Abstract
Study Objectives:
To analyze cyclic alternating pattern (CAP) in restless legs syndrome (RLS) and the eventual changes induced by the acute administration of pramipexole.
Setting:
Sleep clinic in a scientific research institute.
Interventions:
Placebo or pramipexole 0.25 mg.
Methods:
Thirty-four patients were included: 19 patients received 0.25 mg of pramipexole and 15 were given placebo. The control group included 13 normal subjects. Nocturnal polysomnography was carried out in all subjects, and a second night was recorded after pramipexole or placebo was administered to patients with RLS. Sleep stages, CAP, and leg movement activity were scored following standard criteria.
Measurements and Results:
At baseline, rapid eye movement sleep latency was significantly longer in patients with RLS than in normal control subjects, and the periodic leg movement during sleep index (PLMS) was also significantly higher. On the contrary, many CAP parameters appeared to be significantly different, with a general increase in CAP rate in patients with RLS. Acute administration of pramipexole induced moderate changes in sleep architecture (increased number of stage shifts/h, sleep efficiency, and percentage of stage 2 sleep; decreased wakefulness after sleep onset; and a lower PLMS index. No effects of treatment on CAP were observed.
Conclusion:
Patients with RLS show significant abnormalities in sleep microstructure, represented by an excessive sleep instability/discontinuity. Acute pramipexole administration seems to exert no action on these abnormalities; the moderate effects seen on sleep architecture might be interpreted as the beneficial consequence of the removal of presleep RLS symptoms and PLMS.
Citation:
Ferri R; Manconi M; Aricò D; Sagrada C; Zucconi M; Bruni O; Oldani A; Ferini-Strambi L. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. SLEEP 2010;33(6):793-800.
Keywords: Restless legs syndrome, dopamine agonist, cyclic alternating pattern, pramipexole, periodic leg movements
RESTLESS LEGS SYNDROME (RLS) IS OFTEN ASSOCIATED WITH INSOMNIA, IN TERMS OF DIFFICULTY IN FALLING ASLEEP, SLEEP MAINTENANCE, AND SLEEP duration.1 Thus, sleep disruption mainly comes from the subjective report of patients and from the clinical experience of physicians. The severity of RLS-related insomnia ranges widely, from patients who seek treatment only for insomnia and underestimate their sensory symptoms to those who have no sleep complaints at all because the sensory symptoms are mild, rare, or occur prior to bedtime. Thus, insomnia is not considered necessary for or supportive of the diagnosis of RLS.1
Only a few studies have focused on the objective polysomnographic features of RLS; they have found an increase in sleep latency and a decrease in sleep efficiency only in patients with the most severe RLS symptoms.2–6 Except for the generic amount of arousals, no extensive data are available on sleep microstructure in RLS, as compared with age-matched normal control subjects. A very recent study demonstrated that sleep microstructure analysis by means of the cyclic alternating pattern (CAP)7 is helpful in understanding the mechanisms of subjective sleep perception (and misperception) in insomniacs.8 In addition, emerging evidence is available on a possible role for CAP in sleep-related cognitive processing, and sleep loss is thought to be an important factor affecting cognitive performance of patients with RLS. Periodic leg movements during sleep (PLMS) represent a very frequent objective finding in RLS, the contribution of PLMS to sleep disruption and sleep quality is an ongoing discussion.9,10
Dopamine-receptor agonists are remarkably effective and well-tolerated agents for the treatment of RLS; low evening doses of the D3-agonists pramipexole and ropinirole have become the first-line treatment for RLS.11,12 Controlled studies that include polysomnography recordings have provided evidence that ropinirole and pramipexole are effective in reducing both subjective symptoms of RLS and PLMS, even after a first single administration, in patients with RLS. On the contrary, the effects on sleep architecture have been reported in only a few studies, which failed to find extensive modifications of objective polysomnography-derived measures, other than PLMS.2–6 Even with successful treatment of the sensory symptoms of RLS, sleep problems may persist.13,14
Response to dopaminergic medications, together with positive family history and presence of PLMS, is considered to be an important supportive criterion for the diagnosis of RLS.1 The placebo effect in RLS treatment is a major issue that can impair the clinical judgment. The primary outcome measure in most studies, the International Restless Legs Syndrome Rating Scale, has been shown to yield a large placebo effect.15 In brief, the placebo effect seems to be large for subjective parameters but much smaller for objective parameters derived from polysomnography. To our knowledge, there are no studies on CAP in RLS and on the therapeutic effect of dopamine agonists on CAP in these patients. For all of these reasons, the aim of the present investigation was to analyze, in detail, the eventual baseline differences in CAP between normal control subjects and patients with RLS and the eventual changes in sleep architecture and instability induced by the acute administration of pramipexole in patients with RLS.
