Abstract
Purpose
This report provides an overview of current childhood cancer statistics to facilitate analysis of the impact of past research discoveries on outcome and provide essential information for prioritizing future research directions.
Methods
Incidence and survival data for childhood cancers came from the Surveillance, Epidemiology, and End Results 9 (SEER 9) registries, and mortality data were based on deaths in the United States that were reported by states to the Centers for Disease Control and Prevention by underlying cause.
Results
Childhood cancer incidence rates increased significantly from 1975 through 2006, with increasing rates for acute lymphoblastic leukemia being most notable. Childhood cancer mortality rates declined by more than 50% between 1975 and 2006. For leukemias and lymphomas, significantly decreasing mortality rates were observed throughout the 32-year period, though the rate of decline slowed somewhat after 1998. For remaining childhood cancers, significantly decreasing mortality rates were observed from 1975 to 1996, with stable rates from 1996 through 2006. Increased survival rates were observed for all categories of childhood cancers studied, with the extent and temporal pace of the increases varying by diagnosis.
Conclusion
When 1975 age-specific death rates for children are used as a baseline, approximately 38,000 childhood malignant cancer deaths were averted in the United States from 1975 through 2006 as a result of more effective treatments identified and applied during this period. Continued success in reducing childhood cancer mortality will require new treatment paradigms building on an increased understanding of the molecular processes that promote growth and survival of specific childhood cancers.
INTRODUCTION
Childhood cancer is a success story of modern medicine in which effective treatments have been identified for previously untreatable diseases. Pediatric cancer statistics are widely reported with conflicting inferences, creating questions and uncertainty. Are childhood cancers increasing in incidence? If so, does this increase apply to all cancer types or just a few? Are improvements in childhood cancer outcome stalled? If so, does this apply uniformly or are there some cancers for which outcomes continue to improve? What are the major causes of childhood cancer mortality and how have these changed over the past 30 years?
This report provides an overview of current childhood cancer statistics. The data underscore progress for multiple cancer types and focus attention on diagnoses for which current treatments remain inadequate. Understanding incidence, survival, and mortality data is important for analyzing the impact of past research discoveries on outcome and provides essential information for prioritizing future research directions.
METHODS
Study Populations
The surveillance period included the years from 1975 through 2006. Incidence and survival rates were based on data from the Surveillance, Epidemiology, and End Results 9 (SEER 9) registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah), which cover approximately 10% of the U.S. population.1 Deaths in the United States were reported by states to the Centers for Disease Control and Prevention by underlying cause. Rates were age-adjusted to the U.S. 2000 standard population.2 The 2005 and 2006 population estimates were adjusted to account for hurricane-related shifts in the Gulf Coast area. Rates were examined by age group: 0, 1 to 4, 5 to 9, 10 to 14, 15 to 19, < 15, and < 20 years of age. Age-adjusted incidence and mortality rates and relative survival rates were calculated.
Incidence Data
Incident cancer cases were defined according to the third edition of the International Classification of Childhood Cancer (ICCC)3 for the following cancer types: cancer of the CNS, lymphoid leukemias, all other cancers, and all cancers combined.
Mortality Data
Age-adjusted cancer mortality rates were examined for leukemia and lymphoma combined and all other cancers combined, as well as for all cancers. We determined the proportions of childhood cancer deaths in 1975 and 2006 due to cancers of the following sites: brain and other nervous system, leukemia (including acute lymphoblastic leukemia [ALL] and acute myeloid leukemia [AML]), lymphomas (with Hodgkin's lymphoma and non-Hodgkin's lymphoma [NHL] separately), bones and joints, soft tissue (including heart), gonads (ovary and testis), liver and intrahepatic bile duct, kidney, neuroblastoma, and other cancers combined.
Incidence and Mortality Trends
Long-term trends (1975-2006) in age-standardized cancer incidence and death rates were described using join point regression analysis (Joinpoint 3.3; Information Management Services, Silver Spring, MD), which fits a series of joined straight lines on a logarithmic scale to annual age-standardized rates.4 A maximum of four join points were allowed.4 Trends of varying time periods were described by annual percentage change (APC), that is, the slope of the line segment.4
Deaths Averted
The number of childhood malignant cancer deaths averted in the United States from 1975 through 2006 was estimated on the basis of observed deaths per year versus expected deaths, had there been no decrease in rate since 1975. Observed annual age-specific counts of deaths due to malignant cancer were determined by age group: 0, 1 to 4, 5 to 9, 10 to 14, and 15 to 19 years of age. Age-specific expected deaths were estimated by multiplying 1975 age-specific rates by annual age-specific populations from 1975 through 2006. Estimated deaths averted were differences between expected and observed deaths. Total childhood cancer deaths averted were the sum across age strata.
Survival Data
By using the ICCC classification system,3 5-year survival rates for selected childhood age groups were examined during successive 4-year periods from 1975-1978 through 1999-2002. The year 2002 was the last year included in survival analyses to allow up to 5 years of follow-up from time of diagnosis. For all malignant cancers combined, age groups of interest were < 1, 1 to 14, and 15 to 19 years. When survival rates were examined by cancer site, age groups of interest were generally < 15 and 15 to 19 years. Five-year survival data were presented for the following cancer sites: lymphoid leukemia, AML, NHL (including Burkitt's lymphoma, miscellaneous lymphoreticular lymphomas, and unspecified lymphomas), and Hodgkin's lymphoma. Survival rates were presented for malignant bone tumors, including Ewing tumor and related sarcomas of the bone and all cancers of the bone combined, rhabdomyosarcoma, and for germ cell and trophoblastic tumors of the gonads by primary site. Neuroblastoma survival was examined for children younger than 1 year of age and children 1 to 14 years of age. Five-year survival rates were examined for Wilms tumor (0 to 14 years of age) and for brain and CNS tumors including medulloblastoma only, and other cancers of the CNS (0 to 4, 5 to 14, and 15 to 19 years of age).
