Abstract
Purpose
To determine whether acupuncture reduces pain and dysfunction in patients with cancer with a history of neck dissection. The secondary objective is to determine whether acupuncture relieves dry mouth in this population.
Patients and Methods
Patients at a tertiary cancer center with chronic pain or dysfunction attributed to neck dissection were randomly assigned to weekly acupuncture versus usual care (eg, physical therapy, analgesia, and/or anti-inflammatory drugs, per patient preference or physician recommendation) for 4 weeks. The Constant-Murley score, a composite measure of pain, function, and activities of daily living, was the primary outcome measure. Xerostomia, a secondary end point, was assessed using the Xerostomia Inventory.
Results
Fifty-eight evaluable patients were accrued and randomly assigned from 2004 to 2007 (28 and 30 patients on acupuncture and control arms, respectively). Constant-Murley scores improved more in the acupuncture group (adjusted difference between groups = 11.2; 95% CI, 3.0 to 19.3; P = .008). Acupuncture produced greater improvement in reported xerostomia (adjusted difference in Xerostomia Inventory = –5.8; 95% CI, –0.9 to –10.7; P = .02).
Conclusion
Significant reductions in pain, dysfunction, and xerostomia were observed in patients receiving acupuncture versus usual care. Although further study is needed, these data support the potential role of acupuncture in addressing post–neck dissection pain and dysfunction, as well as xerostomia.
INTRODUCTION
Cancers arising in the head and neck comprise a heterogeneous group of malignancies. The annual burden of new head and neck cancers in the United States collectively exceeds 100,000, and the upper aerodigestive and thyroid gland cancers by themselves accounted for an estimated 85,000 new cases in 2009.1
Surgery figures prominently in the curative management of the majority of patients with these cancers. Because spread of disease to the lymph nodes of the neck is frequent, neck dissection is commonly necessary. Various types of neck dissections exist, which vary in their extensiveness, and dissections are most simply classified as either comprehensive or selective.2 Classic radical neck dissection is an example of the former. Among the structures removed are the lymph nodes from the submandibular triangle, along the internal jugular vein, and in the posterior triangle (levels 1 to 5); the sternocleidomastoid muscle; the internal jugular vein; the spinal accessory nerve (cranial nerve XI); and the submandibular gland on one side of the neck.
The removal of the spinal accessory nerve leads to shoulder problems, characterized by shoulder droop, winged scapula, weak abduction, inability to shrug, and a dull ache with pain localized to the shoulder. Even dissection with preservation of the nerve may lead to sequelae. As such, complaints of neck or shoulder pain and dysfunction are common in 30% to 70% of patients after classical radical neck dissection, depending on how symptom severity is defined.3–5 Quality of life and employability also may be adversely affected.6 Less extensive procedures, such as modified radical neck dissections that spare the spinal accessory nerve or other structures, decrease the likelihood of such difficulties but do not prevent them entirely.7–13 Indeed, patients experience symptoms and dysfunction after neck dissection, even after selective procedures, that cannot be entirely attributed to physical damage to the spinal accessory nerve.14–16
Although physical therapy exercises and anti-inflammatory drugs are widely prescribed to address the pain and shoulder dysfunction after neck dissection, their efficacy is often disappointing or incomplete. Extensive searches of MEDLINE and the Cochrane Controlled Trials Registry located only one controlled trial specifically examining this condition, and that trial compared progressive resistance exercise training versus a more standardized therapeutic exercise approach for 12 weeks, reporting superior outcomes with the former.17
Acupuncture is a safe and well-tolerated treatment, and clinical research from randomized controlled trials supports its efficacy for the treatment of acute and chronic pain.18–20 Understanding of the physiologic basis for this efficacy is growing.21–23 Of particular interest to patients suffering from pain and dysfunction after neck dissection are modern randomized trial studies that evaluated the efficacy of acupuncture for neck and shoulder pain attributed to etiologies not related to malignancy or its treatment. In these settings, acupuncture was associated with significant improvement in pain,24–29 and some studies also demonstrated an improvement in function.24,25 Of note, in three studies, a sham acupuncture arm was included.24,27,29
We report here the results of a randomized trial of acupuncture versus usual care to treat pain and dysfunction after neck dissection for cancer. In addition, an exploratory assessment of acupuncture as a treatment for xerostomia, or extreme dry mouth, was incorporated into the design of the study because dry mouth from adjunctive radiation therapy is a frequent, well-recognized treatment sequelae in this population. The application of newer, targeted radiation techniques is able to decrease post-treatment xerostomia compared with conventional radiation but does not eliminate the problem.30–32 Available therapies for xerostomia have modest efficacy and adverse effects33–35; preliminary data suggest that acupuncture may alleviate these symptoms.36–38
PATIENTS AND METHODS
Study Design
The primary objective of the study was to determine whether acupuncture reduces pain or dysfunction in patients with cancer with a history of neck dissection. The secondary objective was to determine whether acupuncture relieves dry mouth in this population.
