Abstract
In spite of partial success in treating Parkinson's disease using ectopically placed grafts of dopamine-producing cells, restoration of the original neuroanatomical circuits, if possible, might work better. Previous evidence of normal anatomic projections from ventral mesencephalic (VM) grafts placed in the substantia nigra (SN) has been limited to neonatal rodents and double grafting or bridging procedures. This study attempted to determine whether injection of a potent growth promoting factor, glial cell line-derived neurotrophic factor (GDNF), into the target regions or placement of fetal striatal co-grafts in the nigrostriatal pathway might elicit neuritic outgrowth to the caudate nucleus. Four adult St. Kitts green monkeys received embryonic VM grafts into the rostral mesencephalon near the host substantia nigra, and injections of AAV2/GDNF or EIAV/GDNF into the caudate. Three adult monkeys were co-grafted with fetal VM tissue near the substantia nigra and fetal striatal grafts (STR) 2.5 mm rostral in the nigrostriatal pathway. Before sacrifice, the striatal target regions were injected with the retrograde tracer fluorogold (FG). FG label was found in tyrosine hydroxylase-labeled neurons in VM grafts in the SN of only those monkeys that received AAV2/GDNF vector injections into the ipsilateral striatum. All monkeys showed FG labeling in the host substantia nigra when FG labeling was injected on the same side. These data show that grafted dopaminergic neurons can extend neurites to a distant target releasing an elevated concentration of GDNF, and suggest that grafted neurons can be placed into appropriate loci for potential tract reconstruction.
Keywords: dopaminergic neurons, neurite extension, parkinsonian non-human primate, FG
Introduction
Reconstruction of the pathway that connects the dopamine-producing neurons of the substantia nigra with their targets in the striatum has been a goal for anatomic and functional repair for Parkinson’s disease. This goal has been elusive, and almost all of the functionally successful experiments in rodents, primates, and humans have placed tissue in various locations in the striatal target regions. In part, this was due to pessimism that the adult brain was no longer capable of eliciting and directing axonal outgrowth over such distances, and the optimism that local presynaptic feedback controlled dopamine release in the target regions would be effective in the absence of regulatory inputs in the substantia nigra. In fact, significant functional effects have been shown with these placements (Bjorklund and Dunnett, 2007; Redmond, 2002), and some patients have shown significant benefits and cell survival over many years (Kordower et al., 2008; Li et al., 2008; Mendez et al., 2008). In the neonatal rat, grafts placed into the SN were shown to have functional effects and projections to the striatum at postnatal day 3 and 10, but not by day 20 (Bentlage et al., 1999), confirming the suspicion that placements in the SN would not be effective in adult animals or patients.
The only successful efforts in older animals have required the placement of double VM grafts (in the SN and the striatum) or "bridging grafts, " both of which have functional benefits and demonstrate retrograde transport of FG to label grafts in the SN. Mendez and his group (Mendez et al., 2000; Mendez and Hong, 1997; Mendez et al., 1996) suggested that the double VM grafts were producing unknown growth factors and that the presence of the VM grafts in the SN added to the functional benefits, a procedure which has also been taken into the clinic (Mendez et al., 2002). Brecknell et al. showed that long oblique "bridge" grafts of fibroblast growth factor-4-transfected RN-22 schwannoma cells stretching from the site of the neuronal grafts to the striatum led to successful labeling of VM graft cells with FG, an increase in neuronal graft survival, and improved functional effects in 6OHDA treated rodents (Brecknell et al., 1996a; Brecknell et al., 1996b).
Our group has used a number of methods to augment graft survival and to attempt to elicit axonal outgrowth in MPTP-parkinsonian monkeys. In our initial study showing successful placement and survival of VM grafts in the vicinity of the SN, we did not see convincing anatomical evidence of rostral projections toward the striatum, but we did not use any special retrograde tracing techniques (Collier et al., 2002). In several studies, we used fetal lateral ganglionic eminence (anlage of the striatum) selected to be more developed than the source for VM tissue in various co-grafting paradigms. The timing of the two sources (i.e E 44–45 for SN and E 50–55 for striatum) was influenced by the later development of the striatum as the target for dopaminergic neurites from the SN. If taken from the same donor the striatal anlagen likely would be too primitive for optimal graft survival. We recently demonstrated the potential for VM grafts placed in the SN to extend neurites into the diencephalon and striatum to reach more distant grafts of embryonic striatal tissue in adult monkeys. The latter were utilized as “helper or bridge grafts” in an attempt to create attractant steps to guide the growth of neurites from grafted dopaminergic neurons. Axons appeared to be attracted to the striatal co-grafts and extended 5–7 mm, almost the full distance to the caudal portion of the caudate in the St. Kitts green monkey (Sladek et al., 2008), but these extensions were not confirmed with any retrograde tracing method.
The glial derived neurotrophic factor, GDNF, is essential for the survival and outgrowth of developing midbrain dopamine neurons (Lin et al., 1993). Consistent with this role, GDNF is highly expressed in the fetal striatum (Stromberg et al., 1993), with lower levels in the mature brain (Barroso-Chinea et al., 2005; Choi-Lundberg and Bohn, 1995). It was soon shown that GDNF injections into the nigrostriatal tract of 6-hydroxydopamine lesioned rats improved functional recovery and increased dopamine release, but without specific retrograde labeling of nigral grafts (Tang et al., 1998). We have also found and confirmed that GDNF has an effect to elicit directional neuritic outgrowth of fetal dopamine neurons, grafted into the striatum, and was enhanced when GNDF overexpression was induced at an adjacent site in the monkey striatum by injection of an AAV2 vector harboring the GDNF gene (Elsworth et al., 2008). Based upon these results, it seemed possible that a similar effect could occur over the relatively longer distances between the SN and the striatum, as an increasing concentration gradient of GDNF resulted from release from cells in the target regions into interstitial fluid.
The present experiment was designed to determine whether these concentrations of GDNF, released from the striatum, were effective in attracting the dopaminergic axons of the grafted VM cells placed in the SN, as determined by retrograde transport of FG injected into the mid to rostral portion of the caudate nucleus.
