Abstract
Previous studies demonstrate increased levels of 4-hydroxynonenal (HNE) and acrolein in vulnerable brain regions of subjects with mild cognitive impairment (MCI) and late-stage Alzheimer’s disease (AD). Recently preclinical AD (PCAD) subjects, who demonstrate normal antemortem neuropsychological test scores but abundant AD pathology at autopsy, have become the focus of increased study. Levels of extractable HNE and acrolein were quantified by gas chromatography mass spectrometry with negative chemical ionization and protein-bound HNE and acrolein was quantified by dot-blot immunohistochemistry in the hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of 10 PCAD and 10 age-matched normal control (NC) subjects. Results of the analyses show a significant (p < 0.05) increase in levels of extractable acrolein in HPG of PCAD subjects compared to age-matched NC subjects and a significant decrease of extractable acrolein in PCAD CER. A significant increase in protein-bound HNE in HPG and a significant decrease in CER of PCAD subjects compared to NC subjects was observed. No significant alterations were observed in either extractable or protein-bound HNE or acrolein in the SMTG of PCAD subjects. Additionally, no significant differences in levels of protein carbonyls were observed in the HPG, SMTG, or CER of PCAD subjects compared to NC subjects.
Keywords: Preclinical Alzheimer’s disease, Lipid peroxidation, Oxidative damage
Introduction
Considerable evidence supports a role for oxidative damage in the pathogenesis of AD. Multiple studies show increased oxidation of nucleic acids [1–4], decreased activity of base excision repair [5–7], increased oxidative protein modification [8–11], and increased lipid peroxidation products [9, 12–17], in late-stage Alzheimer’s disease (LAD) subjects compared to age-matched normal control (NC) subjects. Multiple studies also demonstrate alterations in subjects with MCI, the earliest clinical manifestation of AD [18–21].
There are two broad outcomes of lipid peroxidation: structural damage of membrane integrity and the generation of aldehydic by-products, particularly α,β-unsaturated aldehydes, including 4-hydroxynonenal (HNE) and acrolein. These aldehydes react quickly with nucleophilic sulfhydryl groups, and are toxic to primary hippocampal neuron cultures, [14] and are elevated in vulnerable areas of the brain in LAD [13–15] and mild cognitive impairment (MCI) [9, 12]. However, there has been little study of the levels of aldehydic by-products of lipid peroxidation in preclinical AD (PCAD), a likely prodromal phase of AD. Although criteria for the identification of PCAD subjects is not well defined, PCAD generally refers to subjects who demonstrate normal antemortem neuropsychological test scores adjusted for age and education, but who demonstrate pronounced AD pathology at autopsy and meet intermediate or high likelihood criteria for the histopathological diagnosis of AD by National Institute on Aging Reagan Institute (NIA-RI) criteria [22]. To determine if oxidative damage to lipids and proteins is increased in PCAD we quantified levels of the lipid peroxidation by-products HNE and acrolein in the hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of PCAD subjects compared to NC subjects using gas chromatography mass spectrometry with negative chemical ionization (GC/MS/NCI) for extractable HNE and acrolein and immunochemistry for protein-bound HNE and acrolein. Protein carbonyl content was quantified by immunochemistry.
Current evidence suggests HNE and acrolein are by-products of oxidative damage to polyunsaturated fatty acids (PUFAs). HNE and acrolein by-products of ω-6 PUFAs, whereas, 4-hydroxyhexenal is derived from ω-3 PUFAs[23–26]. Docosahexaenoic acid (DHA), an ω-3 PUFA, and arachidonic acid (ARA), an ω-6 PUFA are the predominate PUFAs in grey matter[27, 28]. To determine the rate of HNE and acrolein production in the presence of AD physiologically relevant oxidative insults ARA was treated with Aβ1–40, Aβ1–42, and iron(II)/ascorbic acid. Levels of extractable HNE and acrolein generated from ARA when treated with Aβ1–40, Aβ1–42, or iron(II)/ascorbic acid were determined by GC/MS/NCI.
Materials & Methods
Materials
Butylated hydroxytoluene, desferal, O-(2,3,4,5,6-pentafluoro-benzyl) (PFB) hydroxylamine hydrochloride, EDTA, sodium sulfate, N,O-bis(trimethylsilyl)trifluoroacetaminde (BSTFA)/1% trimethylchlorosilane (TMCS), hexane, acrolein, 13C3-acrolein, arachidonic acid sodium salt, ammonium iron (II) sulfate hexahydrate, ascorbic acid, bovine serum albumin, and bicinochoninic acid (BCA) protein assay kits were obtained from Sigma Chemical (St. Louis, MO, USA). HNE and d3-HNE was obtained from Cayman Chemical (Ann Arbor, MI, USA). Monoclonal rabbit anti-HNE and monoclonal mouse anti-acrolein antibodies were obtained from Abcam (Cambridge, MA, USA). OxyBlot™ Protein Detection kits were obtained from Millipore (Billerica, MA, USA). Aβ1–40 and Aβ1–42 were a generous gift from Dr. Harry LeVine III Sanders-Brown Center on Aging, University of Kentucky.
Brain Specimen Sampling
Tissue specimens from the HPG, SMTG, and CER of short post-mortem interval (PMI) autopsies of 10 PCAD subjects (1M: 9W) and 10 age-matched NC subjects (2M: 8W) were obtained through the Neuropathology Core of the University of Kentucky Alzheimer’s Disease Center (UK-ADC). These samples were immediately frozen in liquid nitrogen and maintained at −80°C until processed for analysis.
PCAD and NC subjects were followed longitudinally in the UK-ADC Normal Control Clinic and underwent neuropsychological testing, physical and neurological examinations annually. All NC subjects had neuropsychological test scores in the normal range and showed no evidence of memory decline. Although there are not well defined criteria for the identification of PCAD subjects, the UK-ADC tentatively describes PCAD subjects as those with sufficient AD pathologic alterations at autopsy to meet intermediate or high NIA-RI criteria, moderate or frequent neuritic plaque scores according to the Consortium to Establish a Registry for AD (CERAD) with Braak scores of III–VI, and antemortem psychometric test scores in the normal range when corrected for age and education [22].
