Abstract
A novel selenium-containing compound having a selenium atom in the imidazole ring, 2-selenyl-Nα,Nα,Nα-trimethyl-l-histidine, 3-(2-hydroseleno-1H-imidazol-5-yl)-2-(trimethylammonio)propanoate, was identified from the blood and other tissues of the bluefin tuna, Thunnus orientalis. The selenium-containing compound was purified from the tuna blood in several chromatographic steps. High resolution mass spectrometry and nuclear magnetic resonance spectroscopy showed that the exact mass of the [M+H]+ ion of the compound was 533.0562 and the molecular formula was C18H29N6O4Se2. Its gross structure was assigned as the oxidized dimeric form of an ergothioneine selenium analog in which the sulfur of ergothioneine is replaced by selenium. Therefore, we named this novel selenium-containing compound “selenoneine.” By speciation analysis of organic selenium compounds using liquid chromatography inductively coupled plasma mass spectrometry, selenoneine was found widely distributed in various tissues of the tuna, with the highest concentration in blood; mackerel blood contained similar levels. Selenoneine was measurable at 2–4 orders of magnitude lower concentration in a limited set of tissues from squid, tilapia, pig, and chicken. Quantitatively, selenoneine is the predominant form of organic selenium in tuna tissues.
Keywords: Amino Acid, Antioxidant, Glutathione, Oxidative Stress, Radicals, Reactive Oxygen Species (ROS), Selenium, Zebrafish, Betaine, Ergothioneine
Introduction
Selenium, an essential micronutrient for humans and animals (1–3), is a trace element nutrient that functions as selenocysteine for the reduction of antioxidant enzymes such as glutathione peroxidases (4, 5), thioredoxin reductase (6), and thyroid hormone deiodinases (7). The entire selenoprotein gene population, designated the selenoproteome, has been identified in humans and rodents (8), and numerous selenoprotein genes have functions in development and health (9). Selenoproteins are also involved in different human genetic disorders (9). The antioxidant activity of selenium plays a protective role in 50 human diseases, including prostate, lung, and intestine/colon cancer, immunodeficiency, and heart diseases (10–13). Selenium deficiency is also linked to animal diseases such as myopathy, exudative diathesis, and pancreatic degeneration in domestic animals and birds (9). Selenium also protects against mercury toxicity in many different organisms (14, 15).
Fish consumption is a major source of selenium in the human diet in Japan. Red muscle and other tissues of tuna contain organic selenium in excess of 1 ppm, making this the highest selenium content in food. However, the molecular mechanism of the antioxidant activity of dietary selenium in fish muscle has been poorly characterized. Here, we identified and characterized the chemical structure of the major organic selenium compound in the blood, muscles, and other tissues of bluefin tuna, Thunnus orientalis. A novel selenium-containing compound, 2-selenyl-Nα,Nα,Nα-trimethyl-l-histidine, a selenium analog of ergothioneine we call “selenoneine,” was identified as a strong antioxidant in tuna tissues. This compound has a unique chemical structure and is the first example of a natural heterocyclic compound with a selenoketone derived from animals.
EXPERIMENTAL PROCEDURES
Materials
Blood and other tissues were collected from cultured and wild bluefin tuna, T. orientalis, and stored frozen at −80 °C until use. The cultured bluefin tuna (average body weight ∼39 kg) were obtained at an aquaculture farm in the Kyushu Island. The wild bluefin tuna (average body weight 5.2 kg) were caught by set net in the Aomori Prefecture. Wild Pacific mackerel, Scomber japonicus (average body weight 843 g), were caught by set net in the Shizuoka Prefecture. Wild Japanese common squid, Todarodes pacificus (average body weight ∼200 g), were caught by long line in the Sea of Japan. Tilapia, Oreochromis niloticus (average body weight 405 g), were reared with an artificial feed at 20 °C for 5 years in the National Research Institute of Fisheries Science before use. Fresh tissues of pig (average body weight ∼115 kg) and chicken (average body weight ∼3 kg) were obtained at the Yokohama Wholesale Market. Dithiothreitol (DTT),2 reduced glutathione, selenite, 1-diphenyl-2-picrylhydrazyl (DPPH), and tetrahydrofuran (THF) were purchased from Wako Pure Chemical (Osaka, Japan). Acetonitrile was obtained from Kanto Chemical (Tokyo, Japan). Glutathione peroxidase (GPx) from bovine erythrocyte, l-selenocysteine, and l-ergothioneine were acquired from Sigma-Aldrich. l-Selenomethionine and 2, 3-diaminonaphthalene were purchased from Tokyo Kasei (Tokyo, Japan). TroloxTM (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was bought from Roche Diagnostics (Tokyo, Japan).
