Abstract
The prefrontal cortex (PFC) has been implicated in cognitive and affective responses to acute and chronic stress; however, direct evidence for the reactivity or adaptability of PFC neurons to stress is lacking. We followed the unit activity of medial PFC (mPFC) neurons in awake rats during two consecutive exposures to restraint stress or to a non-aversive novel object. The majority (75%) of mPFC neurons had significant responses to the initial restraint that was differentiated into one of three temporal patterns: (i) phasic increase in firing rate during the restraint period, (ii) slow onset increase in firing rate that was sustained for >2 h after restraint, and (iii) brief bi-phasic responses to initiation and termination of restraint. Exposure to a novel object elicited an exposure-locked phasic response in 40% of the neurons. None of the neurons displayed the sustained activation that was prominent after restraint. A second exposure to the object no longer elicited this phasic response while neurons in the three restraint-responsive groups modified their firing rate during the second restraint in a manner that was specific to their pattern of response to the first restraint. These findings demonstrate that whereas some mPFC neurons respond phasically to novel stimuli irrespective of their aversive nature, a separate population of PFC neurons responds to a stressful stimulus with a sustained increase in firing rate that persists in the absence of that stimulus. These neurons may be a substrate for adaptive responses that are necessary for behavioral modification.
Keywords: anxiety, electrophysiology, hypothalamic–pituitary–adrenal axis, post-traumatic stress disorder, schizophrenia
Introduction
Behavioral responses to stress may be categorized into two temporal components (Sapolsky, 2003; Korte et al., 2005). Fast (millisecond to second) responses range from simple reflexive movements to complex cognitive processing such as decision making based on contextual and previously stored information. Slower (minutes to hours) responses include activation of neuroendocrine systems and related events that may mediate adaptive responses to stress. A well-coordinated stress response is, of course, critical to an organism’s survival while an inappropriate stress response may be detrimental to health and contributes to stress-related disorders including post-traumatic stress disorder, mood disorders and addiction (Marinelli & Piazza, 2002; Moghaddam, 2002; de Kloet et al., 2005).
The prefrontal cortex (PFC) is an integral component of the stress-responsive circuitry and is well positioned to coordinate acute and adaptive behavioral responses to stress (Duncan, 2001; McDougall et al., 2004). Medial regions of the PFC (mPFC) have reciprocal projections to the hypothalamus and other brainstem nuclei that regulate autonomic and neuroendocrine responses to stress. Furthermore, the mPFC closely interacts with other association cortices, thalamus and corticolimbic regions, including the amygdala and hippocampus (Bacon et al., 1996; Carr & Sesack, 1996), which are critical for sensory and contextual processing, emotional learning, and behavioral flexibility (Phelps et al., 2004; Wood et al., 2004; Amat et al., 2005). Evidence suggests that the mPFC mediates normal and maladaptive responses to stress. For example, stress causes neurochemical and genomic changes in mPFC that include increased expression of intermediate early genes (Campeau et al., 2002; Figueiredo et al., 2003), increased translocation of glucocorticoid receptors (Kitchener et al., 2004) and increased release of many neurotransmitters including dopamine (Thierry et al., 1976). Inactivation of the mPFC increases reactivity to fear (Morgan & LeDoux, 1995) and amygdala stimulation (Jackson & Moghaddam, 2001), consistent with findings that mPFC neurons may encode fear extinction memories (Milad & Quirk, 2002). Furthermore, repeated stress causes sustained changes in the release of glutamate and catecholamines (Gresch et al., 1994; Bagley & Moghaddam, 1997) and alters mPFC dendritic morphology (Cook & Wellman, 2004; Radley et al., 2004; Brown et al., 2005). Stress also impairs cognitive functions that are dependent on the functional integrity of the mPFC (Arnsten, 2000).
Despite the explicit assumption in the literature that the mPFC is ‘activated’ by stress, and the plethora of behavioral and neurochemical data implicating mPFC in stress-related functions, direct evidence demonstrating that mPFC neurons respond to stress and whether those responses change after repeated stress is lacking. The aim of this study was to determine (i) what, if any, are the patterns of mPFC neuronal responses to stress and (ii) are these responses plastic, i.e. does the response of individual neurons to a subsequent exposure to the same stress change? Thus, the activity of the same mPFC neurons was followed during an initial exposure to restraint and a subsequent exposure in the same context 2 h later. To control for the effects of novelty, we also measured the activity of mPFC neurons to consecutive exposures to an unfamiliar non-aversive object.
Methods
Subjects
Thirteen adult male Sprague–Dawley rats weighing 340–420 g were used in this study (seven for stress studies and six for novel-object studies). Animals were housed individually on a 12-h light/dark cycle, and experiments were performed during the light phase. All experimental protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrode implantation
Chronic microelectrode arrays (NB Laboratories, Denison, TX, USA) were implanted under halothane anesthesia into the right mPFC (target coordinate for the center of array at AP +3.0, ML 0.7, and DV −3.5, Fig. 1a), according to the atlas of Paxinos & Watson (1998). Microelectrode arrays consisted of eight 50-µm-diameter Teflon-insulated, stainless steel wires arranged in a 2 × 4 pattern measuring approximately 0.25 × 0.7 mm. The electrode array was secured onto the cranium with dental cement using eight screws as anchors. A silver wire was connected to one of the screws to use as a recording ground.