RESULTS
Incidence
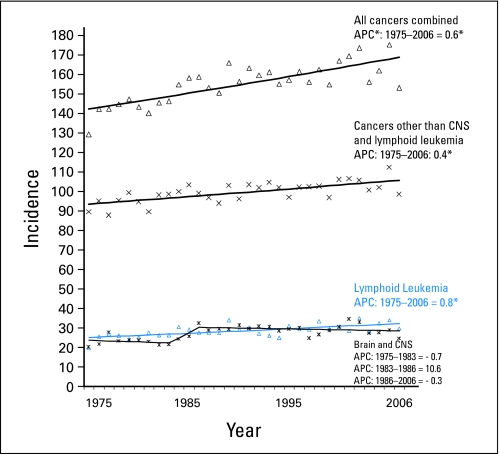
Incidence rates of all cancers combined increased significantly (P ≤ .05) among children younger than 20 years from 1975 through 2006 (Fig 1). One join point segment fit the trend (Table 1). The average annual percentage changes for the recent 5-year and 10-year surveillance periods and the 32-year APC were identical (0.6%). Lymphoid leukemia incidence rates increased significantly from 1975 through 2006 (APC = 0.8%). For CNS and miscellaneous intracranial and intraspinal (CNS) cancers, a three–join point segment model fit incidence trends. A nonsignificant increase in CNS cancer incidence rates (APC = 10.6%) occurred in the mid-1980s, with stable rates for the 21-year period from 1986 through 2006. After exclusion of lymphoid leukemia and CNS tumors, a modest, statistically significant increase was seen for all other cancers between 1975 and 2006 (APC = 0.4%).
Fig 1.
Incidence rates for all cancers combined, cancers other than CNS and lymphoid leukemia, lymphoid leukemia, and brain and CNS cancers among children younger than 20 years of age, according to data from Surveillance, Epidemiology, and End Results 9 (SEER 9) Registries, 1975 through 2006. (*) The slope of the join point regression trend line is significantly different from zero (P ≤ .05). The 95% CIs of annual percentage changes (APCs) are presented in Table 1.
Table 1.
Malignant Cancer Incidence Trends Among Children Younger Than Age 20 Years in the SEER 9 Registries From 1975 Through 2006
| Cancer Site | Joinpoint Trend 1 |
Joinpoint Trend 2 |
Joinpoint Trend 3 |
1997-2006 |
2002-2006 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | 95% CI | Years | APC | 95% CI | Years | APC | 95% CI | AAPC | 95% CI | AAPC | 95% CI | |
| All cancers combined | 1975-2006 | 0.6 | 0.4 to 0.7 | 0.6 | 0.4 to 0.7 | 0.6 | 0.4 to 0.7 | ||||||
| Lymphoid leukemias | 1975-2006 | 0.8 | 0.5 to 1.2 | 0.8 | 0.5 to 1.1 | 0.8 | 0.5 to 1.1 | ||||||
| CNS and miscellaneous intracranial and intraspinal neoplasms | 1975-1983 | −0.7 | −3.9 to 2.6 | 1983-1986 | 10.6 | −16.9 to 47.2 | 1986-2006 | −0.3 | −1.0 to 0.4 | −0.3 | −1.0 to 0.4 | −0.3 | −1.0 to 0.4 |
| All other cancers | 1975-2006 | 0.4 | 0.2 to 0.5 | 0.4 | 0.2 to 0.5 | 0.4 | 0.2 to 0.5 | ||||||
NOTE. Joinpoint 3.3 analyses allowed up to four join points. Best fitting models are presented.
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; APC, annual percentage change; AAPC, average annual percentage change.
Cancer Mortality and Causes of Cancer Death in 1975 and 2006
Mortality rates for all malignant childhood cancers combined declined by more than 50% between 1975 and 2006 (Table 2). The decline was led by a 64% reduction in leukemia mortality rate, an 85% reduction in gonadal cancer mortality rate, 75% declines in NHL and Hodgkin's lymphoma mortality rates, and 35% to 40% declines in neuroblastoma and bone cancer mortality rates. In both 1975 and 2006, leukemia (including AML and ALL) was the leading cause of cancer death in children, followed by cancer of the brain and other nervous system tumors (Table 2). The proportion of cancer deaths due to brain and other nervous system tumors increased from 17.8% in 1975 to 25.7% in 2006. The proportion of deaths due to leukemia declined from 38.9% to 30.4%, and for NHL, it declined from 8.8% to 4.4%. Compared with NHL as a cause of childhood death in 1975, NHL was surpassed by three cancer sites—neuroblastoma (9.1%), bones and joints (8.6%), and soft tissues (6.9%)—as a cause of childhood death in 2006. In addition to Hodgkin's lymphoma, cancers of the kidney and renal pelvis, liver and intrahepatic bile duct, and gonads each accounted for less than 3% of cancer deaths in 1975 and 2006. All other cancers accounted for less than 10% of cancer deaths in both years.
Table 2.
Cancer Deaths
| Cancer Site | Cancer Deaths per 100,000 |
|||
|---|---|---|---|---|
| 1975 | % | 2006 | % | |
| All malignant cancers | 5.14 | 100.0 | 2.48 | 100.0 |
| Leukemia | 2.03 | 38.9 | 0.75 | 30.4 |
| Brain and other nervous system | 0.93 | 17.8 | 0.64 | 25.7 |
| Lymphoma | 0.56 | 11.4 | 0.14 | 5.5 |
| Non-Hodgkin's | 0.44 | 8.8 | 0.11 | 4.4 |
| Hodgkin's | 0.12 | 2.6 | 0.03 | 1.1 |
| Neuroblastoma | 0.36 | 6.6 | 0.23 | 9.1 |
| Bones and joints | 0.35 | 7.2 | 0.21 | 8.6 |
| Soft tissue including heart | 0.17 | 3.4 | 0.17 | 6.9 |
| Kidney and renal pelvis | 0.14 | 2.6 | 0.07 | 2.7 |
| Liver and intrahepatic bile duct | 0.09 | 1.7 | 0.05 | 2.2 |
| Gonad (ovary and testis) | 0.13 | 2.7 | 0.02 | 0.9 |
| All other cancer sites | 0.38 | 7.6 | 0.2 | 8.1 |
NOTE. There were 3,992 malignant cancer deaths reported in children younger than 20 years of age in the United States during 1975 compared with 2,035 such deaths during 2006.
Mortality Rates and Deaths Averted
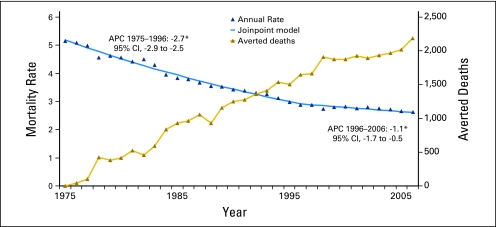
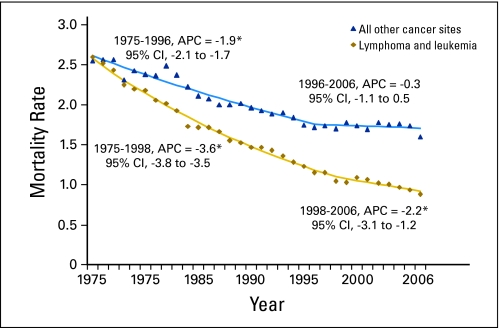
The age-adjusted mortality trend for cancer deaths among children had one join point in 1996, with significant (P ≤ .05) decreasing rates from 1975 to 1996 (APC = −2.7) and 1996 to 2006 (APC = −1.1; Fig 2). The decline was more pronounced for leukemias and lymphomas than for other cancers (ie, solid and CNS tumors). For leukemias and lymphomas (Fig 3), significantly decreasing death rates were observed throughout the 32-year period, though the rate of decline slowed somewhat in recent years (1998-2006, APC = −2.2) versus earlier years (1975-1998, APC = −3.6). For remaining childhood cancers (Fig 4), significantly decreasing rates were observed from 1975 to 1996 (APC = −1.9%), with stable mortality rates from 1996 to 2006 (APC = −0.3%).
