Abstract
Study Objectives:
Obstructive sleep apnea (OSA) impacts on macrovasculature and autonomic function and may therefore interfere with ocular microvascular regulation. We hypothesized that choroidal vascular reactivity to hyperoxia and hypercapnia was altered in patients with OSA compared with matched control subjects and would improve after treatment with continuous positive airway pressure (CPAP).
Methods:
Sixteen healthy men were matched 1:1 for body mass index, sex, and age with 16 men with newly diagnosed OSA without comorbidities. Subjects underwent sleep studies, 24-hour blood pressure monitoring, arterial stiffness measurements, and cardiac and carotid echography. Overall, patients were middle-aged, lean, and otherwise healthy except for having OSA with a limited amount of desaturation, with, at most, subclinical lesions of the cardiovascular system, stage 1 hypertension, or both. Choroidal laser Doppler flowmetry provides a unique opportunity to assess microvascular function by measuring velocity, (ChBVel), volume (ChBVol), and relative subfoveal choroidal blood flow (ChBF). Vascular choroidal reactivity was studied during hyperoxia and hypercapnia (8% CO2) challenges before and after treatment with nasal CPAP.
Results:
Patients with OSA and control subjects exhibited similar choroidal reactivity during hyperoxia (stability of choroidal blood flow) and hypercapnia (significant increases in ChBVel of 13.5% and in ChBF of 16%). Choroidal vasoreactivity to CO2 was positively associated with arterial stiffness in patients with OSA. Gas choroidal vasoreactivity was unchanged after 6 to 9 months of CPAP treatment.
Conclusion:
This study showed unimpaired choroidal vascular reactivity in otherwise healthy men with OSA. This suggests that patients with OSA, without comorbidities, have long-term adaptive mechanisms active in ocular microcirculation.
Citation:
Tonini M; Khayi H; Pepin JL; Renard E; Baguet JP; Lévy P; Romanet JP; Geiser MH; Chiquet C. Choroidal blood-flow responses to hyperoxia and hypercapnia in men with obstructive sleep apnea. SLEEP 2010;33(6):811-818.
Keywords: Sleep apnea, eyes, microcirculation, laser flowmetry, CPAP
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON DISEASE, WITH AN ESTIMATED PREVALENCE OF 3% TO 7% IN THE GENERAL POPULATION.1 OSA is characterized by recurrent episodes of partial or complete upper airway obstruction during sleep that are nearly always associated with a desaturation-reoxygenation sequence, which detrimentally stimulates the cardiovascular system. OSA per se has been shown to generate hypertension,2 atherosclerosis,3 endothelial dysfunction,3,4 and autonomic dysfunction (high sympathetic tone, increase in baseline heart rate, elevated muscle sympathetic nerve activity, increased plasma levels of catecholamines, and reduced α- and β2-adrenergic vascular responses),2,4,5 all of which may interact with ocular vascular regulation. Continuous positive airway pressure (CPAP), the first-line therapy for OSA, suppresses abnormal respiratory events, restores sleep quality, reverses partly or completely acute and chronic cardiovascular modifications,6 and may improve vascular reactivity.7,8 For these reasons, CPAP may also have a significant impact on choroidal vasculature.
Most vascular changes associated with OSA have been studied with regard to macrovasculature,9 and several reports suggest that it alters endothelium-dependent vasodilatation.4 Abnormal vascular reactivity has been described in cerebral circulation10,11 and in forearm circulation after infusion of acetylcholine.9,12 Regarding this vascular dysfunction, OSA has also been reported as being associated with vascular eye diseases, such as nonarteritic anterior ischemic optic neuropathy13,14 and glaucomatous optic neuropathy.15,16
Complementary to macrocirculation, laser Doppler flowmetry (LDF) provides a unique opportunity to investigate microvascularization of the choroid, being a noninvasive and reproducible technique17–19 previously validated in healthy humans. In the absence of retinal vessels within the fovea, blood flow in the choroid, the tissue beneath the retina, may be accurately and directly measured. This tissue also exhibits autonomic regulation,20,21–23 and its blood flow shares some regulatory properties with cerebral blood flow, such as hypercapnia-induced vasodilatation.24,25
Our hypothesis was that patients with OSA differ from control subjects in choroid blood flow regulation. The purpose of our study was to characterize choroidal vascular reactivity to hyperoxia and hypercapnia (8% CO2 breathing) in patients with OSA compared with matched healthy control subjects, before and after CPAP.
MATERIALS AND METHODS
Studied Population
Patients with OSA
Sixteen men with newly diagnosed OSA without associated comorbidities were included in this prospective study. The study was conducted in accordance with the Declaration of Helsinki for research involving human subjects and with Good Clinical Practice guidelines. Informed consent was obtained from the subjects after explanation of the study. The study protocol was approved by the local institutional review board (Comité de Protection des Personnes, Sud-Est V, #6705) and was registered on ClinicalTrials.gov (NCT00874913).
Inclusion criteria were patients presenting with OSA, defined by an apnea-hypopnea index of greater than 15 per hour (number of episode of partial [hypopnea] or complete [apnea] upper airway obstruction), aged 18 to 80 years, and affiliated with the healthcare system. Exclusion criteria were ocular disease (including cataract or retinal disease, ametropia > 3 diopters, optic neuropathy), uncontrolled high blood pressure (> 140/90 mm Hg with at least 2 drugs),26 cardiovascular treatment (vasoconstrictors, vasodilators, β- and α-receptor agonists or antagonists, nitric-derived medication), corticosteroids, theophylline, sildenafil, immunosuppressors, neuroleptics, nonsteroidal antiinflammatory agents, estroprogestative treatment, hypnotics (benzodiazepines), and local treatment for ocular hypertension or glaucoma.
CPAP compliance was considered acceptable if the device was used for at least 4 hours per night.27
Control subjects
Sixteen control subjects—matched 1:1 with patients with OSA for body mass index (BMI), sex, and age—were assessed by complete overnight polysomnographic study to rule out OSA. Exclusion criteria were similar to those for the patients with OSA.
At the screening visit, within 2 weeks before LDF measurement, each subject underwent a general examination and cardiovascular and neurologic examinations. A blood sample was analyzed to characterize cardiovascular and metabolic profile (Table 1).
