Abstract
Purpose
To use patient self-report to provide more valid estimates of whether radiotherapy (RT) is underutilized than possible with registry data, as well as to evaluate for disparities and the influence of preferences and provider interactions.
Methods
We considered 2,260 survey respondents who had nonmetastatic breast cancer, were age 20 to 79 years, were diagnosed between July 2005 and February 2007 in Detroit and Los Angeles, and reported to Surveillance, Epidemiology and End Results (SEER) registries (72% response rate). Survey responses were merged with SEER data. We assessed rates and correlates of RT receipt among all patients with invasive cancer receiving breast-conserving surgery (BCS) and among patients undergoing mastectomy with indications for RT (ie, positive lymph nodes or T3-4 tumors).
Results
Among 904 patients undergoing BCS with strong indications for RT, 95.4% received RT, and 77.6% received RT among the 135 patients undergoing mastectomy with strong indications (P < .001). Among 114 patients undergoing BCS with weaker indications (ie, elderly) for RT, 80.0% received treatment, and 47.5% received RT among the 164 patients undergoing mastectomy with weaker indications (T1N1, T2N1, or T3N0 disease; P < .001). On multivariate analysis, surgery type (P < .001), indication strength (P < .001), age (P = .005), comorbidity (P < .001), income (P = .03), patient desire to avoid RT (P < .001), level of surgeon involvement in decision to have radiation (P < .001), and SEER site (P < .001) were significantly associated with likelihood of RT receipt.
Conclusion
RT receipt was consistently high across sociodemographic subgroups after BCS but was lower after mastectomy, even among patients with strong indications for treatment, in whom clinical benefit is similar. Surgeon involvement had a strong influence on RT receipt.
INTRODUCTION
Radiotherapy (RT) is an integral component of the multidisciplinary management of breast cancer. RT significantly reduces locoregional recurrence and improves overall survival both for patients undergoing breast-conserving surgery (BCS) and for patients with node-positive disease who undergo mastectomy.1
Previous studies have suggested underutilization as well as disparities in the use of adjuvant RT among patients with breast cancer. However, most of these studies relied on registry data,2–6 which may be incomplete.7,8 The few exceptions have become dated9,10 or have focused on centers of excellence that may not be representative of the care received by most patients.11,12 Furthermore, few have considered the receipt of RT after mastectomy,12–14 even though many of these patients are candidates for treatment.
To address these gaps in the literature, we surveyed a population-based sample of patients identified by two Surveillance, Epidemiology and End Results (SEER) registries and assembled a unique data set by supplementing registry data with patient self-report. We considered three research questions. First, what proportion of patients who might benefit from RT actually receive it? Second, what clinical and sociodemographic factors are associated with RT receipt? Third, how do patient preferences and surgeon recommendations affect the receipt of RT?
METHODS
Details of the study design have been published elsewhere.15–17 Questionnaires were developed after considering existing literature and a theoretical model, and standard techniques of content validation were employed (including systematic review by design experts and cognitive pretesting with patients).18
Study Population
Women in the metropolitan areas of Los Angeles (LA) and Detroit who were age 21 to 79 years and who were diagnosed with stage 0 to 319 primary ductal carcinoma in situ or invasive breast cancer from June 2005 through February 2007 were eligible for sample selection. Latina (in LA) and African American (in both LA and Detroit) patients were oversampled.
Population Sampling and Data Collection
Eligible patients were selected by using rapid case ascertainment as they were reported to the LA Cancer Surveillance Program (LACSP) and the Metropolitan Detroit Cancer Surveillance System SEER program registries. This method yields a study sample representative of the two metropolitan areas. We selected all African Americans diagnosed during the diagnosis period. We then selected all Hispanic patients in LA, and selection was followed by a random sample of non–African American/non-Hispanic patients in both regions to achieve the target sample size.17 Asian women in LA were excluded, because these women were being enrolled on other studies.
Physicians were notified of our intent to contact patients, which was followed by a patient mailing that included an introductory letter, survey materials, and a $10 cash gift to eligible participants. The patient questionnaire was translated into Spanish,20 and the Dillman survey method was employed to encourage response.21 The protocol was approved by the institutional review boards of the University of Michigan, University of Southern California, and Wayne State University.
During the study period, 3,252 eligible patients were accrued. This included approximately 70% of the Latina and African American patients and approximately 30% of non-Latina white patients diagnosed in LA and Detroit during the study period. After initial contact, another 119 were excluded because the physician refused permission to contact (n = 20); the patient did not speak English or Spanish (n = 17); the patient was too ill or incompetent to participate (n = 59); or the patient denied having cancer (n = 23). Of the 3,133 patients included in the final accrued sample, 2,260 (72.1%) completed surveys and had information merged to SEER data.
