Abstract
Individuals with hemiparetic stroke often exhibit an abnormal coupling between the frontal plane of the hip and saggital plane of the knee during gait. The purpose of this study was to determine if stretch sensitive reflexes, which are known to be altered following stroke, exhibit similar coupling between the muscles of the hip and knee in the post-stroke population. Eighteen subjects were recruited for this study including ten with hemiparesis resulting from stroke and eight unimpaired, age-matched controls. A servomotor was used to apply ramp and hold perturbations to both the hip and knee joints in separate sessions and electromyographic activity was recorded in eight muscles of the lower limb. Hip abduction perturbations elicited abnormal activation in rectus femoris (RF) in seven of ten stroke subjects with amplitudes ranging from 3.2 to 12.5% of the maximum voluntary contraction (MVC). Only two of eight control subjects exhibited any activity in RF and these responses were only 2.1 and 2.7% of MVC. To determine if the responses in the stroke group were a result of muscle stretch, a musculoskeletal model was used to simulate the experimental abduction perturbations and estimate muscle length changes. The simulation revealed that RF should be shortened by the perturbations and this suggests that the response was not likely due to direct stretch. Moreover, knee flexion perturbations elicited responses in the hip adductors (AL) with a mean amplitude of 5.1 ± 3.8% of MVC across all stroke subjects while no significant responses were recorded in controls. The presence of a reciprocal, reflex-mediated coupling between RF and AL following stroke suggests that changes in the excitability of spinal networks may contribute to the development of abnormal inter-joint coordination patterns observed during hemiparetic gait.
Introduction
One of the common features of hemiparetic gait involves an impaired dynamic coupling between the frontal plane of the hip and the saggital plane of the knee. This coupling is evidenced by excessive hip circumduction and a reduction in knee flexion during swing (Kerrigan et al. 1991, 2000). Biomechanical simulations investigating potential muscular contributions to this impaired knee flexion have revealed that it may be attributed to abnormal activation of the quadriceps (Piazza and Delp 1996; Riley and Kerrigan 1998; Goldberg et al. 2004). Indeed, analysis of muscle activity during hemiparetic gait has revealed that abnormal activation of the quadriceps prior to and during the swing phase appears to be consistent across subjects (Yelnik et al. 1999; Den Otter et al. 2006). The goal of this study was to determine if there is evidence for a spinal contribution to this abnormal pattern of muscle activity following stroke.
Abnormal regulation of afferent feedback has classically been hypothesized to be a significant contributor to abnormal motor behavior in individuals with stroke. These abnormalities are characterized by a lower velocity threshold for eliciting a stretch reflex (Powers et al. 1989) and heightened reflex responses in comparison to healthy subjects (Thilmann and Fellows 1991). Possible mechanisms mediating this increase in sensitivity include a loss of presynaptic inhibition of Ia afferents (Faist et al. 1994) or facilitation of group I and group II activated, lumbar interneuronal pathways (Marque et al. 2001b). Furthermore, a reduced contribution of afferent feedback to locomotor activity in the soleus has been demonstrated following stroke (Mazzaro et al. 2007). The correlation between this depression and the level of spasticity suggests that abnormal processing of afferent input may indeed contribute to functional impairments. Although a number of studies have characterized the changes in monosynaptic reflexes within a single joint, there is a lack of research addressing the role of multi-joint reflexes following stroke and how these reflexes are differentially modulated with respect to control subjects.
Heteronymous reflexes, which occur when afferent activity from a muscle evokes an excitation or inhibition of a different muscle, serve as a direct, neural coupling between muscles and as such, these reflexes have the potential to contribute to multi-joint coordination. In the human lower limb, stimulation of group-I and group-II afferents from intrinsic foot muscles has been shown to produce widespread reflex excitation throughout the lower limb (Marque et al. 2001a, 2005). Furthermore, activity in hip muscle spindle afferents during gait appears to be critical in the initiation of both the stance to swing transition (Grillner and Rossignol 1978; Hiebert et al. 1996) and the swing to stance transition (McVea et al. 2005). What remains to be seen is how potential changes in these afferent-mediated, inter-joint connections contribute to leg muscle coordination following stroke.
Given the evidence for impaired single joint reflexes in the lower limb of individuals with stroke and the suggested role for heteronymous reflexes during unimpaired locomotion, we sought to investigate how stroke influences heteronymous reflex coordination in the human lower limb. Specifically, we quantified the reflex coupling between muscles acting in the frontal plane of the hip and saggital plane of the knee due to the functional significance of these muscles in post-stroke gait. We hypothesized that following stroke, activity in the quadriceps muscles would be facilitated by mechanical stimulation of hip adductor afferents. This hypothesis was evaluated using hip abduction perturbations to compare reflex responses between stroke survivors and unimpaired subjects. In addition, both patellar tendon taps and matched knee flexion perturbations were used to investigate if a mechanical stimulus to the quadriceps would elicit reflex activity in the hip adductors. Our results revealed a bi-directional reflex coupling between the hip adductors and knee extensors and may provide insight to the neural mechanisms responsible for the abnormal patterns of coordination seen during hemi-paretic gait.
Materials and methods
Subjects
Eighteen subjects were recruited for this study including ten with unilateral stroke (Tables 1, 2) and eight unimpaired, age-matched control subjects (age 54 ± 9 years, range 36–65). Subjects from the stroke population were recruited if they had a unilateral stroke, presented right side hemiparesis, and had no cognitive deficits that would prevent them from performing the experimental protocol. Subjects in both the control and stroke groups had no prior history of injury or surgery to their hip or knee. All experimental procedures were approved by the Institutional Review Board of Northwestern University and complied with the principles of the Declaration of Helsinki. Informed consent was obtained prior to testing.
Table 1.