Selenium Determination
To determine the total selenium content, each sample was digested in 1 ml of nitric acid and perchloric acid (1:2 in volume) at 200–220 °C. The selenium concentration was measured using a fluorometric assay with 2,3-diamino-naphthalene (16). Chromatographic separation was carried out using a high-performance liquid chromatography (HPLC) pump (712p, GL Sciences, Tokyo, Japan). The analytical column was an Ultrahydrogel 120 (7.8 × 250 mm, Nihon Waters, Tokyo, Japan) equilibrated with 100 mm ammonium formate buffer. The injection volume was fixed at 20 μl. The mobile phase was delivered at 1 ml/min isocratically, and selenium was detected using online liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP-MS; ELAN DRC II, PerkinElmer Life Sciences), with a concentric nebulizer (WE02-4371, quartz) and a sample injector (2-mm inner diameter, quartz) monitoring 82Se according to the method described previously (17). The plasma and auxiliary argon gas flow rates were 17 and 1.3 liters/min, respectively. The nebulization argon gas flow rate was 1.03 liters/min. The radio-frequency power was 1500 watts. During this separation, GPx, selenite, selenocysteine, selenomethionine, and selenoneine were eluted at retention times of 5.4, 7.4, 7.8, 9.8, and 10.5 min, respectively, and the selenium concentration was determined using the respective compounds as standards.
Purification of the Selenium-containing Compound
First, 0.1 g of DTT was added to 100 g of tuna blood and then homogenized in 10 volumes of acetonitrile. The homogenate was centrifuged at 6000 × g for 10 min, and the supernatant was then concentrated in vacuo. After adding acetonitrile/THF (1:1 in volume), the concentrated extract was solvent-partitioned to obtain acetonitrile-soluble organic fractions, and then the acetonitrile-soluble fraction was concentrated in vacuo. The concentrated extract was mixed with 5 ml of cold distilled water, and the water-soluble material was applied to an Atlantis dC18 300 Å column (19 × 150 mm, Nihon Waters) equilibrated with 0.1% acetic acid. The desired fraction containing total selenium at more than 10 μg/ml was eluted at an elution volume of 40–50 ml with a linear 0–50% acetonitrile gradient. The fraction containing selenium was applied to an Ultrahydrogel-120 column (7.8 × 300 mm, Nihon Waters) equilibrated with 0.1% acetic acid/30% acetonitrile, and the elution volume of 7.5–9.0 ml containing the selenium-containing compound was collected. The concentrated eluent was applied to an Ultrahydrogel-120 column equilibrated with 0.1% acetic acid, and the elution volume of 9.0–13 ml containing the selenium-containing compound was collected. The targeted component was also purified from the red muscles of tuna and swordfish and used in further experiments.
Spectroscopy and Spectrometry
UV spectra were recorded with a UV-visible spectrometer (DU640, Beckman-Coulter). Fluorescence spectra were recorded with a fluorescence spectrometer (model 850, Hitachi, Tokyo, Japan). The specific rotation was measured with a circular dichroism spectrometer (J-720 WI, JASCO, Tokyo, Japan). Mass spectrometry (MS) spectra were recorded on a Quattro II (Micromass, Manchester, UK) equipped with HPLC (HP 1100, Hewlett Packard, Palo Alto, CA) and a JMS-700 (JEOL, Tokyo, Japan). NMR spectra were recorded on a 500-MHz spectrometer (ECA500, JEOL) in D2O as the solvent. The signals of the deuterated solvents were used as references. The chemical shifts of the purified selenium-containing compound for both 1H-NMR and 13C-NMR were observed: 1H-NMR (D2O) δ 7.00 (s), 3.80 (dd, 3.7 and 11.2), 3.16 (s), 3.08 (d, 3.4); 13C-NMR (D2O) δ 170.1 (C-1), 155.8 (C-5′), 120.8 (C-2′), 115.3 (C-4′), 77.2 (C-2), 52.2 (N-CH3), and 23.5 (C-3).