Measures
RT receipt was measured by asking patients, “Did you or are you going to have radiation therapy to treat your breast cancer?” Patients were also asked about the timing of treatment (ie, completed, started, planned). We determined the ultimate surgical procedure by asking about the initial surgical procedure after biopsy and whether subsequent procedures were performed. We also assessed age, ethnicity, comorbidities, insurance status at time of diagnosis, total household yearly income at time of diagnosis, and educational attainment through separate questions. For age and ethnicity, we used SEER data for the few patients (< 1%) missing data by self-report. We used SEER data for clinical information on tumor size and nodal status.
We defined two levels of clinical indication strength—strong and weaker—on the basis of consensus guidelines and evidence that had been published by 2005. We considered patients older than 70 years undergoing breast conserving surgery (BCS) for stage I, estrogen receptor–positive tumors to have weaker indications for RT.22 We categorized all other patients undergoing BCS as having strong indications for RT.23,24 For patients undergoing mastectomy, we categorized those with N2 or greater disease, T4 disease, or T3N1 disease as having strong indications for RT.25–28 Those undergoing mastectomy for T1-2, N1 disease29,30 or T3N0 disease31 were considered to have weaker indications.
We assessed patient preferences regarding radiation by asking, “When decisions were being made about your surgery, how important was it that the type of surgery you had would allow you to avoid exposing yourself to radiation?” (and five response categories ranged from not at all to very much). We assessed patient-provider interactions by asking (with the same five categories), “How much did your surgeon participate in the decision about whether to have radiation?” We collapsed response categories to high (ie, quite a bit or very much) and lower (ie, not at all to somewhat) for analysis. We also asked patients who did not receive or plan to receive radiation to indicate reasons.
Analysis
We included all patients with invasive breast cancer who should have considered RT (N = 1,317): those receiving BCS and the subset of patients undergoing mastectomy with indications for RT (positive lymph nodes or T3-4 primary tumors). We calculated proportions of patients who received RT when grouped by clinical and sociodemographic characteristics, as well as by patient preferences and interactions with providers. All results were weighted to account for the sampling design and differential nonresponse. Results are presented as unweighted values, with weighted percentages. We performed univariate analyses by using χ2 testing, and we defined a separate category to indicate missing values for variables with more than 5% missing data (for income and patient preferences). We then constructed two multivariate models. First, to examine socioeconomic differences, we regressed RT receipt on surgery type, indication strength, age, comorbidity, ethnicity, education, income, insurance, and SEER site as independent variables. We evaluated all first-order interactions between significant variables, as well as all interactions with surgery type, and none were significant except as reported. Next, we added two mechanistic variables—surgeon participation in the radiation decision and patient desire to avoid radiation—to this model, and again we assessed for first-order interactions. Finally, we described reasons for RT nonreceipt as reported by patients who did not receive treatment.
RESULTS
Table 1 lists patient characteristics and rates of RT receipt. The majority of patients received BCS (79.1%). Most of these (93.3%) received RT (79.1% started or completed, 14.2% planned). Fewer patients who underwent mastectomy received RT (60.2%; 41.1% started or completed, 19.1% planned). Surgery type and indication strength were each strongly associated with RT receipt on univariate analysis.
Table 1.
Characteristics of the Patient Population and Rates of RT Receipt
| Variable | Patients |
Weighted % | % Receiving RT* | P† | |
|---|---|---|---|---|---|
| No. | % | ||||
