Abstract
Two missense variants (D299G and T399I) of TLR4 are cosegregated in individuals of European descent and, in a number of test systems, result in reduced responsiveness to endotoxin. How these changes within the ectodomain (ecd) of TLR4 affect TLR4 function is unclear. For both wild-type and D299G.T399I TLR4, we used endotoxin·CD14 and endotoxin·MD-2 complexes of high specific radioactivity to measure: 1) interaction of recombinant MD-2·TLR4 with endotoxin·CD14 and TLR4 with endotoxin·MD-2; 2) expression of functional MD-2·TLR4 and TLR4; and 3) MD-2·TLR4 and TLR4-dependent cellular endotoxin responsiveness. Both wild-type and D299G.T399I TLR4ecd demonstrated high affinity (Kd ~ 200 pM) interaction of endotoxin·CD14 with MD-2·TLR4ecd and endotoxin·MD-2 with TLR4ecd. However, levels of functional TLR4 were reduced up to 2-fold when D299G.T399I TLR4 was coexpressed with MD-2 and >10-fold when expressed without MD-2, paralleling differences in cellular endotoxin responsiveness. The dramatic effect of the D299G.T399I haplotype on expression of functional TLR4 without MD-2 suggests that cells expressing TLR4 without MD-2 are most affected by these polymorphisms.
Potent inflammatory responses to Gram-negative bacterial endotoxins are mediated by TLR4. TLR4 does not bind endotoxin directly, but depends on three other extracellular and/or cell surface proteins: LPS-binding protein (LBP), CD14 and MD-2 (1–3). LBP catalyzes extraction and transfer of LPS (endotoxin) monomers from LPS-rich particles to CD14 (4), forming monomeric endotoxin (E)·CD14 complexes that efficiently transfer endotoxin monomers to secreted MD-2 and to preassociated MD-2·TLR4 heterodimers (5, 6). Whereas MD-2 can bind to TLR4 in the absence of endotoxin (2, 7), a complex of one molecule of endotoxin bound to one molecule of MD-2 is the activating ligand for TLR4 (5, 8), triggering TLR4 dimerization and signaling (7, 9).
Two missense mutations, A896G (D299G) and C1196T (T399I), have been identified in the human TLR4 gene at frequencies up to 18% (10). The single D299G mutation is prevalent in African populations; however, when present in individuals of European descent, D299G is usually cosegregated with T399I. These coding region mutations are located within the extracellular domain of TLR4 (7). The significance of the D299G.T399I haplotype was first suggested in studies investigating the basis of individual differences in responsiveness to inhaled endotoxin (11). These studies demonstrated a nearly 4-fold higher frequency of the D299G.T399I haplotype among individuals who were hyporesponsive to inhaled endotoxin and reduced endotoxin responsiveness of primary cultures of airway epithelial cells bearing a wild-type (wt)/D299G.T399I rather than wt/wt TLR4 genotype. The in vitro observations were extended to other primary and transfected epithelial and myeloid cell lines (12–14). However, several other studies of the responsiveness of human PBMCs and whole blood to endotoxin in vitro and ex vivo failed to observe significant effects of the D299G.T399I polymorphism (15). These latter observations have raised questions about the circumstances required for the D299G.T399I polymorphism to affect TLR4 function; they also underscore the lack of understanding as to how these two amino acid changes within the TLR4 ectodomain (ecd) could affect cellular endotoxin responsiveness.
Arbour et al. (11) showed, by immunostaining using an anti-TLR4 Ab, a marked reduction of surface expression of TLR4 in airway epithelial cells cultured from individuals who expressed D299G.T399I rather than exclusively wt TLR4. Markedly reduced surface expression of immunoreactive variant (versus wt) TLR4 has also been shown in transfected human bronchial epithelial cells (14). In contrast, differences in surface expression of wt and D299G.T399I TLR4 observed in monocytes were apparently more modest (14), and essentially no difference was seen in transiently transfected human embryonic kidney (HEK) 293 cells coexpressing epitope-tagged TLR4 with MD-2 and CD14 (13). Although these studies do not permit a precise quantitative comparison of surface expression of wt and variant TLR4 in these different settings, evidence of reduced surface expression of D299G.T399I (versus wt) TLR4 seems most compelling in cells (e.g., airway epithelial cells) that express TLR4 with little or no coexpression of MD-2 (16). Transit and maturation of TLR4 through the cellular secretory pathway and surface expression is enhanced by coexpression of MD-2 (17, 18). However, TLR4 can be expressed at the cell surface in the absence of MD-2 (16, 19–21), consistent with the sensitivity of these cells to added E·MD-2 complex (5, 16). One possible explanation for the apparently divergent effects in different biologic settings of expression of D299G.T399I TLR4 is that secretion and surface expression of this variant TLR4, in comparison with wt TLR4, is more dependent on coexpression of MD-2.