Fig 2.
Age-specific mortality trends for all malignant cancers combined among children younger than 20 years of age in the United States from 1975 through 2006. An estimated 38,032 childhood malignant cancer deaths were averted from 1975 through 2006, assuming the 1975 baseline rate persisted through 2006. (*) The slope of the join point segment is statistically different from zero (P ≤ .05). APC, annual percentage change.
Fig 3.
United States age-adjusted childhood mortality trends for lymphoma and leukemia, and all other cancer sites combined, with annual percentage changes (APCs) for join point segments for males and females younger than 20 years of age, from 1975 though 2006. (*) The slope of the regression line significantly differs from zero; P < .05.
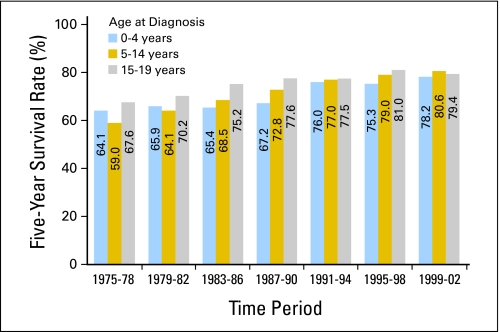
Fig 4.
Five-year survival rates for all cancers combined in children by age group and period of diagnosis from 1975 to 2002, with follow-up of vital status through 2006, according to data from the Surveillance, Epidemiology, and End Results 9 (SEER 9) Registries.
When 1975 age-specific death rates for children were used as a baseline, an estimated 38,032 childhood cancer deaths were averted in the United States from 1975 through 2006. Of the estimated averted deaths, 56% were in children younger than 10 years of age and 44% were in children 10 to 19 years of age.
Survival
Among children younger than 1 year of age, 5-year survival rates for all cancers combined in the late 1970s and 1980s exceeded 60% and reached 78.2% by 1999-2002 (Fig 4). Among children 1 to 14 years of age, 5-year survival rates increased from approximately 60% in 1975-1978 to 80.6% in 1999-2002. Among children 15 to 19 years of age, 5-year survival rates increased from 67.6% in 1975-1978 to 79.4% in 1999-2002.
The 5-year and 10-year survival rates for all cancers combined for children diagnosed in 1994-1997 were similar (ie, values within approximately 3%; Appendix Table A1, online only). Greater differences in 5-year and 10-year survival rates, generally in the 5% to 10% range, were observed for cancers of the brain, osteosarcoma, and Ewing sarcoma. Further discussion focuses on 5-year survival rates, since for most diagnoses, this is a relatively good reflection of long-term survival. As described below, the overall changes in survival rates by age group over time mask differences in survival by age and cancer type.
ALL.
Five-year survival rates for children younger than age 15 years with ALL improved from 61.0% in 1975-1978 to 88.5% in 1999-2002 (Fig 5). Adolescents 15 to 19 years of age also showed improvement in survival over the same period, although their outcome in recent periods (eg, 50.1% 5-year survival in 1999-2002) was lower than that among children younger than age 15 years. This lower survival rate partially reflects differences in tumor biology between children and older adolescents and likely also reflects differences in the way medical oncologists and pediatric oncologists have historically treated ALL arising in this age group.5,6 Survival for infants remains poor compared with that for children 1 to 14 years of age, although 5-year survival rates have increased from 22% in 1975-1978 to 62% in 1999-2002 (data not shown). Poor outcome is largely attributable to the high percentage of ALL cases with mixed lineage leukemia (MLL) gene rearrangements among infants.7,8
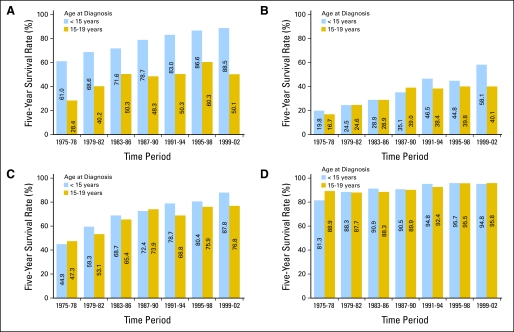
Fig 5.
Five-year survival rates for (A) acute lymphoblastic leukemia, (B) acute myeloid leukemia, (C) non-Hodgkin's lymphoma, and (D) Hodgkin's lymphoma among children by age group and period of diagnosis, 1975 through 2002, with follow-up of vital status through 2006, according to data from Surveillance, Epidemiology, and End Results 9 (SEER 9) Registries.
The improved survival for children with ALL reflects the impact of well-designed clinical trials conducted during this period, with treatment based on risk assessment (including response to initial treatment). While clinical features (eg, age and WBC at presentation) were primarily used for risk stratification before the 1990s, risk-based treatment assignment now considers the prognostic significance of specific cytogenetic abnormalities and other biologic characteristics of patients' leukemia cells. In the early 1980s, the Berlin-Frankfurt-Muenster (BFM) group showed that the use of intensive induction, consolidation, and a delayed intensification phase for patients with ALL produced an approximately 70% cure rate.9 Subsequent trials have refined this treatment strategy, identifying the superior outcome achieved with dexamethasone versus prednisone and identifying the superior outcomes associated with specific augmentations of the BFM regimen in postinduction phases of therapy.10–12 Importantly, improvements in survival have been achieved while reducing the percentage of children who receive cranial radiation as a component of treatment.
AML.
Five-year survival rates for children younger than age 15 years with AML increased from < 20% in 1975-1978 to 58% in 1999-2002 (Fig 5). Pediatric AML clinical trials during this period generally evaluated increasingly intensive regimens. Factors leading to improved outcome likely include incorporation of high-dose cytarabine as postconsolidation therapy and improved supportive care allowing more intensive induction and consolidation therapy to be administered with relative safety. Other contributory factors include more effective use of allogeneic stem-cell transplantation for selected patients with AML and the decreased transplantation-related mortality during recent time periods.13 Improvement in outcome for children with acute promyelocytic leukemia through the introduction of all-trans retinoic acid into front-line therapy14 and recognition that most children with Down syndrome and AML can be successfully treated with less aggressive regimens15 also contributed to improved outcome for childhood AML. For older adolescents (age 15-19 years), outcome was similar to that of younger children in 1975-1978 but has remained lower at 35% to 40% since the late 1980s.
