Abstract
Homogentisate solanesyl transferase (HST) catalyzes the prenylation and decarboxylation of homogentisate to form 2-methyl-6-solanesyl-1,4-benzoquinol, the first intermediate in plastoquinone-9 biosynthesis. In vitro, HST from Spinacia oleracea L., Arabidopsis thaliana, and Chlamydomonas reinhardtii were all found to use not only solanesyl diphosphate but also short chain prenyl diphosphates of 10–20 carbon atoms as prenyl donors. Surprisingly, with these donors, prenyl transfer was largely decoupled from decarboxylation, and thus the major products were 6-prenyl-1,4-benzoquinol-2-methylcarboxylates rather than the expected 2-methyl-6-prenyl-1,4-benzoquinols. The 6-prenyl-1,4-benzoquinol-2-methylcarboxylates were not substrates for HST-catalyzed decarboxylation, and the enzyme kinetics associated with forming these products appeared quite distinct from those for 2-methyl-6-prenyl-1,4-benzoquinol formation in respect of catalytic rate, substrate Km value, and the pattern of inhibition by haloxydine, a molecule that appeared to act as a dead end mimic of homogentisate. These observations were reconciled into a simple model for the HST mechanism. Here, prenyl diphosphate binds to HST to form at least two alternative complexes that go on to react differently with homogentisate and prenylate it either with or without it first being decarboxylated. It is supposed that solanesyl diphosphate binds tightly and preferentially in the mode that compels prenylation with decarboxylation.
Keywords: Enzyme Inhibitors, Enzyme Kinetics, Isoprenoid, Membrane Enzymes, Quinones, Aromatic Prenyltransferase, Herbicide, Homogentisate Prenyltransferase, Plastoquinone
Introduction
Plastoquinone-9 (PQ-9)2 is the major prenylated quinone in chloroplasts. In the thylakoid membrane, it mediates electron flow from photosystem II to the cytochrome b6f complex, and the redox state of the PQ-9 pool regulates the expression of a number of nuclear and plastidial genes as well as the activity of some plastidial enzymes (1). Moreover, PQ-9 is required as a cofactor for phytoene desaturation in carotenoid biosynthesis (2). Reflecting its central and unique role in higher plants, a defect in PQ-9 biosynthesis cannot be remedied by other plastidial prenylquinones such as phylloquinone or any of the structurally related intermediates of the vitamin E biosynthetic pathway. Thus the respective mutants of Arabidopsis thaliana display a seedling-lethal phenotype in soil and an albino phenotype on medium supplemented with sucrose (3, 4).
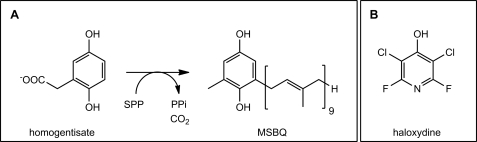
PQ-9 comprises an aromatic head group derived from homogentisate and an isoprenoid side chain derived from solanesyl diphosphate (SPP). An HST located in the inner envelope membrane of chloroplasts catalyzes the prenylation and decarboxylation of homogentisate to form 2-methyl-6-solanesyl-1,4-benzoquinol (MSBQ) (Fig. 1) (5), which is then methylated to the reduced form of PQ-9. The HSTs from Chlamydomonas reinhardtii and A. thaliana have been expressed in Escherichia coli and shown to require divalent cations for catalytic activity (6). Other membrane-bound aromatic prenyltransferases exhibit a similar requirement (7). Recent structural studies of the 4-hydroxybenzoate prenyltransferase, UbiA, from E. coli, which catalyzes the prenylation of 4-hydroxybenzoate in the course of ubiquinone biosynthesis, suggest that the divalent metal ion fulfils an essential role (8). Two conserved aspartate-rich motifs complex the diphosphate group of the prenyl donor via a bridging Mg2+ ion as well as provide part of the binding site that orients the 4-hydroxybenzoate aromatic substrate. It seems likely that the equivalent conserved aspartate-rich motifs of HST and essential Mg2+ ion fulfil an analogous role in HST catalysis. Again, similar to the 4-hydroxybenzoate prenyltransferases (9, 10, 11), it appears that a range of polyunsaturated prenyl donors of various chain lengths can serve as substrates for HST (6).