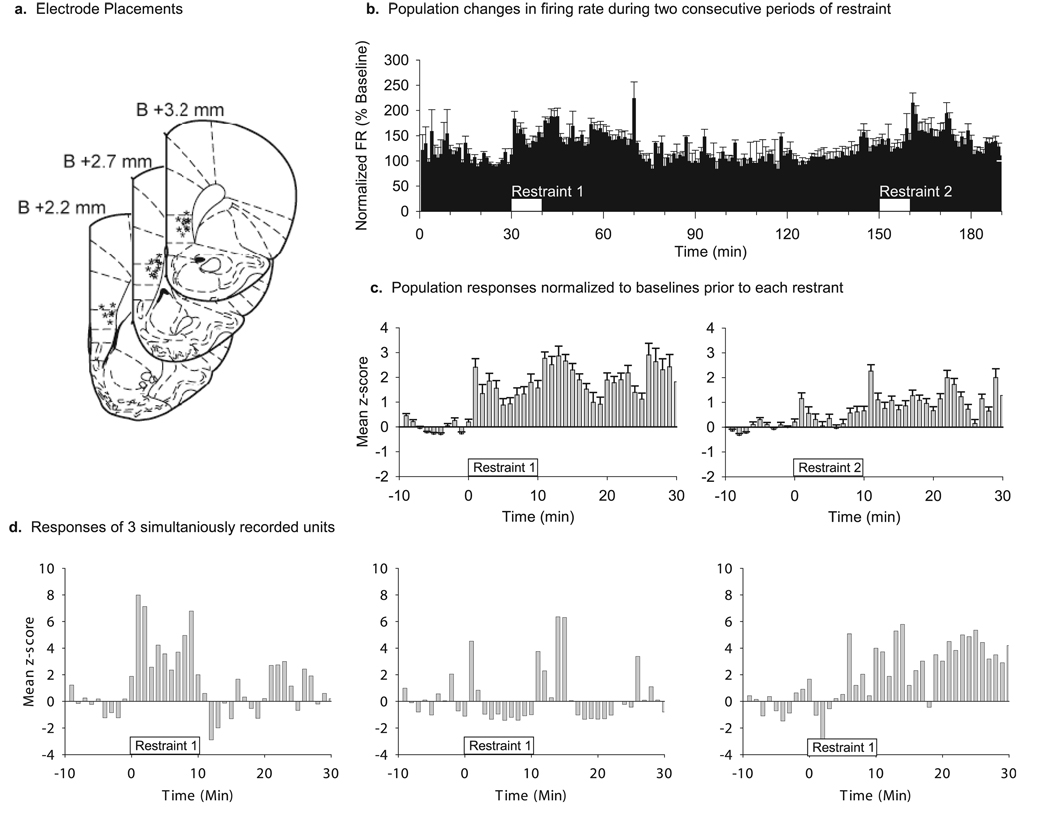
FIG. 1.
(a) Histological verification of electrode locations. All recordings were made from deep layers of the mPFC. Asterisks indicate verified locations of electrode tips. (b) Mean population changes in FR of 115 single-unit neurons recorded from seven rats during two consecutive 10-min episodes of restraint 2 h apart (white boxes). Firing rates were normalized (% baseline) to the 30-min period prior to the first restraint. Dashed line indicates baseline FR (100%). Bin size is 60 s. Error bars are standard error. (c) Population responses during the two episodes of restraint shown as z-scores. Restraint began at time 0 and lasted for 10 min. Peri-event rate histograms were calculated using 60-s time bins. The mean and standard deviation of the rate histograms during the 30-min period prior to each restraint was used to calculate z-scores. Restraint 1 caused a significantly higher increase in FR compared with restraint 2. (d) Examples of FR changes during restraint in three mPFC neurons recorded simultaneously in the same rat. It is clear from these examples that there is a heterogeneous population of responses among mPFC neurons.
Recording procedures
All recordings were performed in a clear polycarbonate cage (44 × 22 × 42 cm) with a modified open top extending an additional 42 cm above the cage. Animals were habituated to the recording cage in daily sessions for 5 days prior to the restraint sessions. Animals were connected to a unity-gain FET headstage (NB Laboratories) by means of lightweight cabling which passed through a commutator (NB Laboratories) that allowed them to move unrestricted during recording. Extracellular single unit activity was recorded using multiple-channel amplifiers with 500× gain and 220–5.9 kHz band pass filters (Plexon, Dallas, TX, USA). The amplified signal from each electrode was digitized (40 kHz sampling rate) and 1.0-ms waveform segments of all events that crossed an experimenter-defined threshold were saved to a PC computer hard disk for offline spike sorting. Spike sorting was performed with Off-Line Sorter software (Plexon) using a combination of automatic and manual sorting techniques. Units were labeled as single units only if autocorrelograms and interspike interval (ISI) histograms indicated that there were no pairs of spikes with ISI < 1.0 ms (absolute refractory period), which is inconsistent with good isolation. Typically, two or three neurons could be isolated from each electrode.
Experimental procedures
Animals were allowed at least 1 week to recover from surgery before experiments began. Animals were then habituated to the recording environment by daily transportation from the housing facility to the recording room where they were placed into the recording cage and connected to the recording apparatus for 5 h. Following at least 5 days of habituation, animals were subjected to 10-min episodes of either restraint stress or the placement of a novel object into the cage. Animals then experienced a second exposure to the same event 2 h later. Recording began 30 min prior to event 1 and continued throughout the intervening 2-h period until 30 min after the termination of event 2.