To test this hypothesis, we have compared the effects of expression of wt or D299G.T399I full-length TLR4 and soluble TLR4 ectodomain (TLR4ecd) in transiently transfected HEK293T cells ± coexpression of MD-2. HEK293 cells express no detectable TLR4, MD-2 (or mCD14) (5, 13), providing a controlled experimental setting in which to compare the extracellular accumulation or surface expression of functional wt and variant TLR4ecd or full-length TLR4, respectively, ± MD-2. Moreover, HEK293 cells expressing TLR4 alone are highly sensitive to TLR4-activating E·MD-2 complexes (5, 22), consistent with the ability of these cells to express wt TLR4 on the cell surface when produced with or without MD-2 (21). We used purified E·sCD14 and E·MD-2 complexes of high and uniform specific radioactivity (25,000 cpm/pmol) (23), to sensitively and quantitatively assay functional MD-2·TLR4 and TLR4, for both wt and variant forms of TLR4 and TLR4ecd. We show that the interaction of MD-2·TLR4ecd with E·CD14 and of TLR4ecd with E·MD-2 is unaffected by the D299G.T399I substitutions in TLR4. However, accumulation of functional extracellular TLR4ecd and full-length transmembrane TLR4 is diminished when D299G.T399I TLR4 is expressed, especially in the absence of MD-2. Reduced expression of functional TLR4 parallels reduced cellular responsiveness to endotoxin, particularly at low agonist concentrations. Our findings suggest that the effects of expressing D299G.T399I TLR4 may be greater in cells, such as airway epithelial cells, in which TLR4 is expressed without MD-2. This may contribute to the variable effects observed on cellular endotoxin responsiveness that have been previously reported.
Materials and Methods
Metabolically labeled endotoxin ([3H] lipooligosaccharide [LOS], 25,000 cpm/pmol) was isolated from Neisseria meningitidis serogroup B as previously described (24). LBP and sCD14 were gifts from Xoma (Berkeley, CA) and Amgen (Thousand Oaks, CA), respectively. Human serum albumin (HSA) was an endotoxin-free, 25% stock solution from Baxter Health Care (Glendale, CA). ANTI-FLAG M2 Affinity Gel was acquired from Sigma-Aldrich (St. Louis, MO). Cell culture media, DMEM supplemented with 4 mM L-glutamine, and 293 SFM II were from acquired Invitrogen (Carlsbad, CA). Sephacryl S200 HR resin size exclusion gel was purchased from GE Healthcare (Piscataway, NJ). The HEK293 T cells were provided by Dr. Fabio Re (University of Tennessee Health Sciences Center, Memphis, TN). BD OptEIA Human IL-8 ELISA Set was acquried from BD Biosciences (San Diego, CA).
Production of recombinant secreted proteins
Recombinant human MD-2 and the extracellular domain of human TLR4 (TLR4ecd; residues 24-631) were transiently expressed in HEK293T cells transfectedwithpEF-BOS-MD-2Flag.His6 and pFLAG-CMV-TLR4ecd, respectively(6). pFLAG-CMV-TLR4ecd was constructed using pFLAG-CMV-TLR4 (gift from S. Vogel, University of Maryland, Baltimore, MD) as a template to amplify the extracellular domain of TLR4. Primers used were 5′-CTTGCGGCCGCGGAAAGCTGGGAGCCCTGCGT-3′ and 5′-CAGAGTCGACTCACTTATTCATCTGACAGG-3′. Underlined sequences represent NotI and SalI restriction recognition sites used to subclone TLR4ecd into pFLAG-CMV. The coding sequences of all plasmid constructs were confirmed. HEK293T cells grown in DMEM with 10% FCS were seeded in T75 flasks at ~80% confluency. After 18–24 h, the cells were washed with PBS (pH 7.4) and transfected with the indicated amount of DNA and PolyFect reagent from Qiagen (Valencia, CA) according to the manufacturer’s protocol. After transfection, the cells were supplemented with 8 ml DMEM/10% FCS. After 12 h, the medium was replaced with 293 SFM II. Conditioned media containing secreted proteins were collected every 24 h for 5 d, and cells were replenished with fresh serum-free medium each day. The collected conditioned media were pooled, concentrated 10× using Millipore Centricon Plus-80, sterile filtered (0.2 μm filters) and stored at 4°C. Conditioned medium from mock-transfected cells was used as a negative control (Figs. 1, 2).
FIGURE 1.
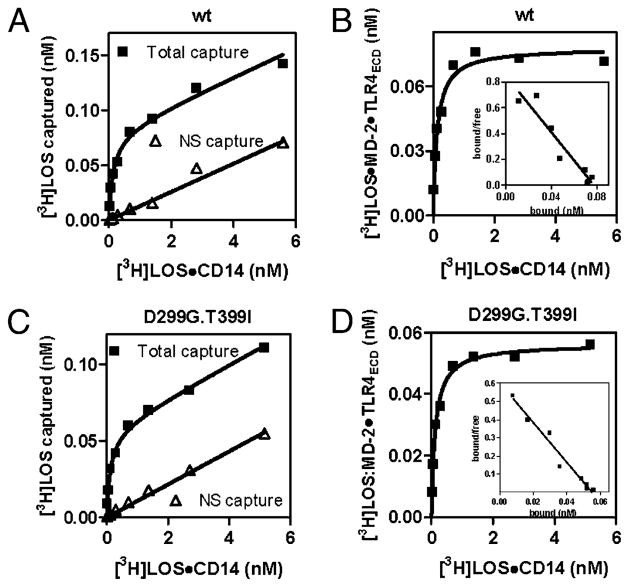
Comparison of dose-dependent transfer of [3H]LOS from [3H]LOS·sCD14 to soluble MD-2·TLR4ecd (wt [A, B] versus D299G.T399I variant [C, D]). Increasing concentrations of [3H]LOS·sCD14 were incubated for 30 min at 37°C in PBS/0.1% HSA supplemented with conditioned medium from HEK293T cells expressing MD-2Flag.His6 and FlagTLR4ecd (wt or D299G.T399I variant) or from mock-transfected cells. Cocapture of [3H]LOS with ANTI-FLAG affinity gel was measured as described in Materials and Methods. Results in A and C show dose-dependent [3H]LOS capture after incubation of [3H]LOS·sCD14 with conditioned medium containing MD-2·TLR4ecd (total capture) and after incubation with conditioned medium from mock-transfected cells (non-specific [NS] capture). Specific capture [i.e., formation of ([3H]LOS·MD-2·TLR4ecd)2] shown in B and D was calculated as the difference between total and non-specific capture. B and D show Scatchard analysis of this data used to determine apparent Kd (slope) and Bmax (x-intercept). Data are from one experiment, representative of three closely similar experiments.