Neuropathological examination of multiple sections of neocortex, hippocampus, entorhinal cortex, amygdala, basal ganglia, nucleus basalis of Meynert, midbrain, pons, medulla, and CER using the modified Bielschowsky stain, hematoxylin, and eosin stains, and Aβ and α-synuclein immunostains was performed for all subjects. Braak staging scores were determined using the Gallyas stain on sections of entorhinal cortex, hippocampus, and amygdala, the Bielschowsky stain on neocortex. Histopathological examination of NC subjects showed only age-associated changes and Braak staging scores of I–II.
Tissue Processing
Tissue samples were prepared as described by Fitzmaurice et al. [29] with modification. Briefly, approximately 70–80 mg of each tissue specimen was homogenized by sonication in 1 mL distilled/deionized water containing EDTA (400 µM), butylated hydroxytoluene (20 µM), and desferal (20 µM) to minimize artefactual oxidation. Following homogenization, 1 nmol of stable labeled HNE (d3-HNE) and acrolein (13C3-acrolein) were added as internal standards. For quality control in human tissue, blank, spiked, and duplicate analyses were carried out for representative samples from each brain region.
In vitro Oxidation of Arachidonic Acid (ARA)
Arachidonic Acid (ARA) was oxidized as described by Kawai et al [24]. Briefly ARA (3 µmol) was oxidized at 37°C with ammonium iron (II) sulfate hexahydrate (10 mM) and ascorbic acid (1 mM), Aβ1–40 (19 nM), or Aβ1–42 (19 nM), for 0, 2, 4, 8, and 24 hours in sodium phosphate buffer (pH 7.4). Following incubation, 1 nmol of stable labeled HNE (d3-HNE) and acrolein (13C3-acrolein) were added as internal standards. For quality control of in vitro oxidation of ARA triplicate analyses were carried out for each treatment at each incubation time.
Aldehyde Derivatization
Aldehdyes were derivatized by the addition of O-(2,3,4,5,6-pentafluoro-benzyl) hydroxylamine hydrochloride (PFB) (0.05 M, 200 µL) at room temperature for 1 hr. Tissue proteins in human samples were precipitated with 1 mL of absolute ethanol, and O-PFB-oxime aldehyde derivatives were extracted into 2 mL hexane, vortexed for 1 min and centrifuged for 5 min at 3,000 RPM. The hexane layer was transferred to clean glass centrifuge tubes, and the hexane extraction repeated. The two hexane phases were combined, and residual water removed with sodium sulfate (0.5 g). The hexane layer was transferred to a clean glass centrifuge tube and dried in 37°C water bath under a stream of nitrogen using an Organomation Associates OA-SYS Heating System (Berlin, MA, USA). Dried PFB-oxime derivatives were further derivatized with 50 µL BSTFA/1% TMCS for 15 min at 80°C. Hexane (50 µL) was added to each sample, the tube vortexed, and the solution transferred to GC auto sampler vials.
Gas Chromatography/Mass Spectrometry
Derivatized samples were analyzed using an Agilent 7890A gas chromatograph on a HP-5ms capillary column (0.25-mm internal diameter, 0.25-µm film thickness, and 30-m length; Hewlett Packard, Palo Alto, CA, USA). Chromatographic parameters were as follows: an initial oven temperature of 50°C was maintained for 1 min followed by an increase to 240°C at 10°C/min and to 300°C at 20°C/min and maintained at 300°C for 1 min. The injector temperature was maintained at 250°C, the GC to MS transfer line at 280°C, and the source temperature at 150°C. Mass analysis was performed using negative chemical ionization with methane (1.2 mL/min) as the reagent gas on an Aligent 5975C mass spectrometer. For analysis, 1 µL of each sample was introduced using a pulsed splitless mode with 99.999% helium carrier gas set at a constant flow rate of 1 mL/min. Total analysis time was 24 min. Derivatized aldehyde mass spectra were acquired in selective ion monitoring (SIM) mode at m/z ratios of 234 for 13C3-acrolein, 231 for acrolein, 336 for d3-HNE, and 333 for HNE. Instrument response plots of integrated peak area of unlabeled analyte signal (231 for acrolein or 333 for HNE) was normalized to integrated peak area of labeled analyte signal (234 for 13C3-acrolein or 336 for d3-HNE ) as a function of unlabeled analyte added over a range of 30 pmol to 1 nmol in a tissue matrix in duplicate. Plots of instrument response versus concentration showed a positive significant correlations (r = 0.99) for HNE and (r = 0.98) for acrolein. Instrument response plots of integrated peak area of unlabeled analyte signal (231 for acrolein or 333 for HNE) was normalized to integrated peak area of labeled analyte signal (234 for 13C3-acrolein or 336 for d3-HNE ) as a function of unlabeled analyte added over a range of 30 pmol to 1 nmol in sodium phosphate buffer matrix at pH 7.4. Plots of instrument response versus concentration showed a positive significant correlations (r = 0.99) for HNE and (r = 0.99) acrolein. The integrated area of each analyte signal was normalized with respect to the integrated area of the corresponding internal standards for all samples.