Table 1.
General characteristics of 16 men with OSA and 16 healthy control subjects
| Patients with OSA (n = 16) | Healthy control subjects (n = 16) | P value | |
|---|---|---|---|
| Age, y | 50.9 ± 2.1 | 51.2 ± 1.7 | 0.4 |
| BMI, kg/m2 | 25.9 ± 0.5 | 25.0 ± 0.5 | 0.17 |
| PtcCO2, mm Hg | 38.5 ± 0.5 | 37.9 ± 0.9 | 0.7 |
| IOP, mm Hg | 13.9 ± 0.3 | 16.3 ± 0.7 | 0.01 |
| OPP, mm Hg | 53.7 ± 1.3 | 46.9 ± 1.0 | 0.001 |
| Sleep evaluation | |||
| AHI, no./h of sleep | 40.1 ± 3.9 | 4.91 ± 0.9 | < 0.001 |
| Mean nocturnal SaO2,% | 94.0 ± 0.5 | 93.7 ± 0.4 | 0.9 |
| Time spent at SaO2 < 90%, min | 25.8 ± 7.8 | 8.2 ± 6.9 | 0.001 |
| Cardiovascular phenotype | |||
| Systemic hypertensiona | 7 (43.7) | 3 (18.7) | 0.2 |
| Blood pressure, mm Hg | |||
| Systolic | 136.6 ± 3.8 | 129.2 ± 1.5 | 0.08 |
| Diastolic | 86.4 ± 3.1 | 83.3 ± 2.0 | 0.34 |
| Mean | 102.4 ± 6.7 | 95.7 ± 1.6 | 0.01 |
| Physiologic dipping | 5 (31.2) | 6(37.5) | 0.3 |
| Arterial stiffness, m/sec | 10.2 ± 0.6 | 9.2 ± 0.6 | 0.06 |
| Carotid IMT, μm | |||
| Right | 0.62 ± 0.02 | 0.57 ± 0.02 | 0.04 |
| Left | 0.64 ± 0.04 | 0.62 ± 0.03 | 0.9 |
| Carotid plaque | 3 (18.7) | 0 | 0.2 |
| LVEF, % | 66 ± 0.01 | 66 ± 0.019 | 0.9 |
| Dyslipidemia | 2 (11.1) | 2 (11.1) | 0.99 |
All data are expressed as mean ± SEM or number (%). OSA refers to obstructive sleep apnea; BMI, body mass index; PtcCO2; transcutaneous carbon-dioxide tension; IOP, intraocular pressure; OPP, ocular perfusion pressure; IMT, intima-media thickness; LVEF, left ventricular ejection fraction;
As determined by ambulatory blood pressure monitoring.
Ophthalmic Examination and Intraocular Pressure Measurement
Each patient had a complete ocular examination (visual acuity, slit-lamp examination, intraocular pressure [IOP], gonioscopy, funduscopy). IOP was measured using a Goldmann applanation tonometer and a Tonopen XL® portable tonometer after instillation of a contact anesthetic (Oxybuprocaine, Novartis Pharma Rueil-Malmaison). The eye examination was completed by visual-field tests (Humphrey® 24/2 and 10/2 SITA-standard visual field) and measurements of retinal nerve fiber layer thickness using optical coherence tomography (OCT 3, Zeiss, Oberkochen, Germany). Ocular examination of all patients with OSA and healthy subjects was normal.
Polysomnography
Continuous recordings were taken with electrode positions C3/A2-C4/A1-Cz/01 of the international 10-20 electrode placement system, eye movements, chin electromyogram, and electrocardiogram with modified V2 lead. Sleep was scored manually according to standard criteria.28 Air flow was measured with nasal pressure associated with the sum of buccal and nasal thermistor signals. Respiratory effort was monitored with abdominal and thoracic bands. An additional indicator of respiratory effort (ie, pulse transit time) was recorded concurrently. Oxygen saturation was measured using a pulse oximeter (Biox-Ohmeda 3700®, Ohmeda, Liberty Corner, NJ). Respiratory events were scored in line with clinical research recommendations.29
Cardiovascular Phenotype of Patients With OSA and Control Subjects
Ambulatory blood pressure monitoring was carried out with a Diasys Integra® device (Novacor SA, France) every 15 minutes during daytime and every 30 minutes during nighttime. The following blood-pressure parameters were studied: systolic blood pressure (SBP), diastolic BP (DBP), mean blood pressure (MBP), and heart rate over the 24 hours and over the daytime (07:00-22:00) and nighttime (22:00 to 07:00).30 Diurnal hypertension was defined as daytime SBP of at least 135 mm Hg, or daytime DBP of at least 85 mm Hg, or both, and nocturnal hypertension as nighttime SBP of at least 120 mm Hg, nighttime DBP of at least 70 mm Hg, or both. Nocturnal dip was calculated using the following formula:
A normal nocturnal dip was defined as a mean of 10% to 20%.31
The echocardiogram was carried out using an HP Sonos 2500 (Hewlett-Packard, Santa Clara, CA) machine with a 2.5-MHz probe. The left ventricular mass (LVM) was measured as previously described.30 Carotid B-mode ultrasonography, performed with an HP Sonos 2500 (Hewlett Packard), was used to ascertain the mean common carotid intima–media thickness and luminal diameter, as previously reported.32 To determine the carotid-femoral pulse wave velocity (PWV), 2 pulse transducers were fixed on the skin over the right common carotid and femoral arteries. The carotid-femoral PWV was calculated as the distance between the arterial sites divided by the time delay30 measured with a Complior device (Artech Medical, Pantin, France).