SUBJECTS AND METHODS
Subjects
A prospective single-blind placebo-controlled study with consecutive enrollment of subjects affected by idiopathic RLS was carried out on patients admitted to the Sleep Disorders Center, Ospedale San Raffaele, Milan. The diagnosis of RLS was established following the International RLS Study Group criteria.1 In addition, for patients to be included in the study, they had to have a mean frequency of symptoms during the last 6 months more often than twice per week, with a score of at least 20 on the International Restless Legs Syndrome Rating Scale (corresponding to severe RLS).16 Only patients who were between the ages of 35 and 85 years, were not taking any medication at the time of the study, and had never been treated for RLS (including with dopaminergic agents, benzodiazepines, opioids, or anticonvulsants) were included in the study. Furthermore, subjects suffering from known causes of secondary RLS (renal failure, anemia with iron deficiency, pregnancy, rheumatoid arthritis, recent anesthesia, or clinical myelopathy and peripheral neuropathy), other sleep disorders (e.g., narcolepsy, sleep terrors, sleepwalking, sleep disordered breathing), or other movement disorders or who were had any other medical conditions that would affect the assessment of RLS were excluded from the study.
All patients underwent a neurologic examination, routine blood tests (including serum iron and ferritin, B12 vitamin, and folate concentrations), electromyography (EMG), and electroneurography of the lower limbs. Patients with any abnormality at the above-mentioned tests or with an apnea-hypopnea index greater than 5 were also excluded.
All subjects underwent 2 nocturnal polysomnographic recordings, after 1 adaptation night, and were randomly subdivided into 2 subgroups: pramipexole and placebo groups. No medication was administered before the first night recording (baseline). Before the second night of recording, 1 group received a single oral dose of 0.25 mg of pramipexole at 21:00, whereas the other group received a placebo (single-blind procedure). In the morning, after each polysomnography recording, all patients evaluated the severity of their previous evening presleep RLS symptoms by means of a visual-analog scale (VAS)17 and were also asked to describe the quality of their sleep as improved, not improved, or worsened. All patients gave their written consent for these procedures and were unaware of the content (drug or placebo) of the medication. The local ethics committee approved the study.
Finally, a group of normal control subjects also underwent only 1 nocturnal polysomnographic recording, after 1 adaptation night. Control subjects were screened to exclude sleep disorders such as RLS and had to be in general good health; exclusion criteria were otherwise the same as those used for patients with RLS.
Nocturnal Polysomnography
Nocturnal polysomnography was carried out after 1 adaptation night in a standard sound-attenuated (noise level to a maximum of 30 dB normal hearing level) sleep-laboratory room. Subjects were not allowed to consume caffeinated beverages the afternoon preceding the recordings and were allowed to sleep until their spontaneous awakening in the morning. Lights-out time was based on individual habitual bedtime and ranged between 22:30 and 23:00. The following signals were recorded: electroencephalogram (EEG; at least 6 channels, including C3 and C4, referred to the contralateral mastoid); electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to the left mastoid), EMG of the submentalis muscle, EMG of the right and left tibialis anterior muscles (bipolar derivations with 2 electrodes placed 3 cm apart on the belly of the tibialis anterior muscle of each leg, impedance was kept less than 10 KΩ), and electrocardiogram (CM4 derivation: anode in position V4 and cathode attached to the manubrium of the sternum). Sleep signals were sampled at 200 Hz and stored on hard disk in European data format for further analysis. EMG signals, in particular, were digitally band-pass filtered at 10 to100 Hz, with a notch filter at 50 Hz. The sleep respiratory pattern of each patient was monitored using oral and nasal airflow thermistors and/or nasal pressure cannula, thoracic and abdominal respiratory effort strain gauge, and oxygen saturation (pulse oximetry). This was performed in all subjects in a previous recording (within 1 week) or during the study recording.
Sleep Scoring and CAP Analysis
Sleep stages were scored following standard criteria on 30-second epochs.18 Subsequently, each CAP A phase was detected in each recording on the C3/A2 or C4/A1 derivation, based on the availability in the polysomnographic recording and on the absence of long periods with artifacts; the side of this EEG channel should not influence the detection of CAP because its components have been shown to map symmetrically over the scalp.19 All CAP phases during non-rapid eye movement sleep (NREM) were detected and classified into 3 subtypes (A1, A2, and A3) according to Terzano et al.7
CAP was detected by the sleep-analysis software Hypnolab 1.2 (SWS Soft, Italy), which allows computer-assisted detection of CAP-A phase subtypes. With this software, detection is performed by means of a human-supervised automatic approach controlled by the scorer. The performances of this system have been evaluated and validated,20 but, for this study, the scorer visually edited the detections proposed by the automatic analysis before the computation of the various CAP parameters, which were automatically generated by the same software and used for statistical analysis. To reduce the effects of interrater variability expected for this type of analysis,20 all recordings were scored by 1 of the authors of this study (DA).
Scoring Rules for the CAP
CAP is organized in sequences of 2 or more CAP cycles. Each CAP cycle consists of a phase A and a phase B, each lasting between 2 and 60 seconds. All CAP sequences begin with a phase A and end with a phase B. In NREM sleep, the phase A patterns comprise (1) intermittent alpha rhythms and sequences of vertex sharp waves in stage 1 sleep; (2) sequences of 2 or more K-complexes alone or followed by alpha-like components and beta rhythms in stage 2 sleep; (3) delta bursts that exceed by at least one-third the amplitude of the background activity in stages 3 and 4 sleep; and (4) transient activation phases and EEG arousals in all sleep stages.
CAP phase B is defined as the intervening background, typical of each sleep stage, identified by the interval separating the repetitive elements (A phases of CAP). The period of sleep between 2 successive A phases separated by an interval more than 60 seconds is scored as non-CAP (NCAP). CAP appears as a synchronous and widely diffuse EEG activity on both hemispheres with minor differences in morphology and amplitude across the various leads. A CAP sequence can bridge across different sleep stages showing different stage-related patterns. Multiple EEG derivations are indicated for accurate visual analysis.