Dot Blot Analysis
Total levels of protein-bound HNE and acrolein were determined by dot blot immunochemistry using a Schleicher & Schuell Dot-Blot apparatus as described by Saiki et al[30]. Briefly, 20 µg of protein, from a 200 µL aliquot of the solution used for GC/MS analysis, in a total volume of 40 µL were loaded in triplicate onto nitrocellulose membranes under vacuum. Following air-drying, blots were blocked for 1 hr at room temperature in 5% dry milk in TTBS and 3.3% goat serum. Blots were probed with 1:1500 dilution of primary rabbit anti-HNE and primary mouse anti-acrolein antibodies overnight at 4°C. Blots were washed 3× 10 min each with TTBS at room temperature and incubated with either horseradish peroxidase conjugated goat anti-mouse secondary antibody for acrolein or goat anti-rabbit secondary antibody for HNE at a 1:3000 dilution for 2 hr at room temperature. Blots were washed 3× 10 min each in TTBS. Dots were visualized using enhanced chemiluminescence per manufacturer’s instructions and intensities quantified using Scion Image Analysis Software (Scion, Fredrick, MD, USA). The dot intensities of replicates were averaged and average dot intensity for each PCAD and NC subject was normalized to mean NC levels for each blot. To verify the specificity of HNE and acrolein antibodies used in this study, bovine serum albumin (BSA) was treated with HNE or acrolein for 16 hr at 37°C at a 1:1 ratio molar ratio. Samples of HNE or acrolein modified BSA were loaded in triplicate and subjected to dot blot analyses as described above with the following modification; blots were incubated either with antibodies for HNE or acrolein or with HNE or acrolein antibodies pre-incubated with HNE or acrolein. To determine a linear response of HNE and acolein immunoreactivity BSA was treated with increasing concentrations of HNE or acrolein (3.125, 6.25, 12.5, 25 µM) for 16 hr at 37°C and subjected to dot blot analyses as described above.
Protein Carbonyl Content
Protein carbonyl content was determined using an OxyBlot™ Protein Detection Kit per manufacturer’s instructions. Briefly, 20 µg of protein, from a 200 uL aliquots of the solution used for GC/MS analysis, in a total volume of 5 µL was denatured with SDS (12%) and derivitized with either 2,4-dinitrophenylhydrazine (DNPH) or a derivatization-control solution for 15 min at room temperature. Samples were neutralized with supplied Neutralization Buffer and loaded onto Whatman Protran BA 85 nitrocellulose membranes (Picastaway, NJ, USA) using a Schleicher & Schuell Dot-Blot apparatus as described above. A control derivatization sample was analyzed for each sample and each sample was derivatized in triplicate. Following air-drying, blots were blocked in TTBS with 3.3% goat serum for 1 hr at room temperature and probed with a 1:750 dilution of rabbit anti-DNPH antibody overnight at 4°C. Blots were washed with TTBS 3 ×10 min at room temperature and incubated with horseradish peroxidase conjugated goat anti-rabbit secondary antibody at a 1:3000 for 2 hr at room temperature and subsequently washed. Dots were visualized using enhanced chemiluminescence per manufacturer’s instructions and quantified using Scion Image Analysis Software (Scion, Fredrick, MD, USA). Dot intensity for each control derivatization was subtracted from the corresponding DNPH-derivatized samples. The dot intensities of replicates were averaged and average dot intensity for each PCAD and NC subject was normalized to mean control levels for each blot.
Statistical Analysis
