Abstract
Study Objectives:
Prior studies have suggested that the prevalence of sleep disordered breathing (SDB) among players in the National Football League (NFL) is disproportionately high. SDB can increase cardiovascular disease risk and is correlated with hypertension. NFL players have a higher prevalence of hypertension, and we sought to determine the prevalence of SDB among players the NFL and the associations of SDB with anthropometric measures and cardiovascular risk factors.
Design:
Cross-sectional cohort study.
Setting:
NFL athletic training facilities from April to July 2007.
Participants:
A total of 137 active veteran players from 6 NFL teams.
Measurements:
This evaluation of SDB among players in the NFL used a single-channel, home-based, unattended, portable, sleep apnea monitor. Multiple domains of self-reported sleep were assessed. Weight, body mass index, body fat percentage, neck circumference, waist circumference, and waist-to-hip ratio, as well as blood pressure, cholesterol, and fasting glucose concentrations were measured.
Results:
The mean respiratory disturbance index was 4.7 (± 12), with a median (interquartile range) of 2 (1,4). The prevalence of at least mild SDB (RDI ≥ 5) was 19% (95% confidence interval, 12.8%-26.6%). Only 4.4% (95% confidence interval, 1.6%-9.2%) of participants had respiratory disturbance index of 15 or greater. Linemen and non-linemen were not different in their prevalence or severity of SDB. No single anthropometric measure was highly associated with SDB, and SDB was not well correlated with cardiovascular risk factors.
Conclusions:
The prevalence of SDB in active NFL players was modest, predominately mild, and positively associated with several measures of adiposity. SDB did not account for excess cardiovascular risk factors.
Citation:
Rice TB; Dunn RE; Lincoln AE; Tucker AM; Vogel RA; Heyer RA; Yates AP; Wilson PWF; Pellmen EJ; Allen TW; Newman AB; Strollo PJ. Sleep-disordered breathing in the National Football League.
Keywords: Sleep disordered breathing, sleep apnea, obesity, National Football League, cardiovascular risk
A 1994 NATIONAL INSTITUTE FOR OCCUPATIONAL SAFETY AND HEALTH REPORT CONCLUDED THAT THE 6848 RETIRED PROFESSIONAL FOOTBALL PLAYERS studied were 46% less likely to die in middle-age, as compared with a general population of similarly aged men.1 Linemen, however, were noted to be at 50% greater risk of death due to heart disease than the general population and at a 3.7 times higher risk when compared with non-linemen who were retired. These findings provided an impetus for wide-ranging studies to better determine the prevalence of cardiovascular disease (CVD) risk factors and disease among active and retired National Football League (NFL) players.
A number of factors—including obesity, hypertension, impaired glucose tolerance, and lipid abnormalities—have been implicated in the pathogenesis of increased cardiovascular death among former linemen. A recent study has confirmed a near 2-fold increase in the metabolic syndrome in former linemen versus non-linemen.2 These athletes have echocardiographic changes, with higher wall thickness and larger chamber size, which are correlated with body size and strength training.3,4 These findings often persist into retirement and are most striking in retired linemen or retirees with a body mass index (BMI) greater than 35.
Over the 15-year period since the National Institute for Occupational Safety and Health report was published, sleep-disordered breathing (SDB) has been identified as being independently associated with death and CVD.5–7 The adjusted hazard ratios for all-cause and cardiovascular mortality are a striking 3.8 (95% confidence interval [CI], 1.6-9) and 5.2 (95% CI, 1.4-19.2), respectively, in those with severe untreated SDB (respiratory disturbance index [RDI] ≥ 30) compared with those without SDB (RDI < 5) in the 18 years of follow-up of the Wisconsin sleep cohort.5 SDB is associated with insulin resistance and glucose intolerance independent of obesity.8,9 Population studies have repeatedly shown anthropometric measures, including BMI and neck and waist circumference, to be strong predictors of SDB.10,11
Prior research that was limited to individuals from a single NFL team showed that SDB may be more prevalent among players in the NFL, particularly in those that that would be considered to be at high risk for having SDB.12 Given this finding and the current trend toward increasing body size in NFL players, this study was undertaken.
We sought to characterize the cross-sectional burden of SDB in active NFL athletes and its association with cardiovascular risk. Associations of anthropometric measures with SDB were also examined in this population with elevated BMI but variable body composition.
METHODS
This sleep examination is part of a larger cross-sectional cardiovascular evaluation of active, veteran football players from 12 of the 32 NFL teams, data from which have been previously reported.13 The NFL subcommittee on Cardiovascular Health oversaw the study. Medstar (Baltimore, MD) Institutional Review Board approval was obtained.