| Site | < .001 | ||||
| LA | 729 | 55.4 | 55.4 | 78.8 | |
| Detroit | 588 | 44.6 | 44.6 | 82.4 | |
| Surgery type | < .001 | ||||
| BCS | 1,018 | 77.3 | 79.1 | 93.3 | |
| Mastectomy | 299 | 22.7 | 21.0 | 60.2 | |
| Indication strength | < .001 | ||||
| Strong | 1,039 | 78.9 | 70.6 | 93.0 | |
| Weaker | 278 | 21.1 | 17.2 | 59.3 | |
| Comorbidity | .017 | ||||
| None | 523 | 39.7 | 41.1 | 83.3 | |
| 1 | 396 | 30.1 | 29.8 | 81.0 | |
| ≥ 2 | 398 | 30.2 | 29.1 | 73.2 | |
| Age, years | .017 | ||||
| < 40 | 82 | 6.2 | 5.0 | 83.0 | |
| 40-49 | 276 | 21.0 | 19.8 | 77.7 | |
| 50-59 | 377 | 28.6 | 28.5 | 81.2 | |
| 60-69 | 341 | 25.9 | 28.0 | 82.6 | |
| ≥ 70 | 241 | 18.3 | 18.7 | 74.9 | |
| Ethnicity | .140 | ||||
| Black | 352 | 26.7 | 15.8 | 79.2 | |
| White | 605 | 45.9 | 66.3 | 81.4 | |
| Latina | 332 | 25.2 | 16.9 | 73.8 | |
| Other or unknown | 28 | 2.1 | 0.9 | 84.2 | |
| Income, $ | .028 | ||||
| < 20,000 | 234 | 17.8 | 14.9 | 70.8 | |
| 20,000-69,999 | 510 | 38.7 | 36.2 | 80.1 | |
| ≥ 70,000 | 316 | 24.0 | 29.4 | 81.2 | |
| Unknown | 257 | 19.5 | 19.5 | 83.8 | |
| Education | 0.264 | ||||
| Not HS graduate | 227 | 17.2 | 12.9 | 75.7 | |
| HS graduate | 285 | 21.6 | 19.6 | 81.3 | |
| Some college | 464 | 35.2 | 38.5 | 78.2 | |
| College graduate | 320 | 24.3 | 27.0 | 82.5 | |
| Unknown | 21 | 1.6 | 2.0 | 87.3 | |
| Insurance | 0.105 | ||||
| None | 92 | 7.0 | 4.7 | 78.2 | |
| Medicaid | 118 | 9.0 | 7.1 | 75.8 | |
| Medicare | 332 | 25.2 | 25.6 | 76.8 | |
| Other | 731 | 55.5 | 58.1 | 81.2 | |
| Unknown | 44 | 3.3 | 4.5 | 85.9 | |
Abbreviations: RT, radiotherapy; LA, Los Angeles; BCS, breast-conserving surgery; HS, high school.
Percent receiving RT calculated within the weighted sample. Patients were categorized as receiving RT if they reported that they had or were going to have radiation therapy to treat their breast cancer (n = 1,136). Of these, 188 patients reported that they had yet to start radiation.
P values for differences in the proportion of RT receipt by the categories are shown; separate category included for unknown when unknown values exceeded 5% (ie, for income).
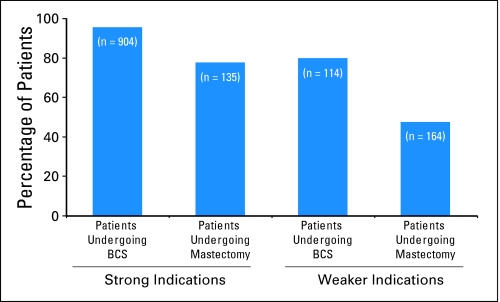
Figure 1 shows adjusted rates of RT utilization within the two surgical groups by indication categories. Nearly all (95.4%) of the 904 patients undergoing BCS with strong indications received RT, compared with only 77.6% of the 135 patients undergoing mastectomy. Of the 114 patients undergoing BCS with weaker indications, 80.0% received RT, whereas 47.5% of 164 patients undergoing mastectomy with weaker indications received RT (P < .001)
Fig 1.
Rates of radiotherapy (RT) receipt by surgery type and indication strength. Higher proportions of patients receive RT after breast-conserving surgery (BCS) than after mastectomy, both among those with strong indications and among those with weaker indications. Twenty-three patients had missing information. Data adjusted for age, comorbidity, site, ethnicity, and education.
Table 2 shows that provider interactions and patient preferences were also strongly correlated with RT receipt. Half (51.2%) of respondents reported high surgeon participation in the decision to receive RT, and these patients were more likely to receive radiation than those whose surgeon participated less (86.5% v 74.7%; P < .001). A substantial minority of respondents (22.7% of those undergoing BCS and 32.8% of patients with mastectomy; P < .001) expressed a high desire to avoid RT exposure as an influence on their surgical decision. As might be expected, patients with high desire to avoid RT were less likely to receive it than those with lower desire (61.6% v 87.5%; P < .001).
Table 2.
Patient Preferences and Interactions With Providers
| Variable | Patients |
Weighted % | % Receiving RT* | P† | |
|---|---|---|---|---|---|
| No. | % | ||||
| Surgeon participation in radiation decision | < .001 | ||||
| Lower | 592 | 45.0 | 44.9 | 74.7 | |
| High | 690 | 52.4 | 51.2 | 86.5 | |
| Unknown | 35 | 2.7 | 49.0 | ||
| Desire to avoid radiation exposure | < .001 | ||||
| Lower | 841 | 63.9 | 62.9 | 87.5 | |
| High | 370 | 28.1 | 27.6 | 61.6 | |
| Unknown | 106 | 8.0 | 9.5 | 80.8 | |
Abbreviation: RT, radiotherapy.