Antioxidant Assay
The radical scavenging activity against DPPH in 0.1 m sodium phosphate buffer (pH 6.8) was measured according to the method described by Oki et al. (18) using the water-soluble vitamin E-like compound Trolox as the standard antioxidant.
RESULTS
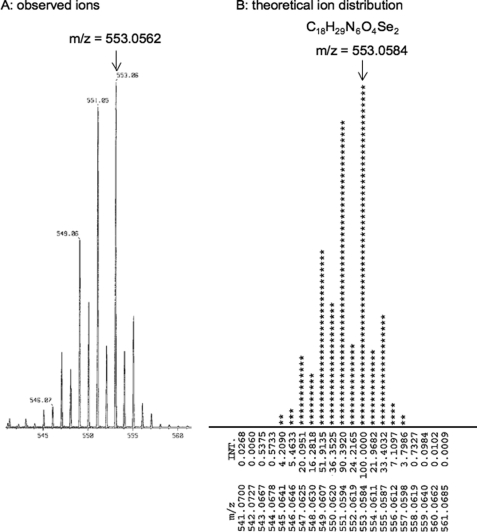
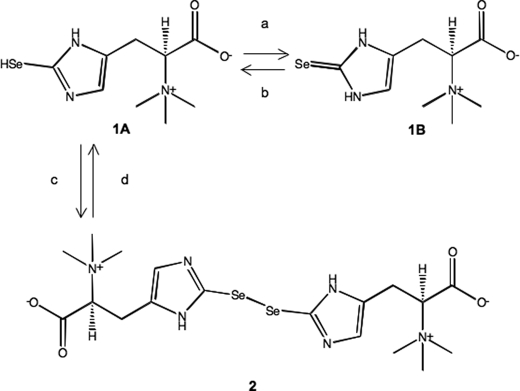
The selenium-containing compound in the tuna blood was purified in several chromatographic steps of C-18 reverse phase HPLC and gel filtration chromatography. A yellow oily compound of 0.2 mg was obtained from 100 g of the tuna blood. High resolution mass spectroscopy using electrospray ionization showed a molecular ion peak at m/z 553.0562, in agreement with the molecular formula C18H29N6O4Se2 (calculated 553.0584) (Fig. 1). The isotopic pattern of the compound matched the theoretical isotopic pattern of two selenium atoms calculated from the natural abundance of selenium isotopes. MS/MS analysis showed fragment ions of 233.0422 predicted for trimethylammonioethyl-imidazole-selenolate (C8H15N3Se), 173.9692 for ethyl-imidazole-selenolate (C5H6N2Se), 79.9165 for selenium, and 59.0730 for trimethylamine (C3H9N), indicating that the compound contained a selenium-containing imidazole ring, a carboxyl group, and a trimethylamino group (supplemental Fig. S1). The molecular ion peak at m/z 553 was converted into that at m/z 278 by LC-electrospray ionization-MS after reducing the selenium compound in the presence of 10 mm DTT or 10 mm glutathione; the compound was found to be an oxidized dimeric form (supplemental Fig. S2). Therefore, from the molecular formula C9H15N3O2Se of the reduced monomeric form, this selenium compound was thought to be a selenium analog of ergothioneine (C9H15N3O2S) in which the sulfur was replaced by selenium. Because the monomeric form of the selenium compound was very unstable during extraction and chromatographic steps under both cold and room temperature conditions (data not shown), we treated the extracted material containing the selenoketone of the selenium compound from the tuna tissues with acetonitrile/THF (1:1 in volume) to accelerate selenoxo-selenol tautomerization (19) and formation of the oxidized dimeric form (Fig. 2). The dimeric form was stable at room temperature and was then used for the structural determination and chemical characterization.