Participants
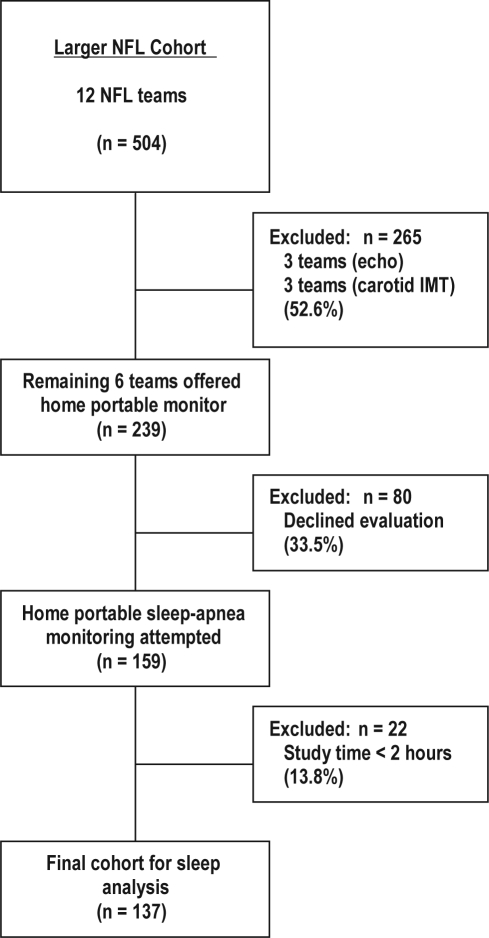
Figure 1 displays how the sleep population was derived from the larger NFL cardiovascular risk factor cohort. Sleep apnea testing was offered to members of 6 of the 12 teams for logistical and financial reasons.13Of the 239 players approached, 159 (66.5%) were agreeable to testing. African Americans were nearly twice as likely as others (42% vs 24%, P = 0.01) to decline sleep testing. Players who declined sleep testing were also, on average, 1 year younger (26 vs 27 years, P = 0.02), 3 cm shorter (188 vs 191 cm, P = 0.04), and 6 kg lighter (110 vs 116 kg, P = 0.02) and had a body fat percentage 2 points lower (16% vs 18%, P = 0.05) than were those who agreed to testing. The BMI of those who declined was 31 kg/m2, compared with 32 kg/m2 (P = 0.02) in the group that agreed to testing. Compared with players who did not agree to participate, those players agreeable to sleep testing were more likely to rate their snoring as loud or very loud (24.3% vs 5.3%, P = 0.02) or to report that their snoring bothered others (75.2% vs 48.7%, P = 0.003).
Figure 1.
Participant flow diagram. NFL refers to the National Football League.
A total of 137 out of 159 participants who underwent portable monitoring testing had at least 2 hours of data, and their data were included for analysis (failure rate 13.8%). The 137 participants were similar to the 22 without 2 hours of data and to the larger NFL cohort, except that Africans Americans made up 57% of the larger cohort but only 45% of the sleep cohort. The players agreeable to sleep apnea testing signed written informed consent prior to evaluation.
Data Collection
The sleep study was conducted during team mini-camps between April and July 2007 and included health history, blood pressure, electrocardiogram, height, weight, neck and waist circumference, waist-to-hip ratio, body fat percentage, and fasting glucose and cholesterol (high-density lipoprotein, low-density lipoprotein, and triglycerides) concentrations. BMI was calculated by dividing weight in kilograms by height in meters squared. Body fat percentage was measured using air displacement with the BOD POD (Life Measurement, Inc., Concord, CA).14,15 The specific methods for the collection of the above data have been previously reported.13
Portable Sleep Apnea Monitoring
Participants who agreed to undergo portable monitoring for sleep apnea were given an ApneaLinkTM (AL; ResMed Corp., Poway, CA) device with instructions for home use.16–18 The AL is a single-channel screening tool for sleep-apnea detection that measures airflow using a nasal cannula with a pressure transducer. SDB is characterized by the continuous variable, RDI, which is defined as the total number of flow-defined apneas and hypopneas measured per hour of AL recording time.
Apnea was defined as a decrease in airflow of 80% of more from baseline that lasted for at least 10 seconds. The AL maximum apnea duration was set at 80 seconds. Hypopnea was defined as a decrease in airflow by 50% to 80% of baseline that lasted at least 10 seconds. The AL maximum hypopnea duration was set at 100 seconds. The AL software automatically analyzed the recording to provide the reported RDI. The AL firmware version 2.97 and the scoring software version 5.13 were used.
The AL device operates on battery power, has a sampling rate of 100 Hz, and has a 16-bit signal processor. The internal memory storage is 15 MB, which allows for approximately 10 hours of data collection. The AL software analyzes data generated by the flow signal, producing a 1-page report. Full disclosure of data is available for review and rescoring by the clinician. All studies were visually reviewed for signal fidelity by the primary investigator (PJS) and sleep study coordinator (OJD). Home use of the AL is a valid method of sleep-apnea detection, the results of which have been highly correlated with laboratory-based polysomnography.16
Patients also completed a sleep-apnea screening questionnaire similar to that used to assess subjective sleep in the CARDIA cohort (Appendix A – see online supplement at www.journalsleep.org). The specific domains assessed were self-reported snoring, apneas, daytime sleepiness; and sleep time.