Choroidal Blood-Flow Measurements
The LDF instrument used in this study (Figure 1) to measure subfoveal choroidal blood flow (ChBF) has been described elsewhere,17 and we give here only a brief summary. The measurement is performed under the fovea, in the choriocapillaris layer, the superficial layer of the choroid with a dense network of capillaries. It is based on the fact that light reflected on moving red blood cells will shift in frequency (Doppler effect). The instrument uses a coherent near-infrared probing beam (785 nm, 90 μW at the cornea) that conforms to the American National Standards Institute standard Z 136.1 for laser safety. Light backscattered by the tissue in the sampled volume is collected by a bundle of optic fibers and guided to an avalanche photodiode. The output photocurrent is sampled at a frequency of 240 kHz with a 16-bit resolution and processed with Labview software on a PC computer to ascertain the ChBF parameters in real time at a rate of 17 Hz, using an algorithm based on photon diffusion and probabilistic theory.33 These parameters are velocity, ChBVel (kHz); volume, ChBVol (in arbitrary units, au); and relative flux, ChBF = ChBVel × ChBVol (au) of the red blood cells within the sampled tissue region. Assuming constant hematocrit levels, the changes in ChBVol and ChBF are proportional to the changes in actual volume and flow of blood, respectively. The software automatically rejects signals for which the light intensity (DC: direct current) is not within ± 20% of its most frequent value, the LDF signal is absent, or the volume is suddenly too large, due to microsaccades, for example. The intensity of the collected backscattered light or DC is a measurement of the optical properties of the local tissue under investigation. A change in that intensity would mean a change in the position of the probing beam and, thus, a change in the blood-flow parameters. In that sense, the stability of the DC value is a sign of good reproducibility. The flowmeter was mounted on a table equipped with a chin rest and head holder. Seated subjects looked into the instrument, directly at the laser beam. The operator aligned the instrument in such a way that the DC signal reached a maximum, and care was taken to keep the DC signal as constant as possible (± 10%) during recording. Two or more continuous 30-second recordings of choroidal circulation were obtained for each measurement. A minimum of 12 seconds of measurement of each eye was analyzed.
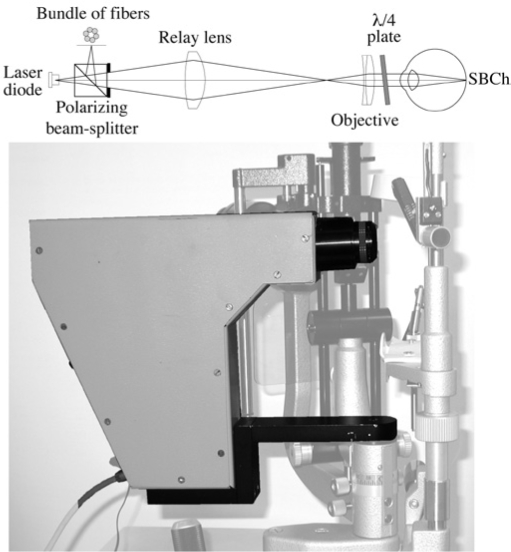
Figure 1.
Optical scheme of the compact confocal flowmeter and approximately scaled photograph of the device for subfoveal choroidal blood flow (SBCh) measurements by laser Doppler flowmetry (LDF)r-t. A polarized diode laser point source is focused on the fovea. The light scattered by the region of the choriocapillaris and possibly by some larger vessels behind this layer is directed along the same optical path to a bundle of fibers arranged on an annulus. The latter is centered on the image of the illuminated volume. The fiber bundle guides the light to an avalanche photo-diode detector. A l/4 plate (an optical element that transforms a linear polarized input light signal into a circular polarized output signal) is placed between the eye and the objective, with the function of preventing the detection of stray light, to improve the relative strength of the signal. (from Riva et al. 2009, courtesy of Acta Ophthalmologica).
Study Protocol
Patients were asked to abstain from alcohol and caffeine for at least 12 hours before the trial. LDF was systematically performed on the right eye. SBP and DBP were obtained (DINAMAP®, Critikon, Tampa, FL) during LDF measurements, then IOP of the fellow eye was immediately measured using the Tonopen XL®. Mean ocular perfusion pressure (OPP) was calculated with the following formula22:
in which MBP was calculated as DBP + 1/3 (SBP – DBP). Resistance was calculated as R = OPP/ChBF.
The study was performed in a randomized, double-blind, 3-way cross-over design. Three inhalation periods of different mixtures of O2 and CO2 were scheduled (Figure 2) for 10 minutes each: 100% O2, Bactal (92% O2 + 8% CO2), and air. Air was considered as a placebo, and measurements during air inhalation allowed calculation of the sensitivity of the gas experiment. All gases were delivered through a partially expanded reservoir bag at atmospheric pressure using a 2-valve system to prevent rebreathing. Transcutaneous CO2 tension (PtcCO2) was measured by the TINA PtcCO2 monitoring system (Radiometer, Copenhagen, Denmark)34 at 5 and 10 minutes and at the end of the experiment.
Figure 2.
Study protocol. Choroidal blood flow parameters, intraocular pressure, and systemic blood pressure were measured at baseline, after 5 and 10 minutes of gas inhalation (air, O2, and CO2), and after 10 minutes of recovery using laser Doppler flowmetry (LDF).
Scheduled resting periods for each subject were at least 20 minutes in a sitting position before the study and 30 minutes between each inhalation period. Stable baseline conditions were established, ensured by repeated measurement of blood pressure. Three LDF measurements of 30 seconds were performed before inhalation, 5 and 10 minutes during the inhalation, and then after resting period of 10 minutes.
Similar experiments were performed for the group of patients with OSA after 6 to 9 months of CPAP treatment. Ten patients were included for this analysis. Six were excluded owing to nonindication of CPAP (n = 1), noncompliance with CPAP (n = 2), or refusal of the second part of the study (n = 3).
Statistical Analysis
Data are presented as mean ± SEM. Normalized data during the experiment at 5 and 10 minutes were calculated according to baseline data. Data analysis was conducted with NCSS 97 software (Kaysville, Utah). Normality was assessed using skewness and kurtosis tests. To check the assumptions of the analysis of variance (ANOVA), variance equality was also tested using the Modified Levene Equal Variance Test. The changes within each group were analyzed by a 1-way repeated ANOVA measure (repeated measure factor: time). Paired t tests were then used for posthoc analysis. The P value was modified using Bonferroni correction. The corrected P value for posthoc analysis was 0.008. A mixed-model ANOVA with 2 factors was also used: the between-subject factor (Group) and within-subject factor (Time). We analyzed the group and time effects as well as the group-by-time interaction. The 2 groups were also compared at baseline for general parameters (Table 1)
Since the LDF signal depends partly on the structural properties of the tissue measured, ie, the submacular choroid here, the absolute values of blood flow parameters in 2 groups of patients at a given time cannot be compared. In contrast, comparisons of relative changes of blood flow parameters are appropriate when the subjects' values are normalized according to baseline values.