Percent receiving RT calculated within the weighted sample. Patients were categorized as receiving RT if they reported that they had or were going to have radiation therapy to treat their breast cancer (n = 1,136). Of these, 188 patients reported that they had yet to start radiation.
P values assessed for differences in likelihood of RT receipt by the categories shown; separate category included for unknown when unknown values exceeded 5% (ie, for both patient preference items).
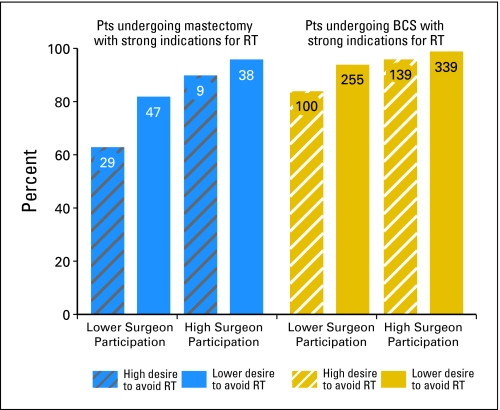
Figure 2 shows the effects of provider interactions and patient preferences on rates of RT receipt among patients with strong indications for adjuvant RT, by surgery type. For patients who received BCS, rates of RT receipt exceeded 90% in all subgroups except for the small minority of patients (n = 100; 10.5% of patients with BCS) who reported a high desire to avoid RT and lower surgeon involvement in the radiation decision (for whom 84.1% received RT). For patients who received mastectomy, rates also exceeded 90% for the 47 patients (38%) who reported high surgeon involvement. However, among patients with mastectomy who reported lower surgeon involvement, 82.1% of those with lower desire to avoid RT received treatment, and only 63.2% of those with high desire to avoid RT received treatment (P < .001).
Fig 2.
Rates of radiotherapy (RT) receipt among patients (Pts) with strong indications, by surgery type, preference to avoid RT, and provider involvement level. Among patients with strong indications undergoing breast-conserving surgery (BCS), even those with a high desire to avoid RT and those reporting lower levels of surgeon involvement in radiation decisions were likely to receive RT. In contrast, among patients with strong indications for RT who underwent mastectomy, a substantial rate of lack of RT receipt was observed in certain subsets, particularly among those expressing high desire to avoid RT who reported lower levels of surgeon involvement in the decision process. Rates adjusted for age, comorbidity, site, ethnicity, and education. Forty-four patients had missing information.
As listed in Table 3 in a multivariate model including all variables in Table 1, surgery type (P < .001), indication strength (P < .001), age (P = .01), comorbidity (P < .001), income (P = .03), and site (P < .001) were significant. When patient preference and surgeon involvement variables were added to this model, the same clinical and sociodemographic variables were significant, and both the patient preference (P < .001) and surgeon involvement (P < .001) variables were also significant correlates of RT receipt. No significant interactions were observed in either model except between site and indication (P = .003 in each model). Patients in LA with weaker indications were less likely to receive treatment than patients in Detroit with weaker indications (75.8% in LA and 84.5% in Detroit after BCS; 40.5% in LA and 54.8% in Detroit after mastectomy). These between-site differences were much smaller for patients with strong indications (94.2% in LA and 96.7% in Detroit after BCS; 74.7% in LA and 80.8% in Detroit after mastectomy).
Table 3.