The identification of CAP should be preceded by the definition of sleep stages according to the conventional criteria. An EEG pattern showing the characteristics of a phase A is initially classified as a phase-A candidate. A phase-A candidate can be scored within a CAP sequence only if it preceded and/or followed by another phase A in the temporal range of 2 to 60 seconds.
In NREM sleep, the CAP sequences may extend across successive sleep stages, and the A phases may present different patterns within the same CAP sequence. In rapid eye movement (REM) sleep, because of the lack of EEG synchronization, the A phases consist exclusively of desynchronized patterns (transient activation phases or arousals).
On the basis of the information derived from EEG activities, muscle tone, and neurovegetative responses, a 3-stage hierarchy of arousal strength can be identified. Subtype A1 is a phase in which EEG synchrony is the predominant activity. In A1 phases, the following activities are included: intermittent alpha rhythm and vertex sharp waves during stage 1 sleep and sequences of K-complexes or delta bursts in other NREM stages. If present, EEG desynchronization occupies less than 20% of the entire phase A. Subtype A1 is generally associated with mild autonomic and somatomotor activity.
Subtype A2 includes phases that contain a mixture of slow and rapid EEG activities. In A2 phases, the following activities are included: K-alpha, arousal preceded by slow wave synchronization, and polyphasic bursts with more than 20% but less than 50% of EEG desynchronization. Subtype A2 is linked with a moderate increase of muscle tone, cardiorespiratory rate, or both.
Subtype A3 are phases with predominant EEG desynchronization, including arousals, transient activation phases, or polyphasic bursts with more than 50% of EEG desynchronization. Subtypes A3 are coupled with a remarkable enhancement of muscle tone, cardiorespiratory rate, or both.
CAP rate is generally calculated as the percentage of NREM sleep or of each NREM sleep stage occupied by CAP sequences; for a complete set of rules and examples for scoring CAP, refer to Terzano et al.7
Analysis of Leg Movements During Sleep
Prior to any recording, we verified that the rectified EMG amplitude recorded from the 2 tibialis anterior muscles was less than 2 μV at rest and exceeded 7 to 10 μV for small voluntary flexions of the foot. Sleep stages were visually scored following standard criteria on 30-second epochs using the sleep-analysis software Hypnolab 1.2. Leg movements during sleep were first detected by the same software that allows their computer-assisted detection. With this software, the detection is performed on the rectified EMG signal, using a human-supervised automatic approach controlled by the scorer (blind to the treatment assignment) that used the newly introduced World Association of Sleep Medicine-International RLS Study Group criteria.21 The performance of this system has been evaluated and validated,22 but, in this study, 1 scorer, unaware of treatment assignment, visually edited the detections proposed by the automatic analysis before computing the final results. In particular, the PLMS index was calculated as the number of leg movements included in a series of 4 of more, separated by more than 5 and less than 90 seconds, per hour of sleep.
Statistical Analysis
The comparison between the different sleep scoring and leg-movement parameters obtained before and after the administration of pramipexole or placebo was carried out by means of the nonparametric Mann-Whitney test for independent datasets and the Wilcoxon test for paired datasets, as required. The Bonferroni correction was also used to take into account the multiple comparisons performed. For this analysis, the commercially available Statistica software package (StatSoft, Inc., Tulsa, OK, STATISTICA data analysis software system, version 6.) was used. Differences were considered significant when they reached the P < 0.05 level.
RESULTS
Thirty-four consecutive untreated patients were included in this study (11 men and 23 women); 19 patients (6 men and 13 women, mean age 57.6 years, 9.44 SD) received pramipexole and 15 (5 men and 10 women, mean age 55.5 years, 11.88 SD) were given placebo. The mean score on the International Restless Legs Syndrome Rating Scale was 26.0 (range 20-37) for the whole group, 25.5 (range 20-34) for the group treated with pramipexole, and 26.5 (range 21-37) for the group receiving placebo. The control group included 13 normal control subjects (5 men and 10 women, mean age 60.7 years, 12.51 SD).
Table 1 shows the baseline sleep-architecture parameters and PLMS index in normal control subjects and the whole group of patients with RLS. Among these parameters, only REM sleep latency was significantly longer in patients with RLS than in normal control subjects; the PLMS index was significantly higher in the patient group, as expected.
Table 1.