Statistical Analysis
Descriptive statistics of baseline characteristics were summarized for the sleep cohort and compared with the larger NFL cohort using Wilcoxon rank sum and Fisher exact tests. Within the sleep study cohort, linemen (offensive and defensive line positions excluding defensive ends) and non-linemen were compared in the same way.
The RDI was summarized with descriptive statistics and categorized at RDI cutoffs of 5, 15, and 30. The RDI data were compared for linemen and non-linemen. The association of the various anthropometric measures with SDB was examined using linear and logistic regression. The RDI was log transformed for linear regression analysis and was categorized as the presence or absence of SDB (RDI ≥ 5) for logistic regression. Anthropometric measures used included weight, BMI, body fat percentage, neck and waist circumference, and waist-to-hip ratio. All models were age- and race- adjusted with single predictor variables listed above.
The association of SDB with cardiovascular risk factors was examined using linear and logistic regression. The systolic blood pressure, presence of hypertension (defined as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg) or prehypertension (defined as systolic blood pressure ≥ 120 ≤ 139 mm Hg and /or diastolic blood pressure ≥ 80 ≤ 89 mm Hg), fasting glucose concentrations, and cholesterol concentrations were used as outcome variables in multivariable adjusted models. All analyses were performed using R statistical software version 2.9.0.19
RESULTS
Players grouped by position category showed significant anthropometric differences, with linemen being generally larger, as expected. Table 1 shows significant differences in height, weight, BMI, body fat percentage, and waist and neck circumference for linemen compared with non-linemen. There were no significant differences in cardiovascular risk factors, with the exception of higher diastolic blood pressure in the linemen. There was a 17.5% (95% CI, 11.6%-24.9%) prevalence of hypertension in the sleep cohort, with an additional 67.9% (95% CI, 59.4%-75.6%) of players defined as prehypertensive.
Table 1.
Demographic, anthropometric, and cardiovascular risk characteristics of study population. Participants grouped by position category.
| Characteristic | Subjects |
P Valuea | ||
|---|---|---|---|---|
| All (n = 137) | Linemen (n = 46) | Non-linemen (n = 91) | ||
| Race | 0.43 | |||
| Black | 61 (44.5) | 17 (37.0) | 44 (48.4) | |
| White | 69 (50.4) | 26 (56.5) | 43 (47.3) | |
| Other | 7 (5.1) | 3 (6.5) | 4 (4.4) | |
| Age, y | 27 ± 3 | 27 ± 3 | 27 ± 3 | 0.39 |
| Height, cm | 189 ± 7 | 193 ± 5 | 187 ± 6 | < 0.001 |
| Weight, kg | 117 ± 20 | 140 ± 8 | 105 ± 13 | < 0.001 |
| BMI, kg/m2 | 32.4 ± 4 | 37.3 ± 2.6 | 30.0 ± 3.0 | < 0.001 |
| Body fat, % | 17.9 ± 6.6 | 24.7 ± 3.3 | 14.3 ± 4.9 | < 0.001 |
| Waist, cm | 101 ± 14 | 116 ± 12 | 94 ± 8 | < 0.001 |
| Neck, cm | 44.5 ± 3.3 | 47.4 ± 2.5 | 43.0 ± 2.6 | < 0.001 |
| BP, mm Hg | ||||
| Systolic | 129 ± 11 | 131 ± 11 | 128 ± 10 | 0.12 |
| Diastolic | 77 ± 8 | 79 ± 9 | 75 ± 7 | 0.006 |
| Use of BP medication | 1 (0.7) | 1 (2.2) | 0 | 0.34 |
| Hypertension | 24 (17.5) | 9 (19.6) | 15 (16.5) | 0.37 |
| Prehypertension | 93 (67.8) | 31 (67.4) | 62 (68.1) | 0.50 |
| NSAID use | 27 (19.7) | 8 (17.4) | 19 (20.9) | 0.82 |
| Fasting glucose, mg/dL | 86 ± 12 | 89 ± 14 | 85 ± 11 | 0.008 |
| LDL, mg/dL | 111 ± 28 | 116 ± 34 | 109 ± 25 | 0.31 |
| HDL, mg/Dl | 47 ± 12 | 43 ± 11 | 49 ± 12 | 0.005 |
Values listed are number (%) for categorical variables or means ± SD for continuous variables.
Comparison of linemen with non-linemen. The Wilcoxon rank sum test was used for continuous variable; Fisher exact test for binary variables. Only 128 observations were available for body fat.
BMI refers to body mass index; BP, blood pressure; NSAID, nonsteroidal antiinflammatory drugs; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
The sleep questionnaire revealed that snoring was reported by 100% (95% CI, 97.7%-100%) of players, and this was reported more than 3 or 4 nights a week in 35.8% (95% CI, 28.4%-43.8%) of players. Observed apneas were reported by 23.9% (95%CI, 17.5%-31.2%) of respondents. The same percentage reported excessive daytime sleepiness. The median (interquartile range) total sleep time was 7 (6, 7.5) hours.