FIGURE 1.
High resolution mass spectrometric identification of the selenium compound. The isotopic pattern of the purified compound (A) matched the theoretical isotopic pattern (B) of two selenium atoms calculated from the natural abundance of selenium isotopes. INT., intensity.
FIGURE 2.
The chemical structure of the selenium-containing compound selenoneine (1A, 1B) and its autoxidized dimer (2). The reagents and conditions are as follows: a, water, methanol or acetonitrile, 0 °C; b, CH2Cl2 or THF, −20 °C; c, CH2Cl2, THF, room temperature; d, water, 10 mm DTT or GSH, room temperature, 30 min.
The 13C-NMR spectroscopy showed nine carbon signals, which were resolved into one carboxylic acid, three methyl, one methylene, three aromatic, and one sp3 methine carbons through distortionless enhancement in polarization transfer experiments. In the 13C-NMR spectrum, the three aromatic carbon signals (δ 155.8, 120.8, and 115.3) were ascribed to a two-substituted imidazole carbon, which was consistent with one proton signal at δ 7.00 (s) in the 1H-NMR spectrum, indicating that the selenol group was within the imidazole ring (supplemental Fig. S3). The observed chemical shifts for both 1H and 13C were closely correlated with those of the structural analysis of ergothioneine: 1H-NMR (D2O) δ 6.70, 3.83, 3.19, 3.10; 13C-NMR (D2O) δ 173.1 (C-1), 158.9 (C-5′), 126.7 (C-2′), 118.1 (C-4′), 79.8 (C-2), 54.3 (N-CH3), and 25.4 (C-3), and the aromatic carbon signal of the selenium compound at C-2′ (δ 120.8) was shifted lower than that of ergothioneine (δ 126.7) (20). Therefore, the selenium compound was confirmed to be the selenium analog of ergothioneine, in which the thiol at position C2′ in the imidazole ring was replaced by selenol. The gross structure of the selenium-containing compound was assigned the chemical form shown in Fig. 2, in which a selenium-containing ergothioneine analog forms a novel selenol or selenoketone structure. We therefore named the compound selenoneine.
The oxidized dimeric form of selenoneine was characterized as absorbing UV at 260 nm (ϵ260 = 525 m−1 cm−1) (supplemental Fig. S4) and fluorescing with maximum emission wavelengths at 240, 280, and 337 nm and a maximum excitation wavelength at 405 nm (ϵem 240/ex 405 = 0.823 × 103 m−1 cm−1, ϵem 280/ex 405 = 1.51 × 103 m−1 cm−1, ϵem 337/ex 405 = 1.46 × 103 m−1 cm−1). Selenoneine is an optically active substance based on the measured specific rotation, α20D: +23°. The measured 50% radical scavenging concentration (RS50) with DPPH for the water-soluble vitamin E-like antioxidant Trolox, l-ergothioneine, and the reduced selenoneine form was 880, 1700, and 1.9 μm, respectively, indicating that selenoneine has greater antioxidant activity than Trolox and ergothioneine.