The study was a prospective, open-label, randomized controlled trial. Random assignment was stratified by neck procedure type (selective, modified, or radical) and baseline Constant-Murley score (> 35 v ≤ 35)39 using blocks of random length. Random assignment was implemented via a secure computerized database, ensuring full allocation concealment. Patient accrual and treatment occurred from 2004 to 2007. Cross over to the acupuncture arm was allowed for the control group after final study assessments were obtained. The study protocol was approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center (MSKCC).
Trial Sample
All patients had undergone neck dissection for cancer; expressed complaints of pain and/or dysfunction in the neck and/or shoulders that the investigator attributed to neck dissection; were at least 3 months since neck dissection and radiation; and had moderate or severe pain and dysfunction (Constant-Murley score ≤ 70). Patients were excluded if they had received acupuncture in the previous 6 weeks. All participating patients signed informed consent.
Potentially eligible patients were identified primarily by review of the MSKCC Head and Neck Surgical Service database. A patient was mailed a recruitment letter after potential eligibility was confirmed with the responsible clinician. Participants were also identified through direct referral to the study and self-referral in response to posted flyers and information on the MSKCC Web site.
At baseline, the following information was recorded for each trial participant: demographic data; histologic diagnosis; pain medication use (yes/no) and quantitation of use using the Medication Quantification Scale40; type of neck dissection; and type of radiation with dosing details. Baseline values for the following outcome measures were also obtained (see Outcome Measures): the Constant-Murley score, modified Constant-Murley score, Numerical Rating Scale of Pain,41 and Xerostomia Inventory.42 The Numerical Rating Scale of Pain and Xerostomia Inventory were completed twice, 7 days apart within 1 week of random assignment, and averaged, both to obtain a more accurate estimate of baseline scores and to increase the efficiency of subsequent statistical analyses.43 The timing of these assessments is summarized in Table 1.
Table 1.
Study Schema
| Study Assessment or Stage | Day (approximate) |
||||
|---|---|---|---|---|---|
| −10 | −3 | 1-28 | 35 | 42 | |
| Numerical Rating Scale of Pain on activity | X | X | Weekly | X | X |
| Constant-Murley score | X | X | |||
| Acupuncture treatments | Weekly | ||||
| Medication use | X | Weekly | X | ||
| Xerostomia Inventory | X | X | X | X | |
| Partial registration | X | ||||
| Full registration/random assignment | X | ||||
Study Interventions
Patients were randomly assigned to acupuncture once a week for 4 weeks versus usual care. Part way through the study, it became apparent that some study participants would not return to complete their final outcome assessments. To enhance compliance, a fifth acupuncture treatment was added, but patients continued to have their final outcome assessments after the fourth treatment but before the fifth.
Acupuncture needles were placed at both standard and customized anatomic points. This allowed the acupuncturists to modify the acupuncture point prescription based on each patient's pain and its location, with the intent of optimizing efficacy while facilitating reproducibility. Standard distal points (LI-4, SP-6, GV-20, luozhen, and auricular shenman) were used in all patients and were chosen on the basis of their classical function. LI-4 is used for pain of the face and neck. SP-6 and GV-20 cross major channels and are used to harmonize the organs and remove obstructions from these channels. Luozhen is an extra point to treat stiffness of the neck and shoulder. Auricular shenmen is traditionally used to calm and to relieve pain. The customized points selected included zone distal points (front, middle, and back) chosen according to the primary zone(s) of pain; local ashi tender points with the greatest sensitivity to palpation pressure; and bilateral point LI-2 in patients with dry mouth. The total number of acupoints (needles) used ranged from a minimum of eight points (14 needles) to a maximum of 26 points (39 needles).