Characteristics of stroke subjects
| Subject | Age (years) |
Gender | Height (in.) |
Weight (lbs) |
Months post stroke |
|---|---|---|---|---|---|
| S1 | 58 | F | 67 | 192 | 56 |
| S2 | 67 | M | 70 | 179 | 139 |
| S3 | 70 | M | 73 | 190 | 228 |
| S4 | 57 | F | 64 | 122 | 32 |
| S5 | 58 | F | 64 | 170 | 49 |
| S6 | 57 | M | 65 | 200 | 168 |
| S7 | 53 | F | 67 | 140 | 240 |
| S8 | 51 | M | 71 | 205 | 32 |
| S9 | 60 | M | 66 | 165 | 120 |
| S10 | 38 | M | 67 | 166 | 79 |
Table 2.
Modified Ashworth scores for stroke subjects
| Subject | Hip extensors |
Hip flexors |
Hip adductors |
Knee extensors |
Knee flexors |
Ankle plantarflexors |
Ankle dorsiflexors |
|---|---|---|---|---|---|---|---|
| S1 | 0 | 0 | 1 | 2 | 2 | 3 | 0 |
| S2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| S3 | 0 | 0 | 0 | 0 | 1+ | 1+ | 0 |
| S4 | 1 | 0 | 0 | 0 | 1+ | 1+ | 0 |
| S5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S6 | 0 | 0 | 2 | 2 | 2 | 1+ | 0 |
| S7 | 0 | 0 | 1 | 2 | 2 | 3 | 0 |
| S8 | 1 | 0 | 0 | 1+ | 1+ | 2 | 0 |
| S9 | 0 | 0 | 0 | 0 | 1+ | 2 | 0 |
| S10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Equipment
A computer-controlled servomotor (Kollmorgen Goldline B-808-A) was used to apply angular stretches to the hip joint. The motor was controlled using a Kollmorgen SR55200 velocity controller, a Galil Motion Control DMC-1710 position controller, and a customized graphical user interface (GUI) which served as the interface between the motor’s control system and the data acquisition system. The subject’s leg was rigidly attached to the motor shaft using a custom fiberglass cast and coupling ring attached to the ankle as shown in Fig. 1a. Given the constraints of the experimental setup, the motor’s axis of rotation was centered at the knee. However, since the perturbation amplitude was small and no restraints were in contact with the knee, the resulting movements occurred primarily in hip abduction and adduction. To quantify the actual amplitude of abduction at the knee, the relative angle of the thigh and shank was measured before and after a 5° perturbation for two subjects. This angle was computed by digitizing the location of markers placed on the iliac crest, lateral femoral epicondyle, and ankle coupling ring using a portable digitizer (MicroScribe G2). These points were then used to create vectors representing the relative orientation of the thigh and shank. The resulting knee abduction amplitude was found to be 0.87° ± 0.54° which is significantly less than the minimum angle used to elicit reflexes during valgus perturbations of the knee (Dhaher et al. 2003).
Fig. 1.
a Experimental setup for applying hip abduction perturbations. Subjects were seated in a Biodex chair with their ankle secured to the motor via a rigid beam. Trunk movement was minimized by securing the torso using two straps which crossed the chest and were secured to the chair. b Illustration of the target matching paradigm used to assist participants in maintaining the desired level of muscle activity
Pre-amplified, surface electrodes (Delsys Bagnoli DE-2.1) were used to record EMG activity in rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), semitendinosus (ST), semimembranosus (SM), medial gastrocnemius (MG), lateral gastrocnemius (LG), and the hip adductors (AL). The electrodes were arranged in a parallel bar configuration with an inter-electrode distance of 1 cm and each electrode had a length and width of 1 cm and 1 mm, respectively. Each active electrode had a preamplifier gain of 10 V/V and a pass band ranging from 20–450 Hz. Each electrode, not including the hip adductors, was located using the surface electromyography for the non-invasive assessment of muscles (SENIAM) standards (Hermens et al. 1999). For the hip adductors, the electrode was placed four fingerbreadths distal to the pubic tubercle while the subject adducted the thigh (Delagi et al. 1981). This location was selected to primarily target adductor longus, however, it is possible that activity from gracilis was measured as well. Correct placement of the surface electrodes was tested by asking the subject to perform moderate contractions to isolate each muscle and verifying that no crosstalk was recorded from electrodes on adjacent muscles. This was quantified by calculating the average rectified EMG amplitude in adjacent muscles over a 50 ms time window and verifying that it was not statistically different than the activity in the respective muscles during a trial where the subject was relaxed.
The EMG signals were additionally amplified using a gain of 100 V/V and both the EMG and resolver signals were low-pass filtered at 227 Hz using 8th-order Bessel anti-aliasing filters (Avens 16 channel 4218) and were notch filtered at 60 Hz. The signals then were sampled at 1,000 Hz using a National Instruments NI PCI-6033E data acquisition board. The data were acquired using the Matlab Data Acquisition Toolbox (The MathWorks, Inc., Natick, MA, USA) and subsequent analyses were performed in Matlab.
Protocol
Maximum voluntary contractions (MVCs) were recorded at the beginning of each experiment and these values were used to normalize EMG activity across subjects. MVCs for the knee and ankle joints were performed against an applied resistance from the experimenter and MVCs in hip adduction were recorded against an applied torque from the motor while it was set in position servo mode. The MVC for each muscle was computed by taking the maximum average rectified EMG amplitude calculated over a 50 ms moving window for each trial. MVC torque in hip adduction was also estimated by multiplying the tangential force at the ankle by each subject’s leg length which was estimated based on height using anthropomorphic data (Winter 2004). The mean MVC values for each subject group are listed in Table 3.
Table 3.