Restraint procedure
Brief restraint is a commonly used mild stress procedure in rodents that is capable of activating the hypothalamic–pituitary–adrenal axis (Pace & Spencer, 2005) and enhancing catecholamine release and c-fos expression in the mPFC (Deutch et al., 1991; Cullinan et al., 1995; Jackson & Moghaddam, 2004). To avoid confounding effects of changes in contextual information, we restrained the animals while they remained in the same cage by using a clear Plexiglas panel to restrict them to a corner of the recording cage. The partition was 25-cm high, and had 1.5-cm clearance on either side to prevent a buildup of bedding material as it was advanced, and a narrow slot at the top to allow the recording cable harness to pass through to the commutator. The partition was advanced until the rat was held firmly between the partition and the end of the cage so that no rearing or lateral movement was possible. After 10 min, the partition was removed and the animal was allowed to move about the cage freely. An identical restraint procedure was repeated 2 h later.
Novelty procedure
Novelty is known to increase activity and enhance catecholamine release and c-fos expression in the mPFC (Handa et al., 1993; Feenstra & Botterblom, 1996; Tulving et al., 1996; Yamasaki et al., 2002; Dobbins & Wagner, 2005). As a control for the response of mPFC neurons to a novel non-aversive object, we recorded from a second group of animals while they were exposed to a novel object in the cage. The basic recording procedure was identical to that used for the restraint procedure. The object was a plastic, brightly coloured child’s toy measuring approximately 7 cm in diameter and 5 cm in height. The object was placed into the center of the cage and the animal was allowed to explore the novel object freely for 10 min, at which time it was removed from the cage. After a 2-h delay, the same object was returned to the animal’s cage for an additional 10 min.
Data analysis
Electrophysiological data was imported into NeuroExplorer (Plexon) for initial firing rate (FR) analysis. Rate histograms of spontaneous spike trains for each neuron were calculated using 60-s bins for the 70min periods surrounding each restraint or novelty exposure epoch. These binned data were then exported to MATLAB (Mathworks, Natick, MA, USA) for further analysis. For each neuron, the baseline FR and the standard deviation (SD) of the baseline FR was calculated by taking the mean and SD during the 30-min period prior to each episode of restraint or novelty exposure. The mean and peak FR during each of the two episodes of restraint stress or novelty exposure were also calculated. In order to compare across the population of neurons with different baseline FRs, we normalized each individual rate histogram using a modified z-score transformation by subtracting the baseline FR from each rate histogram bin and dividing this difference by the standard deviation of the baseline firing rate. Thus, the normalized FR histograms show standard deviations of the FR from the baseline FR. Normalized FR values exceeding 1.67 could then be assumed to have a significance level of P < 0.05 compared with baseline FRs. All data was exported to SPSS (SPSS Inc., Chicago, IL, USA) for further statistical analysis.
Response pattern analysis
Natural clusters of response patterns in the peri-event rate histograms were found by K-means cluster analysis in SPSS. The normalized discharge rate of each neuron (z-scores), measured during sequential 60-s bins starting at the onset of the event (restraint or novelty) and continuing for 10 min post-event (20 min total), was used as an independent variable for the cluster analysis. Thus, the response of each neuron was represented by 20 variables that described the FR change in response to the event (compared with baseline) over time. The number of clusters used for K-means clustering was initially set at two, and the number of seed clusters was incremented in steps of one up to a maximum of ten to find the optimal number of clusters that adequately described the data. Initial cluster centers were chosen at random. A minimum of 20 iterations of the K-means algorithm were used to protect against local minima. The convergence criterion was zero change in cluster distances. In order to determine the proper grouping of neurons and to guard against over-clustering of the data, visual inspection was used to ensure that the defined clusters qualitatively differed from one another. For each clustered grouping of neurons, starting with clusters of two and extending to clusters of ten, the averaged normalized FR of all the neurons contained within each putative grouping were plotted for comparison. Repeated-measures anova, with time as the repeated measure and K-means cluster membership as the grouping variable, was used to determine whether the putative groupings were significantly different from each other (P < 0.05). The number of clusters was sequentially incremented until no new response patterns emerged. Furthermore, the normalized rate histogram of each neuron in isolation was visually compared with the average group waveform to confirm that each neuron was correctly classified. The final determination of the number of restraint response pattern groups involved choosing the fewest number of groups that was capable of expressing the full range of observed response patterns. After neurons were grouped in accordance with response patterns during the first event (i.e. restraint or exposure to novel object), each response group was then individually analysed to determine the response pattern of those neurons to the second event using the same procedures.
Statistical analysis
All statistics were performed in SPSS statistical software. Comparison of stress response or novelty response groups was conducted by repeated-measures anova with time as the repeated measure and response group membership as the between-groups factor, with the Tukey’s post-hoc test used to determine significant differences between individual groups. Comparison of baseline FR and restraint-evoked FR parameter responses between multiple groups was conducted by one-way anova followed by Tukey’s post-hoc test. Comparison of FR parameters in the two groups was by a two-tailed independent samples t-test. Comparisons of within-group FR changes were conducted with a two-tailed paired samples t-test. The level of significance for all comparisons was P < 0.05.
Histology
Animals were anesthetized with an overdose of chloral hydrate and intracardially perfused with saline followed by 10% buffered formalin. The location of the recording electrodes was marked by passing a 25-lμ cathodal current for 30 s. Fixed brains were cut at 200-µm intervals and sections stained with cresyl violet to probe electrode placements. Under a light microscope, tracks of recording electrodes were confirmed to be in the infralimbic mPFC (Fig. 1a).