All data were tested for normality using the Wilkes-Shapiro test. Data for extractable HNE and acrolein and Braak staging scores demonstrated non-normal distributions. All other data demonstrated a normal distribution. Normalized dot blot values of protein-bound acrolein and HNE and total protein carbonyl content, levels of extractable HNE and acrolein within a given incubation period, age, and PMI were compared using a 2-tailed Students t-test. Time dependent levels of extractable HNE and acrolein from the in vitro oxidation of ARA were compared using an ANOVA and are expressed at pmol/µmol of ARA. Levels of extractable HNE and acrolein and Braak staging scores were compared using the Mann-Whitney U-test. Braak staging scores are reported as median values and levels of extractable HNE and acrolein are reported as median values with range. All statistical comparisons were carried out using ABSTAT software (AndersonBell, Arvada, CO, USA). Statistical significance was set at P ≤ 0.05.
Results
Subject demographic data are shown in Table I. There were no significant differences in age or PMI between PCAD and NC subjects. Median Braak staging scores were significantly (P < 0.05) higher in PCAD subjects (IV) compared to NC subjects (I).
Table 1.
Subject demographic data
| Group | Mean ± SEM age (years) |
Sex | Mean ± SEM PMI (h) | Median Braak Score |
|---|---|---|---|---|
| NC | 85.4 ± 1.9 | N=10; 1M, 9W | 2.8 ± 0.7 | I |
| PCAD | 86.0 ± 2.1 | N=10; 2M, 8W | 2.9 ± 0.6 | IV* |
P < 0.05
NC = normal control; PCAD = preclinical Alzheimer’s disease; PMI = postmortem interval; SEM = standard error of the mean
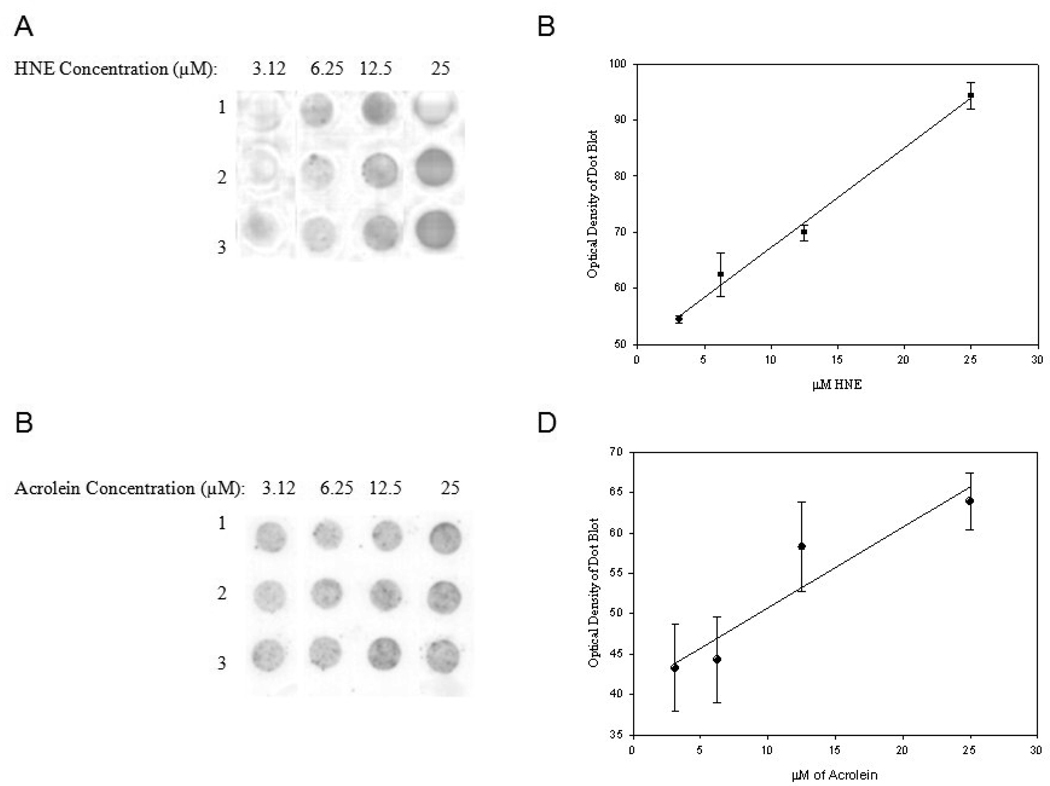
To assess the specificity of HNE and acrolein antibodies used in this study BSA was treated with HNE or acrolein and subjected to dot blot analysis using antibodies without pre-incubation with HNE or acrolein. Figure 1 A shows pre-incubation of HNE antibody with HNE completely eliminates recognition of HNE modified BSA compared to antibody without pre-incubation with HNE (Fig. B). Similarly pre-incubation of the anti-acrolein antibody with acrolein significantly diminished recognition of acrolein modified BSA (Fig. 1C) compared to antibody without pre-incubation with acrolein (Fig. 1D). Suggesting the antibodies are specific for HNE and acrolein. Figs. 2 A and B show a statistically significant linear response between immunostaining intensity and increasing HNE levels (r = 0.99, P < 0.05). A similar statistically significant linear response for acrolein (r = 0.94, P < 0.05) was observed (Figs 2 C and D).
Figure 1.
Antibody specificity of HNE and acrolein. (A) HNE modified BSA loaded in triplicate in Row 1 and HNE modified BSA loaded in triplicate in Row 2 incubated with anti-HNE pre-incubated with HNE. (B) HNE modified BSA loaded in triplicate in Row 1 and HNE modified BSA loaded in triplicate in Row 2 incubated with anti-HNE. (C) Acrolein modified BSA loaded in triplicate in Row 1 and HNE modified BSA loaded in triplicate in Row 2 incubated with anti-acrolein pre-incubated with acrolein. (D) Acrolein modified BSA loaded in triplicate in Row 1 and HNE modified BSA loaded in triplicate in Row 2 incubated with anti-acrolein antibody.
Figure 2.
HNE and acrolein immunochemical staining of protein samples incubated with increasing concentrations of HNE or acrolein. Each modified BSA sample was loaded in triplicate. (A) Representative dot blots of HNE modified BSA with increasing HNE concentrations (3.125, 6.25, 12.5, and 25 µM). (B) Calibration curve of HNE concentration vs. immunochemical response (r = 0.99, P ≤ 0.05). (C) Representative dot blots of acrolein modified BSA with increasing acrolein concentrations 3.125, 6.25, 12.5, and 25 µM). (D) Calibration curve of acrolein concentration vs. immunochemical response (r = 0.94, P ≤ 0.05).
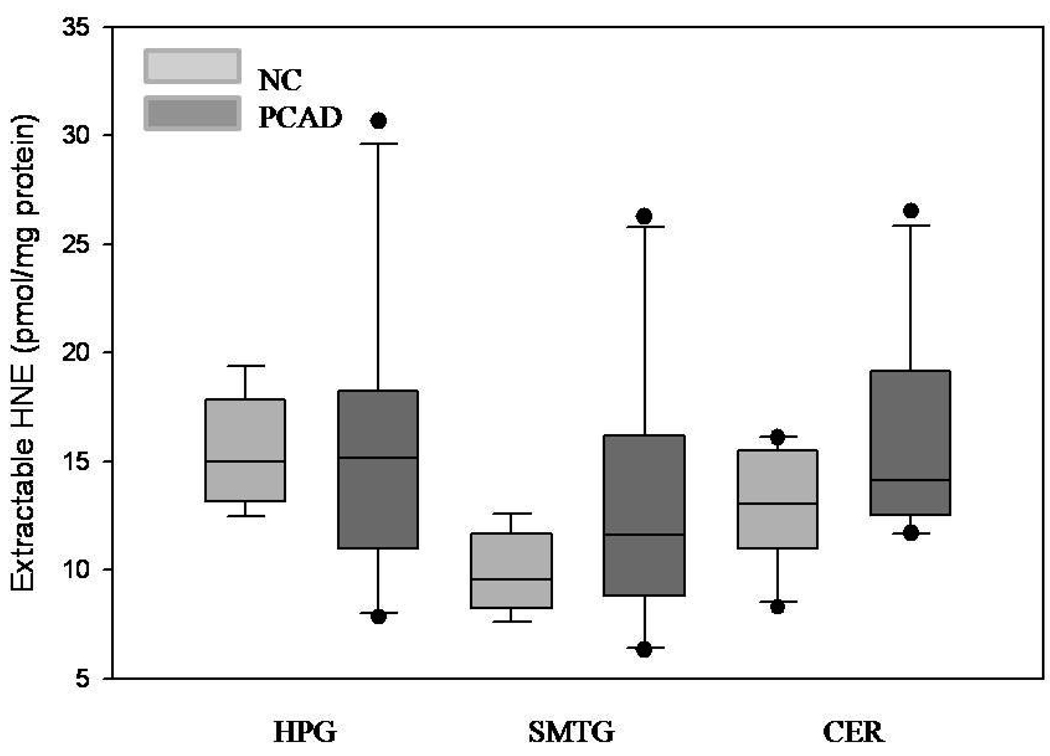
Median extractable acrolein levels were significantly (P < 0.05) higher in the HPG of PCAD subjects (4.9 nmol/mg protein) compared to NC subjects (1.9 nmol/mg of protein) (Fig. 3). In contrast, extractable acrolein was significantly (P < 0.05) decreased in the CER PCAD subjects (1.1 nmol/mg of protein) compared to NC subjects (5.1 nmol/mg of protein). Levels of extractable acrolein were not significantly altered in the SMTG of PCAD subjects compared to NC subjects. Levels of extractable HNE were not significantly different in PCAD subjects compared to NC subjects in HPG, SMTG, or CER (Fig. 4).