Baseline sleep-architecture parameters and PLMS index in normal control subjects and patients with RLS
| Controls (n = 13) | RLS (n = 34) | P Value | |
|---|---|---|---|
| TIB, min | 503.2 ± 78.87 | 525.9 ± 67.36 | NS |
| SPT, min | 463.0 ± 58.40 | 488.6 ± 70.89 | NS |
| TST, min | 376.7 ± 57.02 | 390.0 ± 79.83 | NS |
| Sleep latency, min | |||
| Overall | 28.2 ± 30.10 | 25.4 ± 28.75 | NS |
| REM | 67.8 ± 37.85 | 113.8 ± 61.22 | 0.018 |
| Stage shift, no/h | 9.4 ± 2.17 | 11.8 ± 4.19 | NS |
| Awakenings, no/h | 4.6 ± 2.86 | 4.4 ± 2.57 | NS |
| Sleep efficiency,% | 76.1 ± 14.44 | 74.4 ± 13.18 | NS |
| WASO, % | 17.7 ± 14.68 | 20.1 ± 12.81 | NS |
| Sleep stage, % | |||
| 1 | 4.4 ± 3.82 | 5.8 ± 4.72 | NS |
| 2 | 50.5 ± 13.11 | 42.0 ± 10.31 | NS |
| SWS | 11.2 ± 9.25 | 15.8 ± 8.36 | NS |
| REM | 16.2 ± 6.87 | 16.3 ± 6.11 | NS |
| PLMS index | 9.2 ± 10.40 | 41.0 ± 28.41 | 0.00002a |
Data are presented as mean ± SD. The Mann-Whitney test was used to determine the P values. The percentages of wakefulness after sleep onset (WASO) and sleep stages are referred to sleep period time (SPT).
RLS refers to restless legs syndrome; PLMS, periodic leg movements of sleep; TIB, time in bed; TST, total sleep time; REM, rapid eye movement; SWS, slow wave sleep
Significant after Bonferroni correction.
The mean VAS score for presleep RLS symptoms before and after treatment changed from 6.8 ± 1.47 to 1.3 ± 1.74 (P < 0.0035 Wilcoxon test) in the pramipexole group and from 7.0 ± 1.58 SD to 5.8 ± 2.35 (not significant) in the placebo group. The self-reported quality of nocturnal sleep was not improved in the majority of patients in both subgroups.
Table 2 shows the effects of the first night of treatment with pramipexole or placebo on sleep-architecture parameters and the PLMS index in the 2 subgroups of patients with RLS. Patients treated with pramipexole showed a mild but significant increase in number of stage shifts per hour, which was accompanied by an increase in sleep efficiency and percentage of stage 2 sleep and by a decrease in the amount of wakefulness after sleep onset and a very evident decrease in the PLMS index. On the contrary, patients with RLS who were treated with placebo did not show any significant change in sleep-architecture parameters or the PLMS index.
Table 2.
Sleep-architecture parameters and PLMS index in patients with RLS at baseline and after pramipexole or placebo treatment
| PRAMIPEXOLE (n=19) | Baseline | Treatment | P Value |
|---|---|---|---|
| TIB, min | 532.3 ± 65.00 | 523.2 ± 45.69 | NS |
| SPT, min | 495.5 ± 71.76 | 498.6 ± 49.69 | NS |
| TST, min | 382.4 ± 80.71 | 415.7 ± 62.51 | NS |
| Sleep latency, min | |||
| Overall | 25.3 ±33.28 | 15.2 ± 14.94 | NS |
| REM | 125.4 ±72.59 | 131.1 ± 80.11 | NS |
| Stage shift, no/h | 11.4 ±4.13 | 13.7 ± 4.44 | 0.028 |
| Awakenings, no/h | 4.6 ±2.61 | 5.6 ± 3.63 | NS |
| Sleep efficiency,% | 72.1 ±14.06 | 79.4 ± 9.28 | 0.022 |
| WASO, % | 23.0 ± 12.14 | 16.6 ± 9.06 | 0.036 |
| Sleep stage, % | |||
| 1 | 5.1 ± 5.11 | 5.5 ± 5.22 | NS |
| 2 | 40.3 ± 9.40 | 51.2 ± 12.59 | 0.0017a |
| SWS | 16.4 ± 7.33 | 15.1 ± 8.03 | NS |
| REM | 15.2 ± 6.52 | 11.6 ± 4.88 | NS |
| PLMS index | 40.2 ± 30.20 | 7.9 ± 10.66 | 0.00013a |
| PLACEBO (n=15) | |||
| TIB, min | 517.8 ± 71.66 | 474.4 ± 98.64 | NS |
| SPT, min | 479.9 ± 71.27 | 444.9 ± 94.95 | NS |
| TST, min | 399.7 ± 80.40 | 353.7 ± 81.39 | NS |
| Sleep latency, min | |||
| Overall | 25.5 ± 22.90 | 19.8 ± 21.87 | NS |
| REM | 99.1 ± 40.56 | 125.6 ± 65.84 | NS |
| Stage shift, no/h | 12.2 ± 4.37 | 12.1 ± 4.32 | NS |
| Awakenings, no/h | 4.3 ± 2.59 | 4.3 ± 2.50 | NS |
| Sleep efficiency,% | 77.4 ± 11.79 | 75.3 ± 12.20 | NS |
| WASO, % | 16.4 ± 13.08 | 19.5 ±13.24 | NS |
| Sleep stage, % | |||
| 1 | 6.7 ± 4.16 | 6.1 ± 4.74 | NS |
| 2 | 44.3 ± 11.30 | 43.4 ± 10.52 | NS |
| SWS | 14.9 ± 9.71 | 16.2 ± 6.54 | NS |
| REM | 17.7 ± 5.43 | 14.7 ± 4.67 | NS |
| PLMS index | 42.0 ± 26.97 | 54.4 ± 33.42 | NS |
Data are presented as mean ± SD. The Wilcoxon test was used to determine the P values. The percentages of wakefulness after sleep onset (WASO) and sleep stages are referred to sleep period time (SPT).