Results
We recorded 115 mPFC neurons in seven rats during two consecutive exposures to restraint stress. We first examined the average FR of the population of neurons through both periods of restraint by considering percentage change from the prerestaint 1 baseline (Fig. 1b). The mean population FR increased during both periods of restraint (144.3 ± 8.7% and 111.4 ± 6.2%, respectively), but the magnitude of the response to restraint 2 was significantly smaller than that for restraint 1 (paired samples t-test, t = 4.297, P < 0.001). We also compared the responses to each episode of restraint by normalizing to the baseline measured for 30 min immediately before each restraint and converting the results to z-scores (Fig. 1c). The mean population response to restraint 1 showed an overall increase during and after restraint. The population response during and after restraint 2 was significantly reduced compared with restraint 1 (mean z-scores 0.42 ± 0.16 and 1.38 ± 0.23, respectively; repeated-measures anova, time–stress interaction, F = 5.8, P < 0.001; main effect of time, F = 28.7, P < 0.001; main effect of restraint, F = 54.1, P < 0.001). The average peak z-score during restraint 1 was also higher than the peak z-score during restraint 2 (4.94 ± 0.23 and 2.35 ± 0.32, respectively; paired-samples t-test, t = 7.95, P < 0.001). There was no significant difference in baseline FR prior to restraint 1 and restraint 2 (2.68 ± 0.19 and 2.93 ± 0.23 spikes/s, respectively).
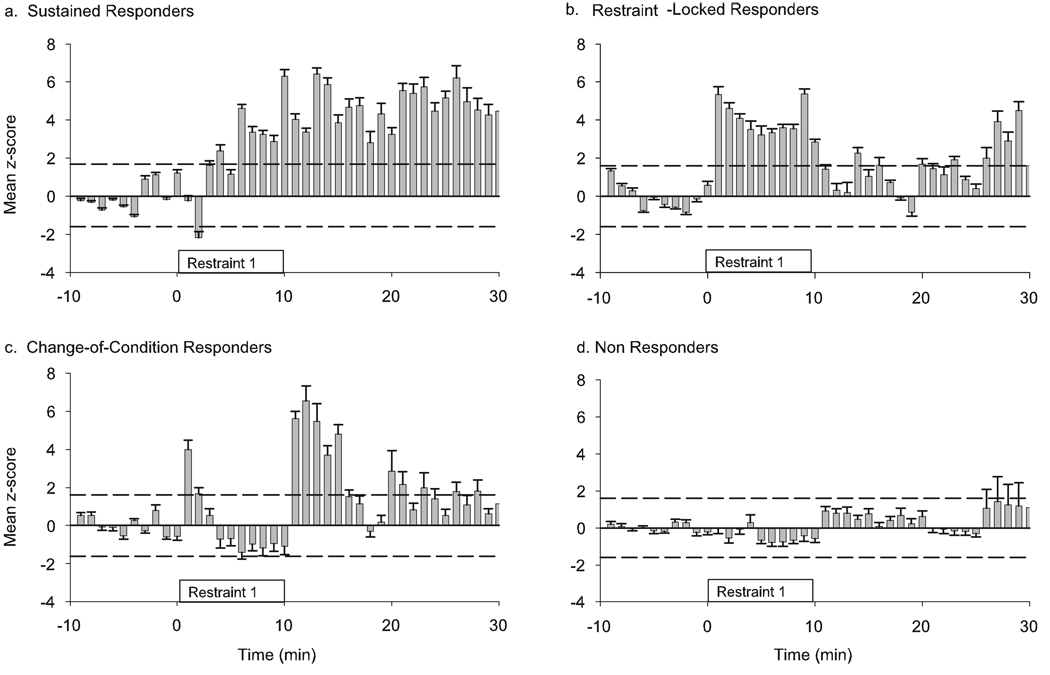
The population response suggested that restraint 1 produced an overall increase in FR during the 10 min of restraint. The temporal pattern of individual neuron responses, however, was heterogeneous (Fig. 1d). To identify different patterns of response, we used K-means cluster analysis on the normalized FR histograms of all 115 neurons recorded during restraint 1. This analysis identified four groups of neurons with distinct response patterns. One group of neurons (18%) had an increase in FR that was slower in onset (significant increase in FR became apparent 3.4 ± 0.5 min after the onset of restraint) but this increase was sustained after the termination of restraint (Fig. 2a). These neurons were classified as ‘sustained responders’. The second group (25%) exhibited an increase in FR that coincided with the restraint duration and were hence designated as ‘restraint-locked responders’ (Fig. 2b). The third group of neurons (20%) had a brief initial increase in FR at the beginning of restraint, and a second response after the termination of restraint (Fig. 2c). This group was called the ‘change-of-condition responders’. The fourth group of neurons (37%) was classified as ‘non-responders’ because they displayed no significant change in FR either during or after restraint (Fig. 2d). Other than the response pattern and expected differences in mean FR changes during restraint, the only difference between these groups of neurons was that the change-of-condition responders had a lower baseline FR than the non-responders (1.67 ± 0.32 vs. 3.29 ± 0.40 spikes/s, respectively; anova, F = 3.91, P = 0.011, Tukey’s post-hoc P < 0.05). The baseline FR of sustained responders and restraint-locked responders (2.26 ± 0.36 and 2.96 ± 0.23 spikes/s, respectively) were not different from other groups.