FIGURE 2.
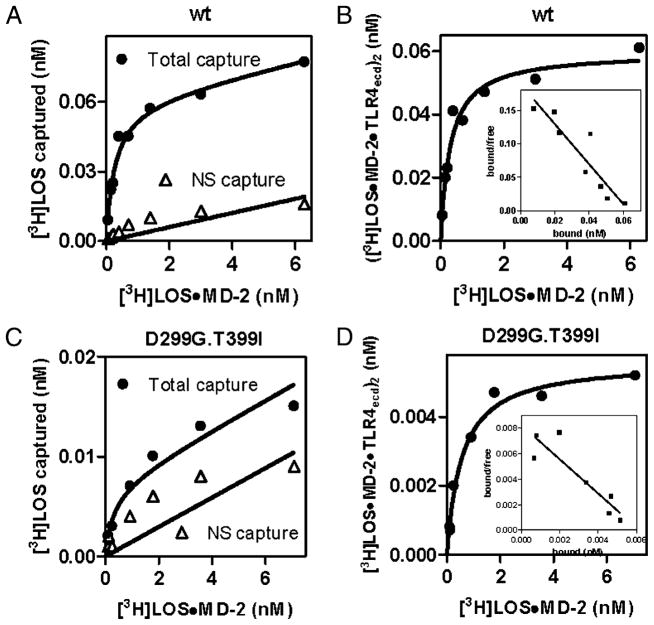
Comparison of dose-dependent binding of [3H]LOS·MD-2 to soluble TLR4ecd (wt [A, B] versus D299G.T399I variant [C, D]). Increasing concentrations of [3H]LOS·MD-2 were incubated for 30 min at 37°C in PBS/0.1% HSA supplemented with conditioned medium from HEK293T cells expressing FlagTLR4ecd (wt or D299G.T399I) or from mock-transfected cells. Cocapture of [3H]LOS with ANTI-FLAG affinity gel was measured as described in Materials and Methods and data were analyzed as described in the legend to Fig. 1. Data are from one experiment, representative of three closely similar experiments.
Preparation of LOS·protein complexes
[3H]LOS·sCD14 and [3H]LOS·MD-2 complexes were prepared as previously described (5, 25). Monomeric [3H]LOS·CD14 complexes were prepared by treatment of [3H]LOS aggregates for 30 min at 37°C with sub-stoichiometric LBP (molar ratio, 200:1 LOS:LBP) and 1.5× molar excess of sCD14 followed by size exclusion chromatography (Sephacryl S200, 1.6 × 70-cm column) in PBS (pH 7.4) supplemented with 0.03% HSA to isolate monomeric [3H]LOS·sCD14 (Mr, ~60,000). Monomeric [3H]LOS·MD-2 (Mr, ~25,000) was generated by treatment of [3H]LOS·sCD14 (30 min at 37°C) with High Five (Invitrogen) conditioned insect cell medium containing sMD-2His6 generated as has been described previously (5), followed by Sephacryl S200 chromatography as described above. Fractions containing the desired [3H]LOS·protein complexes were combined, sterile-filtered, and stored at 4°C. The concentration of the [3H]LOS·protein complexes was calculated based on the known specific radioactivity of the [3H]LOS.
Anti–FLAG-agarose cocapture of [3H]LOS bound to FlagTLR4ecd (wt or D299G.T399I)
Assay of reaction of [3H]LOS·sCD14 with MD-2Flag.His6/FlagTLR4ecd
Increasing concentrations of [3H]LOS·sCD14 (25,000 cpm/pmol LOS) were incubated for 30 min at 37°C in conditioned medium of HEK293T cells either from mock-transfected cells or cells expressing and secreting MD-2 and TLR4ecd (wt or D299G.T399I) supplemented with PBS/0.1% HSA to a final volume of 0.4 ml. Transfer of [3H]LOS from LOS·sCD14 to MD-2Flag.His6/FlagTLR4ecd to form ([3H]LOS·MD-2Flag.His6/FlagTLR4ecd)2 was measured by cocapture of [3H]LOS to ANTI-FLAG M2 Affinity Gel (20 μl). The affinity gel pre-equilibrated in PBS/0.1% HSA was incubated with the sample for 1 h with rotation at 20–25°C. Samples were spun for 5 min at 2000 ×g to sediment the gel and captured [3H]LOS. The gel was washed twice with 0.4 ml ice cold PBS/0.1% HSA. Radioactivity associated with the affinity gel (i.e., captured [3H]LOS) was measured by liquid scintillation spectroscopy. In the harvested conditioned medium containing secreted MD-2 and TLR4ecd, only MD-2 that was associated with TLR4ecd reacted with added [3H]LOS·sCD14 (6, 22).
Assay of reaction of [3H]LOS·MD-2 with FlagTLR4ecd
Increasing concentrations of [3H]LOS·MD-2 (25,000 cpm/pmol LOS) were incubated as described above with conditioned medium of HEK293T cells from mock-transfected cells or cells expressing and secreting TLR4ecd (wt or D299G. T399I). Binding of [3H]LOS·MD-2 to FlagTLR4ecd to form ([3H]LOS·MD-2His6/FlagTLR4ecd)2 was measured by cocapture of [3H]LOS to ANTI-FLAG M2 affinity gel (20 μl) as described above.