Figure 3.
Levels of extractable acolein expressed as the median and range (nmol/mg of protein) in hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of preclinical Alzheimer’s disease (PCAD) and NC subjects. There was a significant (P < 0.05) increase in extractable acrolein in the HPG of PCAD subjects compared to NC subjects. There was a significant (P < 0.05) decrease in acrolein in the CER of PCAD subjects compared to NC subjects.
Figure 4.
Levels of extractable HNE expressed as the median and range (pmol/mg of protein) in hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of preclinical Alzheimer’s disease (PCAD) and NC subjects. There were no significant differences in levels of extractable HNE in any brain region studied.
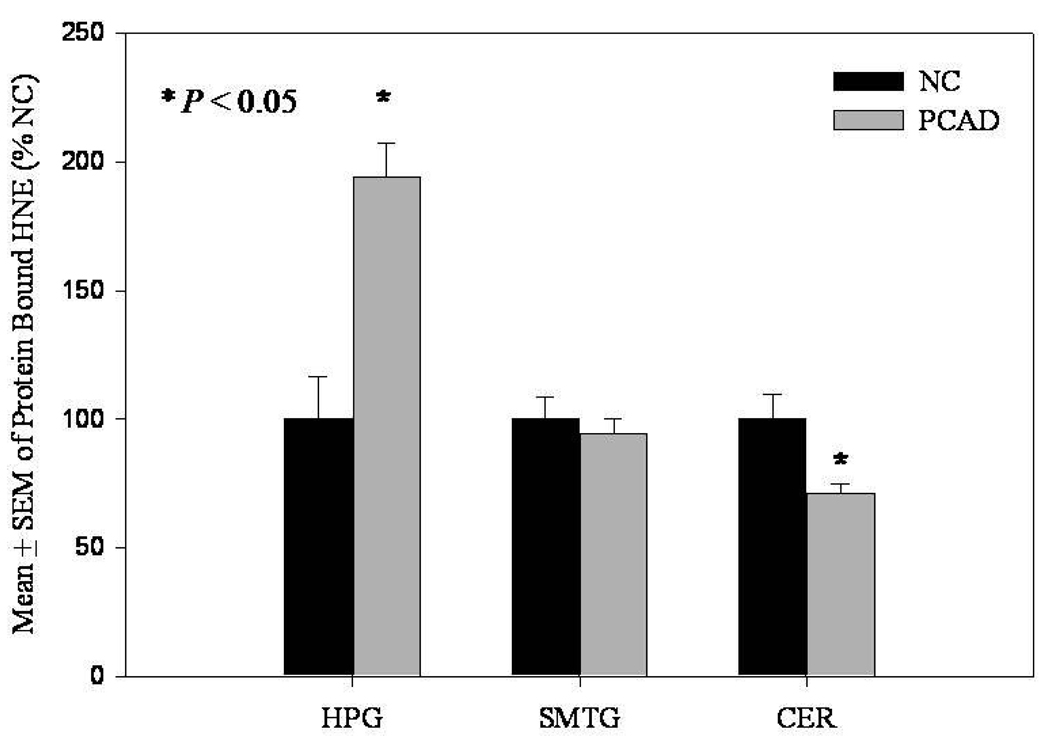
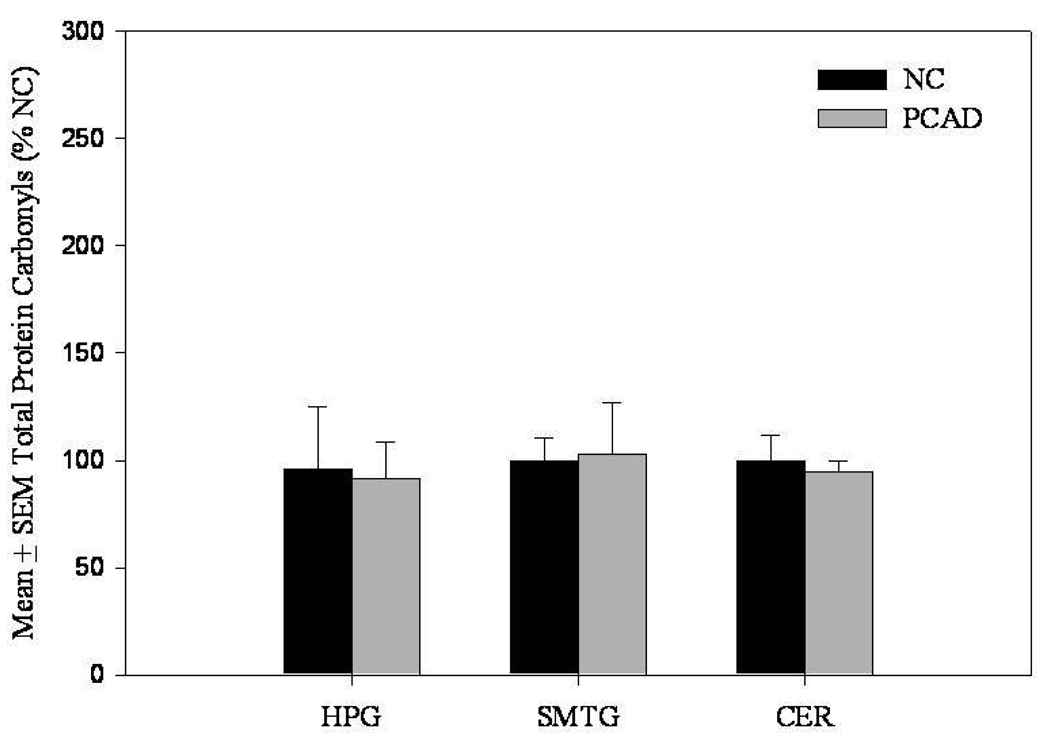
In contrast to levels of extractable HNE, levels of protein-bound HNE were significantly (P < 0.05) increased in the HPG of PCAD subjects (194.2 ± 12.9% control) compared to NC subjects (100 ± 16.3% control) (Fig. 5). Protein-bound HNE was significantly (P < 0.05) decreased in the cerebellum of PCAD subjects (70.9 ± 3.9%) compared to NC subjects (100 ± 9.9) (Fig. 5). There were no significant differences in levels of protein-bound HNE in the SMTG of PCAD subjects compared to NC subjects (Fig. 5). No significant differences were observed in levels of protein-bound acrolein in the HPG, SMTG, or CER (Fig. 6). Additionally, protein carbonyls were not significantly different in the HPG, SMTG, or CER of PCAD subjects compared to NC subjects (Fig. 7). There was a significant positive correlation (r = 0.6, P ≤ 0.005) between the levels of protein-bound HNE and Braak Staging scores in the HPG. Further correlation analysis of neuritic plaque counts in the CA1/subiculum, and SMTG showed no significant relationship with either extractable or protein-bound HNE or acrolein. There were no significant correlations between SDS or formic acid soluble Aβ levels and levels of either extractable or protein-bound HNE and acrolein.