FIGURE 1.
Aromatic prenyltransferase reaction in PQ-9 biosynthesis (A) and structure of the herbicide haloxydine (B). PPi, diphosphate.
In the present study, we further explore the catalytic mechanism and reactions catalyzed by HST. A herbicide, haloxydine (Fig. 1, 3,5-dichloro-2,6-difluoro-4-haloxypyridine), discovered here to be a potent inhibitor of HST, provided an essential tool for this study.
EXPERIMENTAL PROCEDURES
Test Chemicals for Inhibition Studies
Haloxydine and [2-((R)-1-phenyl-ethylamino)-1-phosphono-ethyl]-phosphonic acid were obtained from Syngenta Ltd. and were synthesized as described previously (12, 13). The purity of haloxydine was 96%, and that of the bisphosphonate was 84% as determined by reverse-phase HPLC and proton NMR spectroscopy.
Heterologous Expression and Preparation of Enzyme-enriched Fractions
The cDNA sequences for the mature homogentisate prenyltransferase proteins from C. reinhardtii CC-1690 (AM285678) and from A. thaliana ecotype Columbia (At3g11945, previously At3g11950) were expressed with an N-terminal glutathione S-transferase (GST) tag in E. coli BL21AI as described previously (6). The open reading frame for a 4-hydroxybenzoate prenyltransferase was amplified from the E. coli BL21AI genome (ubiA, NC_012947) by using the forward primer 5′-CAC CAT GGA GTG GAG TCT GAC GC-3′ and the reverse primer 5′-TCA GAA ATG CCA GTA ACT CAT TGC CAG-3′. The PCR product was sequenced using standard techniques and cloned into the expression vector pDEST14 (Invitrogen). Membranes of the E. coli clones expressing the aromatic prenyltransferase encoding sequences were prepared as described by Ref. 6. GST-tagged proteins were detected on Western blot membranes using mouse GST tag antibodies (Novagen) and goat anti-mouse IgG-peroxidase conjugate antibodies (Qiagen) with Lumi-Light Plus (Roche Applied Science) according to the manufacturer's instructions.
Preparation of Chloroplast Envelope Membranes
Chloroplast envelope membranes were isolated from spinach leaves essentially as described by Ref. 14. Intact chloroplasts were purified from a crude chloroplast preparation by isopycnic centrifugation in a Percoll gradient and subsequently lysed in hypotonic medium. From the lysate, envelope membranes were purified in a three-step sucrose gradient.
Enzyme Assays
Homogentisate prenyltransferase activities were measured by determining the incorporation rates of [U-14C]homogentisate and prenyl diphosphates into lipophilic products as described by Ref. 6. [U-14C]Homogentisate (radiochemical purity 85–95%) was synthesized enzymatically from [U-14C]tyrosine as described earlier (6). Unlabeled substrates were purchased from Sigma-Aldrich and American Radiolabeled Chemicals, respectively. E. coli-expressed HST from C. reinhardtii was assayed for 15 min at 28 °C in a 50-μl reaction volume with 30 μg of membrane proteins, 20 mm magnesium acetate, 100 μm [U-14C]homogentisate (∼80 dpm/pmol), and 200 μm prenyl diphosphate (generally farnesyl diphosphate, FPP) in 50 mm Tricine-NaOH buffer, pH 8.5. Spinach envelope membranes (30 μg of total protein) were incubated for 30 min at 28 °C in a 50-μl reaction mixture with 50 mm Bis-Tris propane-HCl, pH 9.0, 50 mm magnesium acetate, 30 μm [U-14C]homogentisate (∼520 dpm/pmol), and 200 μm prenyl diphosphate. In general, enzyme reactions were started with the addition of prenyl donor to the reaction mixture. Assays were stopped and extracted with 200 μl of chloroform:methanol (1:1) and 50 μl of 0.9% sodium chloride solution. Labeled products were visualized on silica gel TLC plates (MACHERY-NAGEL) with a bioimager (FLA3000, raytest), and data were processed with the AIDA software (raytest). The amount of radioactivity was quantified by scintillation counting. Kinetic data were graphed and analyzed using GraphPad Prism (GraphPad Software). Error limits around Km and Vmax represent the bounds of the 95% confidence limits. Where there were six data points, error bounds for Ki values were the upper and lower values derived from the standard errors of the slopes and Y intercepts of the linear regression fits to replots of the estimated values of 1/Vmax or Km/Vmax versus inhibitor concentration. With only three data points, the error bounds simply represent the maximum and minimum slopes between any two. 2-Methyquinol (Alfa Aesar) was oxidized with potassium dichromate in dilute sulfuric acid for co-chromatography analysis.