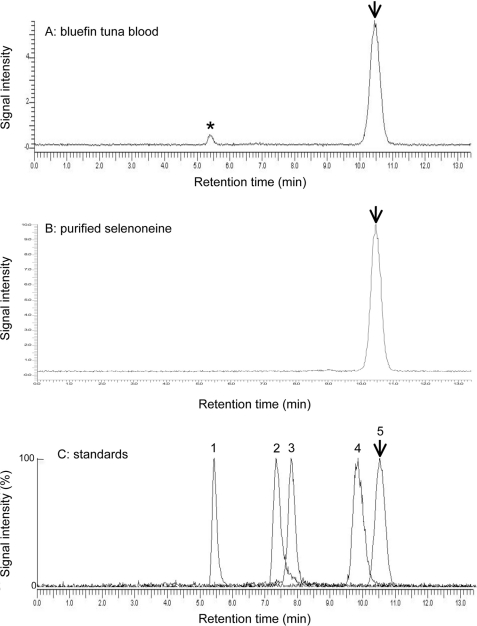
To determine the tissue distribution of selenoneine, we developed a speciation analysis method for organic selenium in animal tissues based on monitoring 82Se using LC-ICP-MS with a GPC column (Fig. 3). Selenium compounds, such as GPx, selenite, selenocysteine, selenomethionine, and selenoneine, were used as standards. The contents of selenoneine, selenoproteins including GPx and selenoprotein P, and other unidentified selenium compounds were determined in various animal tissues, whereas selenite, selenocysteine, and selenomethionine were not detected in the tissues that we examined in this study (Table 1, supplemental Figs. S5–S7). Because selenoneine was associated with the gel matrix, it was eluted 10.5 min after the bed volume of the column. The tuna red muscle contained selenoneine at 190 nmol of selenium/g and selenoproteins at 4.5 nmol of selenium/g, indicating that almost all of the selenium (98%) was present as selenoneine. Other tissues, such as the spleen, hepatopancreas, heart, white muscle, and blood, also contained high levels of selenoneine at 2.4–430 nmol of selenium/g. Pacific mackerel blood, tilapia blood, porcine kidney, chicken heart, gizzard, liver, and squid hepatopancreas also contained selenoneine and selenoproteins, whereas porcine liver contained only selenoproteins and not selenoneine. In the tilapia blood, selenoneine was contained in erythrocytes, but not in serum (supplemental Fig. S6). Therefore, selenoneine is distributed in various animal tissues and at especially high levels in tuna tissues, indicating that it is the predominant form of selenium in tuna tissues.
FIGURE 3.
Speciation analysis of organic selenium in the fish and other animal tissues by LC-ICP-MS. The speciation of water-soluble selenium compounds in the tissues of bluefin tuna and other animal tissues based on LC-ICP-MS analysis is shown. 0.1 g of sample was homogenized with 5× water, and 20 μl of supernatant was injected to LC-ICP-MS after 2–200× dilution with mobile phase (0.1 m ammonium formate). A, the blood of farm-raised bluefin tuna. B, purified selenoneine from the tuna blood. C, standards. For example, peak 1, bovine erythrocyte GPx; peak 2, selenite; peak 3, selenocysteine; peak 4, selenomethionine; peak 5, selenoneine. An Ultrahydrogel 120 (7.8 × 250 mm) column equilibrated with 0.1 m ammonium formate buffer (flow rate at 1 ml/min) was used. An asterisk indicates selenoproteins including GPx eluted near the void volume of the column. An arrow shows the elution of selenoneine.
TABLE 1.
Speciation of water-soluble selenium compounds in the tissues of bluefin tuna and other animals based on LC-ICP-MS analysis
Contents of selenoneine, selenoproteins including GPx and selenoprotein P, and unidentified selenium compounds in the tissues of bluefin tuna, Pacific mackerel, tilapia, chicken, pig, and Japanese common squid are shown. 0.1 g of sample was homogenized with 5× water, and 20 μl of supernatant was injected to LC-ICP-MS after 2–200× dilution with mobile phase (0.1 m ammonium formate). Values are the mean ± S.D. of three individuals.
| Species and tissue | Selenoneine | Selenoproteinsa | Unidentified selenium compounds |
|---|---|---|---|
| nmol of Se/g | nmol of Se/g | nmol of Se/g | |
| Bluefin tuna, farm-raised | |||
| Blood | 430 ± 82 | 24 ± 6.1 | NDb |
| Spleen | 41 ± 16 | 14 ± 2.5 | 4.0 ± 1.5 |
| Hepatopancreas | 39 ± 7.5 | 28 ± 12 | 2.2 ± 0.6 |
| Heart | 15 ± 15 | 4.8 ± 2.3 | 1.5 ± 1.0 |
| Red muscle | 190 ± 8.0 | 4.5 ± 0.5 | 5.3 ± 1.7 |
| White muscle | 2.4 ± 0.3 | 1.4 ± 0.2 | 0.5 ± 0.1 |
| Bluefin tuna, wild | |||
| Blood | 83 ± 29 | 28 ± 6.5 | NDb |
| Pacific mackerel | |||
| Blood | 437 ± 159 | 56 ± 10 | NDb |
| Tilapia | |||
| Blood | 0.9 ± 0.6 | 6.8 ± 3.2 | 0.55 ± 0.36 |
| Chicken | |||
| Liver | 0.3 ± 0.1 | 6.7 ± 1.0 | NDb |
| Heart | NDb | 2.0 ± 0.4 | 1.8 ± 0.4 |
| Gizzard | NDb | 2.7 ± 0.5 | NDb |
| Pig | |||
| Liver | NDb | 8.0 ± 1.2 | 5.0 ± 1.1 |
| Kidney | 0.36 ± 0.1 | 3.3 ± 1.5 | NDb |
| Japanese common squid | |||
| Hepatopancreas | 9.3 ± 3.5 | 5.0 ± 1.1 | 7.0 ± 1.1 |
a Selenoproteins eluted near the void volume of the column were estimated using bovine GPx as the standard.