Assay of binding of [3H]LOS to transfected HEK293T cells
HEK293T cells grown in D-MEM supplemented with 10% FCS were transfected with 12 μg of pFLAG-CMV1-TLR4 [wt or D299G.T399I (13)] with or without 6 μg pEF-BOS-MD-2 using PolyFect reagent (Qiagen) or treated with PolyFect reagent without plasmid as a negative control as described above. After 12 h, the cells were washed with PBS and grown in 293 SFM II for additional 24 h. The cells were then dislodged by scraping in PBS/0.1% HSA and washed twice with PBS/0.1% HSA. The recovered cells (3 × 106/sample) were incubated in PBS/0.1% HSA + increasing concentrations of [3H]LOS·MD-2 or [3H]LOS·CD14 (25,000 cpm/pmol LOS) in 1 ml final volume. After 30 min rotating at 37°C, the cells were spun (1000 rpm, 2 min), the supernatant removed and the cells washed twice with cold PBS/0.1% HSA. Radioactivity associated with the cells was measured by liquid scintillation spectroscopy (Beckman LS liquid scintillation counter). Binding of [3H]LOS·MD-2 and [3H]LOS·sCD14 to the mock transfected cells was <0.5% and 4–5%, respectively, of the total radioactivity added at each dose This was subtracted from the total [3H] LOS bound to the transfected cells (Fig. 3).
FIGURE 3.
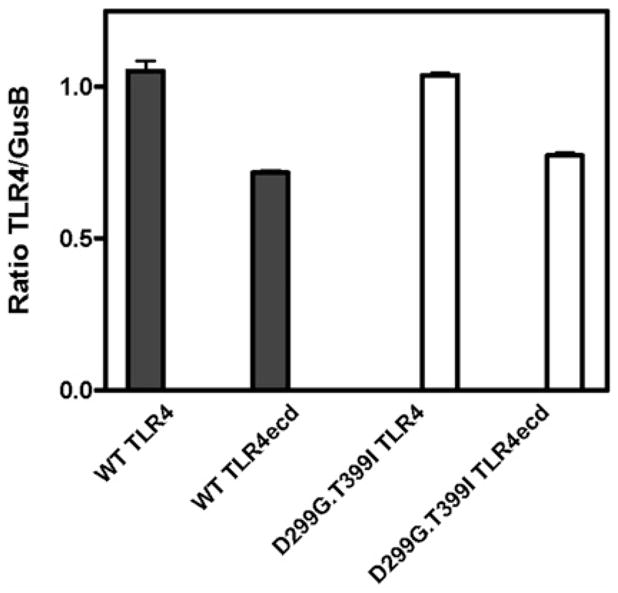
Comparison of levels of TLR4 mRNA in HEK293T cells transiently transfected with full-length and ectodomain of wt and D299G. T399I TLR4. RNA was extracted and mRNA levels of TLR4 and GusB, a control gene, were quantified by RT-PCR as described in Materials and Methods. Levels of TLR4 mRNA is expressed as the ratio of TLR4 copies normalized to copies of gusB mRNA. Data are mean ± SEM of triplicate determinations.
Assay of cell activation by purified LOS·protein complexes
HEK293T cells grown in D-MEM/10% FCS were seeded in a 6-well plate at ~80% confluency and transiently transfected with 18 ng/well pFLAG-CMV1-TLR4 (wt or D299G.T399I) ± 7 ng/well pEF-BOS-MD-2. After 12 h, the medium was removed and 293 SFM II was added for additional 24 h. Cells were transferred to a 96-well plate at ~4 × 104 cells per well and stimulated with increasing concentrations of LOS·CD14 (for cells transfected with TLR4 and MD-2) or LOS·MD-2 (for cells transfected with TLR4 alone). After 24 h, secreted IL-8 was measured in the medium, using a BD OptEIA Human IL-8 ELISA Set from BD Biosciences (San Diego, CA) according to the manufacturer’s protocol.
Real-time RT-PCR
HEK293T cells were transfected as described above in six-well plates. After the 24 h incubation in serum-free medium, cells were dislodged, spun, and the pellet was resuspended in cell lysis buffer (RNeasy RTL buffer, Qiagen). Total RNA was extracted using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instruction. The quantity and purity of the RNA was measured by ThermoScientific Nanodrop1000 apparatus (Wilmington, DE). Equal amounts of RNA from transfected cells and control samples were reverse-transcribed into cDNA using AMV reverse transcriptase (Roche Diagnostics, Mannheim, Germany). Real-time PCR was performed using SYBR Green Master-Mix (ABGene, Foster City, CA) for 40 cycles on an ABI Prism 7000 sequence detector (Applied Biosystems, Foster City, CA) according to the manufacturer’s instruction. Amplification was achieved using an initial cycle of 50° for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and then 60°C for 1 min. Melting curve analyses were done at the completion of the cycling. Primers were designed using the Primer Express (Applied Biosystems) software and are as follows: tlr4-forward 5′-CCT GCG TGG AGG TGG TTC CTA ATA-3′; reverse 5′-AAG CTC AGG TCC AGG TTC TTG GTT-3′ and as a control gene gusB-forward 5′-CCT ATG CCA TCG TGT GGG TGA A-3′; reverse 5′-TGA TGG CGA TAG TGA TTC GGA GC-3′.