Methods
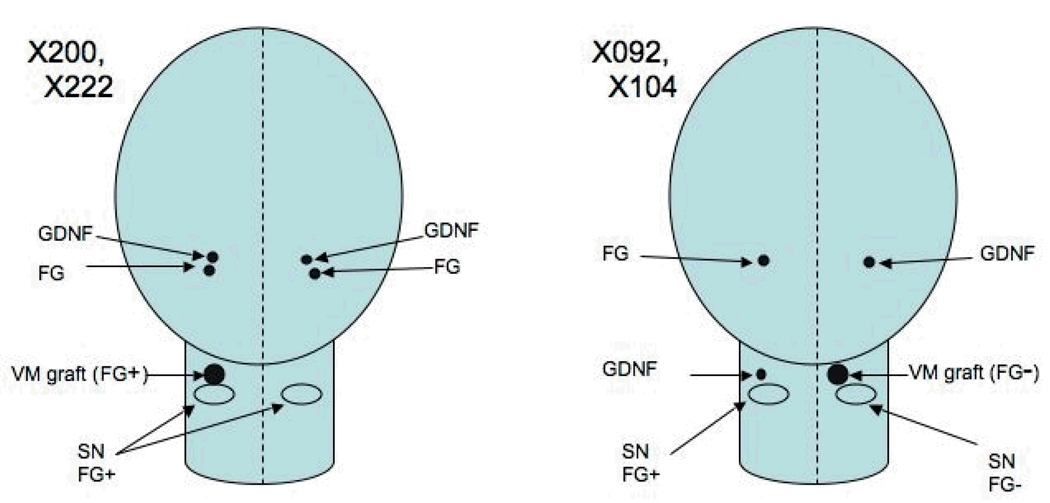
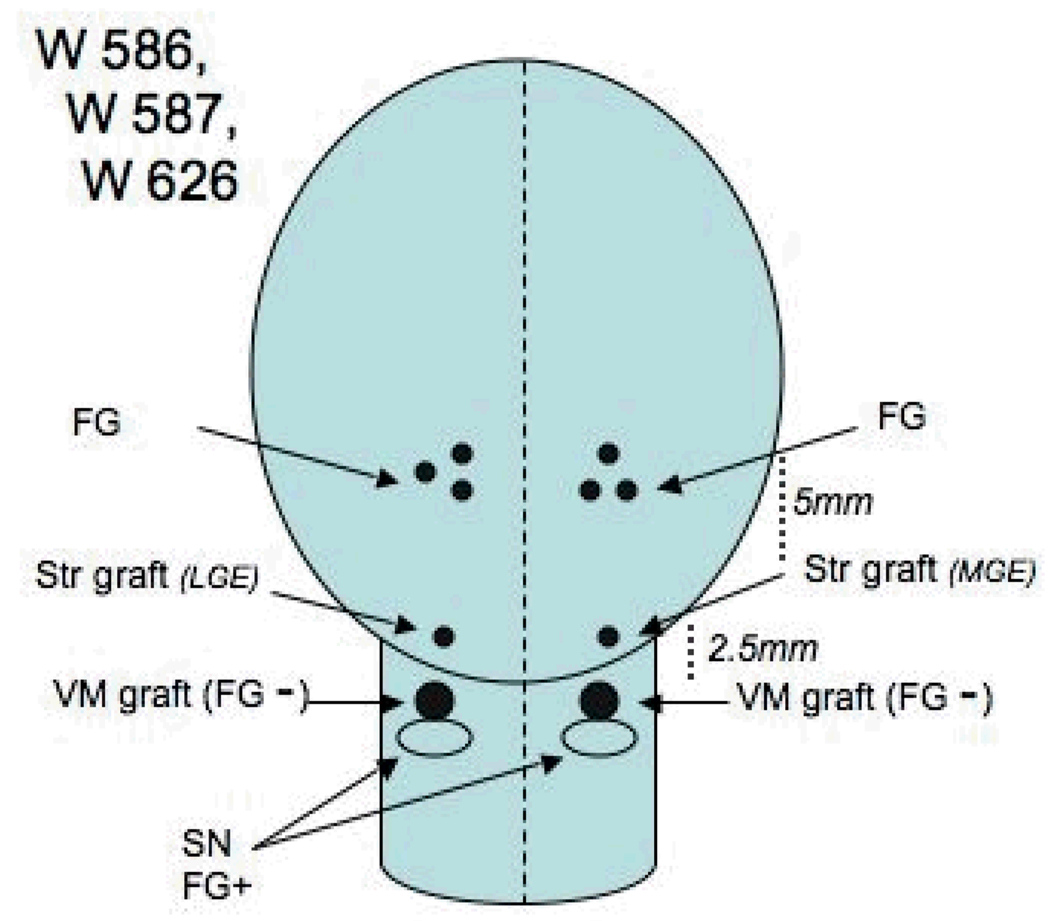
Experimental subjects consisted of seven adult male St. Kitts/African green monkeys (Chlorocebus sabaeus) from the colony at the St. Kitts Biomedical Research Foundation, St. Kitts, West Indies. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. To produce a dopamine-depleted host environment, all of the monkeys were treated with five 0.4 mg/kg intramuscular doses of MPTP over a five day period, as described in detail (Redmond et al., 2007). Tissue for grafting into the SN was obtained and dissected freshly from fetuses of embryonic age 44 days, and for striatal co-grafting from embryonic age 52–56 (Sladek, 1995) based upon ultrasonography and direct measurements after hysterotomy vs prior morphometric tables. Four monkeys (Group1: X222, X200, X092, and X104) received unilateral small solid grafts of ventral mesencephalic tissue (VM) into the rostral mesencephalon immediately dorsal to or within the host substantia nigra. Kopf Instruments stereotaxic devices (Tujunga, CA, USA) were used to target coordinates AP 11.1, Lateral 3.5, and Vertical 12.1 mm from actual ear bar zero), and vector delivered GDNF was injected in the ipsilateral striatum via a 22 gauge needle attached to a Hamilton Syringe (Reno, NV, USA) driven by a microprofusion pump (Stoelting Instruments, Wood Dale IL, USA) at a rate of 1 µl/minute (Figure1). Two of these animals also received GDNF in the contralateral striatum (X222, X200). Prior to sacrifice FG was injected into the identical striatal targets on the ipsilateral and contralateral sides to the SN grafts in these two animals, but only into the contralateral side in the other two animals to serve as controls (X092, X104) for potential spread of the label. Three additional animals (Group2: W586, W587, W626) received striatal and nigral co-grafts at increasing distances apart. The nigral grafts were positioned similarly to those in the first group whereas the striatal grafts were located more rostral along the trajectory of the ascending pathway to the striatum from the SN (each 2.5 mm more rostral and 1 mm more dorsal). Two of these three animals received FG injections bilaterally into the striatum, the remaining animal received a single FG injection as a control for the contralateral nigral graft (Figure 2).
