-free MEM; AI, affinity index; TCID50, 50% tissue culture-infective dose; MSX, methionine sulfoximine.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kozlowski PA, Neutra MR. The role of mucosal immunity in prevention of HIV transmission. Curr Mol Med. 2003;3:217–228. doi: 10.2174/1566524033479852. [DOI] [PubMed] [Google Scholar]
- 2.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 3.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–156. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 6.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 7.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 8.Enose Y, Ui M, Miyake A, Suzuki H, Uesaka H, Kuwata T, Kunisawa J, Kiyono H, Takahashi H, Miura T, Hayami M. Protection by intranasal immunization of a nef-deleted, nonpathogenic SHIV against intravaginal challenge with a heterologous pathogenic SHIV. Virology. 2002;298:306–316. doi: 10.1006/viro.2002.1440. [DOI] [PubMed] [Google Scholar]
- 9.Ferrantelli F, Ruprecht RM. Neutralizing antibodies against HIV–back in the major leagues? Curr Opin Immunol. 2002;14:495–502. doi: 10.1016/s0952-7915(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 10.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 11.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 13.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 14.Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 15.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 17.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, VanCott TC, Trabattoni D, Sannella E, Mestecky J. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 21.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 22.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 23.Berdoz J, Blanc CT, Reinhardt M, Kraehenbuhl JP, Corthesy B. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc Natl Acad Sci USA. 1999;96:3029–3034. doi: 10.1073/pnas.96.6.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bebbington CR, Renner G, Thomson S, King D, Abrams D, Yarranton GT. High-level expression of a recombinant antibody from myeloma cells using a glutamine synthetase gene as an amplifiable selectable marker. Biotechnology. 1992;10:169–175. doi: 10.1038/nbt0292-169. [DOI] [PubMed] [Google Scholar]
- 25.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1988. [Google Scholar]
- 26.Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol. 2001;167:5185–5192. doi: 10.4049/jimmunol.167.9.5185. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D. Using Antibodies. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1999. [Google Scholar]
- 28.Grimsley GR, Pace NC. Spectrophotometric determination of protein concentration. In: Coligan JE, Dunn BM, Speicher DW, Wingfield PT, editors. Current Protocols in Protein Science. John Wiley & Sons; Hoboken: 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MJ, Pleass RJ, Batten MR, Atkin JD, Woof JM. Structural requirements for the interaction of human IgA with the human polymeric Ig receptor. J Immunol. 2005;175:6694–6701. doi: 10.4049/jimmunol.175.10.6694. [DOI] [PubMed] [Google Scholar]
- 30.Piskurich JF, France JA, Tamer CM, Willmer CA, Kaetzel CS, Kaetzel DM. Interferon-γ induces polymeric immunoglobulin receptor mRNA in human intestinal epithelial cells by a protein synthesis dependent mechanism. Mol Immunol. 1993;30:413–421. doi: 10.1016/0161-5890(93)90071-i. [DOI] [PubMed] [Google Scholar]
- 31.Tamer CM, Lamm ME, Robinson JK, Piskurich JF, Kaetzel CS. Comparative studies of transcytosis and assembly of secretory IgA in Madin-Darby canine kidney cells expressing human polymeric Ig receptor. J Immunol. 1995;155:707–714. [PubMed] [Google Scholar]
- 32.Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 33.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 34.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 35.Hetherington S. Solid phase disruption of fluid phase equilibrium in affinity assays with ELISA. J Immunol Methods. 1990;131:195–202. doi: 10.1016/0022-1759(90)90190-7. [DOI] [PubMed] [Google Scholar]
- 36.Janoff EN, Hardy WD, Smith PD, Wahl SM. Humoral recall responses in HIV infection: levels, specificity, and affinity of antigen-specific IgG. J Immunol. 1991;147:2130–2135. [PubMed] [Google Scholar]
- 37.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobardt MD, Salmon P, Wang L, Esko JD, Gabuzda D, Fiala M, Trono D, Van der Schueren B, David G, Gallay PA. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J Virol. 2004;78:6567–6584. doi: 10.1128/JVI.78.12.6567-6584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobardt MD, Armand-Ugon M, Clotet I, Zhang Z, David G, Este JA, Gallay PA. Effect of polyanion-resistance on HIV-1 infection. Virology. 2004;325:389–398. doi: 10.1016/j.virol.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dezzutti CS, Guenthner PC, Cummins JE, Jr, Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 42.Stubbe H, Berdoz J, Kraehenbuhl JP, Corthesy B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J Immunol. 2000;164:1952–1960. doi: 10.4049/jimmunol.164.4.1952. [DOI] [PubMed] [Google Scholar]
- 43.Johansen FE, Braathen R, Brandtzaeg P. Role of J chain in secretory immunoglobulin formation. Scand J Immunol. 2000;52:240–248. doi: 10.1046/j.1365-3083.2000.00790.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiser JN, Bae D, Fasching C, Scamurra RW, Ratner AJ, Janoff EN. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc Natl Acad Sci USA. 2003;100:4215–4220. doi: 10.1073/pnas.0637469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fantini J, Cook DG, Nathanson N, Spitalnik SL, Gonzalez-Scarano F. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with the cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc Natl Acad Sci USA. 1993;90:2700–2704. doi: 10.1073/pnas.90.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furuta Y, Eriksson K, Svennerholm B, Fredman P, Horal P, Jeansson S, Vahlne A, Holmgren J, Czerkinsky C. Infection of vaginal and colonic epithelial cells by the human immunodeficiency virus type 1 is neutralized by antibodies raised against conserved epitopes in the envelope glycocprotein. Proc Natl Acad Sci USA. 1994;91:12559–12563. doi: 10.1073/pnas.91.26.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolbank S, Kunert R, Stiegler G, Katinger H. Characterization of human class-switched polymeric (immunoglobulin M (IgM) and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol. 2003;77:4095–4103. doi: 10.1128/JVI.77.7.4095-4103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delacroix DL, Dive C, Rambaud JC, Vaerman JP. IgA subclasses in various secretions and in serum. Immunology. 1982;47:383–385. [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F, Bergami PL, Duval M, Kuhrt D, Posner M, Cavacini L. Expression and functional activity of isotype and subclass switched human monoclonal antibody reactive with the base of the V3 loop of HIV-1 gp120. AIDS Res Hum Retroviruses. 2003;19:597–607. doi: 10.1089/088922203322230969. [DOI] [PubMed] [Google Scholar]
- 50.Cavacini L, Duval M, Song L, Sangster R, Xiang SH, Sodroski J, Posner M. Conformational changes in env oligomer induced by an antibody dependent on the V3 loop base. AIDS. 2003;17:685–689. doi: 10.1097/00002030-200303280-00006. [DOI] [PubMed] [Google Scholar]
- 51.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 52.Morton HC, Atkin JD, Owens RJ, Woof JM. Purification and characterization of chimeric human IgA1 and IgA2 expressed in COS and Chinese hamster ovary cells. J Immunol. 1993;151:4743–4752. [PubMed] [Google Scholar]