EMGs recorded during MVC trials and maximal moment recorded during hip adduction
| Group | RF (mV) | VL (mV) | VM (mV) | ST (mV) | BF (mV) | MG (mV) | LG (mV) | AL (mV) | Adduction (Nm) |
|---|---|---|---|---|---|---|---|---|---|
| Control (SD) | 1.24 (0.57) | 1.23 (0.64) | 1.11 (0.52) | 1.06 (0.47) | 0.84 (0.50) | 1.11 (0.58) | 0.87 (0.38) | 0.70 (0.19) | 77.6 (15.8) |
| Stroke (SD) | 0.45 (0.28) | 0.68 (0.40) | 0.65 (0.40) | 0.57 (0.73) | 0.29 (0.26) | 0.33 (0.41) | 0.50 (0.51) | 0.23 (0.11) | 53.7 (30.4) |
| P value | 0.002 | 0.040 | 0.048 | 0.12 | 0.008 | 0.004 | 0.10 | <0.001 | 0.062 |
Ramp and hold perturbations were used to perform controlled, repeatable stretch of the hip adductors and knee extensors to investigate the presence of heteronymous reflexes in the lower limb. Each experimental trial consisted of a sequence of 20 ramp and hold perturbations. For the hip abduction trials, the velocity and amplitude were set to 80°/s and 5°, respectively; these perturbation parameters were sufficient to elicit consistent reflex responses in the stretched muscles of both populations. Within each trial, perturbation direction was randomly alternated between abduction and adduction to minimize the probability that subjects would systematically activate their muscles in preparation for the oncoming perturbation. Six randomly selected stroke subjects were asked to return for a second visit to participate in the knee flexion protocol. For these trials, perturbation direction was randomly varied between flexion and extension. Perturbation parameters of 130°/s and 5° were chosen to provide the same stretch velocity to the knee extensors as was provided to the hip adductors in the hip perturbation trials (method described in “Biomechanical modeling”). For both sets of trials, the interval between perturbations was varied randomly from 1 to 3 s while the duration of hold was randomized between 200 ms and 1 s. Uniform distributions were used for all randomizations.
Reflexes were elicited at fixed levels of background EMG and quantified relative to the EMG measured at MVC. Subjects were assisted in maintaining the desired level of contraction through real-time visual feedback of muscle activity using the target matching paradigm shown in Fig. 1b. The horizontal position of the ball represented the level of normalized AL activity while the vertical axis represented the normalized activity in RF. The displayed levels of activity were computed using a 100 ms moving average of the rectified EMG to provide a smooth visualization of the current muscle activity. At the beginning of each target matching trial, the subject was instructed to activate their AL or RF to position the ball into a box on the screen and to keep the ball within the box throughout the trial. For the hip abduction and knee flexion experiments, subjects maintained a contraction of AL and RF, respectively, equal to 5% of MVC. To assess the potential confounding effect of volitional coupling on the reflexes observed in the stroke group, a subset of control subjects repeated the hip abduction experiments using co-contraction levels of AL and RF matched to the average levels measured in the stroke group.
Each subject’s reaction time was measured to ensure that all estimates of reflex activity occurred prior to the onset of volitional or triggered reactions to the imposed perturbations. During a single, randomized trial of 20 hip abduction perturbations with a velocity of 80°/s and amplitude of 5°, subjects were instructed to perform a co-contraction of the thigh muscles as quickly as possible each time the motor moved from the starting position and the resulting EMG’s were recorded. The reaction time for each subject was taken to be the minimum latency of EMG onset following each perturbation. Since the responses were simple and independent of the direction of motor movement, they were likely to be pre-programmed, triggered reactions (Crago et al. 1976).
It is possible that the knee flexion perturbations could cause unintended length changes in AL due to vibrations transmitted through the measurement apparatus to the tissues connecting the knee to the hip (Burke et al. 1983). To address this issue, a series of patellar tendon taps was applied to each subject, providing a direct isolated stretch of the quadriceps muscles. During these control experiments, subjects were instructed to stay relaxed while a series of 20 taps were applied using an instrumented tendon tap hammer (Dhaher et al. 2003) and the corresponding EMG responses were recorded. Tap force was manually adjusted for each subject to produce consistent reflex responses in the quadriceps.
Data analysis
The position signal from the resolver was two-way low pass filtered using a digital second order Butterworth filter with a 20 Hz cutoff frequency and the resulting signal was used to calculate the velocity profile. To compute the average reflex response, the EMGs recorded during each perturbation were aligned about the point where the velocity reached 30°/s. These aligned EMGs were then rectified and averaged. To control for changes in volitional activity during the course of a trial, the average EMG amplitude was calculated over a 500 ms window starting 200 ms prior to perturbation onset for each response. Responses whose amplitude was more than 3 standard deviations above the mean amplitude for the remaining responses were removed. In all cases, a minimum of 15 responses were then averaged to estimate the average reflex elicited in each muscle.
Reflex onset latency and amplitude were computed from the averaged data and these measures were used to characterize both the homonymous and heteronymous reflexes. Baseline activity was computed by averaging the rectified EMG during the 100 ms prior to movement onset. Reflex onset was defined as the time following movement onset when the EMG exceeded the average baseline activity by three standard deviations for 5 ms. Reflex latency was then measured as the difference between the movement and reflex onsets. Reflex amplitude was quantified as the mean amplitude of the rectified EMG during the 50 ms following the onset of the response. To minimize the possibility of analyzing higher level triggered responses, reflex amplitude was only quantified if the response onset occurred more than 50 ms before the minimum reaction time measured across all subjects.
Biomechanical modeling
Due to the complex architecture of the musculoskeletal system, it is possible that the applied abduction perturbations could stretch muscles other than the adductors. To account for this possibility, an inverse kinematic simulation using a generic three-dimensional musculoskeletal model was developed to estimate the changes in muscle length resulting from the experimental perturbations. Data from these simulations were used to determine if measured EMG responses could simply result from muscle stretch and not intermuscular, heteronymous connections. The model was constructed using the AnyBody modeling system (Any-Body Technology, Aalborg, Denmark) and the parameters, including muscle lines of actions and bone surfaces were obtained from previous studies (Dostal and Andrews 1981; Delp 1990; Herzog and Read 1993). The model included 26 muscles of the lower limb as well as the bones of the torso, pelvis, thigh, shank, and foot. Perturbations were simulated by positioning the model in a seated configuration and moving the hip joint from the starting position to the final abducted position as measured during the experiments. During the simulation, the hip flexion/extension and internal/external rotation degrees-of-freedom were fixed. This was assumed to best mimic the experiment since the fixation of the ankle did not allow for internal/external rotation and the seating configuration minimized the likelihood of hip flexion or extension. An inverse kinematic analysis was then performed to calculate the resulting change in length for each muscle from which EMGs were measured experimentally.