NHL.
The five-year survival rate for children younger than age 15 years with NHL has improved dramatically, increasing from 45% in 1975-1978 to 88% in 1999-2002 (Fig 5). Throughout the decades covered in this report, a series of clinical trials defined more effective treatments for the types of NHL that predominate in children. A study initiated in the 1970s identified a leukemia-like regimen as more effective for lymphoblastic lymphoma and a four-drug regimen as more effective for nonlymphoblastic lymphoma.16,17 In the 1980s, high-dose methotrexate was incorporated into treatment regimens for B-cell NHL, as exemplified by the “Total B” regimen.18,19 In the past decade, an international clinical trial refining the highly effective Study of the French Society of Pediatric Oncology (SFOP) regimen for Burkitt's lymphoma and diffuse large B-cell lymphoma boosted event-free survival (EFS) rates to more than 90% for many children with B-cell NHL.20–23 For lymphoblastic lymphoma, the application of ALL-based regimens has resulted in EFS rates of approximately 80%.24,25
For 15- to 19-year-olds with NHL, outcome improved from 47% in 1975-1978 to 77% in 1999-2002. An important distinction between NHL in younger children versus 15- to 19-year-old children is that diffuse large B-cell lymphoma constitutes a higher proportion of cases in the latter population.26
Hodgkin's lymphoma.
Outcome for children with Hodgkin's lymphoma was relatively favorable in 1975-1978 (81%). Since the 1990s, 5-year survival rates have exceeded 90% (Fig 5). Outcome for 15- to 19-year-old adolescents is comparable to that of younger pediatric populations, in contrast to differential outcomes for ALL, AML, and NHL among older adolescents versus younger children. In the past decade, clinical trials for Hodgkin's lymphoma have focused on maintaining high survival rates while minimizing risks of long-term sequelae including second cancers, cardiac toxicity, and impaired fertility.
Brain tumors.
Brain tumor survival rates were examined independently for children ages younger than 5, 5 to 14, and 15 to 19 years, given the different treatment strategies for younger versus older children (eg, delay or avoidance of radiation therapy in young children). Five-year survival rates increased substantially for all age groups (Fig 6), with the youngest children showing the greatest improvements. Survival rates exceed 70% for all age groups in the most recent reporting period, with temporal paces of improvement differing by age group.
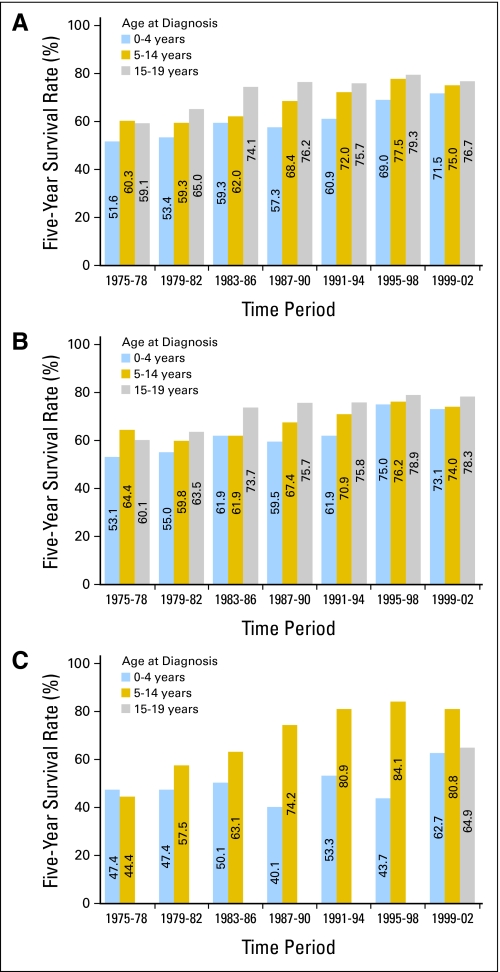
Fig 6.
Five-year survival rates for (A) medulloblastoma and other CNS tumors, (B) CNS tumors other than medulloblastoma, and (C) medulloblastoma among children by age group and period of diagnosis from 1975 through 2002, with follow-up of vital status through 2006, according to data from the Surveillance, Epidemiology, and End Results 9 (SEER 9) Registries. Medulloblastoma survival rates for the 15- to 19-year-old age group were suppressed when there were < 25 patients during the time period.
For children and adolescents with nonmedulloblastoma brain tumors, there were modest improvements in outcome between 1975-1978 and 1999-2002. Younger children (age 0 to 4 years) showed the largest improvement in 5-year survival rates, from 53.1% in 1975-1978 to 73.1% in the most recent reporting period (Fig 6). Because progress in the use of systemic chemotherapy for nonmedulloblastoma brain tumors has been limited, much of the improvement in outcome for these tumors may be attributable to advances in neurosurgery and radiation therapy.
Outcome for patients with medulloblastoma improved between 1975 and 2006, with 5-year survival rates increasing from < 50% in the 1970s to 73% for 1999-2002. The improvement in outcome was most notable for the 5- to 14-year-old age group, with 5-year survival increasing from 44% in 1975-1978 to more than 80% by 1991-1994 (Fig 6). Standard treatment for medulloblastoma during this period evolved from craniospinal radiation without adjuvant chemotherapy to craniospinal radiation with cisplatin-based chemotherapy administered following completion of radiation treatments.27,28 An apparent improvement in outcome for younger children with medulloblastoma for the most recent reporting period may reflect increasing use of intensive chemotherapy, with or without autologous stem cell rescue, to delay or eliminate use of radiation therapy.29,30
Bone sarcomas (osteosarcoma and Ewing sarcoma).