Figure 5.
Levels of protein-bound HNE expressed as mean ± SEM (% of normal control [NC]) in hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of preclinical Alzheimer’s disease (PCAD) and NC subjects. There was a significant (P < 0.05) increase in protein bound HNE in the HPG of PCAD subjects compared to NC subjects. There was a significant (P < 0.05) decrease in protein bound HNE in the CER of PCAD subjects compared to NC subjects.
Figure 6.
Levels of protein-bound acrolein expressed as mean ± SEM (% of normal control [NC]) in hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of preclinical Alzheimer’s disease (PCAD) and NC subjects. There were no significant differences between PCAD and NC subjects for any brain region studied.
Figure 7.
Protein carbonyl content expressed as mean ± SEM (% of normal control [NC]) in hippocampus/parahippocampal gyrus (HPG), superior and middle temporal gyri (SMTG), and cerebellum (CER) of PCAD and NC subjects. There were no significant differences for any brain region studied.
Levels of extractable HNE and acrolein from in vitro oxidation of ARA treated with Aβ1–40, Aβ1–42, or iron (II) ascorbic acid are shown in Table 2. Levels of extractable HNE and acrolein from oxidized ARA were significantly (P ≤ 0.05) higher at 24 hr incubation for all treatments. However, levels of extractable HNE generated by ARA oxidation was significantly (P ≤ 0.05) higher than that of extractable acrolein when treated with Aβ1–40, Aβ1–42, and iron (II) ascorbic acid at all incubation time. Levels of extractable HNE generated from the treatment of ARA with both species of Aβ at 24 hr were comparable to those resulting from treatment with a strong oxidizing system, iron(II)/ascorbic acid.
Table 2.
Levels of Extractable HNE and acrolein generated through oxidation of ARA
| Time | 0 hr | 2 hr | 4 hr | 8 hr | 24 hr | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HNEa | Acroleina | HNEa | Acroleina | HNEa | Acroleina | HNEa | Acroleina | HNEa | Acroleina | |
| Oxidant | ||||||||||
| Aβ1–40 | 41.7 ± 1.6b | N.D. | 187.4 ± 6.4b | N.D. | 198.2 ± 9.3b | N.D. | 583.9 ± 14.8b | N.D. | 12,874.6 ± 753.2b,c | 70.4 ± 13.5 b |
| Aβ1–42 | 44.1 ± 1.5b | N.D. | 172.5 ± 3.8b | N.D. | 138.8 ± 8.3b | N.D. | 649.3 ± 15.4b | N.D. | 16,056.5 ± 595.3b,c | 123.5 ± 5.4b |
| Ascorbic | 45.5 ± 0.9b | N.D. | 437.6 ± 5.7b | N.D. | 1447.4 ± 35.4b | N.D. | 2104.9 ± 71.5b | N.D. | 12,472.9 ± 436.7b,c | 108.9 ± 6.8b |
| Acid/Iron (II) | ||||||||||
Levels of extractable HNE and acrolein are expressed as pmol/µmol ± SD of ARA
HNE levels are significantly greater than acrolein levels within a given treatment period P ≤ 0.05.
Levels are significantly greater than treatment at 0 hr P ≤ 0.05.
N.D. Non-Detectable
Discussion
Preclinical AD is a recent concept that describes cognitively normal individuals who show pronounced AD pathology at autopsy. It is postulated that PCAD individuals would have eventually developed clinical AD if they had lived longer, a concept that is supported by imaging studies [31]. The present study showed protein-bound HNE and extractable acrolein are significantly increased in HPG compared to NC subjects, with acrolein levels similar to those reported in previous studies of MCI and LAD subjects suggesting that lipid peroxidation may play an early role in the progression of AD. These changes were not observed in the SMTG. Thus early changes of lipid peroxidation in the hippocampus correlates with the histopathologic changes in the entorhinal cortex and hippocampus in AD as described by Braak and Braak [32]. Our observations of no significant differences in protein oxidation levels between PCAD and NC suggest lipid peroxidation may precede protein oxidation as the earliest detectable oxidative change in the progression AD. It remains to be determined whether increased RNA or DNA oxidation is present in PCAD brain. While these studies were conducted in bulk brain tissue rather than a homogenous population of neurons, previous immunohistochemical studies indicate HNE and acrolein is largely associated with neurons[33, 34].
