Abstract
Background:
Increasing evidence suggests that blood pressure (BP) is significantly influenced by sleep problems in children, but the association between periodic limb movement during sleep (PLMS) and BP is still unclear. This study aims to compare ambulatory blood pressure (ABP) in children with and without PLMS.
Methods and Results:
A cross-sectional study involving 314 children (mean (SD) age of 10.4 (1.7) years, boys 62.4%). Participants underwent an overnight polysomnographic study and ABP monitoring. Subjects were hypertensive if mean SBP or DBP > 95th percentile and prehypertensive if mean SBP or DBP > 90th percentile of reference. Children with PLMS (n = 17) were at significantly higher risk for nocturnal systolic (adjusted OR (95%CI) = 6.25 [1.87-20.88]) and diastolic (OR (95%CI) = 4.83 [1.66-14.07]) hypertension. However, mean nocturnal BP did not differ between children with and without PLMS. There was a trend for higher daytime BP in patients with PLMS than those children without PLMS (P = 0.084 for systolic BP z score; P = 0.051 for diastolic BP z score; P = 0.067 for systolic prehypertension). There were significant associations between log transformed PLM index and daytime systolic and mean BP z scores (P = 0.03 and 0.033 respectively) as well as that between log transformed PLM related arousal index (PLMSArI) and nocturnal diastolic and mean BP (P = 0.008 and 0.038 respectively).
Conclusions:
PLMS was independently associated with a wide range of BP elevations, especially nocturnal indices. Future studies should examine the underlying pathophysiologic mechanisms and effects of PLMS treatment on BP.
Citation:
Wing YK; Zhang J; Ho CKW; Au CT; Li AM. Periodic limb movement during sleep is associated with nocturnal hypertension in children. SLEEP 2010;33(6):759-765.
Keywords: Periodic limb movements, sleep, children, hypertension
THERE IS INCREASING EVIDENCE TO SUGGEST THAT BLOOD PRESSURE (BP) IS SIGNIFICANTLY INFLUENCED BY SLEEP QUALITY AND SLEEP DISTURBANCES. Short sleep duration, poor sleep quality, and obstructive sleep apnea syndrome (OSA) were found to be associated with elevated BP or hypertension.1–3 However, the association between periodic limb movement during sleep (PLMS) and BP is still unclear. In fact, most of the data comes from adult studies. Restless legs syndrome (RLS), which is closely correlated with PLMS,4,5 has been suggested to be associated with coronary heart disease in previous epidemiologic studies.6–8 Nonetheless, controversial results blur the relationship between RLS and hypertension.6–10 A lower rate of hypertension was found in a study that involved elderly with RLS,10 while 2 other studies did not find any relationship between hypertension and RLS.6,8 On the other hand, a higher rate of hypertension in patients with daily RLS symptoms has been reported elsewhere.7,9 The inconsistency between studies might have arisen due to different methodological approaches. In some studies, hypertension was based on self-report,7–10 or casual BP measurements6 rather than ambulatory BP (ABP) monitoring, which is a more reliable measurement in documenting BP changes.11 In addition, despite a high prevalence of PLMS in patients with RLS,5 none of the previous studies employed polysomnographic studies to investigate for the presence of PLMS.
Sleep laboratory studies found that PLMS were accompanied by significant surges of nocturnal BP,12–14 and the immediate BP fluctuation and variation was suggested to lead to cardiovascular impairment.15 In addition, a higher prevalence of PLMS was found in hypertensive patients with grade III hypertension when compared to those with grade I and II.16 These results have led to the hypothesis that PLMS might precipitate and exacerbate the severity of hypertension. Nevertheless, comorbid PLMS did not aggravate hypertension in adult OSAS patients.17
The impact of PLMS on BP is even more ambiguous in children. PLMS is a common sleep finding in children and adolescents, with prevalence rates of 7.0% to 16.5% in community-based study18,19 and 5.6% to 26% in sleep centers.20–22 PLMS was suggested to have no clinical significance but merely a normal aging process in those subjects without sleep complaints.23 On the other hand, as discussed above, the preliminary association between RLS and cardiovascular disease/hypertension6–9 and immediate surges of BP accompanying periodic limb movements in adults12–14 suggested a probable association between PLMS and cardiovascular disease burden. To the best of our knowledge, the relationship between PLMS and BP and/or hypertension in children has not been investigated. In this study, we sought to examine the association between PLMS and BP in children.