Sensitivity (the minimum statistically significant change in LDF parameters (S) able to be detected) was calculated using the formula35:
where P mean is the mean value of all measurements, SD the standard deviation of the difference between paired measurement for all subjects, and t the 2-tailed value of the t distribution at a 0.05 significance level for the n-1 degree of freedom.
RESULTS
Patient characteristics
The OSA group comprised 16 men with a mean age of 50.9 ± 2.1 years (range 28-64) and a mean BMI of 25.9 kg/m2 ± 0.5 kg/m2 (range 23.5-30.5 kg/m2). The mean apnea-hypopnea index was 40.1 ± 3.9 (range 20-77, mean nocturnal SaO2 of 94% ± 0.5%). General characteristics are summarized in Table 1. Overall, patients were middle-aged, lean, and otherwise healthy except for having OSA with a limited amount of desaturation, with, at most, subclinical lesions of the cardiovascular system, stage 1 hypertension, or both. No patient had diabetes mellitus. No statistical difference was found between the groups of patients with OSA and healthy control subjects in age, BMI, or sex (Table 1). At baseline (Table 1), patients with OSA differed from healthy control subjects by having higher OPP, higher MBP, and lower IOP values.
Air Inhalation (placebo experiment)
Sensitivity of the experiment in patients with OSA (n = 16) and control subjects (n = 16) was 5.9% and 6% for ChBF, respectively. During air inhalation, blood-flow parameters, OPP, and choroidal vascular resistance in patients with OSA and control subjects did not change from baseline (P > 0.2).
CO2 Inhalation (Hypercapnia Experiment)
In patients with OSA, after 5 and 10 minutes of 8% CO2 breathing, the mean increase in PtcCO2 was, respectively, 11 mm Hg (28.6%) and 12.3 mm Hg (38.9%) (P < 0.001). In healthy subjects, a significant increase (P < 0.001) of PtcCO2 was also noted at 5 minutes (8.3 mm Hg, 21.8%) and 10 minutes (10.2 mm Hg, 27.0%). During the recovery period, PtcCO2returned to baseline values.
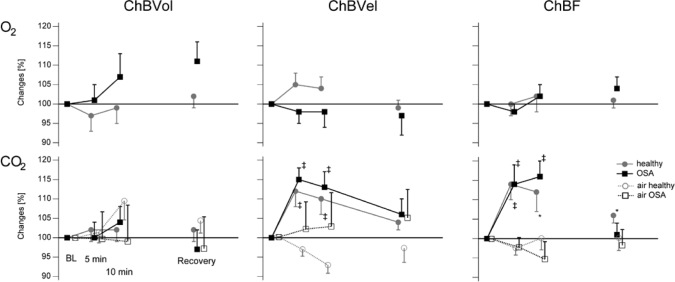
In both populations (Table 2), no significant change in ChBVol and choroidal resistance was found at 5 and 10 minutes. A significant increase in ChBVel (13.5% in both groups) and in ChBF (14% in healthy and 16% in OSA groups) occurred during 10 minutes of inhalation of CO2. In the OSA group, increases in MBP of 6.2% (P = 0.1) at 5 minutes and of 8.3% at 10 minutes (P = 0.008) were noted. In healthy subjects, MBP increased by 7.1% at 5 minutes (nonsignificant) and significantly by 8.2% at 10 minutes (P = 0.003). In both populations, MBP regained baseline values at the end of the experiment. In the healthy group, OPP increased significantly by 13% at 10 minutes (P < 0.001). Figure 3 and Table 3 illustrate that patients with OSA and healthy control subjects showed a similar response in variation of blood-flow parameters (P > 0.2) and OPP (P > 0.9) and in resistance (P > 0.3) to CO2 inhalation for 5 and 10 minutes.
Table 2.
Variation of choroidal blood parameters during CO2 inhalation in 16 men with OSA and 16 healthy matched control subjects
| ChBVol, au | Baseline | 5 min | 10 min | Recovery | P valuea |
|---|---|---|---|---|---|
| Healthy subjects | 124.0 ± 6.6 | 126.7 ± 7.6 | 125.1 ± 6.9 | 124.6 ± 7.9 | 0.87 |
| Patients with OSA | 140.8 ± 11.3 | 134.2 ± 9.5 | 139.1 ± 8.4 | 129.1 ± 10.3 | 0.07 |
| ChBvel, kHz | |||||
| Healthy subjects | 1839.8 ± 138.7 | 2111.4 ± 180.5b | 2082.0 ± 181.2b | 1962.1 ± 168.6 | < 0.001 |
| Patients with OSA | 2085.4 ± 206.2 | 2304.9 ± 184.0b | 2319.1 ± 201.3b | 2198.3 ± 203.0 | 0.002 |
| ChBF, au | |||||
| Healthy subjects | 216.0 ± 15.5 | 251.9 ± 19.4b | 246.3 ± 20.6c | 228.1 ± 17.5c | < 0.001 |
| Patients with OSA | 262.2 ± 21.2 | 287.4 ± 21.2 | 299.9 ± 23.4b | 256.0 ± 18.1 | < 0.001 |
| Resistance | |||||
| Healthy subjects | 0.24 ± 0.02 | 0.22 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.17 |
| Patients with OSA | 0.23 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.24 ± 0.02 | 0.16 |
| OPP, mm Hg | |||||
| Healthy subjects |
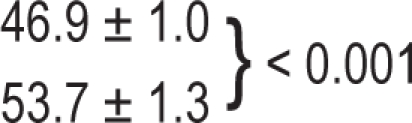 d d
|
51.3 ± 1.6 | 52.8 ± 1.4b | 48.9 ± 1.0 | < 0.001 |
| Patients with OSA | 57.5 ± 2.3 | 58.3 ± 1.8 | 55.1 ± 1.6 | 0.13 |
Data are shown as mean ± SEM). au refers to arbitrary units; ChBvol, choroidal volume, ChBVel, choroidal velocity, ChBF, choroidal blood flow; OPP, ocular perfusion pressure.
P value for all laser Doppler flowmeter (LDF) parameters.
P value = 0.001 using the Wilcoxon test comparing data during inhalation with baseline value.