Figure 1.
The positions of graft, vector and retrograde tracer injection are illustrated schematically for Group 1 animals. The cerebrum is shown as an oval while the rostral brain stem is represented by a tube. In this and the following diagram the presence or absence of FluoroGold (FG) label is designated by plus or minus signs at the graft site. Rostral is to the top of the diagram in this dorsal view.
Figure 2.
Group 2 animals are shown schematically as in figure 1. The relative distances between the co-grafts and the FG injections are indicated. MGE medial ganglionic eminence, LGE lateral ganglionic eminence.
All of the monkeys were without any observable signs of parkinsonism at the time of the grafting experiments and remained asymptomatic throughout the experiment, but based upon prior data of a large number of monkeys treated with identical MPTP doses, had dopamine depletions in the striatum of at least 50% of normals. Group 1 was sacrificed at approximately 6 months after graft implantation while brains for Group 2 were removed after 22 months.
The GDNF gene was delivered using two different vectors – modified adeno-associated virus, serotype 2/, with mouse phosphoglycerate kinase 1 promoter/GDNF (rAAV2/PGK-GDNF) vector, constructed as previously described (Rabinowitz et al., 2002), or the same PGK/GDNF cassette delivered by a modified lentiviral vector derived from equine infectious anemia virus (rEIAV/PGK/GDNF) (Deglon et al., 2000). The AAV2 vector was confirmed to express GDNF in vivo in this species for at least two years, whereas the expression of GDNF from the EIAV vector was significantly attenuated after 3 months. Injections were made bilaterally in the mid to rostral portion of the caudate (stereotaxic coordinate, AP 23.1, Lateral 4.0 mm, vertical 19.0 from ear bar zero), at a rate of 1 µl/minute) at the same surgery when fetal VM tissue was implanted. X200 and X222 were injected bilaterally into the caudate with 5 µl rAAV2/GDNF, and X092 and X104 were injected with 10 µl EIAV/GDNF into the right caudate (ipsilateral to the VM graft in the SN) and near the SN on the left side (see Figures 1–2). The titers of the AAV2 batch (10e11 vg/ml) and EIAV batch (3x10e8 transducing units/ml) were roughly comparable assuming one transducing unit per 300 physical particles for AAV2.
For Group1, FG was injected 7 to 20 days before sacrifice, 10 µl of a 2% solution, infused at a rate of 0.5 µl/minute standard targets for the caudate and putamen.. The striatal/VM co-graft monkeys in Group2 were injected with 5 µl of 2% FG into anterior and middle caudate, and 10 µl into posterior caudate and posterior putamen. Stereotaxic target coordinates for FG injections were for caudate, AP 23.0, 21.0, and 19.0, Lateral 4.0, Vertical 19.0, and for putamen, AP 21.1, Lateral 10.0, Vertical, 19.0.
Animals were killed by pentobarbital overdose and perfused with ice-cold physiological saline, followed by buffered 4% paraformaldehyde solution before brain removal, 12 hours post fixation in paraformaldehyde solution, and storage in 30% sucrose., Brains were then frozen and sectioned into 50 µm thick parasagittal sections using a sliding blade microtome and stored in a cryoprotectant solution. Sections were processed immunohistochemically using double fluorescence labeling for tyrosine hydroxylase (TH) and fluorogold (FG). Sections were incubated with TH antibody (1:1000, Millipore Cat.#MAB318, mouse monoclonal against TH purified from PC12 cells) and FG antibody (1:5000, Millipore Cat.#AB153, rabbit polyclonal against fluorogold). Visualization for FG-labeled cells was with Cy2-conjugated Goat Anti-Rabbit IgG (Jackson Immunoresearch Cat.#111-225-144) and for TH-labelled cells was with Texas Red Anti-Mouse IgG (Vector Laboratories Cat.#TI2000). TH staining was consistent with the classic morphology and distribution of dopamine cells in primate tissue and Cy2 fluorescence was absent in tissue that had not been exposed to FG labeling.
Cell counts were performed using an unbiased optical fractionator sterological method whereby digital images were obtained in selected areas using an Olympus AX-70 microscope equipped with an automated z-stage and MicroSuiteBiological software. Visualization of TH- or FG-labeled cells was done immunohistochemically with diaminobenzidine-nickel sulfate as the chromagen.
Results
Nigral grafts (VM) in all animals appeared similar to those reported in our prior studies. They were distinct and easily identified, even when in apposition with the host SN. These grafts were characterized by a general elongate shape with a longer vertical than horizontal axis (Figure 3). Two animals in Group 1 had grafts in apposition to the rostral pole of the SN, while the other two were located within the rostral one-third of the host SN. VM grafts for all animals in Group 2 were located immediately dorsal to the rostral pole of the host SN. The grafts contained variable numbers of TH positive multipolar neurons with shapes that were characteristic of phenotypes found in the host SN. Neuritic extensions ramified throughout the grafts and were seen to extend into the surrounding neuropil of the ventral mesencephalon.