To account for anatomical differences between the participants in this study and the generic biomechanical model, a sensitivity analysis was performed on the muscle insertion coordinates. The x, y, and z coordinates of each muscle’s insertion and via points were independently and randomly varied between ±10% of the respective muscle’s length using a uniform distribution. Simulations were performed 1,000 times, each using a different set of randomly selected coordinates. The resulting changes in muscle length for each simulation were computed and used to generate 95% confidence intervals for the actual change in length for each muscle of interest.
The musculoskeletal model also was used to match the velocity of the RF muscle to that of the AL during the imposed knee and hip perturbations, respectively. The parameters for the knee flexion perturbations were determined by solving Eq. 1 for perturbation velocity. In this equation, represents the velocity of muscle stretch, accounts for the muscle moment arm about the primary axis of rotation, and represents the angular velocity of the perturbation. The moment arm of the RF was estimated from the model by calculating the slope of the muscle length versus joint angle plot for a 10° knee flexion. The muscle stretch velocity was estimated initially for the AL from the simulation of the hip abduction protocol. This parameter, as well as the duration of stretch, was matched for the knee flexion protocol.
| (1) |
Results
Responses to hip abduction perturbations
The hip abduction perturbations produced consistent reflex responses in the stretched AL for both the stroke and control groups. Figure 2 shows representative EMG responses in individual stroke and control subjects. Both subjects exhibited excitatory responses following muscle stretch, as would be expected from the monosynaptic stretch reflex. As a group, the reflexes elicited in the control subjects had a mean latency and amplitude of 47 ± 6 ms and 8.3 ± 2.5%MVC, whereas those elicited in the stroke subjects had a latency of 42 ± 8 ms and amplitude of 41.3 ± 9.8% MVC. The reflex amplitude was significantly greater in the stroke subjects than in the controls (P = 0.012) while the converse was true for the latency (P = 0.017). To address the possibility that the reduced latency in the stroke subjects was due to a lower reflex threshold, reflexes were also elicited in the stroke group without pre-activation of the AL. These responses occured at a latency equal to that of the control subjects (47 ± 5 ms) suggesting that the observed differences between the groups was likely due to differences in stretch reflex threshold during active contractions.
Fig. 2.
Perturbation profile and corresponding reflex response in the adductor for subjects S5 and C2 from the stroke and control groups, respectively. The perturbation had an amplitude of 5° and a peak velocity of 80°/s. Negative displacement reflects hip abduction. Both subjects exhibit clear reflex responses, but the response in the S5 is significantly larger than the response in C2. EMG traces are the average of 20 trials
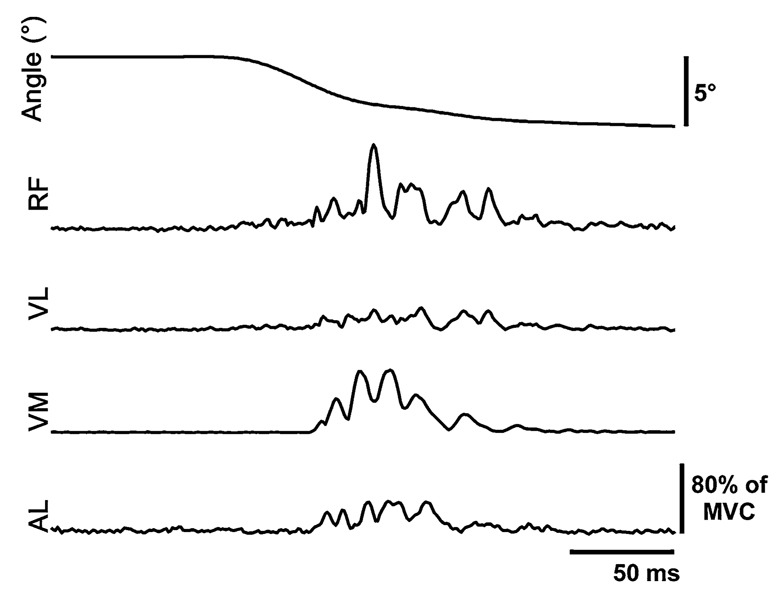
In the stroke subjects, hip abduction perturbations also elicited reflex responses in muscles other than the AL, which were not targeted by the perturbation. Figure 3a shows average EMG responses in eight muscles of representative stroke and control subjects. Some responses, such as those in BF and MG, occurred at a latency near the minimum reaction time of 116 ms and therefore were not considered in the subsequent analyses. The most consistent response in the stroke group other than in the AL was in the RF. Of the ten subjects tested, seven exhibited RF responses, three had responses in ST, and no responses were recorded in other muscles. The amplitude of the RF responses ranged from 3.2 to 12.5% of MVC with a mean latency of 44 ± 7 ms which was not significantly different than that in the AL (P = 0.083).
Fig. 3.
a Normalized EMG responses to a 5° hip abduction at 80°/s in stroke subject S7. The muscles represented are: adductor longus (AL), rectus femoris (RF), vastus lateralis (VL), vastus medialis (VM), semitendinosus (ST), biceps femoris (BF), medial gastrocnemius (MG), and lateral gastrocnemius (LG). The left and right dashed vertical lines indicate perturbation onset and onset of the volitional reaction. The solid vertical bars represent 100% of MVC in AL and 20% of MVC in all other muscles. The perturbation not only elicited a homonymous stretch reflex in AL, but significant responses were also present in RF and ST before the time when the subject could volitionally react to the motor. Responses in other muscles such as BF and MG occur near the volitional reaction latency and were not included in the analyses. b Normalized EMG responses to a 5° hip abduction at 80°/s in control subject C6. The left and right dashed vertical lines indicate perturbation onset and onset of the volitional reaction. The solid vertical bars represent 30% of MVC in AL and 20% of MVC in all other muscles. Not only is the stretch reflex in AL smaller in comparison to S2, but no responses occur in any of the remaining muscles after the perturbation
In contrast to the stroke subjects, individuals in the control group did not show consistent activity in any muscles other than the adductors. Figure 3b shows a representative response for a single control subject and no significant activity is present in any muscles other than AM prior to the subject’s reaction latency. As a group, only two of the eight control subjects exhibited any response in the RF and in each case, they were substantially smaller than the responses in the stroke group. In the subjects who did show this activity, the responses were only 2.1 and 2.7% of MVC. It should be noted that the discrepancy in reflex amplitude between the two groups was not a result of the normalization procedure. The mean absolute RF EMG amplitude for the control group was 11.7 ± 4.6 µV compared to 20.1 ± 2.5 µV for the stroke group (P = 0.025).