b ND, lower than detection limit at 0.25 nmol of Se/g.
DISCUSSION
A novel selenium-containing compound was purified and identified from tuna blood. High resolution mass spectrometry and NMR spectroscopy showed that the compound was the oxidized dimeric form of the ergothioneine selenium analog in which the sulfur was replaced by selenium. Therefore, we propose that this selenium compound, 2-selenyl-Nα,Nα,Nα-trimethyl-l-histidine, be named selenoneine; this name is derived from the terms selenium and ergothioneine, which was previously identified in ergot (21) and blood (22).
Selenium speciation analysis using LC-ICP-MS indicated that selenoneine is the major selenium compound and is widely distributed in animal tissues, including the blood, hepatopancreas, spleen, heart, and skeletal muscle of the tuna, mackerel and tilapia blood, porcine kidney, chicken gizzard, liver, heart, and squid hepatopancreas. The tuna and mackerel blood contained high levels of selenium as selenoneine at 430–437 nmol of selenium/g. Further study is needed to examine whether selenoneine might be a non-toxic organic selenium in animal tissues and cells, in comparison with the high toxicity of known selenium compounds such as selenocysteine (LD50 35.8 mg/kg), selenomethionine (LD50 4.3 mg/kg), and selenite (LD50 3.5 mg/kg) (23–26). Selenoneine was converted from the reduced form to the oxidized dimer in the presence of the nonpolar organic solvent tetrahydrofuran, whereas the oxidized dimeric form was reduced to the monomer in the presence of glutathione or DTT at a concentration of 10 mm. Furthermore, selenoneine had strong radical-scavenging activity against DPPH in vitro. Therefore, selenoneine may be an important member of the redox cycle in animal cells. Our unpublished data3 suggest that selenoneine binds to the heme of hemoglobin and myoglobin to protect it from the autoxidation of iron ions under low oxygen conditions. Previously, GPx and other selenoproteins, which are induced by selenium intake, were believed to enhance the antioxidant ability in animal tissues and cells (1–5, 14, 15). However, because selenoneine is the predominant chemical form in tuna tissues, selenoneine derived from a dietary intake of fish may play the major role as a strong radical scavenger in a variety of physiological and nutritional functions found in previous medical and biochemical studies (1–15). Further studies of the bioaccumulation of selenium as selenoneine, its metabolism, and biological function in the free radical detoxification mechanism using selenoneine as the selenium source should help to characterize the essential role of selenium as selenoneine as a micronutrient for humans and animals.
Supplementary Material
This work was supported in part by grants from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Rural Biomass Research Project, BM-D2300) and the Fisheries Research Agency.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7.
Y. Yamashita, T. Yabu, and M. Yamashita, unpublished data.
- DTT
- dithiothreitol
- DPPH
- 1-diphenyl-2-picrylhydrazyl
- THF
- tetrahydrofuran
- GPx
- glutathione peroxidase
- HPLC
- high-performance liquid chromatography
- LC
- liquid chromatography
- MS
- mass spectrometry
- ICP
- inductively coupled plasma
- em
- emission
- ex
- excitation.