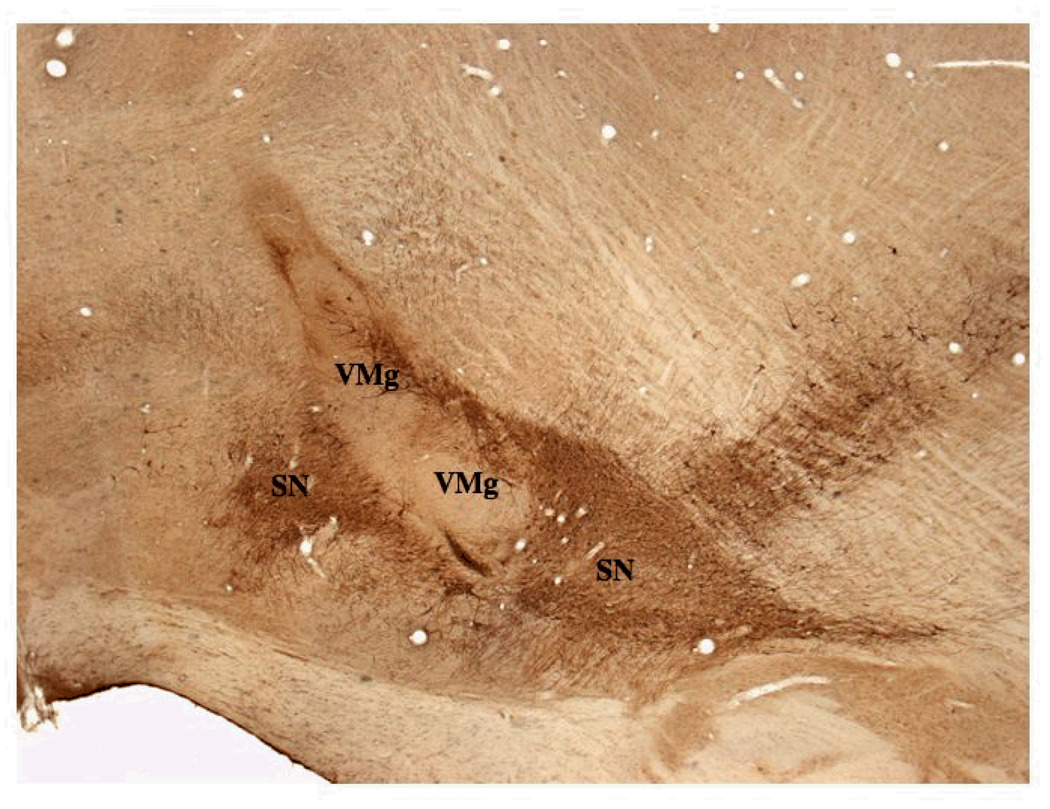
Figure 3.
A. This low power parasagittal view of the rostral brain stem shows the relative position of the VM graft in the SN (VMg) and the host substantia nigra (SN) in a group 1 animal (X200). Rostral is to the left and the injection site for FG is to the left out of the field of view. The VM graft is elongated in the dorso-ventral axis and contains many TH positive neurons which are shown in Figures 4 – 6 at higher power.
FG label was seen in many TH positive neurons in the host SN and in the VM grafts. The variable sized granules were localized to the perinuclear cytoplasm and also extended into proximal portions of neuronal processes (Figures 4, 5 & 6). Retrograde labeled neurons were seen in abundance in the SN on the side of the brain that received intrastriatal injections of FG in all four graft plus vector animals in Group 1 although small numbers of labeled neurons were observed in the contralateral substantia nigra in two animals that did not receive bilateral injections. The host SN of monkey X222 (left side) had 2,233 TH/FG+ labeled of 50,641 total TH+ cells (4.4%), and the graft had 13 TH/FG+ cells labeled of 663 total TH+ cells (2.0%). The second monkey X200 (left side) had 460 TH/FG+ of 42,231 TH+ in the host SN, with 25 TH/FG+ of 2,194 TH/FG+ cells (1.1%) in the VM graft. The label was distinct and confirmed by comparative analysis of host SN neurons that also showed substantial numbers of dual-labeled TH positive neurons on the side that received FG injections. The two animals (i.e. X092, X104) that received FG in the contralateral striatum to the nigral grafts were devoid of FG label in the SN grafts (0 TH/FG+ of 1164 TH+ cells in the graft in S092, right side, and X104, right side, had 0 TH/FG+ of 2765 TH+ in the VM grafts. Moreover, in these control animals FG label was seen only in host dopaminergic neurons on the same side of the brain that received the injection (see Table 1).