It is possible that the presence of a RF reflex response in the stroke subjects reflected a descending co-activation of the RF and AL motoneuron pools. Subjects in the stroke population did maintain a slight co-activation during target matching of the AL such that RF was activated at 2.9 ± 1.5% of MVC. In contrast, the background activity of the RF in the control group was significantly lower (1.4 ± 0.6% MVC; P = 0.017). To verify that the observed reflex coupling between AL and RF did not simply reflect differences in the voluntary activation of the RF by the stroke subjects, five control subjects were asked to co-activate the RF and AL to match the average levels seen in the stroke group. Even at matched levels of back-ground activation, none of these control subjects exhibited significant reflexes in the RF. Across all control subjects, the average RF EMG between 25 and 75 ms following perturbation onset was only 0.6 ± 0.3% MVC.
Estimated muscle length changes
The results of the experimental simulations using the musculoskeletal model showed that hip abduction perturbations were not likely to stretch the RF. Figure 4 shows the normalized change in muscle length for five muscles of the thigh during a biomechanical simulation of a 10° hip abduction. The adductor longus (AL) and gracilis (Gra), which both serve as adductors of the hip, as well as semitendinosus (ST) and biceps femoris (BF) were each lengthened by the abduction movement. In contrast, rectus femoris (RF) was shortened by the simulated hip abduction. This was confirmed by the sensitivity analysis of the muscle insertion coordinates which resulted in a 95% confidence interval of −2.46 to −2.37% change in muscle length.
Fig. 4.
Calculated change in length for five hip muscles in response to a simulated 10° hip abduction using the Anybody Modeling System. The muscles represented are: adductor longus (AL), gracilis (Gra), rectus femoris (RF), semitendinosus (ST), and biceps femoris (BF). Muscle length change is expressed as a percentage of the initial muscle length with positive values indicating stretch. The model results indicate that the RF was shortened by the perturbation while each of the other muscles was stretched. The error bars represent the 95% confidence intervals for length change obtained from the sensitivity analysis of muscle insertion coordinates
Responses to knee flexion and extension perturbations
Responses to knee flexion perturbations in six stroke subjects revealed that the reflex coupling between AL and RF following stroke was reciprocal. These perturbations produced excitatory responses not only in the quadriceps (RF, VL, and VM), but also in AL. Representative data from a single stroke subject, S2, is shown in Fig. 5. The quadriceps stretch reflexes had latencies of 29 ± 8, 33 ± 7, and 37 ± 8 ms for the RF, VL, and VM, respectively while the AL responses, which were present in five of the six subjects, had a mean latency of 47 ± 15 ms. This latency was significantly greater than the RF latency (P = 0.002) suggesting that the response did not result from intermuscular crosstalk, but it was not greater than the homonymous reflex latency in AL following hip abduction perturbations (P = 0.215). In contrast to the knee flexion perturbations, knee extension did not produce excitatory responses in any of the quadriceps.
Fig. 5.
EMG responses to a 5° knee flexion perturbation at 130°/s for subject S2. Negative deflection of the position trace represents knee flexion. Muscle abbreviations are defined in the legend of Fig. 1. The solid vertical bar adjacent to the AL trace represents 20% of MVC for all muscles. Stretch of the knee extensors elicited stretch reflexes in all of the quadriceps as well as a response in AL
Responses to patellar tendon tap
The tendon taps elicited excitatory responses in the quadriceps of all subjects, but concurrent responses in AL were only observed in the stroke population. Typical responses in individual stroke and control subjects are shown in Fig. 6. The stretch reflex in RF had a mean latency of 23 ± 2 and 24 ± 4 ms for the stroke and control groups, respectively, while the AL response in the stroke group had a mean latency of 27 ± 8 ms. Subjects in the stroke group had significantly larger responses in each of the quadriceps muscles as compared to controls (all P<0.05), as well as significantly larger responses in the AL (P<0.05). These differences did not result from differences in the tap force applied to the tendon, as a post hoc analysis indicated that the mean tap force used for the stroke subjects (13.4 ± 3.9 N) was significantly lower than that used for the controls (20.1 ± 6.6 N; P<0.001).
Fig. 6.
Representative example of a patellar tendon tap response in stroke subject S2 (dashed line) and control subject C3 (solid line). Muscle abbreviations are defined in the legend on Fig. 1. The dashed vertical line represents the onset of the tendon tap and the solid vertical line represents 20% of MVC for all muscles. The tendon tap elicited stretch reflexes in RF, VL, and VM for both subjects, but in addition to these responses, subject S2 shows a clear response in AL which was not stretched by the tendon tap
Discussion
Single-joint angular perturbations were applied to the hip and knee to investigate the existence of abnormal multi-joint reflexes in the lower limb of individuals with stroke. Our data revealed that abduction stretches elicited an excitatory response in the hip adductors with a latency consistent with a monosynaptic stretch reflex (Ertekin et al. 2006). In addition, these hip abduction perturbations elicited reflexes in the RF, a knee extensor and hip flexor. This response was reciprocal, such that a stretch of the RF also caused reflex excitation of the AL. These findings are consistent with an increased facilitation of heteronymous reflexes following neurological insult (Marque et al. 2001b; Crone et al. 2003) and may provide insight into the mechanisms contributing to the abnormal dynamic coupling between the hip and knee joints in hemiparetic gait.