METHODS
Subjects and Study Design
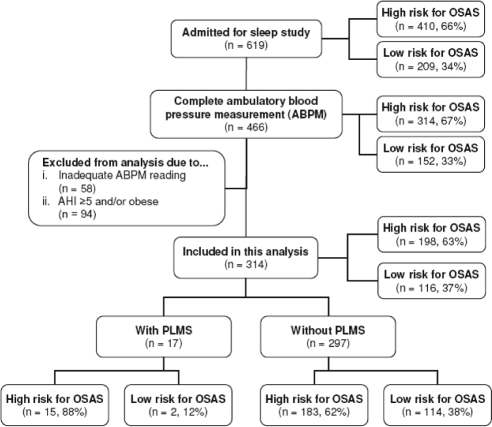
This study was part of an ongoing epidemiologic project investigating sleep problems in Hong Kong Chinese children, which started since late 2003. The protocol was approved by the institutional ethics review committee. The protocol of this study has been described in our previous publications.3,24,25 In brief, a questionnaire consisted of 54 items on demography, sleep environment, family information, sleep habits, problems, and health conditions was completed by parents or caretakers of the children.26 We did not assess specific RLS symptoms among our children. Data from 3 survey questions namely snoring, nocturnal mouth breathing, and night sweating, answered by a 5-point frequency scale (0 = never; 1 = 0-1 night per month; 2 = 1-2 nights per month, 3 = 1-2 nights per week; 4 = ≥ 3 nights per week), were used to classify survey participants as having high (composite score ≥ 7 of a total score of 12) or low risk (score < 7) for OSA.26 Those high risk for OSA (n = 410) and a randomly selected control sample (n = 209) with low risk for OSA were invited to undergo an overnight polysomnographic (PSG) study and ambulatory BP (ABP) monitoring (Figure 1). Due to limited availability of ABP monitoring machines, only 3 of 4 subjects (4 beds unit in our center) were randomly selected for recording. Those children with intercurrent illness within 4 weeks of PSG study, cardiovascular disease, chromosomal abnormalities, or were taking medications that might affect BP measurements were excluded from this study. All the parents and their children gave written informed consent. Anthropometric parameters such as weight, height, waist, and hip circumferences were collected on the day of PSG. Body mass index (BMI) was transformed into BMI z score according to local reference.27 Habitual snoring was defined as snoring ≥ 3 nights /week. Mean sleep duration was calculated from a 1-week sleep log prior to PSG. Insomnia was defined as difficulty in falling asleep, difficulty in maintaining sleep, or early morning awakening ≥ 3 times /week despite adequate opportunity and circumstances for sleep.25 As the effects of OSA3,28 and obesity29 on hypertension were well supported by previous studies, we excluded those children with moderate-severe OSA (apnea-hypopnea index, AHI ≥ 5) and obesity (BMI z score ≥ 1.645, corresponding to the 95th percentile relative to age and gender).
Figure 1.
Flow diagram documenting recruitment of subjects. A total of 619 subjects were admitted for sleep study, 466 of whom also completed ambulatory BP measurement. A total of 152 subjects were excluded from this current analysis because they were (A) AHI ≥ 5 and/or obese (B) inadequate ambulatory BP readings. The PLMS group had a significantly greater proportion of subjects who had been screened as potential high risk for OSAS (X2 = 4.89; P = 0.03)
Polysomnographic Study
All subjects underwent a single overnight PSG.3,26,30 The basic recordings included standard electroencephalogram (C3-A2, C4-A1), electrooculogram (LE-A2, RE-A1), chin EMG, bilateral legs EMG (anterior tibialis muscles), electrocardiogram, nasal airflow pressure, thoracic and abdominal respiratory efforts, oxyhemoglobin saturation, breathing sound, and body posi-tion. All computerized sleep data were analyzed by experienced PSG technologists and clinicians. Sleep stages were scored ac-cording to Rechtschaffen and Kales criteria, using 30-sec epochs.31 Periodic limb movements (PLMs) were defined as limb movements in sleep with EMG bursts of 0.5-5 seconds duration with amplitude ≥ 25% of the physiological calibration signal, and ≥ 4 such events appearing at 5-90 second intervals.32 As respiratory events could lead to arousals associated with limb movements, those movements occurring 0.5 second preceding or following respiratory events (including apnea, hypopnea and respiratory effort related arousal [RERA])32 were excluded. RERA was defined as a sequence of breaths characterized by a noticeable fall in the signal amplitude and flattening of the nasal pressure waveform accompanied by snoring or increasing respiratory effort leading to an arousal from sleep, but not meeting criteria for an apnea or hypopnea.32 The PLM index (PLMI) was defined as the numbers of PLM per hour during sleep.32 Arousals were scored according to American Academy of Sleep Medicine criteria32 and PLMs related arousal was scored when the arousal occurred within 0.5 second preceding or following a PLM. PLMS was diagnosed in subjects with PLMI ≥ 5/hour.