Improved survival has been observed for both osteosarcoma and Ewing sarcoma (Fig 7), although the time periods in which improvements were observed differed by tumor type. For osteosarcoma in children younger than 15 years of age, 5-year survival increased from 40% in 1975-1978 to 68% in 1987-1990, without improvement during more recent time periods. A similar pattern was observed for 15- to 19-year-olds. Thus, there was little improvement in survival for osteosarcoma after the introduction of cisplatin-based adjuvant therapy in the 1980s.31
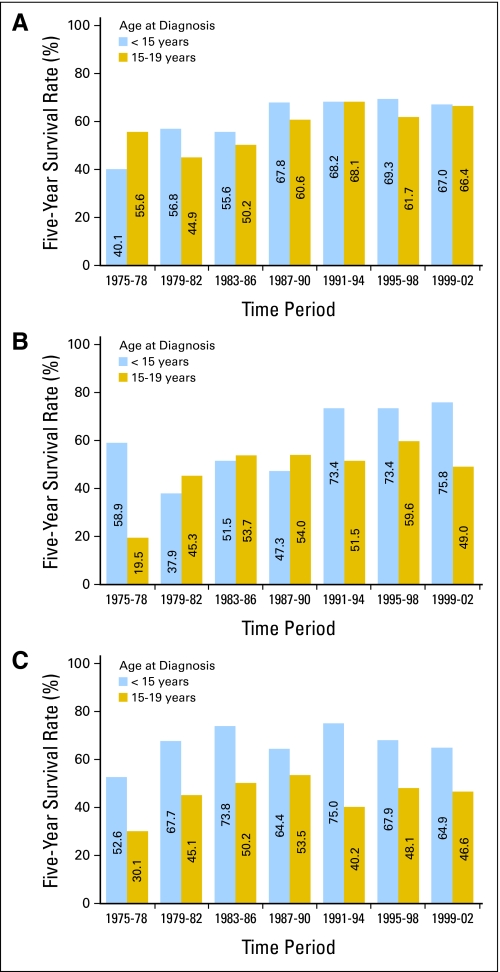
Fig 7.
Five-year survival rates for (A) osteosarcoma, (B) Ewing sarcoma, and (C) rhabdomyosarcoma among children by age group and time period of diagnosis from 1975 through 2002, with follow-up of vital status through 2006, according to data from Surveillance, Epidemiology, and End Results 9 (SEER 9) registries.
Five-year survival rates for older adolescents 15 to 19 years of age with Ewing sarcoma showed steady improvement from approximately 20% in 1975-1978 to 54% in l983-1986, with no additional increases in subsequent years (Fig 7). Prognosis also improved among patients younger than age 15 years, for whom 5-year survival rates increased to 74% in 1991-1994 with stable rates thereafter. The improved outcome noted in the early 1990s and thereafter may reflect benefits of ifosfamide and etoposide, which in a randomized study improved outcome in patients with localized Ewing sarcoma family of tumors.32 For both osteosarcoma and Ewing sarcoma, patients presenting with metastatic disease continue to have poor outcomes.33–35
Soft tissue sarcoma (rhabdomyosarcoma).
Five-year survival rates for children younger than 15 years of age with rhabdomyosarcoma improved from 53% in 1975-1978 to 68% in 1979-1982. Thereafter, rates showed no discernible trend (Fig 7). For 15- to 19-year-olds, the pattern is similar, with 30% 5-year survival in 1975-1978 and rates in subsequent years ranging between 40% and 54%. Children and adolescents with metastatic rhabdomyosarcoma and high-risk clinical features continue to have poor outcomes.36
Germ cell tumors (non-CNS).
Outcome for children age 0 to 14 years and older adolescents age 15 to 19 years with non-CNS germ cell tumors was highly favorable for the period 1999-2002 (Fig 8).37–39 Five-year survival rates among 15- to 19-year-olds increased in the late 1970s and have exceeded 90% since the early 1980s. This corresponds with the time period when cisplatin-based regimens became standard therapy for young adults with testicular and other germ cell tumors.40 Five-year survival for children with gonadal germ cell tumors has exceeded 90% since the early 1980s. For children younger than age 15 years with non-gonadal germ cell tumors, 5-year survival was < 50% in the early 1980s but surpassed 80% by the early 1990s.
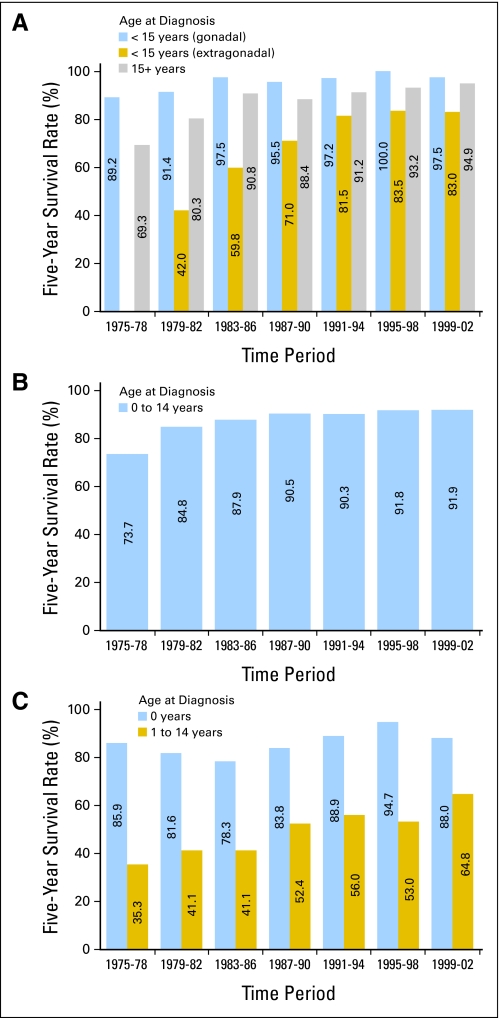
Fig 8.
Five-year survival rates for (A) non-CNS germ cell tumors, (B) Wilms tumors, and (C) neuroblastoma among children by age group and time period of diagnosis from 1975 through 2002, with follow-up of vital status through 2006, according to data from Surveillance, Epidemiology, and End Results 9 (SEER 9) registries.
Renal tumors (Wilms).
Wilms tumor comprises the majority of renal tumors among children. It is notable for the early success achieved in improving outcome through combined multimodality approaches with systematic testing of these approaches in controlled clinical trials (Fig 8). Wilms tumor was also one of the first tumor types that had a research focus on tailoring treatment to avoid late effects of therapy. In 1975-1978, the 5-year survival was 74%, which reflected the use of vincristine and dactinomycin combined with surgery and radiation therapy.39 The improved outcome observed with the addition of doxorubicin to treatment regimens for patients with higher-stage disease likely contributed to the increase in 5-year survival rates to > 90% by 1987-1990.38,41 Since the late 1980s, 5-year survival rates have been consistently above 90%. This favorable outcome occurred despite reductions in the length of therapy, dose of radiation, extent of fields irradiated, and percentage of patients receiving radiation therapy.42,43 Unfortunately, patients with anaplastic Wilms tumors have not benefited from advances in therapy to the same extent as those with more favorable histology.44
Neuroblastoma.