RLS refers to restless legs syndrome; PLMS, periodic leg movements of sleep; TIB, time in bed; TST, total sleep time; REM, rapid eye movement; SWS, slow wave sleep
Significant after Bonferroni correction
Table 3 shows the baseline CAP parameters in normal control subjects and the whole group of patients with RLS. In this table, many parameters appear to be significantly different in the 2 groups of subjects. Total CAP rate is significantly increased, as is the CAP rate during stage 2 sleep and in slow wave sleep, in patients with RLS. Patients with RLS also show a significantly different percentage distribution of the CAP A phase subtypes, with a decrease in A1 and an increase in A2 and A3. The number of A2 and A3 phases per hour of sleep is also significantly increased in patients with RLS, and the B phase of CAP shows a significant decrease in duration. Finally, the mean duration of CAP sequences is significantly longer in patients with RLS than in normal control subjects.
Table 3.
Baseline CAP parameters in normal control subjects and patients with RLS
| CAP rate, % | Controls (n = 13) | RLS (n = 34) | P Value |
|---|---|---|---|
| Total | 42.0 ± 14.67 | 75.2 ± 11.90 | 0.000006a |
| During sleep stage | |||
| 1 | 34.2 ± 24.21 | 67.5 ± 117.83 | NS |
| 2 | 39.2 ± 17.99 | 75.1 ± 13.57 | 0.000013a |
| SWS | 60.5 ± 22.46 | 82.8 ± 14.33 | 0.01 |
| CAP A subtype, % | |||
| A1 | 59.4 ± 21.78 | 31.7 ± 13.40 | 0.0005a |
| A2 | 25.1 ± 11.45 | 35.5 ± 11.12 | 0.017 |
| A3 | 15.5 ± 12.91 | 32.9 ± 16.55 | 0.002a |
| CAP A subtype mean duration, s | |||
| A1 | 7.7 ± 1.79 | 7.5 ± 0.90 | NS |
| A2 | 10.3 ± 3.16 | 8.1 ± 0.78 | 0.038 |
| A3 | 11.4 ± 3.43 | 11.2 ± 1.47 | NS |
| CAP A subtype, no/h | |||
| A1 | 34.1 ± 16.43 | 36.1 ± 17.49 | NS |
| A2 | 15.4 ± 10.47 | 39.6 ± 14.68 | 0.000053a |
| A3 | 8.3 ± 9.26 | 36.6 ± 26.32 | 0.000012a |
| B phase mean duration, s | 22.8 ± 2.81 | 17.6 ± 2.60 | 0.000022a |
| CAP sequence | |||
| Mean duration, s | 209.7 ± 41.45 | 473.3 ± 221.04 | 0.000005a |
| Number | 35.8 ± 9.90 | 32.5 ± 8.95 | NS |
Data are presented as mean ± SD. The Mann-Whitney test was used to determine the P values.
CAP refers to cyclic alternating pattern; RLS, restless legs syndrome; SWS, slow wave sleep
Significant after Bonferroni correction.
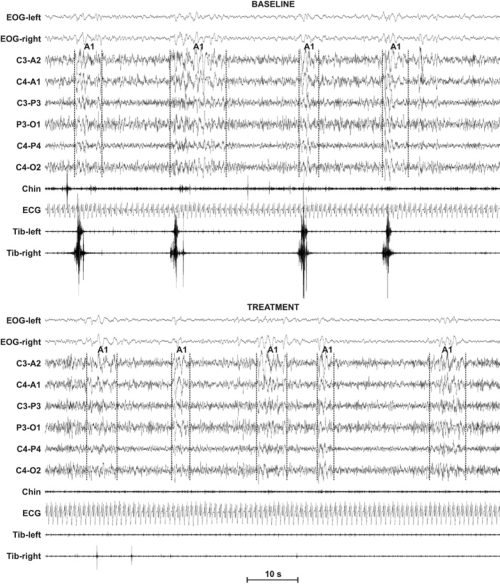
Table 4 shows the effects of the first night of treatment with pramipexole or placebo on CAP parameters in the 2 subgroups of patients with RLS. None of the comparisons contained in this table reached statistical significance; thus, no treatment effects were observed on CAP after the first administration of either pramipexole or placebo. Figure 1 shows an example of polysomnographic recording of one of the subjects treated with pramipexole at baseline, with the presence of CAP accompanied by PLMS, and after treatment, with persistent CAP not accompanied by PLMS.
Table 4.