Ambulatory Blood Pressure (ABP) Measurement
ABP was monitored on the day of sleep study by using a validated oscillometric monitor (SpaceLabs 90217, SpaceLabs Medical, Redmond, Washington, USA).33 The proper cuff placed in the non-dominant arm was chosen according to the length of the arm of the subject. Systolic BP (SBP), diastolic BP (DBP), and mean arterial BP (MAP) were measured hourly during nighttime (from 21:30 to 07:00) and every 30 minutes during daytime (from 7:00 to 21:30). A minimum of 7 successful readings during active wakefulness and at least 7 successful readings during sleep were considered to be adequate and included in the analysis.3 Individual mean SBP, DBP, and MAP were calculated for wake and sleep periods. All mean BP variables were transformed into BP z score according to the “LMS” reference values (relative to gender and height).34 In brief, height and gender specific estimates of the distribution median (M), coefficient of variation (S) and degree of skewness (L) by a maximum likelihood of curve fitness technique was performed. Hypertension and pre-hypertension were defined as mean SBP or DBP values > 95th and 90th percentile of the ABP norm, respectively.34 Nocturnal dipping of systolic BP, diastolic BP, and mean arterial pressure were derived by calculating the difference between mean awake and sleep BP and was expressed as a percentage of mean awake BP. Subjects with nocturnal BP dipping of less than 10% were defined as non-dippers.35
Statistical Analysis
Descriptive statistics were presented as number (percentage) for categorical data and as means (standard deviation) for continuous variables respectively. Parametric and non-parametric data were compared using independent t-test and Mann-Whitney U test respectively. Chi-square or Fisher exact tests were used to analyze the differences in proportions between groups. Those variables with P < 0.10 would be recruited to test the association between PLMS and BP by logistic regression (except for basic demographic variables of age and gender). Finally, age, gender, risk for OSA, and birth history were entered into the model to adjust for the effects of PLMS on BP variables. As previous studies suggested that PLMS with arousal was correlated with higher BP changes,12,14 we also explored the correlation between PLMI and PLMS related arousal index (PLMSArI) with BP respectively. Those children with PLMSArI > 0 were recruited into multiple linear regression model analysis (stepwise method) to examine the associations between PLMSArI with BP. The log transformation for PLMI and PLMSArI was performed in view of the skewed distribution of these data. SPSS 14.0 for windows (SPSS Inc, Chicago, IL) was used for all statistical tests.
RESULTS
Figure 1 depicts the recruitment of subjects for this study. No difference was found in the rate of mild OSA between children with PLMS and without PLMS (Table 2). Apart from the differences in arousal index, PLMI, PLMSArI, and higher percentage of potential risk for OSA, there were no significant differences in demographic, anthropometric, and sleep characteristics between the children with and without PLMS (Tables 1 and 2).
Table 2.
Polysomnographic data in PLMS and non-PLMS groups
| Total N = 314 | PLMS N = 17 | Non-PLMS N = 297 | P | |
|---|---|---|---|---|
| Total sleep time, min | 472.0 ± 58.5 | 459.3 ± 73.5 | 472.7 ± 57.6 | 0.36 |
| Sleep efficiency, % | 82.2 ± 10.1 | 80.8 ± 12.7 | 82.3 ± 9.9 | 0.54 |
| REM sleep latency, min | 133.8 ± 55.4 | 145.3 ± 70.9 | 133.1 ± 54.4 | 0.39 |
| Sleep onset latency, min | 23.7 ± 20.0 | 27.2 ± 27.1 | 23.5 ± 19.6 | 0.46 |
| Stage R, % | 21.1 ± 3.8 | 19.8 ± 4.3 | 21.2 ± 3.8 | 0.14 |
| Stage N1, % | 7.1 ± 3.1 | 7.5 ± 3.3 | 7.1 ± 3.1 | 0.62 |
| Stage N2, % | 48.7 ± 5.6 | 47.9 ± 6.1 | 48.7 ± 5.5 | 0.57 |
| Slow wave sleep, % | 23.1 ± 5.3 | 24.8 ± 6.7 | 23.0 ± 5.2 | 0.17 |
| Apnea hypopnea index, /h | 1.3 ± 1.1 | 1.3 ± 1.2 | 1.3 ± 1.1 | 0.38 |
| OAHI ≥ 1, N (%) | 106 (33.8) | 5 (29.4) | 101 (34.0) | 0.70 |
| AHI ≥ 1, N (%) | 159 (50.6) | 7 (41.2) | 152 (51.2) | 0.42 |
| Oxygen desaturation index, /h | 0.42 ± 0.73 | 0.46 ± 0.45 | 0.42 ± 0.75 | 0.85 |
| Lowest SpO2 during REM | 93.2 (2.0) | 92.2 (2.6) | 93.3 (1.9) | 0.10 |
| Lowest SpO2 during NREM | 93.0 (2.3) | 92.8 (1.9) | 93.0 (2.4) | 0.70 |
| PLMI* | 0.8 ± 2.3 | 8.9 ± 4.0 | 0.4 ± 0.9 | < 0.001 |
| Arousal index, /h* | 6.6 ± 2.6 | 9.4 ± 3.5 | 6.4 ± 2.5 | < 0.001 |
| PLMSArI* | 0.3 ± 0.8 | 2.2 ± 1.9 | 0.2 ± 0.5 | < 0.001 |
Definition of abbreviations: PLMI, periodic limb movement index; PLMSArI, periodic limb movements related arousal index; PLMS, periodic limb movement during sleep;
P < 0.05
Table 1.