Table 2 displays the severity of SDB present for of the overall cohort and by position category. At least mild SDB (RDI of ≥ 5) was present in 19% (95% CI, 12.8%-26.6%) of the total population. Moderate to severe SDB (RDI ≥ 15) was found in 4.4% (95% CI, 1.6%-9.2%) of players. There were no significant differences between linemen and non-linemen. The mean value of the RDI for the cohort was 4.7 ± 12, with a median value of 2. The 25th, 50th, and 75th percentiles for the RDI, total apneas, and total hypopneas are also depicted in Table 2.
Table 2.
Prevalence and standard metrics of sleep disordered breathing among linemen and non-linemen
| Characteristic | Subjects |
P Valuea | ||
|---|---|---|---|---|
| All (n = 137) | Linemen (n = 46) | Non-linemen (n = 91) | ||
| SDB status, number (%)b | 0.63 | |||
| None | 111 (81) | 36 (78.3) | 75 (82.4) | |
| Mild | 20 (14.6) | 9 (19.6) | 11 (12.1) | |
| Moderate | 3 (2.2) | 0 | 3 (3.3) | |
| Severe | 3 (2.2) | 1 (2.2) | 2 (2.2) | |
| RDI | 0.75 | |||
| Median (25th percentile, 75th percentile) | 2 (1,4) | 2 (1,4) | 2 (1,4) | |
| Mean ± SD | 4.7 ± 12 | 5.4 ± 15 | 4.4 ± 11 | |
| Total Apneas, no. | 0.41 | |||
| Median (25th percentile, 75th percentile) | 1 (0,3) | 1 (0,3) | 2 (0,3) | |
| Mean ± SD | 6.7 ± 34 | 7.6 ± 36 | 6.3 ± 34 | |
| Total Hypopneas, no. | 0.72 | |||
| Median (25th percentile, 75th percentile) | 9 (4,14) | 9 (4,14) | 7 (4,14) | |
| Mean ± SD | 13 ± 17 | 15 ± 18 | 13 ± 17 | |
RDI refers to respiratory disturbance index.
Test for significant difference between linemen and non-linemen was a Mantel-Haenszel χ test of linear-by-linear association.
Sleep disordered breathing (SDB) status was determined based on respiratory disturbance index (RDI): none, RDI < 5; mild, RDI 5-14; moderate, RDI 15-29; severe, RDI > 29.
Table 3 displays the β-coefficients for 6 individual, age- and race-adjusted linear models using the different anthropometric measures and the transformed RDI. No single anthropometric measure was highly associated with the RDI. All measures were positively associated with SDB, but the associations were of small magnitude and not statistically significant. When used as an independent variable, the RDI was not significantly associated with systolic blood pressure or the presence of hypertension or prehypertension (Appendix E—see online supplement at www.journalsleep.org). The RDI was not well correlated with fasting glucose, high-density lipoprotein, or low-density lipoprotein concentrations.
Table 3.
Linear regression models for anthropometric associations with the respiratory disturbance index
| Variable | β coefficient (n = 137)a | P Value |
|---|---|---|
| Weight (10 lb) | 0.02b | 0.15 |
| BMI (kg/m2) | 0.03 | 0.11 |
| Body fat, (%) | 0.02 | 0.13 |
| Neck (cm) | 0.01 | 0.80 |
| Waist (cm) | 0.01 | 0.13 |
| Waist:hip (10--1) | 0.01 | 0.28 |
Age- and race-adjusted estimates of the effect of each measure of body size on ln(respiratory disturbance index [RDI]+1).
Sample sizes were smaller for neck circumference, body fat percentage, waist circumference, and waist-to-hip ratio due to missing data.
Because of the properties of the natural logarithm, this corresponds to an approximate 2% greater change in RDI per ± 10-pound difference in weight (and analogously for the other predictors).
DISCUSSION
This cross-sectional evaluation with portable sleep-apnea monitoring in active NFL players found a modest prevalence of SDB, which was predominately mild. In all, 19% of active players had abnormal studies, with at least mild SDB, defined as an RDI ≥ 5. Moderate to severe SDB (RDI ≥ 15) was seen in fewer than 5% of players, despite markedly elevated BMI, as well as universal self-reported snoring. Epidemiologic studies have shown that excess weight, evidenced by increased BMI, contributes to the increased prevalence and severity of SDB.10,11,20 In this population with variable body composition, the BMI and body fat percentage were similarly associated with SDB.