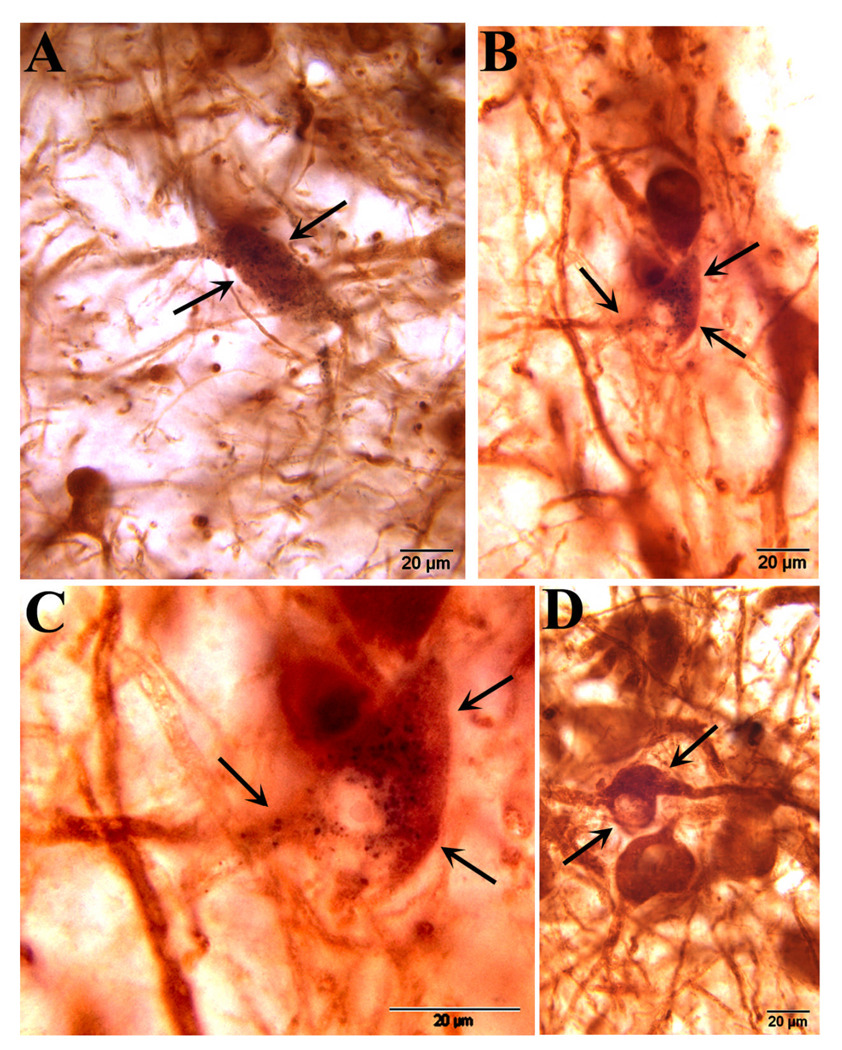
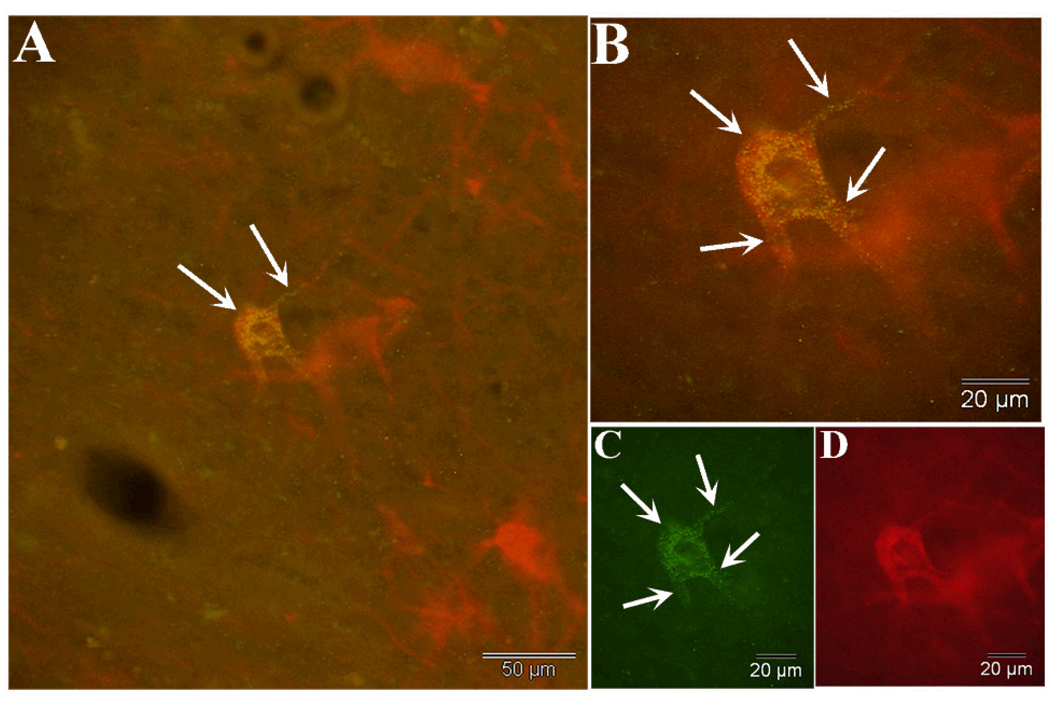
Figure 4.
A and B show two examples of dual-labeled neurons in the host substantia nigra that are characterized by the presence of variably sized FG granules in the perinuclear cytolasm and proximal shafts of neurites (arrows) in X200, also representative of X222. C and D show dopaminergic neurons in the VM graft; a dual labeled neuron similar to that seen in the host SN is shown in C, while unlabeled neurons in the same graft are shown in D.
Figure 5.
Dual immunofluorescence staining for tyrosine hydroxylase reveals a heavily labeled dopaminergic neuron within a VM graft in animal X200. In A, a low power view shows multiple neurons that are stained for tyrosine hydroxylase, and one that also contains fluorogold granules located within the perinuclear cytoplasm and extending into proximal neurites as seen in greater detail in B (arrows) and C which is fluorogold immunofluorescence alone. Fluorescence of tyrosine hydroxylase alone is shown in D.
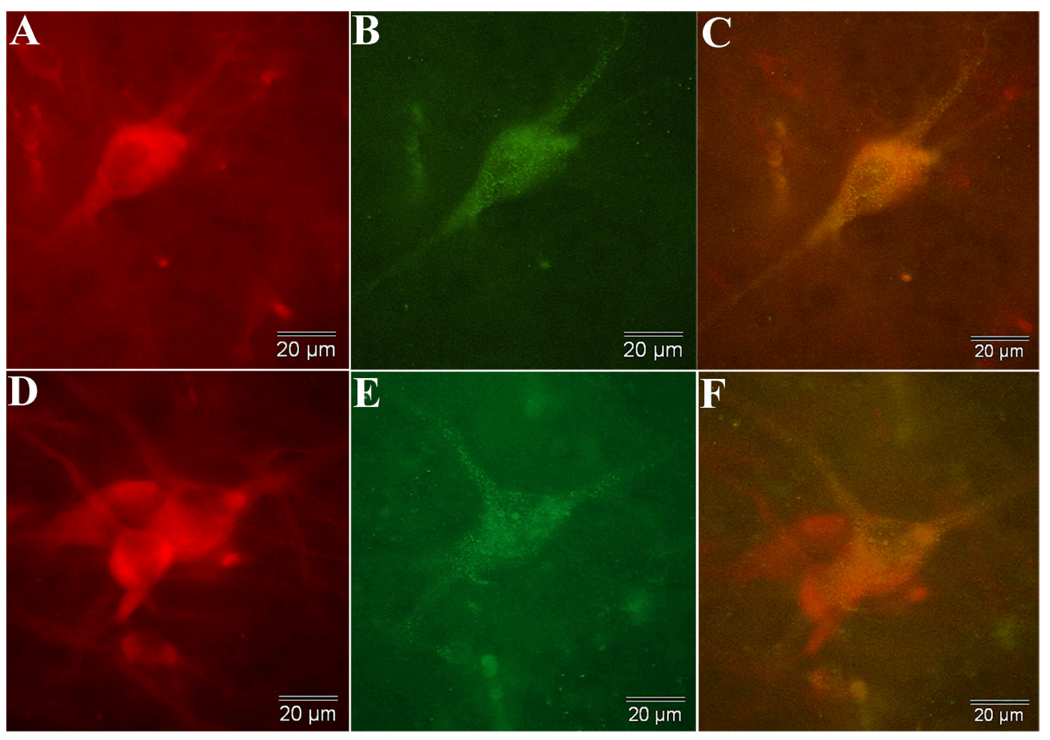
Figure 6.