Demographic, anthropometric data in PLMS and non-PLMS groups
| Total; N = 314 | PLMS; N = 17 | Non-PLMS; N = 297 | P value | |
|---|---|---|---|---|
| Age, y | 10.4 ± 1.7 | 10.2 ± 1.2 | 10.4 ± 1.7 | 0.67 |
| Male gender, N (%) | 196 (62.4) | 14 (82.4) | 182 (61.3) | 0.08 |
| Tanner stage = 1, N (%) | 265 (84.4) | 16 (94.1) | 249 (83.8) | 0.49 |
| Overweight (BMI z score > 1.036), N (%) | 73 (23.2) | 5 (29.4) | 68 (22.9) | 0.56 |
| Body height, cm | 138.6 ± 10.6 | 136.6 ± 8.7 | 138.7 ± 10.7 | 0.43 |
| Body weight, kg | 33.8 ± 8.6 | 32.9 ± 8.9 | 33.9 ± 8.6 | 0.65 |
| Body mass index, m/kg2 | 17.3 ± 2.43 | 17.4 ± 2.8 | 17.3 ± 2.4 | 0.90 |
| Body mass index z score | 0.25 ± 0.91 | 0.22 ± 0.95 | 0.25 ± 0.91 | 0.90 |
| Waist circumference, cm | 61.0 ± 7.2 | 59.9 ± 8.4 | 72.3 ± 8.1 | 0.52 |
| Hip circumference, cm | 72.3 ± 8.1 | 71.9 ± 8.4 | 72.3 ± 8.1 | 0.86 |
| Waist-to-hip ratio | 0.85 ± 0.05 | 0.83 ± 0.04 | 0.85 ± 0.05 | 0.28 |
| Habitual snoring (≥ 3 night/week), N (%) | 96 (30.6) | 4 (23.5) | 92 (31.0) | 0.52 |
| High risk for OSA, N (%) | 198 (63) | 15 (88) | 183 (62) | 0.03* |
| Birth history (full-term baby) (n = 313), N (%) | 292 (93.3) | 14 (82.4) | 278 (93.9) | 0.10 |
| Parent-reported behavioral hyperactivity (n = 313), N (%) | 60 (19.2) | 2 (11.8) | 58 (19.6) | 0.54 |
| ADHD, N (%) | 1 (0.3) | 0 | 1 (0.3) | 0.96 |
| Mean sleep duration (sleep log), hours | 9.44 ± 1.26 | 9.68 ± 1.38 | 0.43 | |
| Usual sleep latency > 30 min, N (%) | 19 (6.1) | 1 (6.3) | 1.00 | |
| Insomnia (≥ 3 times/week), N (%) | 31 (9.9) | 1 (5.9) | 0.88 |
Definition of abbreviations: PLMS, periodic limb movements during sleep; ADHD, clinically diagnosed attention deficit hyperactivity disorder; High risk for OSA, as defined by the composite score of screening questionnaire (please refer to text for details);
P < 0.05
Blood Pressure during Daytime and Nighttime
Table 3 reports the association between PLMS and nocturnal systolic (adjusted OR [95%CI] = 6.25 [1.87-20.88]) and diastolic hypertension (adjusted OR [95%CI] = 4.83 [1.66-14.07]) respectively. In addition, the proportion of nocturnal diastolic non-dippers had a tendency to be greater in PLMS group (adjusted OR [95%CI] = 3.04 [0.95-9.77]); P for adjusted OR = 0.06) (Table 4). However, there were no differences in both nocturnal systolic and diastolic BP absolute values and/or z scores between PLMS and non-PLMS children.
Table 3.
Blood pressure measures in PLMS and non-PLMS groups
| Daytime BP parameters | PLMS; N = 17 | Non-PLMS; N = 297 | P value | Crude OR | Adjusted OR# |
|---|---|---|---|---|---|
| Systolic BP mean (mm Hg) | 115 ± 9 | 111 ± 8 | 0.099 | ||
| Diastolic BP mean (mm Hg) | 73 ± 6 | 71 ± 5 | 0.058 | ||
| Systolic BP z score | 0.23 ± 1.19 | −0.22 ± 1.04 | 0.084 | ||
| Diastolic BP z score | 0.28 ± 0.95 | −0.13 ± 0.83 | 0.051 | ||
| Mean arterial pressure z score | 0.33 ± 0.94 | −0.13 ± 0.83 | 0.077 | ||
| Systolic pre-hypertension, N (%) | 4 (23.5) | 26 (8.8) | 0.067 | 3.21 (0.98-10.55) | 2.84 (0.83-9.72) |
| Diastolic pre-hypertension, N (%) | 2 (11.8) | 19 (6.4) | 0.395 | 1.95 (0.42-9.16) | 1.95 (0.40-9.52) |
| Systolic hypertension, N (%) | 2 (11.8) | 15 (5.1) | 0.233 | 2.51 (0.53-11.98) | 2.44 (0.48-12.48) |
| Diastolic hypertension, N (%) | 1 (5.9) | 10 (3.4) | 0.584 | 1.79 (0.22-14.89) | 1.57 (0.18-13.9) |
| Systolic and diastolic hypertension, N (%) | 1 (5.9) | 4 (1.3) | 0.152 | 4.58 (0.48-43.36) | 6.24 (0.59-66.03) |
| Systolic or diastolic hypertension, N (%) | 2 (11.8) | 21 (7.1) | 0.476 | 1.75 (0.38-8.18) | 1.47 (0.30-7.27) |