Needles were inserted using the traditional Chinese medicine acupuncture technique at a depth of 0.25 to 0.5 inches and retained for 30 minutes. Needles were stimulated manually, but because the sensitivity of acupuncture points may vary especially after surgery, no specific de qi response was elicited. Sterile, stainless steel, single-use, filiform needles (0.20 × 30 mm in width) manufactured by Seirin (Shizuoka, Japan) were used. All treatments were performed at the MSKCC Integrative Medicine Center by staff acupuncturists, all of whom had at least 3 years of formal postgraduate training in traditional Chinese medicine, were certified by the National Commission on Certification of Acupuncture and Oriental Medicine, and had experience ranging from 3 to 20 years. The lead acupuncturist trained all other acupuncturists and observed their technique periodically to ensure uniformity among practitioners.
No formal limitation was placed on other therapy (eg, rehabilitation) the patient could receive while getting acupuncture. However, any additional treatments were recorded.
Usual care entailed no specific treatment, physical therapy, analgesia, and/or anti-inflammatory drugs, per patient preference or physician recommendation. The decision to use a pragmatic control reflected the following factors. A specific active treatment control seemed unwarranted because the efficacy of standard treatment in this setting is not well established. Because acupuncture already had been shown to be superior to placebo in the treatment of neck and shoulder pain of other etiologies,24,27,29 the need for a placebo control was unclear and posed added logistical burdens to patients, which may have adversely affected a patient's willingness to participate. Standards for Reporting Interventions in Controlled Trials of Acupuncture were followed.44
Outcome Measures
The primary outcome measure was a composite score of pain, function, and activities of daily living provided by the Constant-Murley instrument (score of 0 to 100; lower score indicates poorer outcome).39 The four subscales are pain (15 points), activities of daily living (20 points), pain-free range of motion (40 points), and pain-free power (25 points). The scale has low levels of observer error, is often used as a gold standard against which other shoulder instruments are measured, shows good responsiveness,45,46 and was successfully applied in a prior randomized trial of acupuncture for shoulder pain.24 To facilitate a sensitivity analysis, we also scored a modified version of the Constant-Murley instrument, in which items are not scored if restrictions are unrelated to pain (eg, as a result of scarring or fibrosis). The final score is calculated by dividing the score by the maximum possible on scored items and multiplying by 100. Constant-Murley scores were assessed by a researcher who was unaware of the patient's treatment allocation.
In addition, a Numerical Rating Scale of Pain on activity was completed. This scale consists of an 11-point scale (0 to 10) marked with “no pain” and “worst pain” at either end.41 The focus was on pain experienced when using the shoulder. The scale gives scores comparable to those of a visual analog scale but allows for third-party assessment.47,48
The secondary end point of dry mouth was assessed using the Xerostomia Inventory, a validated questionnaire42 that has been used to assess dry mouth in cancer populations.37 It includes 11 questions, with responses ranging from 1 (never) to 5 (very often). The questionnaire was slightly modified for American use. For the study, total scores were normalized to a score of 100.
Both the Numerical Rating Scale of Pain and the Xerostomia Inventory were completed by the patient who was not blinded to treatment arm. The timing of assessments during and after completion of treatments is summarized in Table 1. As was the case at baseline, the Numerical Rating Scale for Pain and Xerostomia Inventory were obtained twice in successive weeks, and the average value was used.43
Sample Size
On the basis of raw data for the Constant-Murley score obtained from the authors of the Kleinhenz et al24 study of acupuncture for shoulder pain, the standard deviation for the post-treatment score was 19.5 and the correlation between pre- and post-treatment score was 0.55. Given these data, we estimated that 58 evaluable patients would be needed for an 80% power to detect a difference between groups of 12 points on the Constant-Murley scale.
Statistical Analysis
All analyses were conducted using Stata 9.2 (StataCorp, College Station, TX) and were based on the intent-to-treat principle for all patients who had evaluable outcome data. Between-group comparisons of post-treatment Constant-Murley, pain, medication, and xerostomia scores were conducted by analysis of covariance with group, baseline score, and type of surgical procedure (selective, modified radical, or radical neck dissection) as covariates. To evaluate the impact of missing follow-up data on our findings, we conducted multiple imputations using the ice command in Stata. In our first model, we used treatment allocation, age at random assignment, baseline Constant-Murley scores, and all pre– and post–random assignment pain scores as predictor variables. We conducted a second analysis to adjust for missing data excluding treatment allocation as a predictor, under the conservative assumption that treatment had no effect in patients with missing data. We stratified the xerostomia analysis by adding as a covariate the following baseline characteristics of radiotherapy history: intensity modulated versus conventional radiotherapy; total radiation dose delivered to the parotids; time since final dose of radiation; and primary site of radiation.