Immunofluorescence of tyrosine hydroxylase (A,D, in red) and fluorogold (B,E, in green) is shown for two neurons within VM grafts in animal X200. Both neurons exhibit shapes that are characteristic of nigral neurons. The combined views are seen in C and F and clearly demonstrate retrograde label in grafted dopaminergic neurons. The two TH positive neurons in D that do not contain FG granules demonstrate that the retrograde label was specific to only some TH positive neurons in the grafts and SN and was not seen ubiquitously throughout the brain.
Table 1.
Th/FG labeled Cells in SN and Grafts
| Animal | Treatment | TH Cells-SN | TH/FG Cells-SN | TH Cells-VMg | TH/FG Cells-VMg |
|---|---|---|---|---|---|
| X092R | STR-EIAV-GDNF | 35,753 | 85 (0.2%) | 1164 | 0 |
| X092L | STR-EIAV-GDNF | 35,240 | 136 (0.4%) | no transplant | no transplant |
| X104R | STR-EIAV-GDNF | 26,870 | 10 (0.04%) | 2765 | 0 |
| X104L | STR-EIAV-GDNF | 17,443 | 16 (0.09%) | no transplant | no transplant |
| X200R | STR-AAV-GDNF | 18,890 | 40 (0.2%) | no transplant | no transplant |
| X200L | STR-AAV-GDNF | 42,231 | 460 (1.1%) | 2194 | 25 (1.1%) |
| X222R | STR-AAV-GDNF | 18,442 | 946 (5.1%) | no transplant | no transplant |
| X222L | STR-AAV-GDNF | 50,641 | 2233 (4.4%) | 663 | 13 (2.0%) |
| W586R | STR co-graft | NC | some - NC | 1550 | 0 |
| W586L | STR co-graft | NC | some - NC | 1679 | 0 |
| W587R | STR co-graft | NC | some - NC | 428 | 0 |
| W587L | STR co-graft | NC | some - NC | 1060 | 0 |
| W626R | STR co-graft | NC | some - NC | 1468 | 0 |
| W626L | STR co-graft | NC | some - NC | 740 | 0 |
"NC"= not counted
The vector and FG injection sites were preserved incompletely during frozen section microtomy and tended to break apart when the tissue was exposed to subsequent fixation due to some necrosis of small to moderate size that was observed at the striatal injection site. Consequently some tissue was absent from the host striatum, primarily about a cubic millimeter emanating from the center of the FG injection site. Several, TH negative neurons also were seen to sequester the FG label, primarily in the mesencephalon rostral and dorsal to the VM grafts, within the noradrenergic locus coeruleus, and also within the more caudal diencephalon. Small, presumably medium spiny neurons of the striatum also were labeled with FG.
Due to the fixation/necrosis or toxicity induced by the FG injections in the striatum, we did not visualize the GDNF. Data reported elsewhere with this same AAV2/GDNF vector has shown that identical injections of the striatum in the same locations will label 35,606 ± 4,387 cells and cover an average volume of 53.393 µm3 ± 11.1 (at 6 months) and will continue to show GNDF expression for up to 24 months (Leichtle et al., 2009) The EIAV/GDNF vector showed a similar number of cells labeled, but a smaller area of expression which lasted only up to three months.
In the Group 2 monkeys that received striatal co-grafts (W586, W587, and W626) at a distance of 2.5 mm from the VM graft in the nigra and that had 4 injections of FG distributed in the striatum, no label was seen in the VM grafts in the SN. The SN grafts were well developed and positioned properly. They had numerous TH positive neurons throughout their rostro-caudal extent. The TH neurons were found both at the periphery as well as the center of the grafts (Figure 7). There were numerous FG labelled neurons in the host striatum, but the striatal co-grafts were devoid of FG even though FG-labeled host neurons were in the immediate vicinity of the striatal grafts. There were some TH/FG labeled neurons in the host SN itself.
Figure 7.
In one of the Group 2 (co-grafted monkeys), A shows the position of a VM graft relative to the host substantia nigra (SN) in a parasagittal plane . Rostral is to the left. The striatal co-graft is located lateral to this histological section. The boxed areas are seen to advantage in B and C. B. The graft contains a dense collection of TH positive neurons and an extensive array of neurites (arrows). In C, many of these neurites (arrows) are seen to exit the graft from its rostral surface, closest to the FG injections which labeled the host substantia nigra, but none of the grafts. OC optic chiasm.