Although no age- and BMI-matched community cohort is available for comparison, this prevalence appears to be in line with estimates of what would be predicted by the participants' BMI distribution, allowing for differences in measurement and uncertainty in this age group.21,22 Bixler and colleagues estimated the prevalence of an AHI of at least 5 in a community-derived cohort of men aged 20 to 39 years to be 7.9% (95% CI 5.0%, 12.1%). But, without knowing the true BMI distribution of their cohort studied, it is impossible to compare the 2. In a single-team study, with players from high (n = 38) and low (n = 14) risk for having sleep apnea, George and colleagues estimated the cross-sectional NFL prevalence of an AHI of at least 10 to be 14% (95% CI, 2%-25%).12 In this 6-team sample of 137 individuals, we found the prevalence at that cutoff (RDI ≥ 10) to be 8% (95% CI, 4.1%-13.9%).
The lack of a stronger association for body fat percentage with SDB is of particular interest given the belief that, in many of these athletes, BMI is a poor surrogate for body composition.15 Unlike what was hypothesized, body fat percentage was not more highly associated with SDB than BMI in a population in which BMI may be misleading. There also was no apparent difference in the prevalence or severity of SDB between linemen and non-linemen, despite striking differences in body composition. This is in contrast with the prior study in which the high-risk group had a prevalence of SDB of 34% compared with 7% in the low- risk group.12 It should be noted that, in this population, the smaller non-linemen still had a mean BMI of 30, a level associated with significant SDB in men.
Because the cohort had a high prevalence of hypertension and prehypertension, an important question was whether or not SDB was contributing to this burden of disease. In this analysis, SDB was unable to account for the elevations of the systolic blood pressure and the presence of hypertension or prehypertension. These differences, with respect to the general population, may be a result of higher weight and other mediators, such as strength training and nonsteroidal antiinflammatory use. There also was no cross-sectional correlation between the presence or severity of SDB and important metabolic parameters, including fasting glucose, low-density lipoprotein, or high-density lipoprotein concentrations. At the position level, differences were noted in diastolic blood pressure, high-density lipoprotein concentration, and fasting glucose concentrations between linemen and non-linemen. This likely represents an obesity effect and is similar to the findings of Miller and colleagues in retired NFL players.2
This current study has several important limitations. The study sample was biased toward players likely to report significant snoring. This study also used a single-channel, portable sleep-apnea monitor in an unattended home study. This posed 2 problems in the measurement of SDB. The first is that the ability to define hypopneas relies solely on airflow. Given that the majority of events measured were hypopneas, the addition of a desaturation threshold may have added precision to this measurement. This is particularly important in defining SDB metrics for correlation with cardiovascular disease, given recently published literature.23 The device is well validated though, without systemic bias in the mild to moderate range of SDB, although it does tend to mildly underestimate the AHI with more severe SDB (AHI ≥ 30).16 This would have little effect on the cohort's overall prevalence of SDB.
The second issue encountered with home-based portable monitoring in this study was missing data. Because the monitoring involved a single channel, loss of the airflow signal led to inadequate data collection in 22 of the 159 individuals who consented to participate in the home-based sleep evaluation. This was further augmented by the fact that this study was underpowered for full analysis. In addition, the failure rate with portable monitoring in this study was 13.8%, which is equivalent to the failure rate of those selected for polysomnography in the prior NFL evaluation (13.3%).12 Last, in addition to the lack of a control group, all comparisons with other populations made in this manuscript are with polysomnographic data and therefore are limited by issues of different measurements.
SDB is more prevalent in active veteran NFL players than age-matched, but not weight-matched, historical cohorts. Surprisingly, body fat percentage was not more closely associated with SDB than was BMI. Despite their large size, these players had favorable metabolic profiles, suggesting a protective effect of exercise. The higher prevalence of hypertension and prehypertension was not explained by SDB. Education and careful monitoring of weight and blood pressure will be essential to offset long-term cardiovascular risk. It is challenging to treat mild OSA, and the long-term consequences of SDB are currently uncertain.24 Further longitudinal study is required to define the risk of SDB in this young and special population, given the understanding that SDB generally increases in prevalence and severity with time.25
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Strollo has received research support from ResMed and Respironics. Dr. Tucker is co chair of the National Football League Subcommittee on Cardiovascular Health and has directed research activities for this subcommittee. The NFL pays the hospital that employs Dr. Tucker for his salary support. Dr. Tucker is on the advisory board of Brainscope a concussion assessment tool.