P value = 0.01 using the paired t test comparing data during inhalation with baseline value.
P value relative to the comparison of OPP values between OSA and healthy subjects.
Figure 3.
Variations of normalized flow parameters, ocular perfusion pressure, and choroidal resistances in 18 patients with OSA and 18 healthy matched control subjects during O2 or CO2 inhalation. ChBvol refers to choroidal volume; ChBVel, choroidal velocity; ChBF, choroidal blood flow. Normalized data are expressed as mean (± SEM). P value for laser Doppler flowmetry parameters using the Wilcoxon test comparing data during inhalation with baseline (BL) values: *P = 0.01, #P = 0.03, †P = 0.005, ‡P = 0.001
Table 3.
Variation of choroidal blood parameters during O2 inhalation in 16 men with OSA and 16 healthy matched control subjects
| Baseline | 5 min | 10 min | Recovery | P value for all measurements | |
|---|---|---|---|---|---|
| ChBVol, au | |||||
| Healthy subjects | 130.3 ± 7.9 | 121.1 ± 6.8 | 123.0 ± 6.7 | 129.8 ± 7.5 | 0.1 |
| Patients with OSA | 125.5 ± 9.9 | 124.3 ± 10.9 | 131.7 ± 11.8 | 138.2 ± 12.9 | 0.14 |
| ChBvel, kHz | |||||
| Healthy subjects | 1845.6 ± 154.4 | 1938.6 ± 155.3 | 1955.9 ± 176.0 | 1820.3 ± 139.5 | 0.06 |
| Patients with OSA | 2118.2 ± 173.3 | 2088.2 ± 178.9 | 2124.9 ± 194.0 | 2111.8 ± 223.2 | 0.97 |
| ChBF, au | |||||
| Healthy subjects | 224.8 ± 17.1 | 221.5 ± 17.7 | 227.2 ± 19.8 | 222.3 ± 16.3 | 0.86 |
| Patients with OSA | 240.1 ± 17.2 | 234.7 ± 17.2 | 247.1 ± 18.0 | 249.8 ± 18.5 | 0.16 |
| Resistance | |||||
| Healthy subjects | 0.24 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.79 |
| Patients with OSA | 0.24 ± 0.02 | 0.25 ± 0.03 | 0.24 ± 0.03 | 0.23 ± 0.02 | 0.57 |
| OPP, mm Hg | |||||
| Healthy subjects |
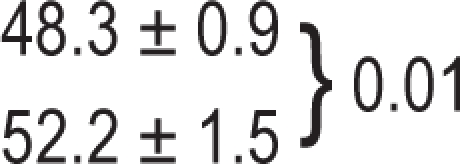 a a
|
48.1 ± 0.9 | 47.3 ± 1.2 | 46.5 ± 1.4 | 0.50 |
| Patients with OSA | 52.2 ± 1.2 | 52.0 ± 1.4 | 53.6 ± 1.9 | 0.29 |
Data are expressed as mean (± SEM). Blood flow parameters did not significantly change during O2 inhalation, and responses in both groups were similar. au refers to arbitrary units; ChBvol, choroidal volume; ChBVel, choroidal velocity; ChBF, choroidal blood flow; OPP, ocular perfusion pressure.
P value relative to the comparison of OPP values between OSA and healthy subjects.
There was a significant correlation between PWV and the increase in ChBVel (r = 0.56, P = 0.02) in patients with OSA. Reactivity to CO2 was higher in patients with OSA, with a VOP > 9.25 (ChBVel +22.3% ± 5.3%) versus VOP < 9.25 (ChBVel +4.8% ± 2.8%, P = 0.01).
After 6 to 9 months of CPAP treatment, reactivity to CO2 at 5 and 10 minutes in 10 patients with OSA was characterized by increases of 14% to 16% in OPP (P = 0.006), of 9% to 11% in ChVel (P = 0.01), and of 12% to 15% in flow (P = 0.007), with no significant change in ChVol (P = 0.2). Reactivity of ChBVel and ChBF was similar to that found before CPAP (P > 0.2).
O2 inhalation (Hyperoxia Experiment)
In patients with OSA and healthy control subjects, blood-flow parameters, OPP, and choroidal resistance did not change during the experiment (P ≥ 0.1, Figure 3; Table 3). The comparison between patients with OSA and healthy control subjects did not show a significant difference (P > 0.1). After CPAP, as in the experiment before CPAP (P > 0.2), patients exhibited no change in ChBF parameters (P > 0.2).
DISCUSSION
This prospective case-controlled study characterized, for the first time, ChBF in patients with OSA. Our data demonstrated that ChBF reactivity to O2 and CO2 in otherwise healthy patients with OSA was similar to that in control subjects and was unchanged after CPAP treatment.
Ocular blood flow at baseline has not previously been studied in patients with OSA. The only published data concern ophthalmic blood velocity at rest, with no difference found between patients with OSA and healthy control subjects.36 Our results showed a higher OPP in patients with OSA, reflecting the higher levels of MBP in this group. However, this higher OPP does not indicate why choroidal or optic nerve blood flow is elevated at baseline because these tissues can autoregulate. Our measurements did not allow comparison of baseline values of flow measured with LDF, as this technique also depends on tissue scattering and absorption properties. Hence, in methodologic terms, only flow variations under stimulation by gas or another stimulus, but not flow per se, can be compared between 2 populations.37
The vascular reactivity of the choroid has previously been characterized in young healthy subjects after inhalation of different mixtures of O2 and CO2.38,39 In our study, the hypercapnia-induced increase in ChBF was similar in patients with OSA and healthy control subjects, about 15%, consistent with that reported in previous studies in healthy subjects.39,40
The mechanisms underlying hypercapnia-induced vasodilatation in the choroid are likely a reduction in arterial pH25,41 and nitric oxide (NO) availability,42 acting on the choriocapillaris,43 which is the choroidal layer measured using the LDF instrument. Hypercapnic cerebral vasodilatation, as illustrated by a significant increase in mean blood flow velocity in the middle cerebral artery,25,42 is also a local phenomenon explained by the involvement of NO, cyclooxygenase, and P-450 oxygenase.44–46 For instance, the NO synthase (NOS) has also been implicated in cerebral blood flow regulation because NOS inhibitors attenuate the increase in cerebral blood flow elicited by hypercapnia.44,47 The normal reactivity of ChBF in our patients with OSA may seem surprising because decreased plasma levels of NO derivatives48,49 and abnormal endothelium-dependent and -independent vasodilatation6,9 have been reported in patients with OSA. The absence of abnormal vascular reactivity of ChBF in OSA is also intriguing given the autonomic regulation of the choroid,22,23 the known reduction in vascular response to α- and β-adrenergic receptors in OSA,50 and the increased sympathetic activation secondary to the desaturation-reoxygenation sequence.51
However, these previous studies included patients who were obese, exhibited severe desaturations, or both11,52,53—unlike our population. In contrast, recent studies in otherwise healthy subjects with OSA10,54 and breath-hold divers55 showed normal cerebral vascular reactivity to hypercapnia. Similarly, our patients with OSA without any other comorbidities should not exhibit severe oxidative stress or lipid peroxidation.56 The absence of comorbidities may be the main reason why OSA per se in otherwise healthy patients was not associated with deregulation of CO2 reactivity in the choroid. The relationship between arterial stiffness and CO2 reactivity found in our study suggests that a vascular remodeling may impact on choroidal vasoreactivity. Our patients had only subclinical changes demonstrated by changes in PWV. In more severely affected patients, with longer disease evolution or comorbidities, changes may be more significant. Another nonexclusive hypothesis for our results is that the choroid is protected against low or moderate OSA by local adaptive mechanisms.