Logistic Regression Models of Radiation Therapy Receipt
| Variable | Model 1 |
Model 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P* | Odds Ratio | 95% CI | P* | |||
| Surgery type | < .001 | < .001 | ||||||
| Mastectomy | 1.00 | — | — | 1.00 | — | — | ||
| BCS | 5.92 | 4.62 | 7.58 | 4.46 | 3.34 | 5.96 | ||
| Indication strength and site† | .003 | |||||||
| Weaker indication, LA | 1.00 | — | — | 1.00 | — | — | ||
| Weaker indication, Detroit | 2.63 | 1.82 | 3.80 | 2.54 | 1.68 | 3.83 | ||
| Strong indication, LA | 6.35 | 4.72 | 8.54 | 7.94 | 5.60 | 11.26 | ||
| Strong indication, Detroit | 7.97 | 4.88 | 13.03 | 8.85 | 5.10 | 15.34 | ||
| Comorbidities | < .001 | < .001 | ||||||
| 0 | 1.00 | — | — | 1.00 | — | — | ||
| 1 | 0.79 | 0.59 | 1.06 | 0.78 | 0.57 | 1.09 | ||
| ≥ 2 | 0.47 | 0.34 | 0.64 | 0.48 | 0.34 | 0.68 | ||
| Age, years | .011 | .005 | ||||||
| < 40 | 1.19 | 0.62 | 2.29 | 0.91 | 0.45 | 1.86 | ||
| 40-49 | 1.27 | 0.76 | 2.11 | 1.22 | 0.69 | 2.17 | ||
| 50-59 | 1.72 | 1.06 | 2.79 | 1.45 | 0.84 | 2.50 | ||
| 60-69 | 0.93 | 0.62 | 1.39 | 0.73 | 0.47 | 1.15 | ||
| ≥ 70 | 1.00 | — | — | 1.00 | — | — | ||
| Ethnicity | .815 | .553 | ||||||
| White | 1.00 | — | — | 1.00 | — | — | ||
| Black | 1.15 | 0.84 | 1.59 | 1.20 | 0.85 | 1.71 | ||
| Latina | 1.11 | 0.78 | 1.58 | 1.23 | 0.84 | 1.81 | ||
| Other | 1.20 | 0.26 | 5.60 | 1.65 | 0.33 | 8.31 | ||
| Income, $ | .027 | .033 | ||||||
| < 20,000 | 1.00 | — | — | 1.00 | — | — | ||
| 20,000-69,999 | 1.22 | 0.87 | 1.71 | 1.04 | 0.71 | 1.52 | ||
| ≥ 70,000 | 1.14 | 0.77 | 1.70 | 1.24 | 0.78 | 1.95 | ||
| Unknown | 1.73 | 1.18 | 2.54 | 1.71 | 1.10 | 2.64 | ||
| Education | .092 | .121 | ||||||
| Not HS graduate | 1.00 | — | — | 1.00 | — | — | ||
| HS graduate | 1.20 | 0.81 | 1.77 | 1.29 | 0.83 | 1.99 | ||
| Some college | 1.22 | 0.84 | 1.77 | 0.88 | 0.59 | 1.33 | ||
| College graduate | 1.63 | 1.08 | 2.47 | 1.16 | 0.73 | 1.82 | ||
| Insurance | .058 | .677 | ||||||
| None | 0.55 | 0.34 | 0.92 | 0.74 | 0.42 | 1.31 | ||
| Medicaid | 1.05 | 0.66 | 1.69 | 1.09 | 0.65 | 1.82 | ||
| Medicare | 1.19 | 0.80 | 1.75 | 1.04 | 0.67 | 1.61 | ||
| Other | 1.00 | — | — | 1.00 | — | — | ||
| Surgeon participation | — | — | — | — | < .001 | |||
| Lower | — | — | — | — | 1.00 | — | — | |
| High | — | — | — | — | 2.33 | 1.80 | 3.02 | |
| Desire to avoid radiation | < .001 | |||||||
| Lower | — | — | — | — | 1.00 | — | — | |
| High | — | — | — | — | 0.35 | 0.27 | 0.44 | |
Abbreviations: BCS, breast-conserving surgery; LA, Los Angeles; HS, high school.
P values for group variables are reported from Wald tests.
Model 1: P value for indication strength < .001; P value for site < .001; P value for interaction = .003. Model 2: P value for indication strength < .001; P value for site < .001; P value for interaction = .003.
Table 4 presents the reasons cited by patients who did not receive RT. Overall, most reported that their doctor said they did not need it (109 of 181 patients; 60%), but some chose not to get it (29 of 181 patients; 16%). Among patients with strong indications, patients with mastectomy were much more likely to report that their surgeon did not discuss RT or that they did not need it than patients with BCS (72.0% v 44.7%; P = .03).
Table 4.
Reasons for Omission of Radiotherapy by Surgical Option and Indication
| Reason | Strong Indications |
Weaker Indications |
||
|---|---|---|---|---|
| BCS (n = 904) | Mastectomy (n = 135) | BCS (n = 114) | Mastectomy (n = 164) | |
| No. not receiving RT | 47 | 25 | 18 | 91 |
| Doctor did not discuss | 6 | 6 | 0 | 5 |
| Doctor said no need | 15 | 12 | 11 | 71 |
| Chose not to | 13 | 3 | 6 | 7 |
| Worry about side effects | 9 | 3 | 1 | 3 |
| Worry about cost | 0 | 1 | 1 | 0 |
| Burden on self or family | 2 | 2 | 1 | 0 |
Abbreviations: BCS, breast-conserving surgery; RT, radiotherapy.