It is unclear why we observed an apparent dichotomy of elevated protein-bound HNE but not extractable HNE and elevated extractable acrolein but not protein-bound acrolein in PCAD HPG. The in vitro oxidation of ARA indicates HNE is generated prior to acrolein when treated with Aβ1–40, Aβ1–42, and iron(II)/ascorbic acid. If a similar pattern is present in PCAD brain it is possible that HNE levels would be increased initially allowing more time for cross-linking reactions to occur with proteins leading to our observation of increased protein bound HNE but not extractable HNE. Similarly, if acrolein production lags HNE generation in the PCAD brain it would likely have less time for interaction with proteins and would be present as the extractable form leading to our observation of elevated extractable acrolein but not protein-bound acrolein. Rajamoorthi et al reported that artificial ARA lipid bilayers were softer and more deformed than artificial DHA lipid bilayers which may contribute to the oxidation of ARA prior to that of the DHA, the dominate PUFA in grey matter[35]. The comparable levels of extractable HNE generated by Aβ species and iron(II)/ascorbic acid system add further support to the hypothesis that may Aβ species play a role in the pathogenesis of AD through the generation of aldehydic by-products of lipid peroxidation.
Levels of extractable HNE and acrolein in the current study were quantified using deuterated or 13C internal standards (13C3-acrolein and d3-HNE) and GC/MS/NCI rather than unlabeled straight chain aldehydes (heptanal and octanal) and using LC/MS as in our earlier studies[12]. The levels of extractable acrolein in NC HPG (1.9 (4.4) nmol/mg protein) in the present study were comparable to those observed previously by Williams at el (1.2 ± 0.3 nmol/mg protein)[12]. Slight variations in the level of extractable acrolein may be due to a combination of variation in analytical methods and subject-to-subject variability. However, in the current study we observed much lower levels of extractable HNE (14.9 (6.9) pmol/mg protein) compared to levels reported by Williams et al (0.4 ± 0.1 nmol/mg protein) in the HPG of NC subjects[12]. However, the levels of HNE that we observed are similar to those reported by Fitzmaurice et al. of the caudate of control subjects (12.0 ± 0.9 pmol/mg protein)[29] using a method from which our current method is derived. The decreased levels of extractable HNE in the current study relative to our previous studies are likely the result of different sample preparation methodology, analysis, or the use of internal standards.
Acrolein (CH2=CH-CHO) and HNE (C5H11-CH(OH)-CH=CH-CHO) are neurotoxic aldehydes that are produced during the peroxidation of polyunstatureated fatty acids. Both aldehydes have been observed by immunohistochemistry in neurofibillary tangles and senile plaques the two hallmark pathological features of AD [36, 37]. In addition, numerous studies demonstrate that acrolein and HNE are highly reactive resulting in the disruption of normal cellular processes and activities through a Michael addition to key amino acids such as lysine, cysteine, and histidine [38]. Modification of proteins by HNE can result in a reduction of activity of several critical enzymes including lactate dehydrogenase and ATP synthase [9].
Acrolein can impair metabolic processes by inhibiting glucose uptake in primary neuronal cultures [39]. In vitro studies also support acrolein’s role in the disruption of glucose metabolism by demonstrating that acrolein when incubated with pyruvate dehydrogenase results in decreased activity [40]. Lovell et al., showed that the activity of glutathione transferase, a detoxification enzyme for HNE and acrolein was significantly decreased in the HPG of LAD subjects [41]. Previous studies showed increased levels of extractable acrolein in the HPG of both MCI and EAD subjects [14]. The median levels of extractable acrolein quantified in the current study in the HPG of PCAD subjects (4.89 nmol/mg protein) are comparable to those reported of the HPG of EAD subjects (2.7 ± 0.2 nmol/mg protein) [12] and LAD subjects (5.0 ± 1.6 mol/mg of protein) [14]. Our observations suggest lipid peroxidation is an early event in the pathogenesis of AD.
Elevations of HNE can have severe consequences on cellular function, such as inhibition of dephosphorylation of microtubule associated protein tau [42] and decreased levels of DNA-histone interaction mediated by a covalent modification of histones particularly at lysine residues [43]. The inhibition of histone DNA interactions may interfere with DNA storage and ultimately contribute to increased DNA oxidation observed in MCI and LAD patients [2, 12, 18, 44]. The elevation of protein-bound HNE in the HPG in the current study are consistent with previous studies by Butterfield et al. who reported a statistically significant increase of protein bound HNE in the hippocampus of MCI subjects [9]. Identification of HNE modified proteins in the HPG of MCI subjects by redox proteomics suggests that HNE modification is common for several proteins involved in energy production [9]. It has been proposed that deficiencies of these proteins as a result of HNE modification may contribute to the generation of reactive oxygen species thus compounding oxidative stress present in the disease [9].
Overall, our data suggest lipid peroxidation occurs early in the progression of AD further supporting the hypothesis that oxidative stress is an early event in the pathogenesis of AD.