FIG. 2.
Different temporal response patterns during restraint 1 as identified by K-means cluster analysis on the normalized FR histograms of 115 neurons. Average normalized rate histograms (z-scores) are shown for each group. Dashed lines indicate a significant deviation from baseline (P< 0.05). Four response patterns were identified. (a) The sustained responders had a slow onset increase in FR that remained elevated after the termination of restraint. (b) The restraint-locked responders increased their FR during the restraint procedure. (c) Change-of-condition responders had a brief increase in FR immediately after the restraint began, and a second increase in FR after the restraint was terminated. (d) Non-responders had no significant response to restraint.
To determine if mPFC neurons modify their response to repeated exposure to the same stressor, we followed the activity of the same neurons during a second episode of restraint stress (restraint 2), 2 h after restraint 1. A separate K-means cluster analysis was performed on restraint 2 responses. This separate analysis allowed us to avoid placing any a priori assumptions about the pattern of restraint 2 responses based on restraint 1 responses.
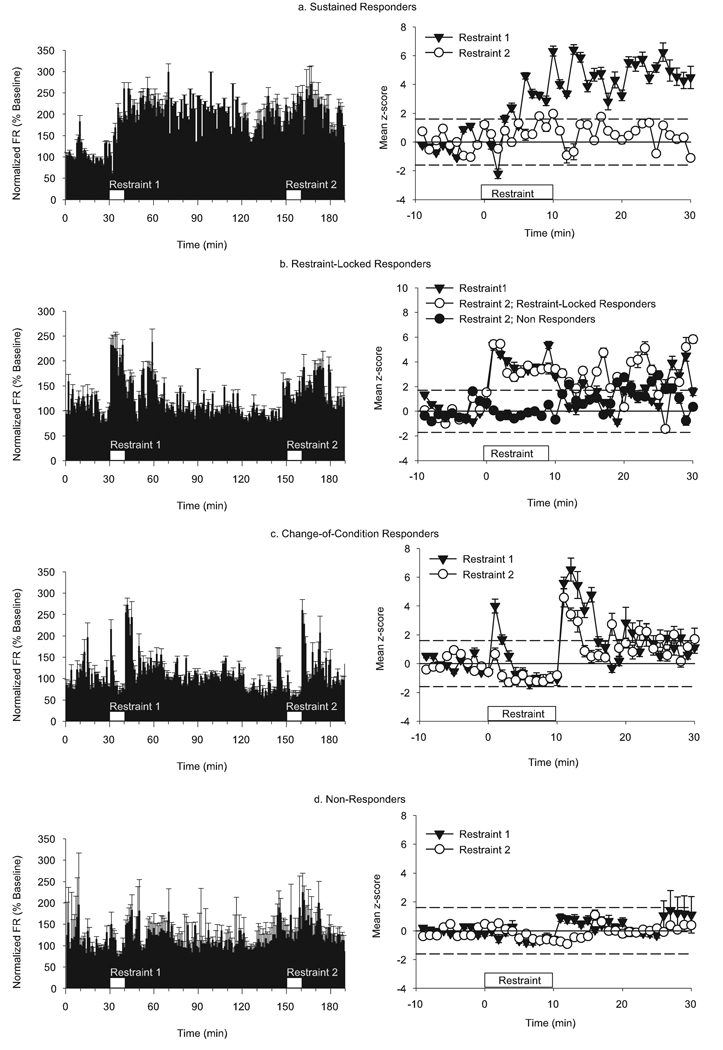
We found that 95% (20 out of 21) of restraint 1 sustained responders became non-responders to restraint 2 (Fig. 3a, right column). At first it appeared that these neurons were not responsive to restraint 2, but when we compared the baseline FR of these neurons before each episode of restraint we saw that these neurons had a significantly higher FR prior to restraint 2 compared with the baseline FR measured before restraint 1 (3.96 ± 0.47 vs. 2.34 ± 0.36 spikes/s, respectively; paired-samples t-test, t = 7.284, P < 0.001). We then re-analysed the restraint 2 response of the sustained-responder group of neurons by normalizing the restraint 2 responses by the prerestraint 1 baseline so that we could see changes during the entire 3-h period of recording (Fig. 3a, left column). The re-normalized data revealed that the sustained increase in FR produced by restraint 1 persisted through the restraint 2 recording period.
FIG. 3.
Modified responses to repeated restraint. The left column shows normalized (% baseline) population responses of the four response groups during the entire 3-h experiment with repeated episodes of restraint (white boxes). The right column shows mean z-score of the responses to restraint 1 and the subsequent response of the same neurons to restraint 2. Dashed lines indicate significant z-scores (P< 0.05). (a) Neurons that had a sustained response to restraint 1 (solid triangles) had no significant response to restraint 2 (open circles). (b) The majority (75%) of the neurons that had restraint-locked response to restraint 1 (solid triangles) exhibited the same duration and magnitude of response to restraint 2 (open circles). Nevertheless, 25% of these neurons became non-responders (solid circles). (c) All neurons in the bi-phasic cluster exhibited the response to restraint 2 (open circles) as they did to restraint 1 (solid triangles); however, the magnitude of their response to changes in either condition was attenuated. (d) All neurons that were non-responders during restraint 1 (solid triangles) remained non-responsive to restraint 2 (open circles).