Results
D299G.T399I mutations in TLR4 ectodomain have little or no effect on interaction of MD-2·TLR4ecd with monomeric endotoxin·CD14 complex
Previous studies in transfected HEK293T cells showed that coexpression of D299G.T399I, rather than wt, TLR4, with MD-2 and mCD14, resulted in ~2-fold reduction in cellular responses to added LPS (13). These studies did not reveal the mechanistic basis of this reduced response. We took advantage of our ability to measure specific and saturable interaction of E·CD14 (e.g., [3H]LOS·sCD14) with MD-2Flag.His6/FlagTLR4ecd (6) to test whether expression of D299G.T399I TLR4ecd, rather than wt TLR4ecd, affected either its reactivity with [3H]LOS·sCD14 or the levels of functional MD-2·TLR4ecd recovered in the extracellular medium. Fig. 1 shows closely similar dose-dependent capture of [3H]LOS by anti-FLAG agarose after incubation of increasing concentrations of [3H] LOS·sCD14 with conditioned culture medium from cells expressing and secreting MD-2Flag.His6 + wt or D299G.T399I FlagTLR4ecd. Comparison of the amount of [3H]LOS captured after incubation of [3H]LOS·sCD14 with conditioned medium containing MD-2Flag. His6/FlagTLR4ecd (total capture) versus negative control medium (nonspecific capture) demonstrated that most [3H]LOS captured, especially at low [3H]LOS·sCD14 concentrations, was dependent on MD-2·TLR4ecd (Fig. 1A, 1C). Harvesting the conditioned medium after prolonged incubation (≥24 h) at 37°C insured that only MD-2Flag.His6 associated with FlagTLR4ecd was reactive with [3H]LOS·sCD14 (6, 22). Thus, the specific (i.e., total–non-specific) capture of [3H]LOS (Figs. 1B, 1D) reflects transfer of[3H]LOS from [3H]LOS·sCD14 to MD-2Flag.His6/FlagTLR4ecd to form ([3H] LOS·MD-2Flag.His6/FlagTLR4ecd)2, as previously described (6, 22).
Scatchard analysis of these data indicated an apparent Kd of [3H] LOS transfer to MD-2· TLR4ecdwt and to MD-2· TLR4ecdD299G.T399I of 196 ±37 pM and 186 ±27 pM, respectively (n = 3). The amount of functional (i.e., [3H]LOS·sCD14-reactive) wt and variant MD-2· TLR4ecd that accumulated in the conditioned media was similar, but ~25% less for the variant complex (272 ±27 fmol MD-2· TLR4ecdwt versus 205 ± 8 fmol MD-2· TLR4ecdD299G.T399I per ml conditioned medium [p < 0.05]).
D299G.T399I mutations in TLR4 ectodomain have little effect on reactivity with monomeric endotoxin·MD-2 complex, but markedly reduce extracellular accumulation of functional TLR4ecd
Similar experiments were performed using conditioned medium from HEK293T cell cultures transiently expressing wt or D299G. T399I TLR4ecd without MD-2 and assaying TLR4-dependent capture of [3H]LOS·MD-2His6. The specificity of capture was demonstrated by comparison of dose-dependent [3H]LOS capture after incubation of [3H]LOS·MD-2His6 with conditioned medium from cells expressing or not expressing FlagTLR4ecd (Figs. 2A, 2C). Scatchard analysis (Figs. 2B, 2D) of the dose-dependent capture of [3H]LOS·MD-2 by wt and D299G.T399I TLR4ecd showed little or no effect of the D299G.T399I substitutions in the TLR4 ectodomain on the high affinity interactions of TLR4ecd with [3H]LOS·MD-2. The calculated Kd of these interactions was 221 ± 43 pM and 403 ± 141 pM for wt and variant TLR4ecd, respectively. However, in contrast to MD-2·TLR4ecd, there was a marked diminution in extracellular accumulation of functional (i.e., [3H]LOS·MD-2–reactive) D299G.T399I versus wt TLR4ecd (196 ± 23 fmol functional wt TLR4ecd versus only 4 ± 1 fmol functional D299G.T399I TLR4ecd per ml conditioned medium). These findings reveal a dramatic effect of substitution of D299G and T399I TLR4ecd on extracellular accumulation of functional TLR4ecd when D299G.T399I TLR4ecd was expressed without MD-2. The differences in functional levels of wt versus variant secreted TLR4 ectodomain and of cell surface TLR4 (see below) were observed under conditions in which the levels of expression (i.e., mRNA) of wt and D299G.T399I TLR4 and TLR4ecd were virtually the same (Fig 3).