| Nighttime BP parameters | |||||
| Systolic BP mean (mm Hg) | 103 ± 12 | 100 ± 8 | 0.176 | ||
| Diastolic BP mean (mm Hg) | 61 ± 8 | 59 ± 5 | 0.308 | ||
| Systolic BP z score | 0.63 ± 1.46 | 0.24 ± 1.03 | 0.292 | ||
| Diastolic BP z score | 0.85 ± 1.46 | 0.24 ± 1.03 | 0.566 | ||
| Mean arterial pressure z score | 0.86 ± 1.29 | 0.71 ± 0.78 | 0.632 | ||
| Systolic pre-hypertension, N (%) | 5 (29.4) | 45 (15.2) | 0.162 | 2.33 (0.78-6.94) | 2.71 (0.88-8.39) |
| Diastolic pre-hypertension, N (%) | 7 (41.2) | 65 (21.9) | 0.077 | 2.50 (0.92-6.82) | 2.55 (0.90-7.22) |
| Systolic hypertension, N (%) | 5 (29.4) | 27 (9.1) | 0.020 | 4.17(1.37-12.72)* | 6.25 (1.87-20.88)* |
| Diastolic hypertension, N (%) | 7 (41.2) | 38 (12.8) | 0.005 | 4.77 (1.71-13.39)* | 4.83 (1.66-14.07)* |
| Systolic and diastolic hypertension, N (%) | 5 (29.4) | 11 (3.7) | 0.001 | 10.83 (3.25-36.13)* | 18.49 (4.60-74.27)* |
| Systolic or diastolic hypertension, N(%) | 7 (41.2) | 53 (17.8) | 0.026 | 3.22 (1.17-8.85)* | 3.34 (1.24-10.02)* |
Pre-hypertension was defined as > 90th percentile of the ABP norm34;
Adjusted for age, gender, risk for OSA, and birth history;
Definition of abbreviations: BP, blood pressure; PLMS, periodic limb movement during sleep;
P < 0.05
Table 4.
Nighttime dipping measures in PLMS and non-PLMS groups
| Nighttime dipping | PLMS; N = 17 | Non-PLMS; N = 297 | P value | Crude OR | Adjusted OR# |
|---|---|---|---|---|---|
| Systolic BP dipping, % | 10.4 ± 6.8 | 10.3 ± 5.6 | 0.981 | ||
| Diastolic BP dipping, % | 17.9 ± 9.0 | 16.9 ± 6.8 | 0.582 | ||
| Mean arterial pressure dipping, % | 13.0 ± 6.9 | 12.3 ± 5.6 | 0.656 | ||
| Systolic BP non-dipper, N (%) | 8 (47.1) | 138 (46.5) | 0.961 | 1.02 (0.39-2.73) | 1.16 (0.42-3.21) |
| Diastolic BP non-dipper, N (%) | 5 (29.4) | 39 (13.1) | 0.072 | 2.76 (0.92-8.25) | 3.04 (0.95-9.77) |
| Mean arterial pressure non-dipper, N (%) | 5 (29.4) | 95 (32.0) | 0.832 | 0.89 (0.30-2.59) | 0.95 (0.32-2.86) |
Non-dipper was difined as nocturnal BP dipping of less than 10%35;
Adjusted for age, gender, risk for OSA, and birth history;
Definition of abbreviations: BP, blood pressure; PLMS, periodic limb movement during sleep
Daytime BP z scores in children with PLMS had a nearly significant trend of higher values than those without PLMS (0.23 ± 1.19 vs −0.22 ± 1.04 in systolic BP and 0.28 ± 0.95 vs −0.13 ± 0.83 in diastolic BP, P = 0.084 and 0.051, respectively). There were no significant differences in daytime systolic and/or diastolic prehypertension and/or hypertension.
Association Between Log Transformed PLMI and PLMSArI with Blood Pressure
The associations between BP and log transformed PLMSArI and PLMI are shown in Table 5. A total of 72 children with PLMSArI > 0 were analyzed. After controlling for potential confounding variables, the models predicted that each 2.72-fold increase in PLMSArI was associated with 2.09 mm Hg increase in nighttime diastolic BP, 1.55 mm Hg increase in nighttime mean arterial BP, and 0.34 unit increase in nighttime diastolic BP z score. The models also predicted that each 2.72-fold increase in PLMI was associated with 8.04 mm Hg increase in daytime systolic BP, 5.28 mm Hg increase in daytime diastolic BP, 5.48 mm Hg increase in daytime mean arterial BP, as well as 0.99 unit increase in daytime systolic BP z score and 0.79 unit increase in daytime mean arterial BP z score (P < 0.05). Systolic and diastolic nighttime BP dipping were not associated with PLMI or PLMSArI (P > 0.05, data not shown).
Table 5.