The other response groups had no difference in baseline FRs before either period of restraint, so all comparisons were made using restraint 1 and restraint 2 data normalized to baseline 1 and baseline 2, respectively. Neurons with the restraint-locked response to restraint 1 had two different responses during restraint 2 (Fig. 3b). About 25% (seven out of 28) became non-responders, and the remaining 75% (21 out of 28) retained a restraint-locked response pattern. There was no difference in the baseline FR of the two subgroups. All of the change-of-condition responders (Fig. 3c, 23 out of 23) displayed the same bi-phasic response to restraint and end of restraint 2. The magnitude of response to restraint 2, however, was considerably reduced in these neurons (repeated-measures anova, time-by-group interaction, F = 8.771, P < 0.001; between groups effect, F = 13.16, P < 0.001). Finally, all of the restraint 1 non-responders (Fig. 3d, 42 out of 42) remained non-responsive to restraint 2.
In a separate group of six rats, we recorded a total of 80 neurons during two consecutive 10-min exposures to an object (a small plastic toy) that was novel to the animal, and that we assumed to be non-threatening and non-aversive to the animal. The population responses to the first presentation of the novel object and the subsequent presentation to the now-familiar object are shown (Fig. 4). There was an overall increase in the FR of mPFC neurons during presentation of the novel object while the second presentation of the now-familiar object was not associated with FR increases. There were no differences in baseline FRs before the two presentations of the object (3.29 ± 0.47 and 3.09 ± 0.39 spikes/s).
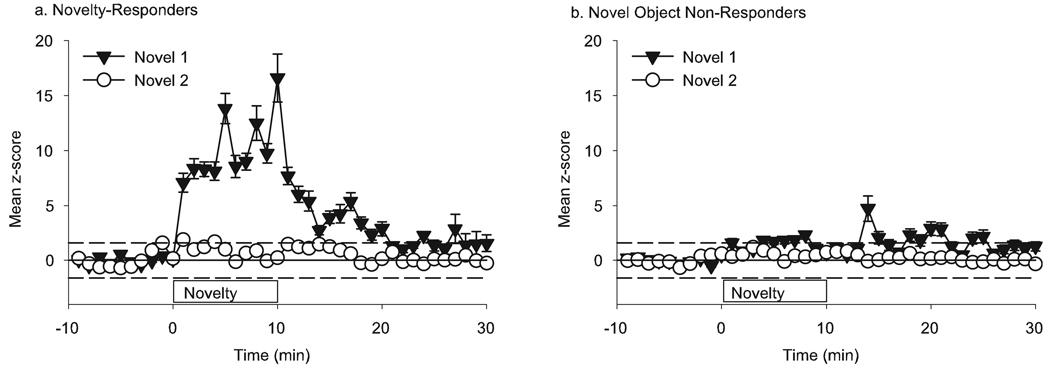
FIG. 4.
Response of mPFC neurons to a non-aversive novel object (n = 80). (a) Some mPFC neurons (40%) had an increase in FR during novel 1 (solid triangles) and were classified as ‘novelty responders’. These neurons did not respond during the second presentation of the now-familiar object (open circles). (b) The majority of mPFC neurons (60%) had no significant change in FR during the 10-min period that the novel object was placed in the cage (solid triangles), nor during the second presentation of the now-familiar object 2 h later (open circles).
Cluster analysis on the 80 neurons recorded during first exposure to the object revealed two types of responses. The majority of mPFC neurons (60%) had no significant change in FR during the 10 min that the novel object was placed in the center of the cage (Novel 1, Fig. 4b), nor during the second presentation of the now-familiar object 2 h later. We classified this group as ‘novelty non-responders’. The remaining 40% of mPFC neurons had an increase in FR during first exposure (Fig. 4a). We classified this group as ‘novelty responders’. There was no difference in the baseline FR of neurons in the novel non-responders or novel responder groups (2.01 ± 0.31 and 2.09 ± 0.34 spikes/s, respectively). The greatest change in FR coincided with the period of time that the novel object was in the cage. During the second presentation of the now-familiar object (Novel 2) there was no significant increase in the FR of these neurons. During the first presentation of the object, rats spent 4.0 ± 0.8 min actively exploring the object, and the remainder of the time was spent grooming or resting. The same animals spent 1.8 ± 0.5 min actively exploring the object during the second presentation, suggesting that mPFC encoding of novelty does not control exploratory responses to the novel stimulus.
Discussion
We find that PFC neurons encode both fast and sustained responses to a novel stressor, and that the response of most stress-sensitive PFC neurons is plastic in that it changes in response to a subsequent exposure to the same stressor. The temporal profile of response to the first stress was diverse but could be differentiated into three patterns: (i) sustained responses in which a delayed increase persisted after the cessation of restraint stress; (ii) restraint-locked responses in which an increase in FR was observed during exposure to restraint stress; and (iii) change-of-condition responses in which neurons briefly activated their FR at the beginning of the restraint procedure and once the animal was unrestrained. Another group of neurons displayed no significant response to restraint. This latter group remained non-responsive to the subsequent restraint procedure, whereas most neurons in the three responsive groups modified their FR in a manner that was specific to their pattern of response to the first restraint. A different pattern of neuronal response was observed when animals were exposed to a non-threatening novel stimulus, suggesting that the pattern of response of PFC neurons to restraint is not due entirely to novelty.