Differences in expression of functional full-length wt and D299G.T399I TLR4 ± MD-2
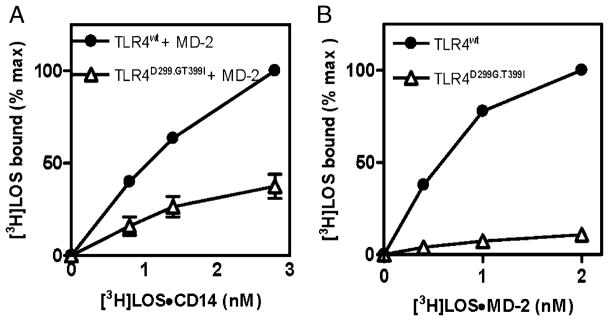
To test the relevance of the above findings to expression of functional full-length TLR4, HEK293T cells were transiently transfected with expression plasmids encoding wt or D299G.T399I full-length TLR4 ± MD-2. Expression of functional TLR4 was assayed by directly measuring cell binding of [3H]LOS after incubation of cells expressing MD-2·TLR4 with [3H]LOS·sCD14 (Fig. 4A) and cells expressing TLR4 alone with [3H]LOS·MD-2 (Fig. 4B). After expression of wt TLR4 alone, there was markedly increased dose-dependent [3H]LOS·MD-2 binding (Fig. 4B). Co-expression of both wt TLR4 and MD-2 was needed for increased cell binding of [3H]LOS during incubation of the cells with [3H] LOS·sCD14 (Fig. 4A). There was ~2-fold reduction in MD-2·TLR4-dependent reaction of [3H]LOS·sCD14 with cells expressing D299G.T399I rather than wt TLR4 (Fig. 4A). TLR4-dependent [3H]LOS·MD-2 binding was reduced to a greater extent (at least 10-fold) when D299G.T399I rather than wt TLR4 was expressed without MD-2. TLR4-dependent [3H]LOS·MD-2 binding was essentially the same at 37 and 4°C (data not shown), confirming that binding was to cell surface TLR4. These findings show decreased surface expression of functional full-length D299G.T399I TLR4 that is much more pronounced when cells express D299G.T399I TLR4 without MD-2.
FIGURE 4.
Comparison of binding of [3H]LOS to HEK293T cells transiently expressing full-length wt (●) or D299G.T399I (△) TLR4 with (A) or without (B) MD-2. Binding of [3H]LOS to transiently transfected HEK293T cells during 30 min incubation with increasing concentrations of [3H]LOS·sCD14 (A) or [3H] LOS·MD-2 (B) was measured as described in Materials and Methods. The results are expressed as percent of the maximum [3H]LOS binding measured (0.11 ± 0.02 pmol bound/5 × 106 cells in A and 0.13 ± 0.02 pmol bound per 5 × 106 cells in B after subtraction of the dose-dependent binding of [3H]LOS to mock-transfected cells. The results shown are the mean ± SEM of two (A) or three (B) independent experiments, each in duplicate.
Comparison of endotoxin responsiveness of HEK293 cells expressing wt or D299G.T399I TLR4 ± MD-2
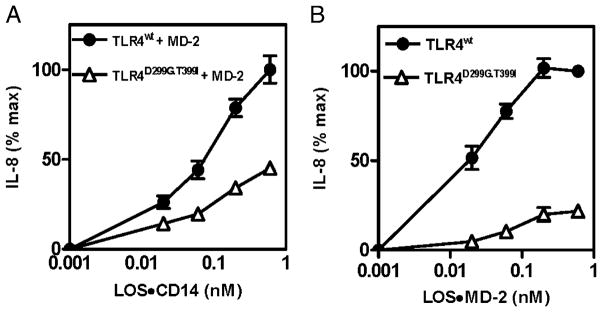
Monomeric endotoxin·CD14 and endotoxin·MD-2 complexes produce, at pM concentrations, dose-dependent activation of cells expressing, MD-2·TLR4 and TLR4 without MD-2, respectively (5, 16). Cells expressing D299G.T399I rather than wt TLR4 with MD-2 showed a 2–3-fold reduction in the magnitude of cell activation induced by increasing concentrations of LOS·sCD14 (Fig. 5A). The less robust cell activation of HEK293T cells expressing D299G.T399I TLR4 closely paralleled the reduced amount of binding of [3H]LOS to these cells during incubations with [3H] LOS·sCD14 (compare Figs. 4A and 5A). Differences in activation by LOS·MD-2 of HEK293T cells expressing wt versus D299G. T399I TLR4 alone were even greater, especially at low concentrations of added LOS·MD-2 (Fig. 5B). Thus, differences in expression of functional wt and variant TLR4 ± MD-2, as reflected by differences in cell binding of [3H]LOS, result in alteration of cellular responsiveness to endotoxin, especially at low agonist concentrations.
FIGURE 5.
Comparison of dose-dependent activation by LOS·sCD14 (A) or LOS·MD-2 (B) of HEK293 T cells transiently expressing full-length wt (●) or D299G.T399I (△) TLR4 with (A) or without (B) MD-2. Cell activation was measured as extracellular accumulation of IL-8 as described in Materials and Methods. Results shown are expressed as the percent of maximum extracellular IL-8—that is, IL-8 induced by treatment of the cells with 0.6 nM LOS·sCD14 (A) or LOS·MD-2 (B) [296 ± 29 pg IL-8 per 5 × 104 cells (A); 754 ± 204 pg IL-8 per 5 × 104 cells (B)]. The results shown are the mean ± SEM of two (A) or four (B) independent experiments, each performed in duplicate.
Discussion
The studies described above provide several novel insights relevant to the effect of D299G.T399I substitutions within the TLR4 ectodomain on cellular responsiveness to endotoxin and possibly other potential agonists of TLR4. Levels of functional TLR4 (i.e., reactive with specific monomeric endotoxin protein complexes), either as the secreted soluble ectodomain or expressed as full-length membrane (cell surface) receptor, were diminished when D299G.T399I rather than wt TLR4 was expressed. Reduced accumulation of functional TLR4 was more profound when D299G. T399I TLR4 was expressed without MD-2. Diminished TLR4-dependent cell binding of endotoxin paralleled reduced cellular responsiveness, especially under conditions (e.g., low endotoxin concentrations) in which limited cell activation may be most closely linked to limited receptor occupancy by agonist.