The E. coli 4-hydroxybenzoate prenyltransferase (UbiA) was assayed in 50 mm Bistris propane, pH 7.0, 20 mm magnesium acetate, 30 μm [carboxy-14C]4-hydroxybenzoate (∼40 dpm/pmol), 50 μm prenyl diphosphate with 5 μg of membrane proteins in a reaction volume of 50 μl. After a 15-min incubation at 30 °C, lipids were extracted as described above and analyzed on silica gel plates in acetone:petrolether (3:7). Specific activities of about 150 pmol min−1 mg−1 protein were determined.
Mass Spectrometry Analyses of Polar HST Reaction Products
To generate adequate amounts of polar products for mass spectrometry analysis, C. reinhardtii HST was incubated for 30 min at 28 °C in a 2-ml reaction volume of 50 mm Tricine-NaOH buffer, pH 8.5, containing 1.2 mg of membrane proteins, 20 mm magnesium acetate, 200 μm [U-14C]homogentisate (∼60 dpm/pmol), and 200 μm geranylgeranyl diphosphate (GGPP). The reaction was stopped and extracted with 1.5 ml of chloroform:methanol (1:1) and 0.5 ml of 0.9% sodium chloride solution. The organic phase was re-extracted with water four times, dried under a stream of nitrogen gas, and redissolved in chloroform. Analysis by TLC using toluene:isoamyl alcohol:acetic acid (80:40:3) as mobile phase confirmed that nearly all of the 14C-labeled product was polar in nature. Further purification was done by HPLC (1100 Series, Agilent Technologies) using a reverse-phase C30 column (250 × 4.6 mm, 5 μm, YMC Co.) and gradient elution between two mobile phases, A and B. Solvent A was methanol:water:formic acid (375:122:2.5), and solvent B was methanol:isopropyl alcohol:formic acid (400:97.5:2.5), B. The gradient was developed as 100% A (initial), 95% A, and 5% B at 2 min, 80% A and 20% B at 5 min, 50% A and 50% B at 12 min, 20% A and 80% B at 35 min, and 10% A and 90% B at 40 min at a flow rate of 0.75 ml/min. Three main radiolabeled fractions containing polar product (as verified by TLC) were collected at 19.5, 21.6, and 22.3 min, respectively, and dried under a stream of nitrogen. High resolution mass spectrometry analysis was performed on samples dissolved in ethyl acetate and injected using an Accela HPLC system coupled to an LTQ Orbitrap XL mass spectrometer (Thermo Scientific) and an FSA 610 TR radiodetector (PerkinElmer Life Sciences). The HPLC system was equipped with a reverse-phase C30 column (250 × 3-mm inner diameter, 3 μm, YMC Co.). Radiolabeled products eluted at ∼4.2 min with the following pump control parameters set for gradient elution: 75% A and 25% B (initial), 70% A and 30% B at 12 min, 0% A and 100% B at 15 min, 0% A and 100% B at 18 min, 75% A and 25% B at 20 min, 75% A and 25% B at 23 min, and a flow rate of 0.5 ml/min with mobile phases consisting of water:methanol:tert-butyl methyl ether (30:60:5) for A and water:methanol:tert-butyl methyl ether (3:7:90) for B. Mass spectrometry was carried out in negative ion mode atmosphere pressure chemical ionization applying a source voltage of 6 kV, source current 20 mA, capillary temperature 180 °C, atmosphere pressure chemical ionization vaporizer temperature 400 °C using a mass resolution of 100,000 (full scan) across a mass range of 100–900 Da. Precursor ion fragmentation was carried out using collision-induced dissociation employing helium as the collision gas operating with a collision energy of 30 eV. In all three fractions, 6-geranylgeranyl-1,4-benzoquinol-2-methylcarboxylate (439.2853 m/z, 0.25 ppm, C28H39O4, 4 min) was detected as the major radiolabeled compound with the lactone derivative (421.2746 m/z, 0.95 ppm, C28H37O3, 6.3 min) as the minor component. Radical anion and other anionic species derived from 6-geranylgeranyl-1,4-benzoquinol-2-methylcarboxylate were detected including the radical anion 440.2928 m/z (−0.47 ppm), the anion 439.2855 m/z (0.25 ppm), and the radical anion 438.2780 m/z (0.90 ppm). It is highly likely that these occur as a result of electrochemical redox reactions in the mass spectrometer.