RESULTS
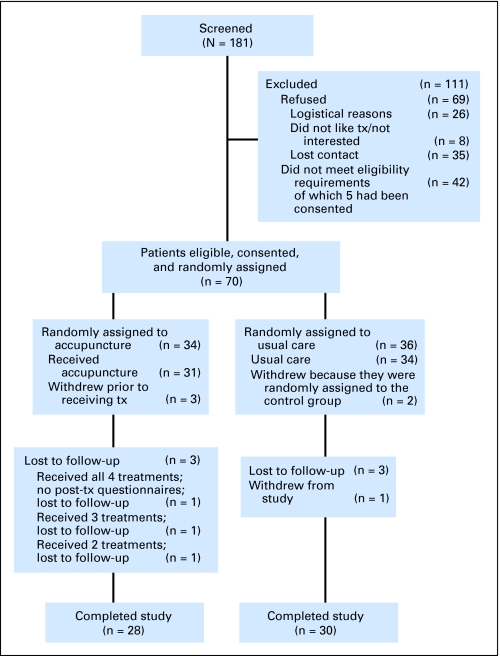
The Consolidated Standards of Reporting Clinical Trials (CONSORT) flowchart for the study is provided in Figure 1.49 The majority of enrolled patients responded to recruitment letters. A total of 58 randomly assigned patients (28 patients assigned to the acupuncture arm and 30 patients assigned to usual care) were evaluable for the primary end point.
Fig 1.
CONSORT flowchart. tx, treatment.
Table 2 lists the baseline characteristics for these 58 individuals. Chance imbalances between treatment arms included a higher proportion of women (46% for acupuncture v 23% for control) and somewhat poorer Constant-Murley scores (41.9 for acupuncture v 48.1 for control) among acupuncture patients. For controls versus acupuncture patients, the use of any type of pain medication before enrollment was more common among controls (53% v 14%, respectively), although scores for the Medication Quantification Scale (5.6 v 2.1, respectively) and Numerical Rating Score of Pain (5.9 v 5.6, respectively) were more similar.
Table 2.
Baseline Characteristics
| Variable | Patients Receiving Acupuncture (n = 28) |
Control Patients (n = 30) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Female | 13 | 46 | 7 | 23 |
| Male | 15 | 54 | 23 | 77 |
| Age, years | ||||
| Median | 61 | 57 | ||
| First quartile | 54 | 50 | ||
| Third quartile | 68 | 63 | ||
| Histologic diagnosis | ||||
| Thyroid cancer | 6 | 21 | 4 | 13 |
| Squamous cell carcinoma | 16 | 57 | 21 | 70 |
| Melanoma | 4 | 14 | 1 | 3 |
| Other | 2 | 7 | 4 | 13 |
| MQS | ||||
| Mean | 2.1 | 5.6 | ||
| SD | 6.1 | 8.9 | ||
| Medication use | 4 | 14 | 16 | 53 |
Abbreviations: MQS, Medication Quantification Scale; SD, standard deviation.
Characteristics of oncologic treatment, as listed in Table 3, were well balanced between the groups. All but seven patients (two in the acupuncture arm and five in the control arm) received radiation.
Table 3.
Treatment Characteristics
| Variable | Patients Receiving Acupuncture (n = 28) |
Control Patients (n = 30) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Time from surgery, months | ||||
| Median | 39 | 34 | ||
| First quartile | 29 | 15 | ||
| Third quartile | 48 | 44 | ||
| Type of neck dissection | ||||
| Selective | 2 | 7 | 4 | 13 |
| Modified radical | 25 | 89 | 23 | 77 |
| Radical | 1 | 3 | 3 | 10 |
| Type of radiation | ||||
| Conventional | 13 | 46 | 14 | 47 |
| Intensity modulated | 3 | 11 | 6 | 20 |
| Radioiodine | 5 | 18 | 4 | 13 |
| Other radiation | 5 | 18 | 1 | 3 |
| No radiation | 2 | 7 | 5 | 17 |
During the study, we tracked the supplemental use of other complementary therapies (such as massage), rehabilitation, or other exercise programs. Twelve patients (40%) on the control arm pursued such options, whereas 20% of patients on the acupuncture arm pursued these options.