We also demonstrated that D299G.T399I substitutions within the TLR4 ectodomain decrease the affinity of neither endotoxin·CD14 for MD-2·TLR4ecd nor endotoxin·MD-2 for TLR4ecd. The normal, high (pM) affinity/reactivity of TLR4ecdD299G.T399I with LOS·MD-2 is consistent with MD-2·TLR4ecd cocrystal structures (7, 9) that show that residues 299 and 399 within the TLR4 ectodomain are not in close proximity to the primary contacts of endotoxin-free MD-2 with TLR4 or the secondary contacts of TLR4-activating E·MD-2 with TLR4 that drive TLR4 dimerization. These residues are also not likely near endotoxin·CD14 docking sites on MD-2·TLR4, consistent with the high-affinity interactions we observed for interaction of MD-2·TLR4ecdD299G.T399I with LOS·sCD14. Our findings also suggest that there are no allosteric effects of these two-point mutations on endotoxin·MD-2 binding by TLR4ecd or on endotoxin·CD14 reaction with MD-2·TLR4ecd.
The normal affinity/reactivity of TLR4ecdD299G.T399I with LOS·MD-2 strongly suggests that the markedly reduced amount of binding of LOS·MD-2 with cells expressing D299G.T399I versus wt TLR4 produced under similar conditions reflects reduced amounts of functional full-length TLR4 when D299G. T399I TLR4 is expressed without MD-2. This was demonstrated directly in studies of TLR4ecd (Fig. 2). We have shown by quantitative RT-PCR that levels of expression of wt and D299G.T399I TLR4, as either the full-length or ectodomain protein, were nearly the same (Fig. 3). The fact that diminished accumulation of functional D299G.T399I TLR4 (full-length or soluble ectodomain) was more pronounced when MD-2 was not coexpressed further argues that the observed differences in functional levels of wt and D299G.T399I TLR4 were not due to reduced production of D299G.T399I TLR4, but instead an altered posttranslational fate (e.g., diminished passage through the secretory pathway and/or reduced [functional] stability of intracellular or surface [secreted] D299G.T399I TLR4). Because TLR4ecd and full-length TLR4 proceed through the same secretory pathway, we expect that reduced extracellular levels of functional D299G.T399I TLR4ecd that we observed are mirrored by reduced levels of functional full-length TLR4 on the cell surface. The reduced amount of binding of [3H]LOS·MD-2 to cells expressing D299G.T399I rather than wt TLR4 is consistent with this prediction and with earlier observations (11) showing reduced surface expression (immunostaining)of D299G. T399I versus wt TLR4 in airway epithelial cells. These earlier studies included individuals who were heterozygous for the wt and D299G. T399I haplotypes, suggesting that the variant allele may have a dominant-negative effect on surface expression of functional wt TLR4 when TLR4 is expressed without MD-2.
In contrast to our observations, Rallabhandi et al. (13) observed no apparent difference in surface expression of wt versus D299G. T399I TLR4/MD-2 in transfected HEK293T cells, as judged by immunochemical detection of epitope-tagged TLR4. Whether or not the modest diminution in functional (i.e., LOS·sCD14-reactive) MD-2·TLR4 we observed when D299G.T399I rather than wt TLR4 was coexpressed with MD-2 reflects greater sensitivity and quantitative precision of [3H]LOS binding assays, versus flow cytometry of FlagTLR4 or a subfraction of surface-expressed D299G.T399I FlagTLR4 that is nonfunctional, cannot yet be judged. However, the close match between reduced MD-2·TLR4-dependent cell binding of [3H]LOS that we observed when D299G.T399I was expressed with reduced activation of these cells by LOS·sCD14 (this study) or by LPS + serum (13) suggest that the differences in [3H]LOS binding that we observed are functionally meaningful.
There have been numerous efforts to relate the structural changes of TLR4 imposed by the 299 and/or 399 polymorphisms to in vitro cellular responsiveness to TLR4 agonists and to various clinical outcomes (15). Attempts to associate specific clinical outcomes with TLR4 genotype is especially difficult, given the uncertain and potentially complex role of TLR4 agonists in many of these clinical scenarios and the possibility that other genotypic differences may be associated with defined TLR4 haplotypes and affect the phenotype under study. However, even in much more focused in vitro studies, comparison of effects of expression of wt versus polymorphic variant TLR4 on TLR4-dependent cell function (e.g., endotoxin responsiveness) have not yielded an entirely clear picture. We believe that our findings may help to explain some of the divergent results that have been reported. Our findings suggest that the phenotype of the cosegregated D299G.T399I polymorphisms will be greatest in those cells expressing TLR4 without MD-2 (e.g., airway epithelial cells), resulting in diminished endotoxin responsiveness when an exogenous source of MD-2 is present (16, 26, 27) as well as, potentially, reduced responses to other putative TLR4 agonists, some of which may not be MD-2–dependent in their interactions with TLR4. Such an effect may help explain the strikingly high association of these TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk, premature infants (28), where diminished TLR4 levels may impact both lung development (29, 30) and early host defense responses to viral infection (31, 32). Effects of D299G.T399I TLR4 expression on cell function are most likely to be manifest at low receptor and agonist concentrations (Fig. 5B), when the number of receptors occupied by agonist is most likely the rate-limiting factor in cell activation. The experimental approaches described in this study should make it possible to extend these observations to primary cells, such as nasal and airway epithelial cells.
Acknowledgments
This work was supported by a Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development grant (to T.L.G.) and U.S. Public Health Service Grants AI18797 (to P.R.), PO144642, and AI59372 (to J.P. W.).