The EMG measure used to compare muscle activity across subjects may be influenced by anatomical factors or the normalization procedure used on the raw EMG. For a given level of muscle activity, comparison of absolute EMG between subjects may be confounded by variations in subject-specific parameters such as adipose thickness (Kuiken et al. 2003), surface impedance (Perreault et al. 1993), and the relative location between the electrode and the motor point (Dimitrova and Dimitrov 2003). Hence, it is beneficial to adopt a normalization procedure to compare measurements on an equivalent scale. However, when using MVC to normalize EMG, hemiparetic subjects may have a substantially reduced MVC amplitude in comparison to able-bodied controls. Indeed, the EMG recorded in the current study during maximum voluntary hip adduction was significantly reduced in our hemiparetic subjects, yet there was no significant difference in the resulting adduction torque during MVC. This differs from previous studies of hemiparetic subjects which have demonstrated a reduction in knee extension torque of up to 80% of control values (Mulroy et al. 2003). The discrepancy between EMG and torque-based measures of MVC reflect the lack of a direct mapping from EMG to functional output. Ultimately, studies quantifying the mechanical contribution of the observed coupling are necessary to determine if these reflexes could contribute to impaired function.
The present investigation of abnormal reflex coupling was motivated by previous evidence of abnormal single-joint reflexes following stroke. These studies indicated that stretch reflexes in stroke survivors are characterized by a lower velocity threshold (Powers et al. 1989), lower amplitude threshold (Levin and Feldman 1994), and heightened responses in comparison to unimpaired controls (Thilmann and Fellows 1991). Although these studies helped to characterize the fundamental properties of spinal reflex pathways, they did not address the possible contribution of these pathways to impaired muscle coordination. Before quantifying this contribution during functional tasks, it was first necessary to demonstrate the existence of abnormal reflex coupling in a controlled setting while also characterizing the properties of this coupling. This process follows the trend of conventional stretch reflex studies which have analyzed simple tendon tap responses before progressing to increasingly complex, functional movements. While the results of this study demonstrate the existence of abnormal reflex coupling in individuals post-stroke, the origin and functional contribution of this impairment remain to be determined.
Potential mechanisms contributing to inter-muscular reflex coupling
Abnormal activation of rectus femoris is consistent with an increased facilitation of lower-limb extensors following neurological disorder. Following stroke, the inhibition in soleus typically produced by rapid plantarflexion perturbations is significantly attenuated during gait (Mazzaro et al. 2007) and may reflect a bias favoring extensors in the lower limb. Facilitation of the lower limb extensors also has been demonstrated in individuals with stroke following stimulation of various heteronymous afferents. For instance, stimulation of the gastrocnemius nerve at group-I intensity typically inhibits soleus motor neurons in able-bodied subjects, but this inhibition is replaced by facilitation in the paretic limb of stroke survivors (Delwaide and Oliver 1988). Likewise, stimulation of heteronymous afferents in the common peroneal nerve produces reciprocal excitation of the soleus in hemiparetic individuals in contrast to the inhibition produced in controls (Crone et al. 2003) and this stimulation also elicits an increased facilitation of the quadriceps (Marque et al. 2001b). In contrast to these previous studies we did not observe activity in any of the monoarticular muscles of the knee or in any of the ankle extensors. This suggests that the coupling observed in the current study is more selective than a general extensor bias. However, at this point, the precise mechanisms contributing to this reflex coupling remain unclear.
The presence of an excitatory response in the rectus femoris following hip abduction perturbations could potentially be explained by afferent activity stemming from a change in the muscle’s length. Given the complex architecture of the musculoskeletal system, it is conceivable that a hip abduction perturbation may lengthen the rectus femoris and produce a monosynaptic stretch reflex. However, our biomechanical simulations revealed that rectus femoris should actually be shortened as the hip adductors are stretched. While this result refutes the idea of a stretch-mediated response, it does not eliminate the possibility that the muscle was activated through other segmental pathways.
Excitation of muscles which are shortened by a perturbation has classically been attributed to shortening reactions or reciprocal excitation. Shortening reactions have been demonstrated following single joint perturbations in the upper and lower limb (Katz and Rondot 1978) and typically have a longer latency than the stretch reflex elicited in the antagonist (Burke et al. 1971; Miscio et al. 2001). The results of the current study differ from this description in two ways. First, there was no difference in latency between the stretch reflex in AL and the response in RF. Second, one also would expect RF to respond when directly shortened by knee extension. This was not the case in our study as knee extension perturbations did not elicit any excitatory response in RF. Alternatively, the RF response to hip abduction could be mediated by reciprocal excitation which is characterized by simultaneous responses in agonists and antagonists at tendon jerk latency (Myklebust et al. 1982). Since the latency of the RF reflex was the same as the stretch reflex in AL, the possibility of reciprocal excitation cannot be ruled out using the methods of the current study. It has been suggested that this phenomenon is mediated by group-Ib afferent activity from antagonist muscles (Laporte and Lloyd 1952), so future studies utilizing varying force levels in antagonists should then be able to address this as a possible mechanism.
Changes in descending motor commands following stroke also could contribute to altered reflex coordination. Following cerebrovascular accident such as stroke, individuals may rely on descending pathways such as the vestibulo-, tecto-, and reticulospinal tracts which have more diffuse connections than the cerebrospinal tract (Gracies 2005). Rearrangement of descending pathways also may include branching of intact corticospinal fibers to new motor neuron groups (Farmer et al. 1991) or branching of spinal interneurons onto motor neurons that are missing descending fibers (Weidner et al. 2001). Thus, reorganization of descending control pathways may contribute to the observed volitional coupling between AL and RF during the target matching task. This co-activation may have resulted in an increased excitability in the motor neurons pools of both muscles such that any afferent input would produce a response in both muscles. The lack of a response in RF in the control group following hip abduction perturbations could then be explained by a lack of co-activation which would leave the RF motoneuron pool in a sub-threshold state. However, since targeted co-activation of RF and AL in the control group did not produce a reflex response in both muscles; it does not appear that the response seen in the stroke group resulted from a descending co-activation bias.