Five-year survival rates for infants with neuroblastoma have been relatively stable since the mid-1970s, ranging from 78% to 95% (Fig 8). Neuroblastoma in infants often has a favorable biology with a high likelihood of disease regression even when little or no cytotoxic chemotherapy is administered.45,46 Older children with neuroblastoma have a less biologically favorable disease than that which occurs in infants, with characteristics including MYCN amplification, deletions on the short arm of chromosome 1, allelic loss on the long arm of chromosome 11, and additional copies of the long arm of chromosome 17.45 Five-year survival rates have improved for older children with neuroblastoma from approximately 40% before 1985 to 65% in the most recent time period (Fig 8). Therapeutic interventions evaluated in phase III trials that may account in part for the improvements in survival for children with high-risk neuroblastoma include high-dose chemotherapy with autologous stem-cell transplantation and the use of isotretinoin as maintenance therapy.47,48
CONCLUSIONS AND FUTURE DIRECTIONS
The primary sustained changes in childhood cancer incidence over the past three decades have been for ALL, with an annual percentage increase approximately twice that for other non-CNS childhood cancers. The reason for the increasing ALL incidence is not known. Hypotheses have focused on the relationship between delayed exposure to infectious agents and leukemia risk and the relationship between high birth weight and ALL risk.49–52 An increasing incidence of ALL is reported for European countries as well, suggesting that causative factors are shared among countries of North America and Europe.53,54 For brain tumors, incidence increased in the mid-1980s but was stable thereafter. This temporal pattern of increase in incidence without a corresponding increase in mortality is consistent with enhanced detection and reporting of low-grade brain cancers in the mid-1980s, perhaps resulting from the dissemination of magnetic resonance imaging of the CNS during this period.55
Survival rates for some childhood cancers, primarily leukemias and lymphomas, have continued to improve over the past decade. Within the coming 5 years, cancers with 5-year survival rates exceeding 90% for children younger than age 15 years may expand to include ALL and NHL, in addition to Hodgkin's lymphoma, non-CNS germ cell tumors, and Wilms tumor.56 By contrast, 5-year survival rates for pediatric and adolescent solid tumors other than neuroblastoma have not changed over the past 10 to 20 years. This is particularly true for pediatric and adolescent sarcomas, including Ewing sarcoma, osteosarcoma, and rhabdomyosarcoma. Similarly, improvements in survival for pediatric brain tumors have been modest over the past 10 to 15 years for most age groups.
Trends in childhood cancer mortality mirror those for survival and are notable for differences in rates of decline in mortality for leukemias and lymphomas compared with those for solid tumors. Declines in mortality for leukemias and lymphomas have slowed in the past decade but continue at an annual decline of approximately 2%. Most childhood leukemias and lymphomas are diseases of lymphoid progenitor cells and thus are predisposed to undergo apoptosis. This lymphoid derivation likely contributes to their responsiveness to DNA damaging agents and antimetabolites and to the success of researchers in identifying effective therapies for these diagnoses using combinations of standard cytotoxic agents. In contrast to mortality rates for leukemia and lymphoma, declines in mortality rates for pediatric solid tumors are modest, and in the past decade, there has been no decline for specific cancers, notably sarcomas of soft tissue and bone.
One explanation for the lack of progress for solid tumors and brain cancers compared with that for leukemias is the lower incidence of the former, which affects the number of phase III studies that can be performed for these diagnoses. For example, during the 17-year period from 1993 to 2009, there were 14 phase III trials activated by the Children's Oncology Group (COG) for ALL, compared with only two each for osteosarcoma and Ewing sarcoma, despite international collaboration. Limited opportunities for defining the effectiveness for new treatment strategies of solid tumors makes it more difficult to refine treatments and to reliably define the contribution of new treatment strategies. While the limited number of clinical trials for pediatric solid tumors and CNS tumors may contribute to the lack of progress, there is little evidence that more effective use of available cytotoxic agents will be sufficient for substantial progress. Given the intensive chemotherapy that is currently used and the dearth of alternative standard or novel cytotoxic agents with compelling single-agent activity, the stable mortality rates suggest that innovative treatment strategies are needed to improve outcome. Hence, childhood cancer clinical researchers will need to focus on developing novel therapies that build on increased understanding of cellular pathways promoting tumor cell growth and survival to reduce cancer-related mortality and to reduce the incidence and severity of late effects that diminish the long-term quality of survivorship.
The premise behind molecularly targeted agents as effective cancer treatments is that cancers have oncogenic dependencies that create “Achilles heels” that can be exploited for therapeutic benefit. Proof of concept for the dramatic impact of effective targeted agents in the pediatric setting is seen for both all-trans retinoic acid for acute promyelocytic leukemia14 and for imatinib for Philadelphia chromosome–positive ALL.57,58 In each case, the targeted agent blocked the activity of a cancer-specific oncogenic protein on which cancer cell growth and survival depended. In the pediatric solid tumor setting, the anti-GD2 antibody ch14.18 was shown to improve EFS when used as part of an immunotherapy regimen after autologous stem-cell transplantation therapy in a randomized trial for children with high-risk neuroblastoma.59 This highlights the potential of immune-based targeted therapy to improve outcome for high-risk pediatric cancers.
One prerequisite for developing molecularly targeted agents for childhood cancers is establishing a comprehensive catalog of the genomic abnormalities present in each childhood cancer. The Childhood Cancer Therapeutically Applicable Research to Generate Effective Treatments (TARGET) Initiative (http://target.cancer.gov/) and related large-scale genomic projects are seeking to accomplish this goal through the systematic application of next-generation sequencing methods and array-based approaches to characterize DNA mutations, gene expression profiles, copy number alterations, and epigenomic profiles of selected childhood cancers. Early results support the potential of this strategy. The TARGET ALL pilot project identified IKZF1 alterations60 and activating mutations in Janus kinase (JAK) family members in leukemia cells from children with high-risk B-precursor ALL.61 The latter discovery will be translated into clinical application by studying JAK inhibitors for children with JAK-mutant ALL.
Clinical trials for both adults and children with cancer will increasingly focus on smaller patient populations that possess specific biologic characteristics and genomic alterations. One implication of the increasing need to study biologically defined patient subsets and to evaluate individualized treatment approaches according to specific genetic lesions is that national and international cooperation by childhood cancer clinical trials groups will be more important than ever, because it will be feasible to study these relatively uncommon patient populations only in the context of such collaborations. Another implication is that it will be impossible to evaluate some of these agents using the historically successful standard phase III clinical trial approach because of the small size of the affected population for which the targeted agent is relevant. This emphasizes the need for accurately defining reliable historical control populations and for optimizing clinical trial designs to support the necessary historical comparisons.