The NFL had no role in the in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
ACKNOWLEDGMENTS
The control of signal integrity of the sleep recording devices was performed by Oliver J. Drumheller, EdD, RRT. Assistance in study design was provided by Elizabeth A. Carter, MPH, and Jianhui Zhu, MD, PhD (Medstar Research Institute, Washington, DC) and by Jason G. Umans, MD, PhD (Penn Medical Lab of MedStar Research Institute, Washington, DC). We thank Marty Lauzon, ATC, and Ron Medlin, MS, ATC (members of the NFL Subcommittee on Cardiovascular Health), for assistance in data collection; the athletic training staffs of the 12 participating teams; and the head athletic trainers and medical team physicians (Bill Tessendorf, MA, ATC; Thomas White, MD; Bud Carpenter, ATC; Ryan Vermillion, ATC; Howard Katz, MD; Tim Bream, ATC; Chris Hanks, ATC; Keith Burch, MD; Al Bellamy, MS, ATC; Dean L. Kleinschmidt, ATC; James Muntz, MD; Kevin Bastin, ATC; Douglas W. Robertson, MD; Hunter Smith, MA, ATC; Dave Hammer, ATC; David T. Murray, MD; Michael D. Ryan, PT, ATC, PES; Gary W. Dorshimer, MD; Rick Burkholder, ATC; Chris Peduzzi, ATC; John A. Norwig, ATC; Daniel Garza, MD; J. D. Ferguson, MS, ATC; Anthony Casolaro, MD; John Burrell, ATC; Bubba Tyer, ATC; John Mellody, MS, ATC; Sam Ramsden, ATC), and numerous assistant athletic trainers from each team. We also thank the NFL Commissioner's Office; the NFL Players Association; the late Gene Upshaw; Thom Mayer, MD; and the participating NFL teams (the Baltimore Ravens, Buffalo Bills, Carolina Panthers, Chicago Bears, Detroit Lions, Houston Texans, Indianapolis Colts, Jacksonville Jaguars, Philadelphia Eagles, Pittsburgh Steelers, San Francisco 49ers, and Washington Redskins).
Dr. Rice's work is supported by NIH T32 HL 082610-02.
Appendix A. Sleep questionnaire
Appendix B. Demographic and other characteristics by overnight sleep-testing group
| Characteristic | ||||
|---|---|---|---|---|
| Race | Sleep testing (n = 159) | No sleep testing (n = 345) | Total (n = 504) | P Valuea |
| Black | 74 (46.5) | 213 (61.7) | 287 (56.9) | 0.001 |
| White | 77 (48.4) | 109 (31.6) | 186 (36.9) | 0.001 |
| Other | 8 (5) | 23 (6.7) | 31 (6.2) | 0.001 |
| Age, y | 27 ± 3 | 27 ± 3 | 27 ± 3 | 0.41 |
| Height, in. | 75 ± 3 | 74 ± 3 | 74 ± 3 | 0.03 |
| Weight, lbs. | 256 ± 44 | 251 ± 51 | 252 ± 49 | 0.13 |
| BMI, kg/m2 | 32 ± 4 | 32 ± 5 | 32 ± 5 | 0.22 |
| Body fat, %b | 18 ± 7 | 17 ± 8 | 17 ± 7 | 0.17 |
| Neck, cm | 45 ± 3 | 44 ± 3 | 44 ± 3 | 0.03 |
| BP, mm Hg | ||||
| Systolic | 129 ± 11 | 126 ± 11 | 127 ± 11 | 0.01 |
| Diastolic | 77 ± 8 | 75 ± 8 | 76 ± 8 | 0.11 |
| BP meds | 1 (0.6) | 6 (1.7) | 7 (1.4) | 0.44 |
| Hypertension | 29 (18.4) | 42 (13) | 71 (14.8) | 0.13 |
| Prehypertension | 102 (67.1) | 203 (61.7) | 305 (63.4) | 0.26 |
| NSAID use | 32 (20.1) | 68 (19.7) | 100 (19.8) | 0.90 |
Data are presented as number (%) or mean ± SD. Denominators for percentages vary somewhat across rows due to missing data.
BMI refers to body mass index; BP, blood pressure; NSAID, nonsteroidal antiinflammatory drugs.
Wilcoxon-Mann-Whitney test for continuous variables; Fisher exact test for binary variables;
Body-fat percentage was calculated using the Brozek formula.
Appendix C. Demographic and other characteristics for those offered in-home sleep testinga
| Characteristic | ||||
|---|---|---|---|---|
| Race | Accepted (n =159) | Declined (n=80) | Total (n=239) | P Valuea |
| Black | 74 (58.3) | 53 (41.7) | 127 (53.1) | 0.01 |
| White | 77 (77) | 23 (23) | 100 (41.8) | 0.01 |
| Other | 8 (66.7) | 4 (33.3) | 12 (5) | 0.01 |
| Age, y | 27 ± 3 | 26 ± 3 | 27 ± 3 | 0.02 |
| Height, in. | 191 ± 8 | 188 ± 8 | 188 ± 8 | 0.04 |
| Weight, lbs. | 116 ± 20 | 110 ± 24 | 115 ± 21 | 0.02 |
| BMI, kg/m2 | 32 ± 4 | 31 ± 5 | 32 ± 5 | 0.02 |
| Body fat, %b | 18 ± 7 | 16 ± 8 | 17 ± 7 | 0.05 |
| Neck, cm | 45 ± 3 | 44 ± 3 | 44 ± 3 | 0.35 |
| BP, mm Hg | ||||
| Systolic | 129 ± 11 | 128 ± 10 | 129 ± 11 | 0.33 |
| Diastolic | 77 ± 8 | 76 ± 8 | 76 ± 8 | 0.58 |
| BP meds | 1 (0.6) | 2 (2.5) | 3 (1.3) | 0.26 |
| Hypertension | 29 (18.4) | 7 (10.1) | 36 (15.9) | 0.17 |
| Prehypertension | 106 (67.1) | 50 (72.5) | 156 (68.7) | 0.44 |
| NSAID use | 32 (20.1) | 15 (18.8) | 47 (19.7) | 0.86 |
Data are presented as number (%) or mean ± SD. Denominators for percentages vary somewhat across rows due to missing data.