DISCUSSION
In this large, population-based study in two metropolitan areas, we found high rates of RT receipt among patients undergoing BCS but lower rates among patients undergoing mastectomy. For patients with strong indications for treatment on the basis of guidelines adopted before the study period, 95.4% of patients undergoing BCS received radiotherapy compared with only 77.6% of patients undergoing mastectomy. Despite deliberate oversampling for minority patients, we did not observe significant differences by ethnicity or education, although we did observe a modest income gradient, as lowest-income patients were somewhat less likely to receive treatment. Patient preferences and provider interactions were strongly associated with likelihood of RT receipt across surgery types and indication strengths. Most notably, among patients who received mastectomy and had strong indications for RT, only 63.2% received RT when desire to avoid radiation was high and reported surgeon participation in the decision was low, compared with greater than 90% when surgeon participation was high. Most patients with strong indications for RT after mastectomy who failed to receive treatment reported that their doctor either did not discuss RT or said it was not needed.
In recent years, attention has been focused on concerns about underutilization of adjuvant RT after BCS. RT receipt after BCS has even been used as a quality indicator.32 The high rate of RT we observed after BCS among patients with strong indications is encouraging, especially because this constitutes the majority of patients with breast cancer who have strong indications for RT. The rate of post-BCS RT in this population-based study was similar to that recently reported within National Comprehensive Cancer Network institutions.11
These results differed, however, from other population-based studies of RT utilization. Not only older2–4 but also recent studies5,6 that used SEER data alone have suggested substantial underutilization of RT after BCS. For example, a recent analysis of SEER data suggested that rates of RT after BCS decreased from 79.4% in 1988 to 66.4% in 2004.6 However, the validity of radiation treatment information in registry data is limited.8 For example, in one study comparing SEER registry data to medical record review, RT use was accurately captured by registry data in only 72% of cases.7 Some researchers have responded to concerns about the limitations of registry data by focusing on the linked SEER-Medicare data set,33,34 but these studies generally include women younger than 65 years old and so have limited generalizability.13,35–39 The few studies that have obtained more complete data suggested a higher rate of RT receipt after BCS than registry studies (84% in a comprehensive chart review study of women treated in New York City in 1999 to 200010 and 86% in a study surveying hospital cancer registrars about patients diagnosed in 19949), but these estimates are now dated. Studies that are dated or that rely on incomplete data cannot provide information to evaluate whether quality initiatives have been successful, nor are they helpful in guiding ongoing quality improvement efforts.
Less attention has been paid to the receipt of radiation after mastectomy for patients who need it. The moderate rate of RT after mastectomy observed even in patients with strong indications suggests that a substantial minority of patients with breast cancer remain undertreated. In our study, we defined a subgroup of patients for whom RT was strongly indicated on the basis of clinical guidelines.25 In these patients, the absolute risk of locoregional recurrence exceeds 30% in the absence of RT and is reduced by two thirds with RT, yielding a survival benefit. Yet, even among patients with strong indications for treatment, those undergoing mastectomy were substantially less likely to receive RT than those undergoing BCS, despite similar expected benefit. This gap was particularly pronounced when provider participation in the radiation decision was reported to be low and patient desire to avoid radiation was high.
Among patients with weaker indications, we also observed higher RT receipt among those undergoing BCS than those undergoing mastectomy. Our finding that receipt of RT for patients with weaker indications was lower in LA than Detroit underscores that there may be controversy among clinicians for this group (in contrast to the group with strong indications, who had similar rates of RT receipt regardless of site).40 In the Cancer and Leukemia Group B (CALGB) trial that defined a group of patients who might consider RT omission after BCS (ie, women age 70 years and older with stage I, estrogen receptor–positive disease), the 5-year risk of local recurrence was only 4% after BCS and tamoxifen alone.22 In contrast, in the largest American series of patients undergoing mastectomy with involvement of one to three axillary nodes (who constitute the majority of patients with mastectomy with weaker indications), the risk of locoregional failure in the absence of RT was 13%.29,30 Thus, although legitimate clinical uncertainty influences decision-making in the group of patients with weaker indications, our finding of substantially higher rates of RT among patients undergoing BCS with weaker indications—rates that exceed even the rates of RT received by patients who received mastectomy with strong indications—seems remarkable, especially because RT for patients undergoing BCS with weaker indications is unlikely to yield a survival benefit, whereas RT after mastectomy yields a survival advantage that could be as high as 10% in patients with strong indications.