The trial's principal results are listed in Table 4. Acupuncture was significantly superior to control for all outcome measures. For the main end point, acupuncture patients scored 11.2 points higher than controls on the Constant-Murley scale (95% CI, 3.0 to 19.3; P = .008). Xerostomia Inventory scores also significantly improved. Adjusting for missing data by multiple imputation had little effect on our results.
Table 4.
Outcome Assessment
| Variable | Baseline Score |
Follow-Up Score |
Difference Between Groups | 95% CI | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture |
Control |
Acupuncture |
Control |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Constant-Murley score (higher scores indicate better outcome) | 41.9 | 13.5 | 48.1 | 16.8 | 55.8 | 20.6 | 49.5 | 18.2 | 11.2 | 3.0 to 19.3 | .008 |
| Modified Constant-Murley score (higher scores indicate better outcome) | 41.7 | 13.7 | 47.8 | 16.5 | 56.6 | 21.6 | 49.6 | 18.3 | 12.0 | 3.6 to 20.4 | .006 |
| Xerostomia Inventory* (lower scores indicate better outcome) | 60.1 | 21.6 | 63.3 | 18.3 | 52.6 | 21.6 | 61.8 | 18.9 | −5.8 | −1.0 to −10.7 | .02 |
| NRS of Pain (lower scores indicate better outcome) | 5.6 | 1.6 | 5.9 | 2.2 | 3.6 | 2.4 | 5.8 | 2.3 | −1.7 | −0.8 to −2.7 | < .001 |
Abbreviations: SD, standard deviation; NRS, Numerical Rating Scale.
Includes two acupuncture patients and one control patient who gave xerostomia data but did not attend follow-up functional testing.
Because patients were not blinded to treatment arm, we reanalyzed the Constant-Murley data limiting our analysis to those components of the scale that lend themselves to more objective assessment (ie, range of motion and power; data on the pain and activities of daily living subscales were excluded because these seem more susceptible to possible biased reporting by unblinded trial participants). The difference between groups was of a similar magnitude (10.1 points) and remained significant (P = .037), favoring the acupuncture arm.
Medication use decreased in both groups during the study. The Medication Quantification Scale scores decreased from 5.6 to 4.5 in control patients and from 2.1 to 1.3 in acupuncture patients. However, the observed differences in medication scores between groups were not statistically significant (P = .4).
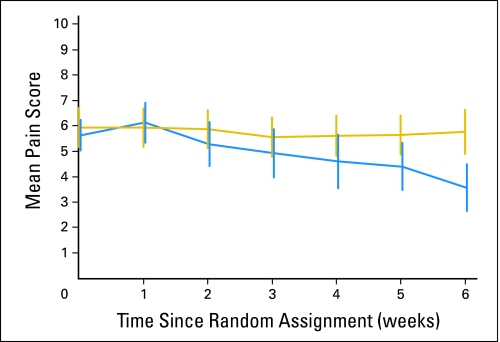
Numerical Rating Scale scores of pain over time are shown in Figure 2. A general estimating equations approach showed a statistically significant interaction between time and acupuncture (P < .001), supporting that pain scores diverge between groups over time as a cumulative effect of acupuncture treatment.
Fig 2.
Numerical rating scale of pain over time. Gold indicates control group; blue indicates acupuncture group. The vertical bars represent 95% CIs.
In preplanned exploratory analysis, we saw no modification of acupuncture effect by either baseline Constant-Murley or procedure type (P > .2 for all analyses). As a post hoc analysis, we adjusted for the large difference between groups in baseline medication use. There was no material effect on our findings (adjusted difference between means = 13.1; 95% CI, 4.1 to 22.2; P = .005). In an interaction analysis, we did see evidence that acupuncture was more effective in patients taking medication at baseline (P = .034).
No serious adverse events were attributed to acupuncture. Twenty-seven minor events were noted on study. The most common included temporary increased pain, minor bruising or bleeding, and constitutional symptoms.
DISCUSSION
Although shoulder pain and dysfunction are common sequelae of neck dissection,3–5 especially when the spinal accessory nerve is removed,7,9 there is a lack of consistently effective treatment. As such, the quality of life and employability of these patients are adversely affected.6,8,11,13 Most of the customary treatment approaches have not undergone assessment in a controlled trial in this setting.