PFC response to stress
Several lines of evidence suggest that the mPFC may be critical for mediating adaptive responses to aversive stimuli (Arnsten, 2000; Garcia, 2002; Milad & Quirk, 2002; Moghaddam, 2002; McDougall et al., 2004; Amat et al., 2005). Although most previous investigations have focused on the impact of chronic, painful or prolonged aversive manipulations such as hours of immobilization, exposure to high-intensity shocks or several weeks of unpredictable or cold stress (Jedema & Grace, 2003; Radley et al., 2004; Amat et al., 2005; Miracle et al., 2006), very little is known about the adaptability of PFC neurons to a single exposure to mild stress. Chronic and severe stressors provide excellent models for studying pathological states such as post-traumatic stress syndrome; however, models of ‘mild stress’ are equally important for understanding the neuronal basis of stress adaptability because these models may be relevant to daily human experiences that require rapid adaptation for proper cognitive and emotional functioning.
Numerous studies have reported changes in immediate early gene and neurotransmitter release in the PFC in response to brief and mild stressors (e.g. Thierry et al., 1976; Deutch & Roth, 1990; Timmerman et al., 1999; Kawahara et al., 2000; Figueiredo et al., 2003). Our present findings, including the overall responsiveness of PFC neurons to restraint and the rapid plasticity of this response, are consistent with previous studies demonstrating that glutamatergic afferents to the PFC are activated by restraint or tail pinch (Lowy et al., 1993; Moghaddam, 1993). This presynaptic glutamatergic response was significantly attenuated when animals were exposed to the same stressor a second or third time (Bagley & Moghaddam, 1997), suggesting that the modified response of PFC neurons observed in the present study may have resulted, in part, from reduced afferent glutamate input. Similar to glutamate, dopamine projections to the PFC are also highly responsive to stress (e.g. Abercrombie et al., 1989). Using the same restraint stress procedure as the one used in the present study, we found that the release of dopamine in response to a second restraint procedure is significantly smaller than the response to the initial exposure (Jackson & Moghaddam, 2004), further supporting the idea that the rapid modification of PFC response to the same stimulus is mediated through reduced afferent drive of these neurons.
The PFC response to stress is often associated with the activation of the neuroendocrine system and secretion of glucocorticoids (Diorio et al., 1993; Crane et al., 2003). Our findings indicate that a component of the mPFC neuronal response to restraint, which was encoded by restraint-locked and change-of-condition responding neurons, occurs too fast to be associated with endocrine-mediated effects of restraint, such as release of glucocorticoids, which occur several minutes after the initiation of restraint (Marinelli & Piazza, 2002). This finding is consistent with previous studies showing that activation of stress-induced afferent activity in the PFC is partially independent of endocrine activity (Dunn, 1988; Moghaddam et al., 1994). Specifically, the initial phase (2–5 min) of the stress-induced increase in glutamate efflux in the PFC is sustained after adrenalectomy (Moghaddam et al., 1994). This is in contrast to the effects of stress on glutamate efflux in other stress-sensitive regions such as the hippocampus, which are entirely dependent on glucocorticoid release (Stein-Behrens et al., 1994). In the PFC, the delayed increase (5–20 min) in stress-activated glutamate efflux is, however, dependent on activation of the neuroendocrine system because it is abolished by adrenalectomy and restored by glucocorticoid replacement. Therefore, the slow onset and long duration of response of neurons that were categorized as sustained-responders is similar to the pattern of increase of the glucocorticoid-sensitive segment of glutamate efflux. Collectively, these findings suggest that there may be two components to the PFC stress response: a fast component that is encoded by stress-locked and change-of-condition responding neurons and a slower but sustained (and possibly glucocorticoid-dependent) component encoded by the slow onset sustained-responding neurons. The fast component would be critical for initiating decision-making and other executive processes that are necessary for rapid behavioral responses to aversive and threatening stimuli. The slow component may be a substrate for stress-induced plasticity, consistent with the well-described role of glucocorticoids in memory consolidation (McGaugh, 2004). Clearly, further work is required to establish the validity of this two-component model.
PFC neuronal responses to novelty
Monoamine projections to the mPFC have been shown to be activated by novel stimuli (Handa et al., 1993; Feenstra & Botterblom, 1996; Tulving et al., 1996; Yamasaki et al., 2002; Dobbins & Wagner, 2005). Furthermore, motivationally salient modulation of mPFC neurons has been reported in rats engaged in cognitive tasks (Jung et al., 1998, 2000; Hok et al., 2005). Therefore, some of the restraintelicited responses in the mPFC neurons may not have been related to restraint per se, but may generalize to other novel or salient stimuli regardless of their aversive or rewarding nature. To control for the effects of novelty, we measured the response of mPFC neurons to repeated presentations of a non-aversive novel object. The majority of mPFC neurons had no significant FR change during presentation of the novel object. The neurons that did respond to novelty had an FR increase that was locked to the presence of the novel object, in a manner very similar to the restraint-locked responses. All of the novelty-responsive neurons, however, failed to respond to the second presentation of the now-familiar object, suggesting that these responses were related to the novelty of the stimulus. Most of the neurons with restraint-locked responses also failed to respond during the second episode of restraint, suggesting that this type of response could also be primarily associated with the novelty of the experience rather than a direct consequence of the aversive stressful experience. This observation, however, does not preclude the possibility that the restraint-locked responses were specific to the emotional salience of the first period of restraint, or that the second restraint was perceived to be less threatening or had a neutral reward value.