In the population in which we observed substantial underutilization—patients with mastectomy—provider involvement was an important correlate of RT receipt. Even patients who expressed preferences to avoid RT were highly likely to receive it if their surgeons were highly involved in the decision process. Patients who did not receive radiation after mastectomy were most likely to report that their providers had either failed to discuss RT or had failed to recommend it. Because most patients with lymph node involvement receive adjuvant chemotherapy before RT, discussions between patients and surgeons may take place many months before the delivery of RT. Therefore, educational efforts targeting both surgeons and medical oncologists may be important in improving rates of RT receipt in this population, as may be the development of tailored decision aids that encourage communication between patients and providers.41,42
This study has several strengths, including a large, diverse patient sample and access to both clinical data and patient reports of treatment receipt, individual socioeconomic characteristics, patient preferences, and provider interactions. Nevertheless, several limitations merit comment. First, the location of the study in the greater Detroit and LA metropolitan areas may limit the generalizability of the findings, particularly to more rural areas. Second, our measures were drawn from patient self-report, which may be prone to bias. Although there is no clear gold standard for comparison, our measure of self-reported RT receipt has strong face validity, because patients surveyed months after diagnosis should accurately recall whether or not they received radiation treatment. Furthermore, self-reported RT receipt was highly and logically correlated with clinical and treatment factors that direct radiation treatment recommendations. Patient recall of communication issues may be more prone to bias, however. Third, although the sample size was adequate to detect substantial differences, power to detect modest differences was more limited. Finally, although the response rate to this survey was high, it is possible that selection bias may also have influenced our results.
Our findings have important implications for physician behavior and clinical policy. The results suggest that we have largely achieved success in the appropriate use of RT after BCS in metropolitan areas like those we studied, but more attention needs to be paid to the use of RT after mastectomy. We found that surgeon participation in the RT decision was a powerful correlate of use. This underscores the need to focus physician attention on potential gaps in treatment delivery. We also found that patient concerns about radiation were negatively associated with RT use. This is important because patients with these concerns may choose mastectomy with the intention of avoiding radiation, resulting in a higher prevalence of these concerns in patients undergoing mastectomy than undergoing BCS.43 Thus, it is important to consider these concerns when informing these patients about RT. Our findings suggest that initiatives to ensure that surgeons are informed about the role of RT after mastectomy, to encourage provider participation in the postmastectomy RT decision, and to improve patient education in this setting, would further optimize care for patients with breast cancer.
Acknowledgment
We thank our project staff for outstanding work: Barbara Salem, MS, MSW, and Ashley Gay, BA (University of Michigan); Ain Boone, BA, Cathey Boyer, MSA, and Deborah Wilson, BA (Wayne State University); and Alma Acosta, Mary Lo, MS, Norma Caldera, Marlene Caldera, and Maria Isabel Gaeta (University of Southern California). We also thank the patients with breast cancer who responded to our survey.
Supported by Grants No. R01 CA109696 and R01 CA088370 from the National Cancer Institute to the University of Michigan; by Mentored Research Scholar Grant No. MRSG-09-145-01 from the American Cancer Society (R.J.); by Established Investigator Award in Cancer Prevention, Control, Behavioral, and Population Sciences Research No. K05CA111340 from the National Cancer Institute (S.J.K.); by California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 (for collection of Los Angeles County cancer incidence data used in this study); by the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) Program Contracts No. N01-PC-35139 (University of Southern California), N01-PC-54404 (Public Health Institute), and N01-PC-35145 (for collection of metropolitan Detroit cancer incidence data); and by the Centers for Disease Control and Prevention National Program of Cancer Registries Agreement No. 1U58DP00807-01 (Public Health Institute).
Footnotes
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Reshma Jagsi, Steven J. Katz
Financial support: Reshma Jagsi, Steven J. Katz
Administrative support: John J. Graff, Ann S. Hamilton, Steven J. Katz
Provision of study materials or patients: John J. Graff, Ann S. Hamilton
Collection and assembly of data: Paul Abrahamse, John J. Graff, Ann S. Hamilton, Steven J. Katz
Data analysis and interpretation: Reshma Jagsi, Paul Abrahamse, Monica Morrow, Sarah T. Hawley, Jennifer J. Griggs, Steven J. Katz
Manuscript writing: Reshma Jagsi, Steven J. Katz
Final approval of manuscript: Reshma Jagsi, Paul Abrahamse, Monica Morrow, Sarah T. Hawley, Jennifer J. Griggs, John J. Graff, Ann S. Hamilton, Steven J. Katz
REFERENCES
- 1.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Lazovich DA, White E, Thomas DB, et al. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991;266:3433–3438. [PubMed] [Google Scholar]
- 3.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. doi: 10.1056/NEJM199204233261701. [DOI] [PubMed] [Google Scholar]
- 4.Nattinger AB, Hoffmann RG, Kneusel RT, et al. Relation between appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. 2000;356:1148–1153. doi: 10.1016/S0140-6736(00)02757-4. [DOI] [PubMed] [Google Scholar]