CAP parameters in patients with RLS at baseline and after pramipexole or placebo treatment
| PRAMIPEXOLE (n = 19) | ||
|---|---|---|
| CAP Rate, % | Baseline | Treatment |
| Total | 73.3 ± 13.71 | 71.8 ± 14.59 |
| During sleep stage | ||
| 1 | 78.8 ± 157.99 | 38.9 ± 24.49 |
| 2 | 73.0 ± 15.90 | 72.0 ± 16.34 |
| SWS | 78.2 ± 17.60 | 80.4 ± 16.11 |
| CAP A subtypes, % | ||
| A1 | 33.3 ± 14.04 | 28.1 ± 16.33 |
| A2 | 30.6 ± 10.83 | 35.4 ± 13.02 |
| A3 | 36.0 ± 17.53 | 36.5 ± 17.39 |
| CAP A subtypes mean duration, s | ||
| A1 | 7.7 ± 0.89 | 7.2 ± 0.94 |
| A2 | 8.1 ± 0.83 | 7.8 ± 0.48 |
| A3 | 10.5 ± 1.29 | 11.2 ± 1.28 |
| CAP A subtype index, no/h | ||
| A1 | 37.5 ± 17.95 | 31.8 ± 21.17 |
| A2 | 33.9 ± 14.40 | 39.5 ± 21.18 |
| A3 | 41.3 ± 31.52 | 36.5 ± 17.88 |
| B phase mean duration, s | 17.2 ± 2.87 | 17.8 ± 2.28 |
| CAP sequence | ||
| Mean duration, s | 406.0 ± 125.98 | 434.1 ± 266.66 |
| Number | 34.4 ± 7.79 | 40.9 ± 11.82 |
| PLACEBO (n = 15) | ||
| CAP Rate, % | ||
| Total | 77.6 ± 9.02 | 75.6 ± 10.91 |
| During sleep stage | ||
| 1 | 53.1 ± 15.39 | 44.1 ± 17.51 |
| 2 | 77.8 ± 9.75 | 75.2 ± 11.87 |
| SWS | 88.4 ± 5.73 | 88.4 ± 8.11 |
| CAP A subtypes, % | ||
| A1 | 29.5 ± 12.70 | 30.3 ± 10.95 |
| A2 | 41.6 ± 8.34 | 42.5 ± 10.64 |
| A3 | 28.9 ± 14.85 | 27.2 ± 11.16 |
| CAP A subtypes mean duration, s | ||
| A1 | 7.3 ± 0.91 | 7.4 ± 0.99 |
| A2 | 8.2 ± 0.73 | 8.3 ± 0.71 |
| A3 | 12.1 ± 1.23 | 12.0 ± 1.33 |
| CAP A subtype index, no/h | ||
| A1 | 34.2 ± 17.32 | 34.8 ± 16.14 |
| A2 | 46.7 ± 11.95 | 46.8 ± 17.33 |
| A3 | 30.7 ± 16.95 | 28.0 ± 12.10 |
| B phase mean duration, s | 18.0 ± 2.24 | 18.4 ± 3.11 |
| CAP sequence | ||
| Mean duration, s | 558.5 ± 284.34 | 498.4 ± 208.12 |
| Number | 30.1 ± 9.96 | 28.7 ± 7.47 |
Data are presented as mean ± SD. The Wilcoxon test was used to determine the P values, all which were nonsignificant.
CAP refers to cyclic alternating pattern; RLS, restless legs syndrome; SWS, slow wave sleep.
Figure 1.
Example of polysomnographic recording of 1 of the subjects treated with pramipexole at baseline. Note the presence of cyclic alternating pattern (CAP) transients accompanied by periodic leg movements of sleep (PLMS) in the baseline recording; the transients are not accompanied by PLMS after treatment. EOG refers to electrooculogram; ECG, electrocardiogram.
DISCUSSION
To our knowledge, this is the first paper looking at CAP in patients with RLS and the therapeutic effect of dopamine-receptor agonists on CAP in these patients. Moreover, as introduced above, there are not many studies in the literature reporting the effect of dopamine-receptor agonist treatment on polysomnographic data of patients with RLS. As an example, Jama et al.23 recently reported that pramipexole is effective for reducing PLMS and decreasing sleep latency after 3 weeks of treatment; other sleep parameters showed lesser, usually statistically nonsignificant, changes, in contrast with the patients' subjective ratings of RLS severity and sleep disturbance, which were significantly improved. Pramipexole has also been shown to be effective after its first administration on the PLMS index and subjective RLS severity; however, concerning polysomnography parameters, Saletu et al.4 reported that increased sleep efficiency was accompanied by an increase in stages 1 and 2 sleep and stage shifts, whereas slow wave sleep and REM sleep decreased. Similar results were reported in 2 additional studies by Manconi et al., with statistical significance only for the increase in stage 2 sleep in 1 report5 and for sleep efficiency and stage 2 sleep in the other.6 In this new study, we have been able to confirm these findings and report an increased number of stage shifts per hour, increased sleep efficiency, decreased wakefulness after sleep onset, and increased percentage of stage 2 sleep. However, it should be noted that, at baseline, these parameters were substantially the same as those previously reported by Montplaisir et al.2 in a large cohort of patients with RLS and were not different from those of normal control subjects. Even if patients tended to have an increased number of stage shifts per hour and wakefulness after sleep onset and decreased percentage of stage 2 sleep in comparison with control subjects, these differences did not reach statistical significance. In fact, overall, the sleep-architecture parameters found at baseline in our patients with RLS do not seem to be substantially different from those expected for the patients' age, and only REM-sleep latency appeared to be significantly longer than normal, beside the expected higher number of PLMS. This first study on CAP in RLS clearly demonstrates that this type of analysis shows many more significant differences, at baseline, between these patients and normal control subjects than do classic sleep-architecture parameters. Patients with RLS show a higher NREM sleep instability, with approximately 75% of this sleep stage occupied by CAP sequences; moreover, both the shorter and more frequent A2 (and A3) subtypes indicate a different CAP time structure between normal control subjects and patients with RLS. This allows us to make some important, even though speculative, considerations. First of all, it is necessary to take into account the role that CAP might have in sleep-related cognitive processing because there is convincing preliminary evidence of its correlation with some sleep-dependent cognitive functions.24–26 Thus, it is possible to speculate that CAP alterations might have a role in the mechanisms of cognitive dysfunction reported in patients with RLS27,28; however, this study does not provide direct evidence of this but does open a possible new field of research. Cognitive alterations in RLS have been indicated to be similar to those expected as a consequence of sleep loss,27,28 even if they might appear to be less pervasive, probably due to adaptation.29 Classic sleep-architecture parameters would seem to be in disagreement with this view, with classic parameters of patients with RLS being very similar to those of control subjects, but CAP alterations clearly provide additional detailed information on sleep microstructure alterations in RLS, which might play an important role.