Similarly, ChBF reactivity to hyperoxia in patients with OSA was similar to that of healthy control subjects, with no significant change of ChBF, which is consistent with previous studies of healthy subjects.38,39 This indicates that even pronounced changes in arterial oxygen tension have little impact on subfoveal ChBF in healthy subjects and patients with OSA, in contrast with retinal and cerebral vasoconstriction.57,58
The investigation of ChBF parameters was initially scheduled after CPAP in patients with OSA because effective CPAP therapy is associated with the reversal of vascular endothelial dysfunction and inflammation.6,32,48 Treatment of OSA with CPAP is known to increase reactive hyperemic blood-flow responses to transient forearm arterial occlusion7 and cerebral blood-flow response to hypoxia to normal levels,4 to normalize cerebrovascular reactivity to hypercapnia,53 and to increase cardiovascular response to hypercapnia.54 As recently has been described for cerebral circulation,54,59 treatment of OSA with CPAP did not change vascular reactivity in response to hypercapnia.
Methodologic Considerations, Study Limitations, and Strengths
Methodologic considerations liable to influence vascular reactivity were largely eliminated from this prospective comparative study. We compared a homogeneous population of otherwise healthy patients with newly diagnosed and untreated OSA with matched control subjects for factors such as sex, obesity and age, which may influence hypercapnia reactivity, blood flow regulation, or both. Our series comprised 16 patients with OSA and 16 healthy control subjects, a significant number of subjects compared with other studies in this field (8-22 patients).10,11,52,54,59 Inclusion criteria were defined to exclude patients with confounding factors liable to influence our assessment of choroidal reactivity to CO2, such as antihypertensive treatment,60 smoking,61 or diabetes.62 Matched healthy subjects were screened for OSA by overnight polysomnography and for cardiovascular lesions by a full examination, ie, ABPM, echocardiography, carotid-femoral PWV, and carotid ultrasonography.
Patients with OSA and control subjects were studied in the morning (08:00-11:00), when hypercapnic cerebral vascular reactivity is known to be reduced.53,63,64 However, our daytime experiment does not exclude a different CO2 vasoreactivity during sleep, as has been previously shown for cerebral circulation.65
One of the possible limitations in analyzing our results was the increase in SBP during hypercapnia (6%-8%) but without significant change in OPP. Given ChBF autoregulation across a wide range of perfusion pressures35,39 and the small change in BP during the experiment, the variation in ChBF during hypercapnia was probably not due to BP variation.
The reproducibility of the measurements of this gas experiment is comparable with that described previously with a similar LDF,39 (7.4%) with a sensitivity of blood flow measurements of 6% in patients with OSA and healthy control subjects. One limitation of our comparison before and after CPAP is the lack of randomization comparing effective CPAP with sham CPAP.54
As described previously37 when using LDF, detecting a 15% difference in flow with 80% power by means of a paired test requires the inclusion of 7 subjects to evaluate changes within 1 session. The number of subjects in each group was therefore sufficient to detect a significant change of flow during the gas experiment.
Conclusion and Perspectives
We report for the first time unimpaired choroidal vascular reactivity to blood-gas perturbation in otherwise healthy patients with OSA. Given the absence of comorbidities in our studied population, our conclusion does not rule out that choroidal vascular reactivity to O2 and CO2 is present in obese patients with severe OSA associated with cardiovascular disease. Another explanation could be that our patients were at the beginning of the time course in the evolution of the disease, with only subclinical cardiovascular lesions that can be counterbalanced by long-term adaptive mechanisms. Further studies are needed to test gas ocular vasoreactivity during short-term exposure to intermittent hypoxia in the model that we have developed in healthy subjects.66 Furthermore, recent progress in LDF for the optic nerve (New optical device for functional studies of the optic nerve head. Geiser MH, Truffer F, Khayi H, Chiquet C. European Association for Vision and Eye Research Congress, Portoroz, Slovenia, October 2008) will allow study of optic nerve circulation, which may be affected in patients with OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEGMENTS
We extend our thanks to Nathalie ARNOL for the statistical analysis. Financial support for this study was provided by Innovation Hospitalière (Grenoble University Hospital), AGIRADOM scientific council, French Hospitals Federation (FHF), Ministry of Foreign Affairs (Egide, Germaine de Staël programme), and HES-SO (higher-education network in Western Switzerland).