- 5.Du XL, Gor BJ. Racial disparities and trends in radiation therapy after breast-conserving surgery for early-stage breast cancer in women, 1992 to 2002. Ethn Dis. 2007;17:122–128. [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman RA, He Y, Winer EP, et al. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713–719. doi: 10.1200/JCO.2008.17.9234. [DOI] [PubMed] [Google Scholar]
- 7.Malin JL, Kahn KL, Adams J, et al. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94:835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- 8.Bickell NA, Chassin MR. Determining the quality of breast cancer care: Do tumor registries measure up? Ann Intern Med. 2000;132:705–710. doi: 10.7326/0003-4819-132-9-200005020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in Stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. doi: 10.1200/JCO.2001.19.8.2254. [DOI] [PubMed] [Google Scholar]
- 10.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 11.Buchholz TA, Theriault RL, Niland JC, et al. The use of radiation as a component of breast conservation therapy in National Comprehensive Cancer Network Centers. J Clin Oncol. 2006;24:361–369. doi: 10.1200/JCO.2005.02.3127. [DOI] [PubMed] [Google Scholar]
- 12.Punglia R, Hughes ME, Edge SB, et al. Factors associated with guideline-concordant use of radiotherapy after mastectomy in the National Comprehensive Cancer Network. Int J Radiat Oncol Biol Phys. 2008;72:1434–1440. doi: 10.1016/j.ijrobp.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punglia RS, Weeks JC, Neville BA, et al. Radiation therapy after mastectomy between 1991 and 1999 in elderly women: Response to clinical trial information. J Clin Oncol. 2006;24:3474–3482. doi: 10.1200/JCO.2006.05.7844. [DOI] [PubMed] [Google Scholar]
- 14.Jagsi R, Abrahamse P, Morrow M, et al. Postmastectomy radiotherapy for breast cancer: Patterns, correlates, communication, and insights into the decision process. Cancer. 2009;115:1185–1193. doi: 10.1002/cncr.24164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendation and receipt of mastectomy for breast cancer. JAMA. 2009;302:1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. 2009;101:1337–1347. doi: 10.1093/jnci/djp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: Population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groves RM, Fowler FJ, Couper MP, et al. Survey Methodology. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. ed 6. Philadelphia, PA: Lippincott Raven Publishers; 2002. [Google Scholar]
- 20.Marin G, Van Oss Marin B. Newbury Park, CA: Sage Publications; 1991. Research With Hispanic Populations: Applied Social Research Methods Series, Issue Number 23. [Google Scholar]
- 21.Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York, NY: John Wiley and Sons; 1997. [Google Scholar]
- 22.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 24.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 25.Recht A, Edge SB, Solin LJ, et al. Postmastectomy radiotherapy: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 26.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 27.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 29.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 30.Taghian A, Jeong J-H, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 31.Taghian AG, Jeong JH, Mamounas EP, et al. Low locoregional recurrence rate among node-negative breast cancer patients with tumors 5 cm or larger treated by mastectomy, with or without adjuvant systemic therapy and without radiotherapy: Results from five national surgical adjuvant breast and bowel project randomized clinical trials. J Clin Oncol. 2006;24:3927–3932. doi: 10.1200/JCO.2006.06.9054. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare and Medicaid Services. Physician Quality Reporting Initiative. http://www.cms.hhs.gov/pqri/
- 33.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(suppl 4):49–54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 34.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: The advantages of the linked Medicare and SEER data: Surveillance, Epidemiology and End Results. J Clin Epidemiol. 1999;52:463–470. doi: 10.1016/s0895-4356(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 35.Ballard-Barbash R, Potosky AL, Harlan LC, et al. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–726. doi: 10.1093/jnci/88.11.716. [DOI] [PubMed] [Google Scholar]
- 36.Gross CP, Smith BD, Wolf E, et al. Racial disparities in cancer therapy: Did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith BD, Haffty BG, Smith GL, et al. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71:98–106. doi: 10.1016/j.ijrobp.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Hampton T. Studies address racial and geographic disparities in breast cancer treatment. JAMA. 2008;300(14):1641. doi: 10.1001/jama.300.14.1641. [DOI] [PubMed] [Google Scholar]
- 39.Smith BD, Smith GL, Roberts KB, et al. Baseline utilization of breast radiotherapy before institution of the Medicare practice quality reporting initiative. Int J Radiat Oncol Biol Phys. 2009;74:1506–1512. doi: 10.1016/j.ijrobp.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma in situ. J Clin Oncol. 2005;23:3001–3007. doi: 10.1200/JCO.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peele PB, Siminoff LA, Xu Y, et al. Decreased use of adjuvant breast cancer therapy in a randomized controlled trial of a decision aid with individualized risk information. Med Decision Making. 2005;25:301–307. doi: 10.1177/0272989X05276851. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien MA, Whelan TJ, Villasis-Keever M, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27:974–985. doi: 10.1200/JCO.2007.16.0101. [DOI] [PubMed] [Google Scholar]
- 43.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]