Acupuncture is well suited to fill this void. It is a relatively safe, inexpensive treatment modality with few adverse effects, demonstrated to benefit acute and chronic pain.18–20 Scientifically rigorous studies have shown benefit in neck and shoulder pain of other etiologies,24–29 as well as improvement in function.24,25
For our control arm, we opted for a pragmatic rather than a placebo control. A pragmatic control arm has the advantage of providing an estimate of effect size most consistent with the questions of greatest interest facing patients and clinicians. These questions include: What effects will an acupuncture referral have on a patient's pain, and will the patient use less pain medication as a result? One disadvantage of a pragmatic comparison of acupuncture with usual care concerns lack of blinding and potential placebo effect. It has been estimated that trials without double blinding exaggerate odds ratios by 17%.50 To address these issues in part, our primary outcome measure, the Constant-Murley scale, was assessed by a third party who was blinded to treatment assignment, and we analyzed separately those components of the Constant-Murley scale that lent themselves to more objective assessment.
Xerostomia is a common and troubling adverse effect of radiation to the head and neck. Our results are consistent with those noted in selected reports,36–38 as well as those reported by Deng et al21 in their analysis of differences on functional magnetic resonance imaging and related saliva production after treatment with true versus sham acupuncture in healthy volunteers. It should be emphasized that the effect on xerostomia was a secondary end point in our study and so should be interpreted cautiously, particularly because assessment was unblinded and measurement of objective saliva production was not performed.
In summary, significant reductions in pain, dysfunction, and xerostomia were observed in study patients receiving acupuncture versus usual care. Acupuncture treatment was well tolerated. Although further study is needed, these data support the potential role of acupuncture in addressing post–neck dissection pain and dysfunction, as well as xerostomia.
Footnotes
Supported by Grant No. CA098792 from the National Institutes of Health (Bethesda, MD).
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00090337.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: David G. Pfister, Barrie R. Cassileth, Gary E. Deng, K. Simon Yeung, Nancy Lee, Jatin Shah, Andrew J. Vickers
Administrative support: David G. Pfister, Barrie R. Cassileth, Gary E. Deng, Jennifer S. Lee, Donald Garrity
Provision of study materials or patients: David G. Pfister, Barrie R. Cassileth, Gary E. Deng, K. Simon Yeung, Jennifer S. Lee, Nancy Lee, Dennis Kraus, Ashok R. Shaha, Jatin Shah
Collection and assembly of data: David G. Pfister, Jennifer S. Lee, Donald Garrity, Ashok R. Shaha
Data analysis and interpretation: David G. Pfister, Gary E. Deng, Jennifer S. Lee, Angel Cronin, Nancy Lee, Dennis Kraus, Jatin Shah, Andrew J. Vickers
Manuscript writing: David G. Pfister, Barrie R. Cassileth, Gary E. Deng, K. Simon Yeung, Nancy Lee, Dennis Kraus, Jatin Shah, Andrew J. Vickers
Final approval of manuscript: David G. Pfister, Barrie R. Cassileth, Gary E. Deng, K. Simon Yeung, Jennifer S. Lee, Donald Garrity, Angel Cronin, Nancy Lee, Dennis Kraus, Ashok R. Shaha, Jatin Shah, Andrew J. Vickers
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Robbins KT, Shaha AR, Medina JE, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134:536–538. doi: 10.1001/archotol.134.5.536. [DOI] [PubMed] [Google Scholar]
- 3.Krause HR. Shoulder-arm-syndrome after radical neck dissection: Its relation with the innervation of the trapezius muscle. Int J Oral Maxillofac Surg. 1992;21:276–279. doi: 10.1016/s0901-5027(05)80735-0. [DOI] [PubMed] [Google Scholar]
- 4.Leipzig B, Suen JY, English JL, et al. Functional evaluation of the spinal accessory nerve after neck dissection. Am J Surg. 1983;146:526–530. doi: 10.1016/0002-9610(83)90246-5. [DOI] [PubMed] [Google Scholar]
- 5.Dijkrstra PU, van Wilgen PC, Buijs RP, et al. Incidence of shoulder pain after neck dissection: A clinical explorative study for risk factors. Head Neck. 2001;23:946–953. doi: 10.1002/hed.1137. [DOI] [PubMed] [Google Scholar]