From 1975 through 2006, approximately 38,000 childhood cancer-related deaths were averted as a result of treatment advances since 1975. This highlights the continuing return on past investments in childhood cancer research and how the benefit of today's discoveries will increase over time. However, childhood cancer clinical research now stands at a crossroad. The era of consistent improvements in outcome from sequential clinical trials by optimizing delivery of standard cytotoxic agents and other conventional therapeutic approaches (eg, surgery, radiation therapy, and hematopoietic stem-cell transplantation) is coming to an end, while the era of targeted therapeutics is in its infancy. Early success in the childhood cancer setting showed improved outcome for targeted agents such as imatinib for Philadelphia chromosome–positive ALL and the anti-GD2 antibody ch14.18 for neuroblastoma highlight the promise of this strategy. Programs like the Childhood Cancer TARGET Initiative and the Pediatric Preclinical Testing Program62 can help usher in the new era by providing the preclinical rationale for prioritizing specific molecular targets and agents for study in selected childhood cancers. At the same time, the pediatric clinical trials infrastructure must become more proficient in defining molecular subclasses and evaluating new therapies in genomically defined subtypes of childhood cancers. This will increasingly involve international collaborations so that sufficient patient numbers can be attained and will require innovative clinical trial designs that can efficiently and effectively evaluate new treatment approaches. These and other adaptations in both the preclinical and clinical arenas will be required so that progress toward the goal of curative therapy with a life free of long-term sequelae for every child diagnosed with cancer can continue in the twenty-first century.
Appendix
Table A1.
5- and 10-Year Relative Survival Rates (1994-1997)
| Age (years) | Diagnosis (ICCC) | 5-Year Survival Rate (%) |
10-Year Survival Rate (%) |
Difference | ||
|---|---|---|---|---|---|---|
| Relative | SE Relative | Relative | SE Relative | |||
| 0-4 | All cancers | 77.9 | 1.1 | 75.9 | 1.1 | 2.0 |
| 5-14 | All cancers | 79.5 | 1.0 | 76.3 | 1.1 | 3.2 |
| 15-19 | All cancers | 80.2 | 1.1 | 77.6 | 1.1 | 2.6 |
| < 15 | I(a) Lymphoid leukemias | 85.1 | 1.3 | 81.9 | 1.4 | 3.2 |
| 15-19 | I(a) Lymphoid leukemias | 65.3 | 5.4 | 63.0 | 5.5 | 2.3 |
| < 15 | I(b) Acute myeloid leukemias | 43.0 | 4.2 | 41.6 | 4.2 | 1.4 |
| 15-19 | I(b) Acute myeloid leukemias | 39.1 | 6.4 | 37.5 | 6.3 | 1.6 |
| < 15 | II(a) Hodgkin's lymphomas | 97.3 | 1.6 | 96.6 | 1.9 | 0.7 |
| 15-19 | II(a) Hodgkin's lymphomas | 95.3 | 1.4 | 94.3 | 1.6 | 1.0 |
| < 15 | II(b,c,d,e) Non-Hodgkin's lymphomas | 81.1 | 2.8 | 81.1 | 2.8 | 0.0 |
| 15-19 | II(b,c,d,e) Non-Hodgkin's lymphomas | 76.4 | 3.7 | 74.3 | 3.9 | 2.1 |
| 0-4 | III CNS without medulloblastomas | 73.0 | 3.0 | 68.5 | 3.2 | 4.5 |
| 5-14 | III CNS without medulloblastomas | 77.6 | 2.2 | 74.0 | 2.4 | 3.6 |
| 15-19 | III CNS without medulloblastomas | 75.9 | 3.8 | 73.7 | 4.0 | 2.2 |
| 0-4 | III(c.1) Medulloblastomas | 46.9 | 7.0 | 42.7 | 7.0 | 4.2 |
| 5-14 | III(c.1) Medulloblastomas | 84.7 | 5.0 | 75.0 | 6.1 | 9.7 |
| 0 | IV Neuroblastoma and other peripheral nervous cell tumors | 92.7 | 3.0 | 91.4 | 3.2 | 1.3 |
| 1-14 | IV Neuroblastoma and other peripheral nervous cell tumors | 55.4 | 3.9 | 51.6 | 4.0 | 3.8 |
| < 15 | VI(a) Nephroblastoma (Wilms) | 91.6 | 2.0 | 90.6 | 2.1 | 1.0 |
| < 15 | VIII(a) Osteosarcomas | 64.8 | 5.3 | 60.0 | 5.4 | 4.8 |
| 15-19 | VIII(a) Osteosarcomas | 62.3 | 6.0 | 51.2 | 6.3 | 11.1 |
| < 15 | VIII(c) Ewing tumor and related sarcomas of bone | 73.9 | 6.2 | 65.8 | 6.8 | 8.1 |
| 15-19 | VIII(c) Ewing tumor and related sarcomas of bone | 58.6 | 8.3 | 50.3 | 8.4 | 8.3 |
| < 15 | IX(a) Rhabdomyosarcomas | 70.1 | 4.3 | 69.3 | 4.3 | 0.8 |
| 15-19 | IX(a) Rhabdomyosarcomas | 43.7 | 10.4 | 43.7 | 10.4 | 0.0 |
| < 15 | X(b) Non-CNS germ cell, extragonadal | 91.8 | 4.8 | 91.8 | 4.8 | 0.0 |
| < 15 | X(c) Non-CNS germ cell, gonadal | 100.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| 15-19 | X(b,c) Non-CNS germ cell tumors | 91.5 | 2.3 | 91.2 | 2.4 | 0.3 |
Abbreviations: ICCC, International Classification of Childhood Cancer; SE, standard error.
Footnotes
Supported in part by Grant No. U10 CA098543 from the National Cancer Institute (NCI), and by NCI contracts with SEER registries.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Malcolm A. Smith, Nita L. Seibel, Sean F. Altekruse, Lynn A.G. Ries, Gregory H. Reaman
Collection and assembly of data: Malcolm A. Smith, Nita L. Seibel, Sean F. Altekruse, Lynn A.G. Ries, Danielle L. Melbert
Data analysis and interpretation: Malcolm A. Smith, Nita L. Seibel, Sean F. Altekruse, Lynn A.G. Ries, Danielle L. Melbert, Maura O'Leary, Franklin O. Smith, Gregory H. Reaman
Manuscript writing: Malcolm A. Smith, Nita L. Seibel, Sean F. Altekruse, Lynn A.G. Ries, Maura O'Leary, Franklin O. Smith, Gregory H. Reaman
Final approval of manuscript: Malcolm A. Smith, Nita L. Seibel, Sean F. Altekruse, Lynn A.G. Ries, Danielle L. Melbert, Maura O'Leary, Franklin O. Smith, Gregory H. Reaman
REFERENCES
- 1.National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Population Estimates Used in NCI's SEER*Stat Software. http://seer.cancer.gov/popdata/methods.html.