Discussion
The finding of FG label in 1 – 2% of grafted dopaminergic neurons in a VM graft in the substantia nigra is evidence of efferent fibers that have reached the striatal injection site. This result was obtained after only 6 months and exclusively in the monkeys that received ipsilateral injections of a AAV2 vector that produces overexpression of GDNF in the target region. The result is consistent with our prior data which produced definite directional outgrowth in VM cells over much shorter distances within the striatum. The FG label was found consistently in cells of the host substantia nigra itself and other locations expected based upon the described anatomy of the nigrostriatal system (Jimenez-Castellanos and Graybiel, 1987; 1989; Langer et al., 1991). The number of cells in the VM grafts which were FG labeled is similar to the number of host SN TH+ neurons that were also labeled, indicating the area from which FG could be transported retrogradely to normally developed nigrostriatal projections. The diffusion of GDNF from overexpression from the vector injection sites seems likely to have covered a larger area than the FG injections, which we were unable to confirm in this study. More extensive labeling with FG and detailed measurements of GDNF concentrations will be needed to evaluate the full extent of reinnervation by the VM grafts as well as the mechanism (diffusion vs. retrograde transport) by which GDNF has an effect on cells placed dorsal to the SN. Further studies are needed to determine whether any other characteristic of the two recombinant vector systems might have contributed to the result beyond their differences in quantity and duration of GDNF expression.
Injection of vector on the opposite side from the graft did not elicit outgrowth based upon the absence of FG labeling. In addition, three co-grafted monkeys which showed evidence of outgrowth from a VM graft in the SN to a striatal graft placed 2.5 mm in the direction of the striatum did not show FG labeling or other evidence that neuritic extensions reached the striatum in spite of evidence that FG labeled the endogenous cells of the striatum and the SN, as expected. Although there is direct anatomical evidence of extension of neurites to the striatal graft at 2.5 mm (and similar extension to striatal grafts placed at 5 mm and 7 mm), this attractant effect was not successful for attracting outgrowth beyond the striatal grafts into the striatum.
It is somewhat unexpected that striatal grafts, which intuitively might be expected to contain or release the necessary factors for building the nigrostriatal projections and circuitry, do not apparently have the ability to direct outgrowth beyond their own location. It is possible though that the obvious reinnervation in the area of the striatal co-grafts stops at the co-grafts themselves. It seems very likely that, if the FG injections had been at the level of the co-grafts (at 2.5 mm distance), the VM grafts would very likely have been labeled. The striatal placed VM grafts reported by Mendez et al, which appear to elicit outgrowth from VM grafts in the SN, may also release GDNF as well as other growth factors. The double grafting strategy, however, by hedging the bet, may be less physiological than one which depends upon full reinnervation of the striatum from the region of the SN, a situation in which the grafted DA neurons may potentially be more normally regulated. Placing double grafts into both the striatum and the SN may produce earlier dopamine delivery and functional effects, but at the risk of disturbances to the normal function of those regions which may lead to dyskinesia (Ma et al., 2002).
The effect we report in this study adds to the evidence that grafted VM cells in the adult mesencephalon have the capacity to reinnervate their normal target cells, if a GNDF signal is supplied. It remains unknown whether synaptic connections would be more appropriate (Leranth et al., 1998), whether afferent regulation might take place within the SN, and whether such a reconstruction might have functional effects that were the same or superior to ectopic graft placements within the striatum. The study does illustrate a reconstruction plan that involves the use of specific genes to elicit effects which might recapitulate normal development and achieve a wider distribution throughout the target areas.
Used alone, the over-expression of GDNF has been shown to have strong dopaminergic-neuroprotective effects in rodents (Choi-Lundberg et al., 1997; Dowd et al., 2005) and primates (Gash et al., 1996; Kordower et al., 2000; Kordower et al., 2006), and a Phase II clinical study for Parkinson's disease was recently carried out using a closely related growth factor, neurturin (Marks et al., 2008), but was discontinued due to lack of efficacy. In addition to mechanisms previously described, it is possible that GDNF overexpression in those animal models and patients acts by also inducing sprouting and re-growth of neurites from damaged, but surviving dopamine neurons. If the potential benefits of growth factor monotherapy are due to neuroprotective effects on surviving dopamine neurons and axons and the prevention of disease progression, then the combination of GDNF delivery with cell replacements (in the SN) might significantly extend the benefits to include patients with more severe disease and greater damage to the dopamine system. Future studies in primates need to replicate the finding reported here and also show greater functional benefits than ectopic graft placements into the striatum or GDNF delivery alone. In the absence of toxicity or side effects (Hovland et al., 2007; Tatarewicz et al., 2007), such a combination treatment might be studied in a clinical Phase I safety trial reasonably soon.
More basic future studies of brain repair will no doubt benefit from greater knowledge of the genetic programs for brain development, additional growth factors, better tools for gene delivery, and a wider array of cells derived and programmed from embryonic and adult sources. Such cells will be programmed (or re-programmed) to the precise level to migrate, reconnect, and synapse appropriately to repair the broken circuits in neurodegenerative disease.
Acknowledgements
We thank staff at St. Kitts Biomedical Research Foundation for their invaluable contributions to this study, especially Clive Wilson, Ernell Nisbett, O'Neal Whattley, Wellington Sutton, Steve D. Whittaker, Shervin Liddie, and Junior Swanston. We thank Dr. Eleni Markakis for help and advice on the use of fluorogold. Supported by NINDS Grants PO1-NS044281, UO1-NS046028, Axion Research Foundation, and the Michael J. Fox Foundation for Parkinson's Research.