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 3.Levy P, Pepin JL, Arnaud C, Baguet JP, Dematteis M, Mach F. Obstructive sleep apnea and atherosclerosis. Prog Cardiovasc Dis. 2009;51:400–10. doi: 10.1016/j.pcad.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol. 2007;92:51–65. doi: 10.1113/expphysiol.2006.035204. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher EC. Cardiovascular disease associated with obstructive sleep apnea. Monaldi Arch Chest Dis. 2003;59:254–61. [PubMed] [Google Scholar]
- 6.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imadojemu VA, Gleeson K, Quraishi SA, Kunselman AR, Sinoway LI, Leuenberger UA. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2002;165:950–3. doi: 10.1164/ajrccm.165.7.2102003. [DOI] [PubMed] [Google Scholar]
- 8.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–5. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 10.Urbano F, Roux F, Schindler J, Mohsenin V. Impaired cerebral autoregulation in obstructive sleep apnea. J Appl Physiol. 2008;105:1852–7. doi: 10.1152/japplphysiol.90900.2008. [DOI] [PubMed] [Google Scholar]
- 11.Loeppky JA, Miranda FG, Eldridge MW. Abnormal cerebrovascular responses to CO2 in sleep apnea patients. Sleep. 1984;7:97–109. doi: 10.1093/sleep/7.2.97. [DOI] [PubMed] [Google Scholar]
- 12.Carlson JT, Rangemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–84. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Palombi K, Renard E, Levy P, et al. Non-arteritic anterior ischaemic optic neuropathy is nearly systematically associated with obstructive sleep apnoea. Br J Ophthalmol. 2006;90:879–82. doi: 10.1136/bjo.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mojon DS, Hedges TR, 3rd, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 2002;120:601–5. doi: 10.1001/archopht.120.5.601. [DOI] [PubMed] [Google Scholar]
- 15.Mojon DS, Hess CW, Goldblum D, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2002;216:180–4. doi: 10.1159/000059625. [DOI] [PubMed] [Google Scholar]
- 16.Mojon DS, Hess CW, Goldblum D, Bohnke M, Korner F, Mathis J. Primary open-angle glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2000;214:115–8. doi: 10.1159/000027478. [DOI] [PubMed] [Google Scholar]
- 17.Geiser M, D U., Riva CE. Compact laser Doppler choroidal flowmeter. Journal of Biomedical optics. 1999;4:459–64. doi: 10.1117/1.429960. [DOI] [PubMed] [Google Scholar]
- 18.Gugleta K, Orgul S, Flammer I, Gherghel D, Flammer J. Reliability of confocal choroidal laser Doppler flowmetry. Invest Ophthalmol Vis Sci. 2002;43:723–8. [PubMed] [Google Scholar]
- 19.Polska E, Polak K, Luksch A, et al. Twelve hour reproducibility of choroidal blood flow parameters in healthy subjects. Br J Ophthalmol. 2004;88:533–7. doi: 10.1136/bjo.2003.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 21.Steinle JJ, Krizsan-Agbas D, Smith PG. Regional regulation of choroidal blood flow by autonomic innervation in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R202–9. doi: 10.1152/ajpregu.2000.279.1.R202. [DOI] [PubMed] [Google Scholar]
- 22.Bill A, Nilsson SF. Control of ocular blood flow. J Cardiovasc Pharmacol. 1985;7(Suppl 3):S96–102. doi: 10.1097/00005344-198500073-00011. [DOI] [PubMed] [Google Scholar]
- 23.Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417. doi: 10.1152/physrev.1975.55.3.383. [DOI] [PubMed] [Google Scholar]
- 24.Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J Appl Physiol. 1996;81:1084–95. doi: 10.1152/jappl.1996.81.3.1084. [DOI] [PubMed] [Google Scholar]
- 25.Bayerle-Eder M, Wolzt M, Polska E, et al. Hypercapnia-induced cerebral and ocular vasodilation is not altered by glibenclamide in humans. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1667–73. doi: 10.1152/ajpregu.2000.278.6.R1667. [DOI] [PubMed] [Google Scholar]
- 26.Guzik P, Wykretowicz A, Krauze T, et al. Add-on therapy with a nighttime dose of doxazosin in patients with uncontrolled hypertension: effects on autonomic modulation of the cardiovascular system. Hypertens Res. 2008;31:443–53. doi: 10.1291/hypres.31.443. [DOI] [PubMed] [Google Scholar]
- 27.Weaver DR, Stehle JH, Stopa EG, Reppert SM. Melatonin receptors in human hypothalamus and pituitary: implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab. 1993;76:295–301. doi: 10.1210/jcem.76.2.8381796. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. Washington, DC: National Institutes of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 29.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 30.Baguet JP. Masked hypertension in obstructive sleep apnea syndrome. J Hypertens. 2008;26:885–92. doi: 10.1097/HJH.0b013e3282f55049. [DOI] [PubMed] [Google Scholar]
- 31.Mallion JM, Baguet JP, Siche JP, Tremel F, De Gaudemaris R. Clinical value of ambulatory blood pressure monitoring. J Hypertens. 1999;17:585–95. doi: 10.1097/00004872-199917050-00001. [DOI] [PubMed] [Google Scholar]
- 32.Baguet JP, Mallion JM, Moreau-Gaudry A, Noirclerc M, Peoc'h M, Siche JP. Relationships between cardiovascular remodelling and the pulse pressure in never treated hypertension. J Hum Hypertens. 2000;14:23–30. doi: 10.1038/sj.jhh.1000933. [DOI] [PubMed] [Google Scholar]
- 33.Bonner RF, Nossal R. Principles of laser doppler flowmetry. In: Shepherd AP, Öberg PA, editors. Developments in Cardiovascular Medicine: Laser Doppler Blood Flowmetry. Boston, MA: Kluwer Academic Publishers; 1990. pp. 73–92. [Google Scholar]
- 34.Rosner V, Hannhart B, Chabot F, Polu JM. Validity of transcutaneous oxygen/carbon dioxide pressure measurement in the monitoring of mechanical ventilation in stable chronic respiratory failure. Eur Respir J. 1999;13:1044–7. doi: 10.1034/j.1399-3003.1999.13e18.x. [DOI] [PubMed] [Google Scholar]
- 35.Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest Ophthalmol Vis Sci. 1997;38:2338–43. [PubMed] [Google Scholar]