In this study, we were also able to confirm that, besides the dramatic decrease of PLMS and the subjectively reported amelioration of RLS symptoms, the first administration of pramipexole is followed by minor changes in classic sleep-architecture parameters4–6 and by no changes in sleep microstructure, which continues to be characterized by a high amount of sleep instability or discontinuity. This also confirms preliminary anecdotal evidence reported in a patient with alternating leg movement activity, who continued to have a high CAP rate after some weeks of treatment with pramipexole.30
Also, in this case, there is an evident discrepancy between classic sleep-architecture parameters and CAP parameters. It should also be noted that, after the first night of treatment with pramipexole, the presleep RLS subjective severity was significantly decreased, as measured by the VAS, but this was not accompanied by a similar improvement in the subjective quality of nocturnal sleep. Thus, CAP parameters seem to correlate better than do the classic sleep-architecture parameters with patients' subjective perception of sleep, which is in agreement with the recent report by Parrino et al. in patients with insomnia.8 Our CAP findings also agree with the results obtained by Jama et al., which reported unchanged or even slightly increased arousals after a 3-week treatment with pramipexole in patients with RLS, despite a clear reduction in PLMS with arousals.23 This was previously noted by Montplaisir et al., who failed to find improvement in sleep polysomnographic parameters in patients with RLS successfully treated, in terms of sensory symptoms and PLMS, with levodopa13 and, later, with pramipexole.14
A possible pathogenetic hypothesis for RLS, supported by theoretical reasoning and experimental evidence, indicates the involvement of the descending inhibitory dopaminergic pathways that from the hypothalamic A11 regions project to the D3 receptors located in the dorsal and intermediolateral nucleus of the spinal gray matter.31–33 Pramipexole, which is a selective D3-receptor agonist, might alleviate RLS symptoms and reduce PLMS by acting on the same D3 receptors of the spinal dorsal and intermediolateral gray matter. With our study, we can confirm that acute pramipexole administration seems to exert very limited action on the central sleep microstructure mechanisms, and the moderate effects seen on sleep architecture might be interpreted as the beneficial consequence of the removal of presleep RLS symptoms and reduction in PLMS.
We did not study, in details, the complex relationship between CAP and PLMS21,34,35 in the present investigation; however, both PLMS and CAP are accompanied by significant changes in autonomic function, as demonstrated by studies on heart rate36–40 and blood-pressure variability,41–43 during sleep in patients with RLS, and we believe that this area deserves further attention and controlled studies.
This study has some limitations. First, the reliability of CAP scoring in patients with RLS who are of the age similar to that of our subjects has not yet been established, although CAP has been evaluated in previous research involving subjects with an age comparable with that of our patients.34,44,45 Finally, our study was performed only after the first acute administration of pramipexole, and these results cannot be extended to the chronic administration of this substance. To obtain a more complete picture, future studies are needed to clarify the long-term effects of pramipexole on CAP in patients with RLS. These studies would potentially provide information useful for programming eventual new or add-on therapeutic strategies in RLS to modify not only subjective RLS symptoms and PLMS, but also sleep microstructure abnormalities, represented by the excessive sleep instability or discontinuity.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The work was performed at the Sleep Disorders Center, Department of Neuroscience, Scientific Institute and University Ospedale San Raffaele, Vita-Salute University, Milan, and at the Sleep Research Centre, Department of Neurology I.C., Oasi Institute (IRCCS), Troina, Italy.