Functional implications
Reflex activation is known to be task specific (Dietz et al. 1994; Zehr and Stein 1999; Dietz and Sinkjaer 2007) and our results must be interpreted in that context. Nevertheless, the observed patterns of reflex coupling may provide insight into the mechanisms constraining muscle activation patterns during volitional movements, including gait. Evidence for the link between reflex excitability under isometric conditions and its function during movement has been provided by a number of studies. In the feline model, the excitatory heteronymous coupling between the vastii and triceps surae, as well as the inhibitory coupling between the vastii and rectus femoris (Wilmink and Nichols 2003), reflect the in-phase movement of the ankle and knee and the independent movement of the hip during locomotion. In humans, H-reflex amplitude in the soleus is facilitated during hip extension and inhibited during hip flexion reflecting its level of activity in stance and swing phase, respectively (Knikou and Rymer 2002). Following stroke, withdrawal reflexes in the upper-limb have been shown to produce an abnormal spatial distribution of torque (Dewald et al. 1999) which was similar in nature to the constrained torque synergies seen during volitional arm movements (Dewald et al. 1995). In the hemiparetic lower limb, the facilitation of quadriceps motoneurons following stimulation of the common peroneal nerve (Marque et al. 2001b) and the facilitation of rectus femoris in the current study may reflect the abnormal activation of the quadriceps prior to and during the swing phase of gait (Yelnik et al. 1999; Den Otter et al. 2006).
The relationship between the function of the muscles involved in the observed reflex responses and the kinematic abnormalities seen during in hemiparetic gait provides further evidence that these reflex abnormalities may be related to functional impairments. The two functions most commonly ascribed to the biarticular rectus femoris are knee extension and hip flexion. Hence, co-activation of rectus femoris and the hip adductors should produce moments in adduction and flexion at the thigh and possibly an overall stiffening of the knee joint. While a knee extension moment would be consistent with previous findings regarding reduced knee flexion in hemiparetic gait (Kerrigan et al. 1991; De Quervain et al. 1996), adduction of the hip might seem to be in opposition to the clinical definition of circumduction. Circumduction, which is defined as an increased coronal thigh angle during swing (Kerrigan et al. 2000), could be explained by an increase in the amplitude of hip abduction. However, it has been shown that the excessive coronal thigh angle is primarily due to hip hiking and the hip angle is actually more adducted in circumducting individuals as compared to controls (Kerrigan et al. 2000). This reduced hip abduction is consistent with the adduction moment created by AL, so it is reasonable that activation of both of the AL and RF may contribute to the impaired hip and knee function seen during hemiparetic gait.
Alternatively, the increased reflex activation of the hip adductors may reflect a beneficial compensatory strategy used by the nervous system to counter the enhanced activity in the knee extensors. Mulroy et al. (2003) recently demonstrated that a subgroup of hemiparetic subjects exhibited increased activation of adductor longus in addition to the commonly reported enhanced rectus femoris activity. This increase in activity in the adductors was accompanied by greater knee flexion during swing, suggesting that the adductor longus may have generated a hip flexion moment that facilitated knee flexion through inertial coupling of the thigh and shank. Moreover, if the hip adductors are activated as a group, contraction of gracilis would further counter the enhanced activity in the knee extensors due to its moment arm in knee flexion (Wretenberg et al. 1996). Studies investigating the relationship between the strength of the reflex coupling reported in the present study and the kinematics of hemiparetic gait will help to distinguish between these alternative functional roles of the enhanced reflex coupling between the rectus femoris and adductor longus.
Acknowledgments
This work was supported by a National Science Foundation Graduate Research Fellowship and the National Institutes of Health.
Contributor Information
James M. Finley, Email: jamesfinley2008@u.northwestern.edu, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60201, USA; Sensory Motor Performance Program, Rehabilitation Institute of Chicago, 345 E Superior St, Chicago, IL 60611, USA.
Eric J. Perreault, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60201, USA Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611, USA; Sensory Motor Performance Program, Rehabilitation Institute of Chicago, 345 E Superior St, Chicago, IL 60611, USA.
Yasin Y. Dhaher, Email: y-dhaher@northwestern.edu, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60201, USA; Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL 60611, USA; Sensory Motor Performance Program, Rehabilitation Institute of Chicago, 345 E Superior St, Chicago, IL 60611, USA.