- 6.Shone GR, Yardley MP. An audit into the incidence of handicap after unilateral radical neck dissection. J Laryngol Otol. 1991;105:760–762. doi: 10.1017/s0022215100117232. [DOI] [PubMed] [Google Scholar]
- 7.Terrell JE, Welsh DE, Bradford CR, et al. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope. 2000;110:620–626. doi: 10.1097/00005537-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz AL, Weymuller EA., Jr Impact of neck dissection on quality of life. Laryngoscope. 1999;109:1334–1338. doi: 10.1097/00005537-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Cheng PT, Hao SP, Lin YH, et al. Objective comparison of shoulder dysfunction after three neck dissection techniques. Ann Otol Rhinol Laryngol. 2000;109:761–766. doi: 10.1177/000348940010900811. [DOI] [PubMed] [Google Scholar]
- 10.Sobol S, Jensen C, Sawyer W, et al. Objective comparison of physical dysfunction after neck dissection. Am J Surg. 1985;150:503–509. doi: 10.1016/0002-9610(85)90164-3. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Nibu K, Saito A, et al. Quality of life after neck dissection. Arch Otolaryngol Head Neck Surg. 2006;132:662–666. doi: 10.1001/archotol.132.6.662. [DOI] [PubMed] [Google Scholar]
- 12.Erisen L, Basel B, Irdesel J, et al. Shoulder function after accessory nerve-sparing neck dissections. Head Neck. 2004;26:967–971. doi: 10.1002/hed.20095. [DOI] [PubMed] [Google Scholar]
- 13.Laverick L, Lowe D, Brown JS, et al. The impact of neck dissection on health related quality of life. Arch Otolaryngol Head Neck Surg. 2004;130:149–154. doi: 10.1001/archotol.130.2.149. [DOI] [PubMed] [Google Scholar]
- 14.Van Wilgen CP, Dijkstra PU, van der Laan BF, et al. Shoulder complaints after neck dissection: Is the spinal accessory nerve involved? Br J Oral Maxillofac Surg. 2003;41:7–11. doi: 10.1016/s0266-4356(02)00288-7. [DOI] [PubMed] [Google Scholar]
- 15.Witt RL, Gillis T, Pratt R., Jr Spinal accessory nerve monitoring with clinical outcomes measures. Ear Nose Throat J. 2006;85:540–544. [PubMed] [Google Scholar]
- 16.Cappiello J, Piazza C, Giudice M, et al. Shoulder disability after different selective neck dissections (levels II-IV versus levels II-V): A comparative study. Laryngoscope. 2005;115:259–263. doi: 10.1097/01.mlg.0000154729.31281.da. [DOI] [PubMed] [Google Scholar]
- 17.McNeely ML, Parliament MB, Seikaly H, et al. Effects of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: A randomized controlled trial. Cancer. 2008;113:214–222. doi: 10.1002/cncr.23536. [DOI] [PubMed] [Google Scholar]
- 18.Acupuncture. JAMA; NIH Consensus Conference; 1998. pp. 1518–1524. [PubMed] [Google Scholar]
- 19.Ernst E, Pittler MH. The effectiveness of acupuncture in treating acute dental pain: A systematic review. Br Dent J. 1998;184:443–447. doi: 10.1038/sj.bdj.4809654. [DOI] [PubMed] [Google Scholar]
- 20.Melchart D, Lende K, Fischer P, et al. Acupuncture for recurrent headaches: A systematic review of randomized controlled trials. Cephalalgia. 1999;19:779–786. doi: 10.1046/j.1468-2982.1999.1909779.x. [DOI] [PubMed] [Google Scholar]
- 21.Deng G, Hou BL, Holodny AI, et al. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at L1-2 acupuncture point: A randomized controlled trial. BMC Complement Altern Med. 2008;8:37. doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomeranz B, Chiu D. Naloxone blockade of acupuncture analgesia: Endorphin implicated. Life Sci. 1976;19:1757–1762. doi: 10.1016/0024-3205(76)90084-9. [DOI] [PubMed] [Google Scholar]
- 23.Peets JM, Pomeranz B. CXBK mice deficient in opiate receptors show poor electroacupuncture analgesia. Nature. 1978;273:675–676. doi: 10.1038/273675a0. [DOI] [PubMed] [Google Scholar]
- 24.Kleinhenz J, Streitberger K, Windeler J, et al. Randomized clinical trial comparing the effects of acupuncture and a newly designed placebo needle in rotator cuff tendonitis. Pain. 1999;83:235–241. doi: 10.1016/s0304-3959(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 25.Sun KO, Chan KC, Lo SL, et al. Acupuncture for frozen shoulder. Hong Kong Med J. 2001;7:381–391. [PubMed] [Google Scholar]