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 3.Saletu B, Gruber G, Saletu M, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 1. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology. 2000;41:181–9. doi: 10.1159/000026658. [DOI] [PubMed] [Google Scholar]
- 4.Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002;252:185–94. doi: 10.1007/s00406-002-0380-7. [DOI] [PubMed] [Google Scholar]
- 5.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Manconi M, Ferri R, Feroah TR, Zucconi M, Ferini-Strambi L. Defining the boundaries of the response of sleep leg movements to a single dose of dopamine agonist. Sleep. 2008;31:1229–37. [PMC free article] [PubMed] [Google Scholar]
- 7.Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2001;2:537–53. doi: 10.1016/s1389-9457(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 8.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: The role of CAP and arousals in sleep misperception. Sleep Med. 2009 doi: 10.1016/j.sleep.2008.12.014. in press. [DOI] [PubMed] [Google Scholar]
- 9.Montplaisir J, Michaud M, Denesle R, Gosselin A. Periodic leg movements are not more prevalent in insomnia or hypersomnia but are specifically associated with sleep disorders involving a dopaminergic impairment. Sleep Med. 2000;1:163–7. doi: 10.1016/s1389-9457(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 10.Carrier J, Frenette S, Montplaisir J, Paquet J, Drapeau C, Morettini J. Effects of periodic leg movements during sleep in middle-aged subjects without sleep complaints. Mov Disord. 2005;20:1127–32. doi: 10.1002/mds.20506. [DOI] [PubMed] [Google Scholar]
- 11.Ferini-Strambi L. Treatment options for restless legs syndrome. Expert Opin Pharmacother. 2009;10:545–54. doi: 10.1517/14656560902793605. [DOI] [PubMed] [Google Scholar]
- 12.Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: An evidence-based review and implications for clinical practice. Mov Disord. 2008 doi: 10.1002/mds.22254. [DOI] [PubMed] [Google Scholar]
- 13.Montplaisir J, Boucher S, Gosselin A, Poirier G, Lavigne G. Persistence of repetitive EEG arousals (K-alpha complexes) in RLS patients treated with L-DOPA. Sleep. 1996;19:196–9. doi: 10.1093/sleep/19.3.196. [DOI] [PubMed] [Google Scholar]
- 14.Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52:938–43. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- 15.Fulda S, Wetter TC. Where dopamine meets opioids: a meta-analysis of the placebo effect in restless legs syndrome treatment studies. Brain. 2008;131:902–17. doi: 10.1093/brain/awm244. [DOI] [PubMed] [Google Scholar]
- 16.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 17.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–36. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 19.Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP) Sleep Med. 2005;6:29–36. doi: 10.1016/j.sleep.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Ferri R, Bruni O, Miano S, Smerieri A, Spruyt K, Terzano MG. Inter-rater reliability of sleep cyclic alternating pattern (CAP) scoring and validation of a new computer-assisted CAP scoring method. Clin Neurophysiol. 2005;116:696–707. doi: 10.1016/j.clinph.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 23.Jama L, Hirvonen K, Partinen M, et al. A dose-ranging study of pramipexole for the symptomatic treatment of restless legs syndrome: polysomnographic evaluation of periodic leg movements and sleep disturbance. Sleep Med. 2009;10:630–6. doi: 10.1016/j.sleep.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Ferri R, Huber R, Aricò D, et al. The slow-wave components of the cyclic alternating pattern (CAP) have a role in sleep-related learning processes. Neurosci Lett. 2008;432:228–31. doi: 10.1016/j.neulet.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep. 2007;30:1577–85. doi: 10.1093/sleep/30.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferini-Strambi L, Ortelli P, Castronovo V, Cappa S. Increased periodic arousal fluctuations during non-REM sleep are associated to superior memory. Brain Res Bull. 2004;63:439–42. doi: 10.1016/j.brainresbull.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 29.Gamaldo CE, Benbrook AR, Allen RP, Oguntimein O, Earley CJ. A further evaluation of the cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2008;9:500–5. doi: 10.1016/j.sleep.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosentino FI, Iero I, Lanuzza B, Tripodi M, Ferri R. The neurophysiology of the alternating leg muscle activation (ALMA) during sleep: study of one patient before and after treatment with pramipexole. Sleep Med. 2006;7:63–71. doi: 10.1016/j.sleep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Ondo WG, He Y, Rajasekaran S, Le WD. Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov Disord. 2000;15:154–8. doi: 10.1002/1531-8257(200001)15:1<154::aid-mds1025>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383–8. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- 33.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 34.Parrino L, Boselli M, Buccino GP, Spaggiari MC, Di Giovanni G, Terzano MG. The cyclic alternating pattern plays a gate-control on periodic limb movements during non-rapid eye movement sleep. J Clin Neurophysiol. 1996;13:314–23. doi: 10.1097/00004691-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Ferri R. Two legs, one heart, one sleeping brain. Sleep Med. 2006;7:299–300. doi: 10.1016/j.sleep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–91. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- 38.Ferri R, Parrino L, Smerieri A, et al. Cyclic alternating pattern and spectral analysis of heart rate variability during normal sleep. J Sleep Res. 2000;9:13–8. doi: 10.1046/j.1365-2869.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferini-Strambi L, Bianchi A, Zucconi M, Oldani A, Castronovo V, Smirne S. The impact of cyclic alternating pattern on heart rate variability during sleep in healthy young adults. Clin Neurophysiol. 2000;111:99–101. doi: 10.1016/s1388-2457(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 40.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000;111:1611–9. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 41.Pennestri MH, Montplaisir J, Colombo R, Lavigne G, Lanfranchi PA. Nocturnal blood pressure changes in patients with restless legs syndrome. Neurology. 2007;68:1213–8. doi: 10.1212/01.wnl.0000259036.89411.52. [DOI] [PubMed] [Google Scholar]
- 42.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Parrino L, Boselli M, Spaggiari MC, Smerieri A, Terzano MG. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. Electroencephalogr Clin Neurophysiol. 1998;107:439–50. doi: 10.1016/s0013-4694(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 45.Moser D, Kloesch G, Fischmeister FP, Bauer H, Zeitlhofer J. Cyclic alternating pattern and sleep quality in healthy subjects--is there a first-night effect on different approaches of sleep quality? Biol Psychol. 2010;83:20–6. doi: 10.1016/j.biopsycho.2009.09.009. [DOI] [PubMed] [Google Scholar]