Acknowledgements
This research was supported by NIH grant 5P01-AG05119 and P30-AG028383. The authors thank Ms. Sonya Anderson for subject demographic data and Ms. Paula Thomason for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. American Neurological Association. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 2.Gabbit SP, Lovell MA, Markesbery WR. Increased Nuclear DNA Oxidation in the Brain in Alzheimer's Disease. Journal of Neurochemistry. 1998;71:2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzherimer's disease. The Journal of Neuroscience. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shan X, Tashiro H, Lin CG. The identification and characterization of oxidized RNAs in Alzheimer's disease. The Journal of Neuroscience. 2003;23:4913–2921. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovell MA, Xie C, Markesbery WR. Decrease base excision repair and increased helicase activity in Alzheimer's disease brain. Brain Research. 2000;855:166–123. doi: 10.1016/s0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- 6.Lovell MA, Gabbit SP, Markesbery WR. Increased DNA oxidative and decreased levels of repair products in Alzheimer's disease ventricular CSF. Journal of Neurochemistry. 1999;72:771–776. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- 7.Shao C, Xiong S, Li G, Gu L, Mao G, Markesbery WR, Lovell MA. Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzherimer's disease brain. Free Radical Biology & Medicine. 2008 doi: 10.1016/j.freeradbiomed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield DA, Poon HF, St. Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiology of Disease. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-noenal, in brain from persons with mild cognitive impairment. Neuroscience Letters. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Gabbit SP, Aksenov MY, Lovell MA, Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. Journal of Neurochemistry. 1999;73:1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 11.Sultana R, Boyd-kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu JP, Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: a redox proteomics analysis. Neurobiology of Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increase levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiology of Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.McGarth LT, McGleenon BM, Brennan S, McColl D, McIlroy S, Passmore AP. Increased oxidative stress in Alzheimer's disease as assessed with 4-hydroxynonenal but not malondialdehyde. Q J Med. 2001;94:385–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- 14.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 15.Markesbery WR, Lovell MA. Four-Hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiology of Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 16.Markesbery WR, Kyyscio RJ, Lovell MA, Morrow JD. Lipid Peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 17.Reich EE, Markesbery WR, Roberts LJ, III, Swifte LL, Morrow JD, Montine TJ. Brain Regional Quantification of F-Ring and D-/E-Ring Isoprostanes and Neuroprostanes in Alzheimer's disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Markesbery WR, Lovell MA. Increase oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. Journal of Neurochemistry. 2006;96:825–832. doi: 10.1111/j.1471-4159.2005.03615.x. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Markesbery WR, Chen Q, Keller JN. Ribosome Dysfunction Is an Early Event in Alzheimer's Disease. The Journal of Neuroscience. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and Increased RNA Oxidation, in Ribosomes from Early Alzheimer's Disease. Neurochem Res. 2006;31:705–710. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 21.Shao C, Xiong S, Guo-Min L, Gu L, Mao G, Markesbery WR, Lovell MA. Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer's disease brain. Free Radic Bio Med. 2008;45:813–819. doi: 10.1016/j.freeradbiomed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclincal" AD Revisited: Neuropathology of cognitively older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer HSRJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 24.Kawai Y, Fujii H, Okada M, Tsuchie U, Uchida K, Osawa T. Formation of nepsilon-(succinyl)lysine in vivo: a novel marker for docosahexaenoic acid-derived protein modification. J. Lipid Res. 2006;47:1386–1398. doi: 10.1194/jlr.M600091-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein isa product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 26.Uchida K. 4-hydroxy-2-nonenal: a product and mediator of oxidative stress. Progress in Lipid Reseach. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 27.Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional Membrane Phospholipid Alterations in Alzheimer's Disease. Neurochemical Research. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- 28.Soderberg M, Kristensson EK, Dallner G. Fatty Acid Composition of Brain Phospholipids in Aging and Alzheimer's Disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice PS, Junchao T, Yazdanpanah M, Liu PP, Kalasinsky KS, Kish SJ. Levels of 4-hydroxynonenal and malondialdehyde are increased in brain of human chronic users of methamphetamine. The Journal of Pharmacology and Experimental Theapeutics. 2006;319:703–709. doi: 10.1124/jpet.106.109173. [DOI] [PubMed] [Google Scholar]
- 30.Saiki RK, Bugawan TL, Horn GT, Mukkis KB, Erlich HA. Analysis of enzymatically amplified beta-globin and hla-dq alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- 31.Smith CD, Chebrolu J, Markesbery WR, Liu J. Improved predictive model for pre-symptomatic mild cognitive impairment and Alzherimer's disease. Neurol Res. 2008;30:1091–1096. doi: 10.1179/174313208X327973. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropatholgical stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Montine KS, Olson SJ, Amarnath V, Whetsell WO, Graham DG, Montine TJ. Immunohistochemical Detection of 4-Hydroxy-2-Nonenal Adducts in Alzheimer's Disease Is Associated with Inheritance of APOE4. American Journal of Pathology. 1997;150:437–443. [PMC free article] [PubMed] [Google Scholar]
- 34.Calingasan NY, Uchida K, Gibson GE. Protein-Bound Acrolein: A Novel Marker of Oxidative Stress in Alzheimer's Disease. Journal of Neurochemistry. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 35.Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF. Packing and Viscoelasticity of Polyunsturated omega-3 and omega-6 Lipid Bilayers as Seen by 2H NMR and X-Ray Diffraction. Journal of American Chemical Society. 2005;127:1576–1588. doi: 10.1021/ja046453b. [DOI] [PubMed] [Google Scholar]
- 36.Calingansan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer's disease. Journal of Neurochemistry. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 37.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK. Oxidative damage is the earliest even in Alzhiemer's disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 38.Catala A. Lipid peroxidation of membrane phopholipids generates hydroxy-alkenals and oxidized phopholipids active in physiological and/or pathological conditions. Chemistry and Physics of Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radical Biology & Medicine. 2000;29:714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- 40.Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotoxicity Research. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- 41.Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology. 1998;51:1562–1566. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- 42.Mattson MP, Fu W, Waeg G, Uchida K. 4-Hydroxynonenal, a product of lipid peroxidation, inhibits dephosphorylation of the microtubule-associated protein tau. NeuroReport. 1997;8:2275–2281. doi: 10.1097/00001756-199707070-00036. [DOI] [PubMed] [Google Scholar]
- 43.Drake J, Petroze R, Castegna A, Ding Q, Keller JN, Markesbery WR, Lovell MA, Butterfield DA. 4-hydroxynonenal oxidatively modifies histones: implications for Alzheimer's disease. Neuroscience Letters. 2004;356:155–158. doi: 10.1016/j.neulet.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 44.Lovell MA, Gabbita SP, Markesbery WR. Increase DNA oxidation and Decreased Levels of Repair Products in Alzheimer's Disease Ventricular CSF. Journal of Neurochemistry. 1999;72:771. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]