Literature Cited
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, Gonzalez-Hernandez T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. The European journal of neuroscience. 2005;21(7):1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- Bentlage C, Nikkhah G, Cunningham MG, Bjorklund A. Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substantia nigra is critically dependent on the age of the host. Experimental neurology. 1999;159(1):177–190. doi: 10.1006/exnr.1999.7110. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Fifty years of dopamine research. Trends in neurosciences. 2007;30(5):185–187. doi: 10.1016/j.tins.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Brecknell JE, Du JS, Muir E, Fidler PS, Hlavin ML, Dunnett SB, Fawcett JW. Bridge grafts of fibroblast growth factor-4-secreting schwannoma cells promote functional axonal regeneration in the nigrostriatal pathway of the adult rat. Neuroscience. 1996a;74(3):755–784. doi: 10.1016/0306-4522(96)00167-4. [DOI] [PubMed] [Google Scholar]
- Brecknell JE, Haque NS, Du JS, Muir EM, Fidler PS, Hlavin ML, Fawcett JW, Dunnett SB. Functional and anatomical reconstruction of the 6-hydroxydopamine lesioned nigrostriatal system of the adult rat. Neuroscience. 1996b;71(4):913–925. doi: 10.1016/0306-4522(95)00509-9. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85(1):80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science (New York, NY. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Sortwell CE, Elsworth JD, Taylor JR, Roth RH, Sladek JR, Jr, Redmond DE., Jr Embryonic ventral mesencephalic grafts to the substantia nigra of MPTP-treated monkeys: feasibility relevant to multiple-target grafting as a therapy for Parkinson's disease. The Journal of comparative neurology. 2002;442(4):320–330. doi: 10.1002/cne.10108. [DOI] [PubMed] [Google Scholar]
- Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L, Zufferey R, Trono D, Aebischer P. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Human gene therapy. 2000;11(1):179–190. doi: 10.1089/10430340050016256. [DOI] [PubMed] [Google Scholar]
- Dowd E, Monville C, Torres EM, Wong LF, Azzouz M, Mazarakis ND, Dunnett SB. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. The European journal of neuroscience. 2005;22(10):2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Redmond DE, Jr, Leranth C, Bjugstad KB, Sladek JR, Jr, Collier TJ, Foti SB, Samulski RJ, Vives KP, Roth RH. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Experimental neurology. 2008;211(1):252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Hovland DN, Jr, Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, Emery MG, Ernst NB, Reed RP, Zeller JR, Gash DM, Masterman DM, Potter BM, Cosenza ME, Lightfoot RM. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF) in rhesus monkeys. Toxicologic pathology. 2007;35(5):676–692. doi: 10.1080/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987;23(1):223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castellanos J, Graybiel AM. Evidence that histochemically distinct zones of the primate substantia nigra pars compacta are related to patterned distributions of nigrostriatal projection neurons and striatonigral fibers. Experimental brain research Experimentelle Hirnforschung. 1989;74(2):227–238. doi: 10.1007/BF00248855. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature medicine. 2008;14(5):504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science (New York, NY. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Annals of neurology. 2006;60(6):706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Langer LF, Jimenez-Castellanos J, Graybiel AM. The substantia nigra and its relations with the striatum in the monkey. Progress in brain research. 1991;87:81–99. doi: 10.1016/s0079-6123(08)63048-4. [DOI] [PubMed] [Google Scholar]
- Leichtle SW, Zufferey R, Bober J, Leranth C, Markakis EA, Samulski RJ, Zhou X, Aebischer P, Vives KP, Redmond DE., Jr AAV2 is superior to EIAV vector for long-term expression of human GDNF in the African green monkey nigrostriatal system. Human gene therapy. 2009 [Google Scholar]
- Leranth C, Sladek JR, Jr, Roth RH, Redmond DE., Jr Efferent synaptic connections of dopaminergic neurons grafted into the caudate nucleus of experimentally induced parkinsonian monkeys are different from those of control animals. Experimental brain research Experimentelle Hirnforschung. 1998;123(3):323–333. doi: 10.1007/s002210050575. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature medicine. 2008;14(5):501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science (New York, NY. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Annals of neurology. 2002;52(5):628–634. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet neurology. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Mendez I, Baker KA, Hong M. Simultaneous intrastriatal and intranigral grafting (double grafts) in the rat model of Parkinson's disease. Brain Res Brain Res Rev. 2000;32(1):328–339. doi: 10.1016/s0165-0173(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Mendez I, Dagher A, Hong M, Gaudet P, Weerasinghe S, McAlister V, King D, Desrosiers J, Darvesh S, Acorn T, Robertson H. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. Journal of neurosurgery. 2000;96(3):589–596. doi: 10.3171/jns.2002.96.3.0589. [DOI] [PubMed] [Google Scholar]
- Mendez I, Hong M. Reconstruction of the striato-nigro-striatal circuitry by simultaneous double dopaminergic grafts: a tracer study using fluorogold and horseradish peroxidase. Brain research. 1997;778(1):194–205. doi: 10.1016/s0006-8993(97)01055-x. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sadi D, Hong M. Reconstruction of the nigrostriatal pathway by simultaneous intrastriatal and intranigral dopaminergic transplants. J Neurosci. 1996;16(22):7216–7227. doi: 10.1523/JNEUROSCI.16-22-07216.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nature medicine. 2008;14(5):507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. Journal of virology. 2002;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond DE., Jr Cellular replacement therapy for Parkinson's disease -- where are we today? Neuroscientist. 2002;8(5):457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR, Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek JR, Jr, Bjugstad KB, Collier TJ, Bundock EA, Blanchard BC, Elsworth JD, Roth RH, Redmond DE., Jr Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in nonhuman primate. Cell transplantation. 2008;17(4):427–444. [PubMed] [Google Scholar]
- Sladek JR, Jr, Elsworth JD, Taylor JR, Roth RH, Redmond DE., Jr . In: Techniques for neural transplantation in non-human primates. C Ricordi RGL, editor. Austin: R.G. Landes; 1995. pp. 391–408. [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Experimental neurology. 1993;124(2):401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Tang FI, Tien LT, Zhou FC, Hoffer BJ, Wang Y. Intranigral ventral mesencephalic grafts and nigrostriatal injections of glial cell line-derived neurotrophic factor restore dopamine release in the striatum of 6-hydroxydopamine-lesioned rats. Experimental brain research Experimentelle Hirnforschung. 1998;119(3):287–296. doi: 10.1007/s002210050344. [DOI] [PubMed] [Google Scholar]
- Tatarewicz SM, Wei X, Gupta S, Masterman D, Swanson SJ, Moxness MS. Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson's disease receiving r-metHuGDNF via continuous intraputaminal infusion. Journal of clinical immunology. 2007;27(6):620–627. doi: 10.1007/s10875-007-9117-8. [DOI] [PubMed] [Google Scholar]