Association between PLMS related arousal index and PLMS with blood pressure (absolute value and z score) (those patients without PLMS related arousal were excluded):
| Ln_PLMI Unadjusted (n = 72) |
Ln_PLMI Adjusted (n = 72) |
Ln_PLMSArI Unadjusted (n = 72) |
Ln_PLMSArI Adjusted (n = 72) |
|||||
|---|---|---|---|---|---|---|---|---|
| Absolute value | B ± SE | P | B ± SE | P | B ± SE | P | B ± SE | P |
| DSBP | 8.28 ± 3.73 | 0.030 | 8.04 ± 3.46 | 0.023 | – | – | – | – |
| DDBP | 5.39 ± 2.55 | 0.038 | 5.28 ± 2.48 | 0.037 | – | – | – | – |
| DMAP | 5.64 ± 2.58 | 0.032 | 5.48 ± 2.42 | 0.026 | – | – | – | – |
| NSBP | – | – | – | – | – | – | – | – |
| NDBP | – | – | – | – | 2.10 ± 0.79 | 0.009 | 2.09 ± 0.77 | 0.008 |
| NMAP | – | – | – | – | – | – | 1.55 ± 0.73 | 0.038 |
| Z score | ||||||||
| DSBP z score | 1.01 ± 0.47 | 0.033 | 0.99 ± 0.45 | 0.030 | – | – | – | – |
| DDBP z score | 0.86 ± 0.42 | 0.043 | – | – | – | – | – | – |
| DMAP z score | 0.81 ± 0.38 | 0.038 | 0.79 ± 0.36 | 0.033 | – | – | – | – |
| NSBP z score | – | – | – | – | – | – | – | – |
| NDBP z score | – | – | – | – | 0.34 ± 0.14 | 0.015 | 0.34 ± 0.14 | 0.013 |
| NMAP z score | – | – | – | – | – | – | – | – |
Age, gender, patent-report hyperactivity, BMI z score, risk for OSA, body height, tanner stage, sleep duration, log transformed AHI, and habitual snoring were included into initial linear regression models (stepwise method).
Definition of abbreviations: DSBP, daytime systolic blood pressure; DDBP, daytime diastolic blood pressure; DMAP, daytime mean arterial blood pressure; NSBP, nighttime systolic blood pressure; NDBP, nighttime diastolic blood pressure; NMAP, nighttime mean arterial blood pressure.
–, could not be recruited into final models of linear regression to predict BP variables.
Additional Analysis
We also reanalyzed the whole dataset (n = 408) by including those children with prominent OSA (AHI ≥ 5) and obesity. Similar results of the association of nighttime BP changes with PLMS were found, but the association between daytime BP and PLMS was attenuated (see supplementary material for more details).
DISCUSSION
Children with PLMS, as defined by PLMI ≥ 5/hour during sleep, had about 4-6 fold increased odds of suffering from nocturnal hypertension (OR 6.25 for systolic hypertension and OR 4.83 for diastolic hypertension). In addition, there was a trend towards higher daytime BP z score in the PLMS group. Although periodic limb movements were accompanied by immediate surges of BP,12–14 there remained uncertainty as to whether these transient fluctuations of BP would be translated to sustained changes. Thus, our study suggested that PLMS can have long-lasting effects on both nocturnal and probably daytime BP.
Non-dipping BP has been associated with hypertensive target organ damage such as greater left ventricular mass index, even in the absence of sustained diurnal or nocturnal hypertension.36 The current study also found that PLMS had a tendency to be correlated with 3-fold (95% CI, 0.95-9.77) increase in non-dipping diastolic BP.
The mechanism of association between PLMS and hypertension is unclear. Arousal might play an important role in this relationship. PLMS with cortical arousal had higher BP changes than PLMS without arousal.12–14 In this study, PLMSArI was positively correlated with sleep diastolic and mean BP changes. The arousal accompanying periodic limb movements could lead to autonomic activation with consequent increase in BP.12–14 Children with PLMS were found to have reduced vagal activity (as measured by heart rate LF/HF ratio) during sleep period with PLMs.37 These studies indicated that arousal and/or autonomic activation might link cardiovascular impairment with PLMS.
On a neurotransmitter level, dopamine plays an important role in the pathogenesis of both hypertension38 and PLMS.39 Renal dopamine dysfunction has been correlated with various forms of hypertension in human.38 In addition, impairment of dopaminergic transmission has been correlated with PLMS and dopaminergic agents reduce the frequency of PLMI.39 Hypofunction of diencephalospinal dopaminergic pathway was proposed to heighten sympathetic efferent drive and suppress sensory afferent drive with consequent sympathetic hyperactivity leading to RLS/PLMS and hypertension.40,41 We speculate that iron deficiency, either via dopaminergic system dysfunction or other pathways,42 might mediate the association between PLMS and hypertension. Interestingly, higher daily dietary iron intake has been found to be correlated with lower systolic BP in a cross sectional epidemiological study including 17 population samples from different countries.43 Further study is needed to clarify the role of iron deficiency and dopaminergic transmission on the relationship between childhood PLMS and hypertension.