References
- Burke D, Andrews CJ, Gillies JD. The reflex response to sinusoidal stretching in spastic man. Brain. 1971;94:455–470. doi: 10.1093/brain/94.3.455. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976;39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78:1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Delagi EF, Perotto A, Iazzetti J, Morrison D. Anatomic guide for the electromyographer. Springfield: Charles C. Thomas; 1981. [Google Scholar]
- Delp SL. PhD Thesis. Stanford University; 1990. Surgery simulation: a computer-graphics system to analyze and design musculoskeletal reconstructions of the lower limb. [Google Scholar]
- Delwaide PJ, Oliver E. Short-latency autogenic inhibition (IB inhibition) in human spasticity. J Neurol Neurosurg Psychiatry. 1988;51:1546–1550. doi: 10.1136/jnnp.51.12.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Otter AR, Geurts AC, Mulder T, Duysens J. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture. 2006;25(3):342–352. doi: 10.1016/j.gaitpost.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF, Given JD, McGuire JR, Rymer WZ. Reorganization of flexion reflexes in the upper extremity of hemiparetic subjects. Muscle Nerve. 1999;22:1209–1221. doi: 10.1002/(sici)1097-4598(199909)22:9<1209::aid-mus7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Dhaher YY, Tsoumanis AD, Rymer WZ. Reflex muscle contractions can be elicited by valgus positional perturbations of the human knee. J Biomech. 2003;36:199–209. doi: 10.1016/s0021-9290(02)00334-2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007;6:725–733. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Dietz V, Discher M, Trippel M. Task-dependent modulation of short- and long-latency electromyographic responses in upper limb muscles. Electroencephalogr Clin Neurophysiol. 1994;93:49–56. doi: 10.1016/0168-5597(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol. 2003;13:13–36. doi: 10.1016/s1050-6411(02)00083-4. [DOI] [PubMed] [Google Scholar]
- Dostal WF, Andrews JG. A 3-dimensional biomechanical model of hip musculature. J Biomech. 1981;14:803–812. doi: 10.1016/0021-9290(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Bademkiran F, Tataroglu C, Aydogdu I, Karapinars N. Adductor T and H reflexes in humans. Muscle Nerve. 2006;34:640–645. doi: 10.1002/mus.20648. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain. 1994;117(Pt 6):1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–1510. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. J Biomech. 2004;37:1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Gracies JM. Pathophysiology of spastic paresis. II: emergence of muscle overactivity. Muscle Nerve. 2005;31:552–571. doi: 10.1002/mus.20285. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G. European recommendations for surface electromyography. Roessingh Research and Development, Enschede; 1999. [Google Scholar]
- Herzog W, Read LJ. Lines of action and moment arms of the major force-carrying structures crossing the human knee-joint. J Anat. 1993;182:213–230. [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Katz R, Rondot P. Muscle reaction to passive shortening in normal man. Electroencephalogr Clin Neurophysiol. 1978;45:90–99. doi: 10.1016/0013-4694(78)90345-0. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Gronley J, Perry J. Stiff-legged gait in spastic paresis. A study of quadriceps and hamstrings muscle activity. Am J Phys Med Rehabil. 1991;70:294–300. [PubMed] [Google Scholar]
- Kerrigan DC, Frates EP, Rogan S, Riley PO. Hip hiking and circumduction: quantitative definitions. Am J Phys Med Rehabil. 2000;79:247–252. doi: 10.1097/00002060-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer Z. Effects of changes in hip joint angle on H-reflex excitability in humans. Exp Brain Res. 2002;143:149–159. doi: 10.1007/s00221-001-0978-4. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Lowery MM, Stoykov NS. The effect of subcutaneous fat on myoelectric signal amplitude and crosstalk. Prosthet Orthot Int. 2003;27:48–54. doi: 10.3109/03093640309167976. [DOI] [PubMed] [Google Scholar]
- Laporte Y, Lloyd DP. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952;169:609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- Levin MF, Feldman AG. The role of stretch reflex threshold regulation in normal and impaired motor control. Brain Res. 1994;657:23–30. doi: 10.1016/0006-8993(94)90949-0. [DOI] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Marchand-Pauvert V, Gautier J, Simonetta-Moreau M, Pierrot-Deseilligny E. Group I projections from intrinsic foot muscles to motoneurones of leg and thigh muscles in humans. J Physiol. 2001a;536:313–327. doi: 10.1111/j.1469-7793.2001.t01-1-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Simonetta-Moreau M, Maupas E, Roques CF. Facilitation of transmission in heteronymous group II pathways in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry. 2001b;70:36–42. doi: 10.1136/jnnp.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Simonetta-Moreau M, Pierrot-Deseilligny E, Marchand-Pauvert V. Group II excitations from plantar foot muscles to human leg and thigh motoneurones. Exp Brain Res. 2005;161:486–501. doi: 10.1007/s00221-004-2096-6. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis. 2007;16:135–144. doi: 10.1016/j.jstrokecerebrovasdis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McVea DA, Donelan JM, Tachibana A, Pearson KG. A role for hip position in initiating the swing-to-stance transition in walking cats. J Neurophysiol. 2005;94:3497–3508. doi: 10.1152/jn.00511.2005. [DOI] [PubMed] [Google Scholar]
- Miscio G, Pisano F, Del Conte C, Pianca D, Colombo R, Schieppati M. The shortening reaction of forearm muscles: the influence of central set. Clin Neurophysiol. 2001;112:884–894. doi: 10.1016/s1388-2457(01)00468-0. [DOI] [PubMed] [Google Scholar]
- Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18:114–125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- Myklebust BM, Gottlieb GL, Penn RD, Agarwal GC. Reciprocal excitation of antagonistic muscles as a differentiating feature in spasticity. Ann Neurol. 1982;12:367–374. doi: 10.1002/ana.410120409. [DOI] [PubMed] [Google Scholar]
- Perreault EJ, Hunter IW, Kearney RE. Quantitative analysis of four EMG amplifiers. J Biomed Eng. 1993;15:413–419. doi: 10.1016/0141-5425(93)90079-e. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. J Biomech. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Powers RK, Campbell DL, Rymer WZ. Stretch reflex dynamics in spastic elbow flexor muscles. Ann Neurol. 1989;25:32–42. doi: 10.1002/ana.410250106. [DOI] [PubMed] [Google Scholar]
- Riley PO, Kerrigan DC. Torque action of two-joint muscles in the swing period of stiff-legged gait: a forward dynamic model analysis. J Biomech. 1998;31:835–840. doi: 10.1016/s0021-9290(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Fellows SJ. The time-course of bilateral changes in the reflex excitability of relaxed triceps surae muscle in human hemiparetic spasticity. J Neurol. 1991;238:293–298. doi: 10.1007/BF00319742. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci USA. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmink RJ, Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hind limb. J Neurophysiol. 2003;90:2310–2324. doi: 10.1152/jn.00833.2002. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. London: Wiley; 2004. [Google Scholar]
- Wretenberg P, Nemeth G, Lamontagne M, Lundin B. Passive knee muscle moment arms measured in vivo with MRI. Clin Biomech (Bristol, Avon) 1996;11:439–446. doi: 10.1016/s0268-0033(96)00030-7. [DOI] [PubMed] [Google Scholar]
- Yelnik A, Albert T, Bonan I, Laffont I. A clinical guide to assess the role of lower limb extensor overactivity in hemiplegic gait disorders. Stroke. 1999;30:580–585. doi: 10.1161/01.str.30.3.580. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]