- 2.National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Limited-Use, November 2007 Sub (1973-2006) [Google Scholar]
- 3.Steliarova-Foucher E, Stiller C, Lacour B, et al. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Nachman J. Clinical characteristics, biologic features and outcome for young adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2005;130:166–173. doi: 10.1111/j.1365-2141.2005.05544.x. [DOI] [PubMed] [Google Scholar]
- 6.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubnitz JE, Link MP, Shuster JJ, et al. Frequency and prognostic significance of HRX rearrangements in infant acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood. 1994;84:570–573. [PubMed] [Google Scholar]
- 8.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: Report on CCG 1953 from the Children's Oncology Group. Blood. 2006;108:441–451. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995: Berlin-Frankfurt-Münster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 10.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: A report from the Children's Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 12.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliansky DM, Appelbaum F, Cassileth PA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myelogenous leukemia in adults: An evidence-based review. Biol Blood Marrow Transplant. 2008;14:137–180. doi: 10.1016/j.bbmt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: Long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100:4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 15.Gamis AS. Acute myeloid leukemia and Down syndrome evolution of modern therapy: State of the art review. Pediatr Blood Cancer. 2005;44:13–20. doi: 10.1002/pbc.20207. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JR, Jenkin RD, Wilson JF, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin's lymphoma: A report of CCG-551 from the Childrens Cancer Group. J Clin Oncol. 1993;11:1024–1032. doi: 10.1200/JCO.1993.11.6.1024. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JR, Wilson JF, Jenkin DT, et al. Childhood non-Hodgkin's lymphoma: The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2) N Engl J Med. 1983;308:559–565. doi: 10.1056/NEJM198303103081003. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SB, Bowman WP, Abromowitch M, et al. Results of treatment of advanced-stage Burkitt's lymphoma and B cell (SIg+) acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. J Clin Oncol. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- 19.Brecher ML, Schwenn MR, Coppes MJ, et al. Fractionated cyclophosphamide and back to back high dose methotrexate and cytosine arabinoside improves outcome in patients with stage III high grade small non-cleaved cell lymphomas (SNCCL): A randomized trial of the Pediatric Oncology Group. Med Pediatr Oncol. 1997;29:526–533. doi: 10.1002/(sici)1096-911x(199712)29:6<526::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Patte C, Auperin A, Michon J, et al. The Société Françcaise d'Oncologie Pédiatrique LMB89 protocol: Highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 21.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: Results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- 22.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: It is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abromowitch M, Sposto R, Perkins S, et al. Shortened intensified multi-agent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: Report from the Children's Oncology Group. Br J Haematol. 2008;143:261–267. doi: 10.1111/j.1365-2141.2008.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abromowitch M, Termuhlen A, Chang M, et al. High-dose methotrexate and early intensification of therapy do not improve 3 year EFS in children and adolescents with disseminated lymphoblastic lymphoma: Results of the randomized arms of COG A5971. Blood. 2008;112 abstr 3610. [Google Scholar]
- 26.Percy CL, Smith MA, Linet M, et al. Bethesda, MD: NIH publication 99-4649; 1999. Lymphomas and Reticuloendothelial Neoplasms (ICCC II): Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995; pp. 35–50. [Google Scholar]
- 27.Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18:3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- 28.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 29.Dhall G, Grodman H, Ji L, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–1175. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 30.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 31.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 32.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 33.Kager L, Zoubek A, Pötschger U, et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 34.Miser JS, Krailo MD, Tarbell NJ, et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide—a Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22:2873–2876. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Miser JS, Goldsby RE, Chen Z, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: Evaluation of increasing the dose intensity of chemotherapy—a report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49:894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- 36.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: Results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms' tumor. Results of the Third National Wilms' Tumor Study. Cancer. 1989;64:349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.D'Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms' tumor: Results of the Second National Wilms' Tumor Study. Cancer. 1981;47:2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.D'Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms' tumor: Results of the national Wilms' tumor study. Cancer. 1976;38:633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 40.Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977;87:293–298. doi: 10.7326/0003-4819-87-3-293. [DOI] [PubMed] [Google Scholar]
- 41.Breslow NE, Ou SS, Beckwith JB, et al. Doxorubicin for favorable histology, Stage II-III Wilms tumor: Results from the National Wilms Tumor Studies. Cancer. 2004;101:1072–1080. doi: 10.1002/cncr.20433. [DOI] [PubMed] [Google Scholar]
- 42.Green DM, Breslow NE, Beckwith JB, et al. Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:3744–3751. doi: 10.1200/JCO.1998.16.12.3744. [DOI] [PubMed] [Google Scholar]
- 43.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: A report from the National Wilms' Tumor Study Group. J Clin Oncol. 1998;16:237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 44.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of anaplastic histology Wilms' tumor: Results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 45.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 46.De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol. 2009;27:1034–1040. doi: 10.1200/JCO.2008.17.5877. [DOI] [PubMed] [Google Scholar]
- 47.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children's Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 49.Gilham C, Peto J, Simpson J, et al. Day care in infancy and risk of childhood acute lymphoblastic leukaemia: Findings from UK case-control study. BMJ. 2005;330:1294. doi: 10.1136/bmj.38428.521042.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma X, Buffler PA, Wiemels JL, et al. Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2005;14:1928–1934. doi: 10.1158/1055-9965.EPI-05-0115. [DOI] [PubMed] [Google Scholar]
- 51.Kamper-Jørgensen M, Woodward A, Wohlfahrt J, et al. Childcare in the first 2 years of life reduces the risk of childhood acute lymphoblastic leukemia. Leukemia. 2008;22:189–193. doi: 10.1038/sj.leu.2404884. [DOI] [PubMed] [Google Scholar]
- 52.Caughey RW, Michels KB. Birth weight and childhood leukemia: A meta-analysis and review of the current evidence. Int J Cancer. 2009;124:2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 53.Spix C, Eletr D, Blettner M, et al. Temporal trends in the incidence rate of childhood cancer in Germany 1987-2004. Int J Cancer. 2008;122:1859–1867. doi: 10.1002/ijc.23281. [DOI] [PubMed] [Google Scholar]
- 54.Kaatsch P, Steliarova-Foucher E, Crocetti E, et al. Time trends of cancer incidence in European children (1978-1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:1961–1971. doi: 10.1016/j.ejca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Smith MA, Freidlin B, Ries LA, et al. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 56.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–1309. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- 57.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 58.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu AL, Gilman AL, Ozkaynak MF, et al. A phase III randomized trial of the chimeric anti-GD2 antibody ch14.18 with GM-CSF and IL2 as immunotherapy following dose intensive chemotherapy for high-risk neuroblastoma: Children's Oncology Group (COG) study ANBL0032. J Clin Oncol. 2009;27(suppl) abstr 10067z. [Google Scholar]
- 60.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houghton PJ, Morton CL, Tucker C, et al. The Pediatric Preclinical Testing Program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]