One might also be concerned that sleep quality and quantity might mediate the effects of PLMS on hypertension in children, as short sleep duration and poor sleep quality were correlated with elevated BP or hypertension in both adolescents and adults.1,2 However, reports of the effects of PLMS on sleep quality and subjective sleep complaints are contradictory.44 In our study, no differences were found between PLMS and non-PLMS children in both sleep duration and quality (Tables 1 and 2). Thus, our results did not support the hypothesis that PLMS lead to hypertension by worsening sleep quality or shortening sleep duration. On the other hand, one might also be concerned that PLMs found in our study might be related to respiratory events. In view of this, we excluded those limb movements following respiratory events. Furthermore, the exclusion of those children with AHI ≥ 5 minimized the potential influences of respiratory events on scoring of PLMs. In fact, the parameters related to OSA (AHI, oxygen desaturation index and lowest SpO2 during sleep) did not differ between PLMS and non-PLMS groups. We also excluded those children with known cardiovascular diseases as pervious studies suggested that PLMS was commonly found in patients with heart failure.45
We did not use the term PLMD (periodic limb movement disorder) as this term implies the presence of sleep disturbances symptoms in addition to PLMS. Unlike adult PLMS, the relationship between childhood PLMS and RLS is not certain.46 Thus, we did not assess RLS symptoms in this study but focused on the relationship between PLMS and BP.
Clinical Implications
PLMS are common in community studies18,19 and in about 5.4% of children of our study. Our findings of increased risk of nighttime hypertension and non-dipping BP among children with PLMS suggested the need for further study of PLMS in children. Childhood elevated BP has been found to predict the development of hypertension and metabolic syndrome in adulthood47 and non-dipping BP has been associated with target organ damage.48 Another striking phenomenon is the similar or even greater magnitude of the association in nocturnal hypertension with PLMS and that of moderate-severe OSA (OR = 6.25 (PLMS) vs. 3.9 (OSA) for systolic hypertension and OR = 4.83 (PLMS) vs. 3.3 (OSA) for diastolic hypertension, respectively).3 Should PLMS be a major cause of hypertension in children, effective management of childhood PLMS, for example by iron therapy,49 might provide a window for the treatment of hypertensive patients with PLMS, especially for those subjects with low ferritin level.49
Limitations
One of the limitations for this study was the unwillingness of some subjects to undergo 24-hour BP monitoring because of school and other social commitments. Nonetheless, all our sleep BP measurements satisfied the criteria as needed for adequate ABP monitoring.35 Second, the nature of cross-sectional study limited the conclusion of cause-effect relationship between PLMS and hypertension. Third, absence of esophageal manometry would not allow us to assess respiratory effort precisely. Forth, serum ferritin levels, caffeine intake, and sympathetic activities were not assessed in our study. Fifth, in view of night to night variability in the PLMI, a single night PSG study may not be the most accurate way to assess PLMS.50,51 Sixth, a trend of more preterm babies were found in children with PLMS. However, we did not ask specifically about birthweight of the children in view of potential reporting/recall bias, albeit low birthweight is proposed as a potential risk factor for future hypertension.52 Finally, our sample was selected from community subjects based on questionnaire screening of high and low risk of obstructive sleep apnea. One should be cautioned when generalizing our conclusions to other population. Nonetheless, our study has several strengths. Firstly, the recruitment of subjects from the community instead of hospital attendants would have minimized referral bias. We had also excluded those obese and moderate to severe OSA subjects from the study cohort as well as controlled for a series of confounding variables. Lastly, the employment of ambulatory measurements rather than casual readings provided a more reliable evaluation of BP changes.
CONCLUSIONS
PLMS are common and their consequences are under-recognized in children. Using ambulatory BP monitoring, PLMS was demonstrated to be associated with nocturnal hypertension and possibly non-dipping BP as well as daytime elevated BP. There was a linear association between PLM index and PLMS arousal index with BP. Further studies with larger datasets across different populations are necessary to confirm our results. Prospective and interventional studies are necessary to delineate the causal-effect relationship between PLMS and hypertension and to clarify the pathophysiological mechanisms and the treatment effects.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Wing has received research support from Sanofi-Aventis. Mr. Ho has participated in speaking engagements for Tyco Healthcare.
ACKNOWLEDGMENTS
We thank the logistic support of all the staff of the Sleep Assessment Unit at Shatin Hospital, the cooperation and participation of all the schools, teachers, children and their parents.
This study was part of the epidemiological study funded by grant (CUHK4161/02M) from the Research Grants Council of the Hong Kong SAR, China.