We thank Xoma (Berkeley, CA) for recombinant LBP and Amgen (Thousand Oaks, CA) for sCD14; DeSheng Zhang for preparation, isolation, and characterization of radiolabeled LOS and LOS·protein complexes; and Dr. Stephanie Vogel (University of Maryland, Baltimore, MD) for helpful discussions and critical reading of the manuscript.
Abbreviations used in this paper
- E
endotoxin
- ecd
ectodomain
- HEK
human embryonic kidney
- HSA
human serum albumin
- LBP
lipopolysaccharide binding protein
- LOS
lipooligosaccharide
- wt
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Beutler B. Innate immune responses to microbial poisons: discovery and function of the Toll-like receptors. Annu Rev Pharmacol Toxicol. 2003;43:609–628. doi: 10.1146/annurev.pharmtox.43.100901.135729. [DOI] [PubMed] [Google Scholar]
- 2.Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol. 2003;3:119–128. doi: 10.1016/s1567-5769(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 3.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 4.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- 5.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin.protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 7.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JO. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Teghanemt A, Widstrom RL, Gioannini TL, Weiss JP. Isolation of monomeric and dimeric secreted MD-2. Endotoxin.sCD14 and Toll-like receptor 4 ectodomain selectively react with the monomeric form of MD-2. J Biol Chem. 2008;283:1881–1889. doi: 10.1074/jbc.M800672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 10.Ferwerda B, McCall MB, Alonso S, Giamarellos-Bourboulis EJ, Mouktaroudi M, Izagirre N, Syafruddin D, Kibiki G, Cristea T, Hijmans A, et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc Natl Acad Sci USA. 2007;104:16645–16650. doi: 10.1073/pnas.0704828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 12.Rallabhandi P, Awomoyi A, Thomas KE, Phalipon A, Fujimoto Y, Fukase K, Kusumoto S, Qureshi N, Sztein MB, Vogel SN. Differential activation of human TLR4 by Escherichia coli and Shigella flexneri 2a lipopolysaccharide: combined effects of lipid A acylation state and TLR4 polymorphisms on signaling. J Immunol. 2008;180:1139–1147. doi: 10.4049/jimmunol.180.2.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 14.Tulic MK, Hurrelbrink RJ, Prêle CM, Laing IA, Upham JW, Le Souef P, Sly PD, Holt PG. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179:132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, Netea MG. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14:346–352. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB., Jr Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 17.da Silva Correia J, Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem. 2002;277:1845–1854. doi: 10.1074/jbc.M109910200. [DOI] [PubMed] [Google Scholar]
- 18.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 19.Konno K, Wakabayashi Y, Akashi-Takamura S, Ishii T, Kobayashi M, Takahashi K, Kusumoto Y, Saitoh S, Yoshizawa Y, Miyake K. A molecule that is associated with Toll-like receptor 4 and regulates its cell surface expression. Biochem Biophys Res Commun. 2006;339:1076–1082. doi: 10.1016/j.bbrc.2005.11.123. [DOI] [PubMed] [Google Scholar]
- 20.Visintin A, Halmen KA, Khan N, Monks BG, Golenbock DT, Lien E. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4) J Leukoc Biol. 2006;80:1584–1592. doi: 10.1189/jlb.0606388. [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi Y, Kobayashi M, Akashi-Takamura S, Tanimura N, Konno K, Takahashi K, Ishii T, Mizutani T, Iba H, Kouro T, et al. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J Immunol. 2006;177:1772–1779. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- 22.Teghanemt A, Re F, Prohinar P, Widstrom R, Gioannini TL, Weiss JP. Novel roles in human MD-2 of phenylalanines 121 and 126 and tyrosine 131 in activation of Toll-like receptor 4 by endotoxin. J Biol Chem. 2008;283:1257–1266. doi: 10.1074/jbc.M705994200. [DOI] [PubMed] [Google Scholar]
- 23.Post DM, Zhang D, Weiss JP, Gibson BW. Stable isotope metabolic labeling of Neisseria meningitidis lipooligosaccharide. J Endotoxin Res. 2006;12:93–98. doi: 10.1177/09680519060120020501. [DOI] [PubMed] [Google Scholar]
- 24.Giardina PC, Gioannini T, Buscher BA, Zaleski A, Zheng DS, Stoll L, Teghanemt A, Apicella MA, Weiss J. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem. 2001;276:5883–5891. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 25.Iovine N, Eastvold J, Elsbach P, Weiss JP, Gioannini TL. The carboxyl-terminal domain of closely related endotoxin-binding proteins determines the target of protein-lipopolysaccharide complexes. J Biol Chem. 2002;277:7970–7978. doi: 10.1074/jbc.M109622200. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy MN, Mullen GE, Leifer CA, Lee C, Mazzoni A, Dileepan KN, Segal DM. A complex of soluble MD-2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J Biol Chem. 2004;279:34698–34704. doi: 10.1074/jbc.M405444200. [DOI] [PubMed] [Google Scholar]
- 27.Vasl J, Oblak A, Gioannini TL, Weiss JP, Jerala R. Novel roles of lysines 122, 125, and 58 in functional differences between human and murine MD-2. J Immunol. 2009;183:5138–5145. doi: 10.4049/jimmunol.0901544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, Hemming VG, Blanco JC, Vogel SN. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 29.Sampath V, Davis K, Senft AP, Richardson TR, Kitzmiller JA, Berclaz PY, Korfhagen TR. Altered postnatal lung development in C3H/HeJ mice. Pediatr Res. 2006;60:663–668. doi: 10.1203/01.pdr.0000246071.50268.51. [DOI] [PubMed] [Google Scholar]
- 30.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol. 2004;140:283–291. doi: 10.1016/j.resp.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]