- 36.Karakucuk S, Goktas S, Aksu M, et al. Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS) Graefes Arch Clin Exp Ophthalmol. 2008;246:129–34. doi: 10.1007/s00417-007-0656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riva C, Geiser M. Report on ocular blood flow assessment using real-time laser Doppler flowmetry. Acta Ophthalmol (Copenh) 2010 doi: 10.1111/j.1755-3768.2009.01621.x. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Kergoat H, Faucher C. Effects of oxygen and carbogen breathing on choroidal hemodynamics in humans. Invest Ophthalmol Vis Sci. 1999;40:2906–11. [PubMed] [Google Scholar]
- 39.Geiser MH, Riva CE, Dorner GT, Diermann U, Luksch A, Schmetterer L. Response of choroidal blood flow in the foveal region to hyperoxia and hyperoxia-hypercapnia. Curr Eye Res. 2000;21:669–76. [PubMed] [Google Scholar]
- 40.Gugleta K, Orgul S, Hasler P, Flammer J. Circulatory response to blood gas perturbations in vasospasm. Invest Ophthalmol Vis Sci. 2005;46:3288–94. doi: 10.1167/iovs.05-0158. [DOI] [PubMed] [Google Scholar]
- 41.Alm A, Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972;84:306–19. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmetterer L, Findl O, Strenn K, et al. Role of NO in the O2 and CO2 responsiveness of cerebral and ocular circulation in humans. Am J Physiol. 1997;273:R2005–12. doi: 10.1152/ajpregu.1997.273.6.R2005. [DOI] [PubMed] [Google Scholar]
- 43.Flower RW, Fryczkowski AW, McLeod DS. Variability in choriocapillaris blood flow distribution. Invest Ophthalmol Vis Sci. 1995;36:1247–58. [PubMed] [Google Scholar]
- 44.Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–52. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- 45.Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–8. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 46.Heinert G, Nye PC, Paterson DJ. Nitric oxide and prostaglandin pathways interact in the regulation of hypercapnic cerebral vasodilatation. Acta Physiol Scand. 1999;166:183–93. doi: 10.1046/j.1365-201x.1999.00558.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Paulson OB, Lassen NA. Effect of nitric oxide blockade by NG-nitro-L-arginine on cerebral blood flow response to changes in carbon dioxide tension. J Cereb Blood Flow Metab. 1992;12:947–53. doi: 10.1038/jcbfm.1992.131. [DOI] [PubMed] [Google Scholar]
- 48.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 49.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: response to CPAP therapy. Thorax. 2000;55:1046–51. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grote L, Kraiczi H, Hedner J. Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:1480–7. doi: 10.1164/ajrccm.162.4.9912028. [DOI] [PubMed] [Google Scholar]
- 51.Lavie L, Hefetz A, Luboshitzky R, Lavie P. Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatment. J Mol Neurosci. 2003;21:57–63. doi: 10.1385/JMN:21:1:57. [DOI] [PubMed] [Google Scholar]
- 52.Placidi F, Diomedi M, Cupini LM, Bernardi G, Silvestrini M. Impairment of daytime cerebrovascular reactivity in patients with obstructive sleep apnoea syndrome. J Sleep Res. 1998;7:288–92. doi: 10.1046/j.1365-2869.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 53.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998;51:1051–6. doi: 10.1212/wnl.51.4.1051. [DOI] [PubMed] [Google Scholar]
- 54.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Ventilatory and cerebrovascular responses to hypercapnia in patients with obstructive sleep apnoea: effect of CPAP therapy. Respir Physiol Neurobiol. 2009;165:73–81. doi: 10.1016/j.resp.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Ivancev V, Palada I, Valic Z, et al. Cerebrovascular reactivity to hypercapnia is unimpaired in breath-hold divers. J Physiol. 2007;582:723–30. doi: 10.1113/jphysiol.2007.128991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svatikova A, Wolk R, Lerman LO, et al. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005;26:2435–9. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 57.Chamot SR, Cranstoun SD, Petrig BL, Pournaras CJ, Riva CE. Blood PO2 and blood flow at the optic disc. J Biomed Opt. 2003;8:63–9. doi: 10.1117/1.1527935. [DOI] [PubMed] [Google Scholar]
- 58.Riva CE, Grunwald JE, Petrig BL. Autoregulation of human retinal blood flow. An investigation with laser Doppler velocimetry. Invest Ophthalmol Vis Sci. 1986;27:1706–12. [PubMed] [Google Scholar]
- 59.Droste DW, Ludemann P, Anders F, et al. Middle cerebral artery blood flow velocity, end-tidal pCO2 and blood pressure in patients with obstructive sleep apnea and in healthy subjects during continuous positive airway pressure breathing. Neurol Res. 1999;21:737–41. [PubMed] [Google Scholar]
- 60.Niknam RM, Schocket LS, Metelitsina T, DuPont JC, Grunwald JE. Effect of hypertension on foveolar choroidal haemodynamics. Br J Ophthalmol. 2004;88:1263–5. doi: 10.1136/bjo.2003.038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wimpissinger B, Resch H, Berisha F, Weigert G, Schmetterer L, Polak K. Response of choroidal blood flow to carbogen breathing in smokers and non-smokers. Br J Ophthalmol. 2004;88:776–81. doi: 10.1136/bjo.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagaoka T, Kitaya N, Sugawara R, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004;88:1060–3. doi: 10.1136/bjo.2003.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qureshi AI, Christopher Winter W, Bliwise DL. Sleep fragmentation and morning cerebrovasomotor reactivity to hypercapnia. Am J Respir Crit Care Med. 1999;160:1244–7. doi: 10.1164/ajrccm.160.4.9810111. [DOI] [PubMed] [Google Scholar]
- 64.Meadows GE, Kotajima F, Vazir A, et al. Overnight changes in the cerebral vascular response to isocapnic hypoxia and hypercapnia in healthy humans: protection against stroke. Stroke. 2005;36:2367–72. doi: 10.1161/01.STR.0000185923.49484.0f. [DOI] [PubMed] [Google Scholar]
- 65.Klingelhofer J, Hajak G, Sander D, Schulz-Varszegi M, Ruther E, Conrad B. Assessment of intracranial hemodynamics in sleep apnea syndrome. Stroke. 1992;23:1427–33. doi: 10.1161/01.str.23.10.1427. [DOI] [PubMed] [Google Scholar]
- 66.Tamisier R, Gilmartin GS, Launois SH, et al. A New Model of Chronic Intermittent Hypoxia in Humans: Effect on ventilation, sleep and blood pressure. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]