BMI refers to body mass index; BP, blood pressure; NSAID, nonsteroidal antiinflammatory drugs.
Wilcoxon-Mann-Whitney test for continuous variables; Fisher exact test for binary variables.
Appendix D. Sleep questionnaire responses by overnight sleep-testing group
| Question | Sleep testing (n = 159) | No sleep testing (n = 80) | Total (n = 239) | P value |
|---|---|---|---|---|
| 1. Do you snore? | ||||
| Yes | 156 (100) | 79 (100) | 235 (100) | 1a |
| No (skip to Q6) | 0 | 0 | 0 | |
| I don't know (skip to Q6) | 0 | 0 | 0 | |
| If you snore: | ||||
| 2. Your snoring is: | ||||
| Slightly louder than breathing | 44 (46.8) | 24 (63.2) | 68 (51.5) | 0.02b |
| As loud as talking | 30 (31.9) | 12 (31.6) | 42 (31.8) | |
| Louder than talking | 12 (12.8) | 2 (5.3) | 14 (10.6) | |
| Very loud – can be heard in adjacent rooms | 8 (8.5) | 0 (0) | 8 (6.1) | |
| 3. How often do you snore? | ||||
| Nearly every day | 29 (29.3) | 8 (21.1) | 37 (27) | 0.86b |
| 3 to 4 nights per week | 28 (28.3) | 14 (36.8) | 42 (30.7) | |
| 1 to 2 nights per week | 26 (26.3) | 12 (31.6) | 38 (27.7) | |
| 1 to 2 nights per month | 10 (10.1) | 4 (10.5) | 14 (10.2) | |
| Never or nearly never | 6 (6.1) | 0 (0) | 6 (4.4) | |
| 4. Has your snoring ever bothered other people? | ||||
| Yes | 76 (75.2) | 19 (48.7) | 95 (67.9) | 0.003a |
| No/I don't know | 25 (24.8) | 20 (51.3) | 45 (32.1) | |
| 5. Has anyone noticed that you quit breathing during your sleep? | ||||
| Nearly every day | 13 (13) | 3 (7.9) | 16 (11.6) | 0.30a |
| 3 to 4 times a week | 5 (5) | 3 (7.9) | 8 (5.8) | |
| 1 to 2 times a week | 12 (12) | 1 (2.6) | 13 (9.4) | |
| 1 to 2 times a month | 8 (8) | 4 (10.5) | 12 (8.7) | |
| Never or nearly never | 62 (62) | 27 (71.1) | 89 (64.5) | |
| 6. How often do you feel tired or fatigued after your sleep? | ||||
| Nearly every day | 16 (10.3) | 12 (15.8) | 28 (12.1) | 0.45b |
| 3 to 4 times a week | 27 (17.4) | 6 (7.9) | 33 (14.3) | |
| 1 to 2 times a week | 50 (32.3) | 22 (28.9) | 72 (31.2) | |
| 1 to 2 times a month | 42 (27.1) | 21 (27.6) | 63 (27.3) | |
| Never or nearly never | 20 (12.9) | 15 (19.7) | 35 (15.2) | |
| 7. During your waking time, do you feel tired, fatigued or not up to par? | ||||
| Nearly every day | 17 (11) | 10 (13) | 27 (11.7) | 0.91b |
| 3 to 4 times a week | 21 (13.6) | 10 (13) | 31 (13.4) | |
| 1 to 2 times a week | 43 (27.9) | 21 (27.3) | 64 (27.7) | |
| 1 to 2 times a month | 48 (31.2) | 19 (24.7) | 67 (29) | |
| Never or nearly never | 25 (16.2) | 17 (22.1) | 42 (18.2) | |
| 8. Have you ever nodded off or fallen asleep while driving a vehicle? | ||||
| Yes | 156 (100) | 77 (100) | 233 (100) | 1a |
| No/I don't know | 0 | 0 | 0 | |
| 9. If YES, How often does this occur? | ||||
| Nearly every day | 3 (4.7) | 2 (6.9) | 5 (5.4) | 0.91b |
| 3 to 4 times a week | 0 | 0 | 0 | |
| 1 to 2 times a week | 5 (7.8) | 0 | 5 (5.4) | |
| 1 to 2 times a month | 15 (23.4) | 8 (27.6) | 23 (24.7) | |
| Never or nearly never | 41 (64.1) | 19 (65.5) | 60 (64.5) | |
| 10. During the past month, how many hours of actual sleep each night did you get? | ||||
| 7 (6-7.5) | 7 (6-7.5) | 7 (6-7.5) | 0.56c | |
Data are presented as number (%), except question #10, which is median (interquartile range). Denominators vary across rows due to missing data.