REFERENCES
- 1.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation. 2008;118:1034–40. doi: 10.1161/CIRCULATIONAHA.108.766410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 3.Li AM, Au CT, Sung RY, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63:803–9. doi: 10.1136/thx.2007.091132. [DOI] [PubMed] [Google Scholar]
- 4.Walters AS, Wagner M, Hening WA. Periodic limb movements as the initial manifestation of restless legs syndrome triggered by lumbosacral radiculopathy. Sleep. 1996;19:825–6. [PubMed] [Google Scholar]
- 5.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997:1261–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 6.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 7.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006:7545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16:1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 10.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54:1064–8. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 11.Urbina E, Alpert B, Flynn J, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–51. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 12.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 13.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 14.Siddiqui F, Strus J, Ming X, Lee A, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Zakopoulos NA, Tsivgoulis G, Barlas G, et al. Impact of the time rate of blood pressure variation on left ventricular mass. J Hypertens. 2006;24:2071–7. doi: 10.1097/01.hjh.0000244957.47114.88. [DOI] [PubMed] [Google Scholar]
- 16.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–7. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Alawi A, Mulgrew A, Tench E, Ryan CF. Prevalence, risk factors and impact on daytime sleepiness and hypertension of periodic leg movements with arousals in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:281–7. [PubMed] [Google Scholar]
- 18.O'Brien LM, Holbrook CR, Faye Jones V, Gozal D. Ethnic difference in periodic limb movements in children. Sleep Med. 2007;8:240–6. doi: 10.1016/j.sleep.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree VM, Ivanenko A, O'Brien LM, Gozal D. Periodic limb movement disorder of sleep in children. J Sleep Res. 2003;12:73–81. doi: 10.1046/j.1365-2869.2003.00332.x. [DOI] [PubMed] [Google Scholar]
- 20.Bokkala S, Napalinga K, Pinninti N, et al. Correlates of periodic limb movements of sleep in the pediatric population. Pediatr Neurol. 2008;39:33–9. doi: 10.1016/j.pediatrneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Martin BT, Williamson BD, Edwards N, Teng AY. Parental symptom report and periodic limb movements of sleep in children. J Clin Sleep Med. 2008;4:57–61. [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk VG, Bohn S. Periodic limb movements in children: prevalence in a referred population. Sleep. 2004;27:313–5. doi: 10.1093/sleep/27.2.313. [DOI] [PubMed] [Google Scholar]
- 23.Carrier J, Frenette S, Montplaisir J, Paquet J, Drapeau C, Morettini J. Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord. 2005;20:1127–32. doi: 10.1002/mds.20506. [DOI] [PubMed] [Google Scholar]
- 24.Chan YS, Li AM, Au CT, et al. Cardiac remodeling and dysfunction in children with obstructive sleep apnea-a community based study. Thorax. 2008;64:233–9. doi: 10.1136/thx.2007.094904. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Li AM, Kong AP, Lai KY, Tang NL, Wing YK. A community-based study of insomnia in Hong Kong Chinese children: Prevalence, risk factors and familial aggregation. Sleep Med. 2009;10:1040–6. doi: 10.1016/j.sleep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Li AM, Cheung A, Chan D, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol. 2006;41:1153–60. doi: 10.1002/ppul.20505. [DOI] [PubMed] [Google Scholar]
- 27.Leung SS, Cole TJ, Tse LY, Lau JT. Body mass index reference curves for Chinese children. Ann Hum Biol. 1998;25:169–74. doi: 10.1080/03014469800005542. [DOI] [PubMed] [Google Scholar]
- 28.Bixler EO, Vgontzas AN, Lin HM, et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52:841–6. doi: 10.1161/HYPERTENSIONAHA.108.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–7. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 30.Wing YK, Hui SH, Pak WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88:1043–7. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/ Brain Research Institute; 1968. UCLA. [Google Scholar]
- 32.American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 33.O'Brien E. The Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Blood Press Monit. 2003;8:17–8. doi: 10.1097/01.mbp.0000057011.67622.05. [DOI] [PubMed] [Google Scholar]
- 34.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–48. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community-dwelling normotensives. Am J Hypertens. 2003;16:434–8. doi: 10.1016/s0895-7061(03)00567-3. [DOI] [PubMed] [Google Scholar]
- 37.Walter LM, Foster AM, Patterson RP, et al. Cardiovascular variability during periodic leg movements in sleep in children. Sleep. 2009;32:1093–9. doi: 10.1093/sleep/32.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–42. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 39.Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:560–83. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- 40.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 41.Walters AS, Rye DB. Review of the relationship of restless legs syndrome / periodic limb movements in sleep to hypertension, heart disease and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Tzoulaki I, Brown IJ, Chan Q, et al. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ. 2008;337:a258. doi: 10.1136/bmj.a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. 2006;106:21–8. doi: 10.1016/j.ijcard.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 46.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 47.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237–46. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- 48.Birkenhager AM, van den Meiracker AH. Causes and consequences of a non-dipping blood pressure profile. Neth J Med. 2007;65:127–31. [PubMed] [Google Scholar]
- 49.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 50.Hornyak M, Kopasz M, Feige B, Riemann D, Voderholzer U. Variability of periodic leg movements in various sleep disorders: implications for clinical and pathophysiologic studies. Sleep. 2005;28:331–5. [PubMed] [Google Scholar]
- 51.Sforza E, Haba-Rubio J. Night-to-night variability in periodic leg movements in patients with restless legs syndrome. Sleep Med. 2005;6:259–67. doi: 10.1016/j.sleep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertens. 2003;5:133–6. doi: 10.1111/j.1524-6175.2003.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]