- 26.Ceccheerelli F, Bordin M, Gagliardi G, et al. Comparison between superficial and deep acupuncture in the treatment of shoulder's myofascial pain: A randomized and controlled study. Acupunct Electrother Res. 2001;26:229–238. doi: 10.3727/036012901816355938. [DOI] [PubMed] [Google Scholar]
- 27.Vickers AJ. Acupuncture for treatment of chronic neck pain: Reanalysis of data suggest that effect is not a placebo effect. BMJ. 2001;323:1306. [PMC free article] [PubMed] [Google Scholar]
- 28.Irnich D, Behrens N, Molzen H, et al. Randomised trial of acupuncture compared with conventional massage and “sham” laser acupuncture for treatment of chronic neck pain. BMJ. 2001;322:1574–1578. doi: 10.1136/bmj.322.7302.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irnich D, Behrens N, Gleditsch J, et al. Immediate effects of dry needling and acupuncture at distant points in chronic neck pain: Results of a randomized, double-blind, sham-controlled crossover trial. Pain. 2002;99:83–89. doi: 10.1016/s0304-3959(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 30.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 31.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Nutting C, A'Hern R, Rogers MS, et al. First results of a phase III multicenter randomized controlled trial of intensity modulated (IMRT) versus conventional radiotherapy (RT) in head and neck cancer (PARSPORT: ISRCTN48243537; CRUK/03/005) J Clin Oncol. 2009;27(suppl):302s. abstr LBA6006. [Google Scholar]
- 33.Johnson JT, Ferrett GA, Nethery WJ, et al. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993;329:390–395. doi: 10.1056/NEJM199308053290603. [DOI] [PubMed] [Google Scholar]
- 34.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 35.Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102–1109. doi: 10.1016/j.ijrobp.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Andersen SW, Machin D. Acupuncture treatment of patients with radiation-induced xerostomia. Oral Oncol. 1997;33:146–147. doi: 10.1016/s0964-1955(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 37.Johnstone PA, Niemtzow RC, Riffenburgh RH. Acupuncture for xerotomia: Clinical update. Cancer. 2002;94:1151–1156. [PubMed] [Google Scholar]
- 38.Frydholm M, Strang P. Acupuncture for patients in hospital-based home care suffering from xerostomia. J Palliat Care. 1999;15:20–23. [PubMed] [Google Scholar]
- 39.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop. 1987;214:160–164. [PubMed] [Google Scholar]
- 40.Masters Steedman S, Middaugh SJ, Kee WG, et al. Chronic-pain medications: Equivalence levels and method of quantifying usage. Clin J Pain. 1992;8:204–214. [PubMed] [Google Scholar]
- 41.Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nurs. 1997;20:88–93. doi: 10.1097/00002820-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Thomson WM, Chalmers JM, Spencer AJ, et al. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community Dent Health. 1999;16:12–17. [PubMed] [Google Scholar]
- 43.Frison L, Pocock SJ. Repeated measures in clinical trials: Analysis using mean summary statistics and its implications for design. Stat Med. 1992;11:1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- 44.STRICTA. Standards for Reporting Clinical Trials of Acupuncture. http://www.stricta.info/
- 45.Skutek M, Fremerey RW, Zeichen J, et al. Outcome analysis following open rotator cuff repair: Early effectiveness validated using four different shoulder assessment scales. Arch Orthop Trauma Surg. 2000;120:432–436. doi: 10.1007/s004020000133. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor DA, Chipchase LS, Tomlinson J, et al. Arthroscopic subacromial decompression: Responsiveness of disease-specific and health-related quality of life outcome measures. Arthroscopy. 1999;15:836–840. doi: 10.1053/ar.1999.v15.015083. [DOI] [PubMed] [Google Scholar]
- 47.Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22–28. doi: 10.1097/00002508-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Bolton JE, Wilkinson RC. Responsiveness of pain scales: A comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther. 1998;21:1–7. [PubMed] [Google Scholar]
- 49.CONSORT. Homepage. http://www.consort-statement.org/
- 50.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]