Pearson χ test.
Mantel-Haenszel χ test of linear-by-linear association.
Wilcoxon-Mann-Whitney test.
Appendix E. Linear/logistic model predictions of blood pressure and lipid and glucose concentrations from RDI
| Outcome | Estimate (coefficient or OR) | 95% CI | P Value |
|---|---|---|---|
| Hypertensiona | 1.10 | 0.62, 1.97 | 0.74 |
| Prehypertensiona | 0.93 | 0.58, 1.49 | 0.77 |
| SBP, mmHgb | 0.42 | -1.91, 2.74 | 0.72 |
| Glucose ≥ 126 mg/dLa | 2.43 | 0.62, 9.52 | 0.20 |
| Glucose, mg/dLb | 0.20 | -2.92, 3.32 | 0.90 |
| Triglycerides ≥ 150 mg/dLa | 0.82 | 0.37, 1.82 | 0.63 |
| Triglycerides, mg/dLb | -12.3 | -27.5, 2.94 | 0.12 |
| LDL ≥ 160 mg/dLa | 1.02 | 0.26, 3.94 | 0.98 |
| LDL, mg/dLb | 0.97 | -6.14, 8.07 | 0.79 |
| HDL < 40 mg/dLa | 0.99 | 0.58, 1.70 | 0.98 |
| HDL, mg/dLb | 1.67 | -0.82, 4.17 | 0.19 |
| Total cholesterol ≥ 240 mg/dLa | 0.74 | 0.27, 2.02 | 0.56 |
| Total cholesterol, mg/dLb | 0.59 | -6.99, 8.16 | 0.88 |
Age, race, body mass index (BMI), and nonsteroidal antiinflammatory drugs (NSAID) use are adjusted estimates of the effects of ln(respiratory disturbance index [RDI]+1) on cardiovascular risk factor outcomes, from linear and logistic regression models.
OR refers to odds ratio; CI, confidence interval; SBP, systolic blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Binary variable;
Continuous variable.
REFERENCES
- 1.Baron S, Rinsky R. Cincinatti: Centers for Disease Control, National Institute for Occupational Safety and Health; 1994. NIOSH Mortality Study of NFL Football Players: 1959-1988; pp. 1–13. [Google Scholar]
- 2.Miller MA, Croft LB, Belanger AR, et al. Prevalence of metabolic syndrome in retired National Football League players. Am J Cardiol. 2008;101:1281–1284. doi: 10.1016/j.amjcard.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Abernethy WB, Choo JK, Hutter AM., Jr Echocardiographic characteristics of professional football players. J AmColl Cardiol. 2003;41:280–284. doi: 10.1016/s0735-1097(02)02633-5. [see comment] [DOI] [PubMed] [Google Scholar]
- 4.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League linemen versus nonlinemen of left ventricular mass and left atrial size. Am J Cardiol. 2008;102:343–347. doi: 10.1016/j.amjcard.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 8.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 9.Shaw J, Punjabi N, Wilding J, Alberti K, Zimmet P. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81:2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Shahar E, Nieto F, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 12.George CFP, Kab V, Kab P, Villa JJ, Levy AM. Sleep and breathing in professional football players. Sleep Med. 2003;4:317–325. doi: 10.1016/s1389-9457(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 13.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301:2111–2119. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 14.McCrory M, Gomez T, Bernauer E, Molé P. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 15.Kraemer WJ, Torine JC, Silvestre R, et al. Body size and composition of National Football League players. J Strength Cond Res. 2005;19:485–489. doi: 10.1519/18175.1. [DOI] [PubMed] [Google Scholar]
- 16.Erman MK, Stewart D, Einhorn D, Gordon N, Casal E. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–392. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Teschler T, Weinreich G, Hess S, Wessendorf T, Teschler H. Validation of microMESAM as screening device for sleep disordered breathing. Pneumologie. 2003;57:734–740. doi: 10.1055/s-2003-812423. [DOI] [PubMed] [Google Scholar]
- 18.Ragette R, Wang Y, Weinreich G, Teschler H. Diagnostic performance of single airflow channel recording (ApneaLink) in home diagnosis of sleep apnea. Sleep Breath. 2009 doi: 10.1007/s11325-009-0290-2. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2004. [Accessed March 3, 2010]. Manual— R: a Language and Environment for Statistical Computing. Available at www.R-project.org. [Google Scholar]
- 20.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 21.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 22.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 23.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–1155. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pack A, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51:434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Young T, Peppard P, Gottlieb D. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]