PFC neuronal responses selective to restraint stress
The other two response patterns to restraint, change-of-condition and sustained responders, may be selective stress responses because these patterns were not observed during exposure to a novel object. The main feature of the change-of-condition neurons was that they showed a phasic activation during transition from one state to another; one that would be considered aversive, i.e. beginning of restraint, and the other during the favorable condition of being freed from restraint. It is possible that these neurons encode for salient (or novel) stimuli, yet this pattern of activity was never seen in response to the non-aversive novel stimuli. Thus, it appears that this response pattern is related to the salience of adversative stimuli. This argument is supported by the observation that the response magnitude of these neurons was lower during the second restraint, which would present a less salient and no longer novel stimulus compared with the first restraint.
The most prominent modified response to restraint 2 was observed in the sustained response group because nearly all neurons in this group failed to exhibit a significant response to restraint 2. Although the observed changes in FR in these neurons were not great in overall magnitude (3–6 spikes/s), they did represent a doubling or tripling of these cells’ baseline FR and were significantly greater than the activation seen in neurons in other clusters. The magnitude of FR changes is consistent with other recordings of rat mPFC neurons during performance of goal-directed activities (Hok et al., 2005) or in response to fear conditioning (Baeg et al., 2001). Furthermore, given that most models of neural plasticity, including long-term depression, require sustained increases in neural activity (Malenka & Bear, 2004), synaptic plasticity is a possible mechanism to account for the decreased responsiveness of these neurons during stress 2. Of note, when we examined the continuous recordings of each neuron and normalized the response magnitudes using the prerestraint 1 baseline, it became evident that the increase in FR produced by restraint 1 persisted through the restraint 2 recording period. Therefore, the apparent lack of additional FR increase in response to restraint 2 may have resulted from a ceiling effect limiting any further FR increase. Alternatively, these neurons may have remained engaged in mediating adaptive processes stemming from the first restraint episode.
Conclusions
Our findings suggest that mPFC neurons may mediate rapid behavioral responses to a stressful situation as well as slow adaptive responses that are necessary for behavioral modification. In response to a novel stressor, the majority of PFC neurons appear to come on line with varying response patterns that encode different temporal aspects of the stress procedure. However, PFC neurons rapidly become less responsive to the same stressor. This plasticity, which represents disengagement of PFC neurons from the aversive stimulus, may be critical for allowing the organism to gain back behavioral flexibility despite repeated exposure to a familiar stressor. Disruption of this adaptive function of the PFC may lead to an abnormal reaction to stress and disease states that are associated with compromised executive and emotional functioning.
Acknowledgements
This work was supported by a National Alliance for Research in Schizophrenia and Depression (NARSAD) Young Investigator Award (M.E.J.) and National Institute of Mental Health awards R37MH48404 and R21MH65026.
Abbreviations
- FR
firing rate
- mPFC
medial PFC
- PFC
prefrontal cortex
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog. Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb. Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. doi: 10.1016/s0306-4522(96)00435-6. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb. Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J. Comp. Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic–pituitary–adrenal axis response to a physical stressor, systemic delivery of interleukin-1beta. Eur. J. Neurosci. 2003;17:1473–1481. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cerebr. Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog. Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 402–363. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney M. The role of the medial preforntal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb. Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J. Neurochem. 1988;51:406–412. doi: 10.1111/j.1471-4159.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur. J. Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Garcia R. Stress, synaptic plasticity, and psychopathology. Rev. Neurosci. 2002;13:195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J. Neurochem. 1994;63:575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Bollnow MR. Induction of c-fos mRNA in the brain and anterior pituitary gland by a novel environment. Neuroreport. 1993;4:1079–1082. [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc. Natl Acad. Sci. USA. 2005;102:4602–4607. doi: 10.1073/pnas.0407332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J. Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Stimulus-specific plasticity of prefrontal cortex dopamine neurotransmission. J. Neurochem. 2004;88:1327–1334. doi: 10.1046/j.1471-4159.2003.02205.x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology. 2003;28:63–72. doi: 10.1038/sj.npp.1300020. [DOI] [PubMed] [Google Scholar]
- Jung MW, Qin Y, Lee D, Mook-Jung I. Relationship among discharges of neighboring neurons in the rat prefrontal cortex during spatial working memory tasks. J. Neurosci. 2000;20:6166–6172. doi: 10.1523/JNEUROSCI.20-16-06166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Qin Y, McNaughton B, Barnes C. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cerebr. Cortex. 1998;8:437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur. J. Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur. J. Neurosci. 2004;19:1837–1846. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Lowy M, Gault L, Yammamato B. Adrenolectomy attenuates stress induced elevation in extracellular glutamate concentration in hippocampus. J. Neurosci. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur. J. Neurosci. 2004;20:2430–2440. doi: 10.1111/j.1460-9568.2004.03707.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn. Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J. Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol. Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Pace TW, Spencer RL. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychological stressor. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1082–E1088. doi: 10.1152/ajpendo.00521.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem. Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Lin WJ, Sapolsky RM. Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J. Neurochem. 1994;63:596–602. doi: 10.1046/j.1471-4159.1994.63020596.x. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Cisci G, Nap A, de Vries JB, Westerink BH. Effects of handling on extracellular levels of glutamate and other amino acids in various areas of the brain measured by microdialysis. Brain Res. 1999;833:150–160. doi: 10.1016/s0006-8993(99)01538-3. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb. Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc. Natl Acad. Sci. USA. 2004;101:3973–3978. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc. Natl Acad. Sci. USA. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]