REFERENCES
- 1.Himeno S., Imura N. (2000) J. Health Sci. 46, 393–398 [Google Scholar]
- 2.Imura N., Naganuma A. (1991) in Advances in Mercury Toxicology ( Suzuki T., Imura N., Clarkson T. W. eds) pp.275– 288, Plenum Press, New York, NY [Google Scholar]
- 3.Combs G. F., Combs S. B. (1986) The Role of Selenium in Nutrition, pp. 1– 532,Academic Press, New York, NY [Google Scholar]
- 4.Brigelius-Flohé R. (1999) Free Radic. Biol. Med. 27, 951–965 [DOI] [PubMed] [Google Scholar]
- 5.Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. (1973) Science 179, 588–590 [DOI] [PubMed] [Google Scholar]
- 6.Mustacich D., Powis G. (2000) Biochem. J. 346, 1–8 [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur J. R., Nicol F., Beckett G. J. (1993) Am. J. Clin. Nutr. 57, 236S–239S [DOI] [PubMed] [Google Scholar]
- 8.Kryukov G. V., Castellano S., Novoselov S. V., Lobanov A. V., Zehtab O., Guigó R., Gladyshev V. N. (2003) Science 300, 1439–1443 [DOI] [PubMed] [Google Scholar]
- 9.Hatfield D. L., Berry M. J., Gladyshev V. N. (2006) Selenium: Its Molecular Biology and Role in Human Health, 2nd Ed., pp. 1–419, Springer, New York, NY [Google Scholar]
- 10.Kiremidjian-Schumacher L., Roy M. (2001) Biofactors 14, 161–168 [DOI] [PubMed] [Google Scholar]
- 11.Salonen J. T., Alfthan G., Huttunen J. K., Pikkarainen J., Puska P. (1982) Lancet 2, 175–179 [DOI] [PubMed] [Google Scholar]
- 12.Ghose A., Fleming J., Harrison P. R. (2001) Biofactors 14, 127–133 [DOI] [PubMed] [Google Scholar]
- 13.El-Bayoumy K. (2001) Mutat. Res. 475, 123–139 [DOI] [PubMed] [Google Scholar]
- 14.Raymond L. J., Ralston N. V. (2004) SMDJ Seychelles Med. Den. J. 7, 72–77 [Google Scholar]
- 15.Cuvin-Aralar M. L., Furness R. W. (1991) Ecotoxicol. Environ. Saf. 21, 348–364 [DOI] [PubMed] [Google Scholar]
- 16.Watkinson J. H. (1966) Anal. Chem. 38, 92–97 [DOI] [PubMed] [Google Scholar]
- 17.Ge H., Cai X. J., Tyson J. F., Uden P. C., Denoyer E. R., Block E. (1996) Anal. Commun. 33, 279 [Google Scholar]
- 18.Oki T., Osame M., Masuda M., Kobayashi M., Furuta S., Nishiba Y., Kumagai T., Sato T., Suda I. (2003) Breed. Sci. 53, 101–107 [Google Scholar]
- 19.Elguero J., Marzin C., Katritzky A. R., Linda P. (1976) The Tautomerism of Heterocycles, Advances in Heterocyclic Chemistry, Supplement No. 1 ( Katritzky A. R., Boulton A. J. eds) Academic Press, New York, NY [Google Scholar]
- 20.Xu J. Z., Yadan J. C. (1995) J. Org. Chem. 60, 6296–6301 [Google Scholar]
- 21.Tanret C. (1909) J. Pharm. Chim. 30, 145–153 [Google Scholar]
- 22.Benedict S. R., Newton E. B., Behre J. A. (1926) J. Biol. Chem. 67, 267–277 [Google Scholar]
- 23.Sasakura C., Suzuki K. T. (1998) J. Inorg. Biochem. 71, 159–162 [DOI] [PubMed] [Google Scholar]
- 24.Wilber C. G. (1980) Clin. Toxicol. 17, 171–230 [DOI] [PubMed] [Google Scholar]
- 25.Sayato Y., Hasegawa T., Taniguchi S., Maeda H., Ozaki K., Narama I., Nakamuro K. (1993) Jpn. J. Toxicol. Environ. Health 39, 289–296 [Google Scholar]
- 26.World Health Organization (WHO) (1987) Environmental Health Criteria 58, selenium, World Health Organization Publication, Geneva, Switzerland [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.