RESULTS
In Vitro Inhibition of Homogentisate Prenyltransferases
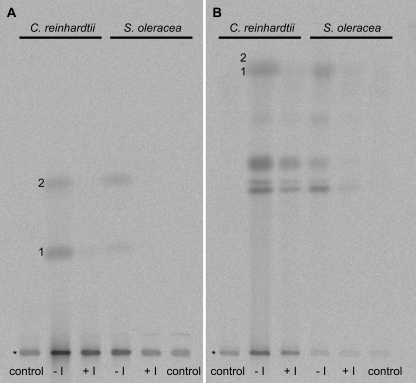
HSTs were assayed with unlabeled FPP and [14C]homogentisate, and the 14C-labeled products were analyzed by extraction into chloroform and subsequent TLC using dichloromethane as mobile phase. As shown in Fig. 2A, the reduced (quinol) and oxidized (quinone) forms of the 2-methyl-6-farnesyl-1,4-benzoquinol/quinone (MFBQ) product resolved into a characteristic pair of radiolabeled bands. Haloxydine was found to be an efficient inhibitor of HSTs; 0.5 mm haloxydine nearly completely inhibited MFBQ formation using either E. coli-expressed C. reinhardtii HST or spinach chloroplast envelope membranes as the source of HST. Similar inhibition was obtained with whole chloroplast HST activity and with A. thaliana HST expressed in E. coli membranes (data not shown). By contrast, haloxydine did not inhibit the E. coli (UbiA) 4-hydroxybenzoate prenyltransferase. Membrane preparations from E. coli cells overexpressing UbiA were incubated in reaction mixtures with [14C]4-hydroxybenzoate and unlabeled geranylgeranyl diphosphate as described under “Experimental Procedures.” The addition of 1 mm haloxydine had no detectable effect (<5%) on the rate of formation of labeled 3-geranylgeranyl-4-hydroxybenzoate.
FIGURE 2.
Inhibition of HSTs by haloxydine. Chloroform extracts from assays with C. reinhardtii HST and native HST in chloroplast envelope membranes of spinach were analyzed via TLC using dichloromethane (A) and toluene:isoamyl alcohol:acetic acid (80:40:3) (B) as mobile phase. The enzymes were assayed in the absence (− I) or presence (+ I) of 0.5 mm haloxydine in standard reaction mixtures as described under “Experimental Procedures.” Control assays lacked prenyl donor. * marks the origin; 1 and 2, MFBQ quinol and quinone form, respectively.
Alternative Products of the HST Reaction
Although the addition of haloxydine inhibited HST and suppressed the appearance of the radiolabeled bands corresponding to MFBQ, the predominant visible effect of adding the inhibitor was on the amount of 14C label retained near the origin of the TLC plate (Fig. 2A). It appeared that haloxydine also inhibited the formation of hitherto unknown major reaction products that were extracted into chloroform and that, on the basis of their relative immobility in dichloromethane, must be more polar than MFBQ. Further TLC using a toluene:isoamyl alcohol:acetic acid system resulted in a separation of the “unknown products” into several bands. As shown in Fig. 2B, similar band patterns were detected for assays with C. reinhardtii HST as well as native HST from spinach. Thus these products seemed to be formed irrespective of the source of HST.
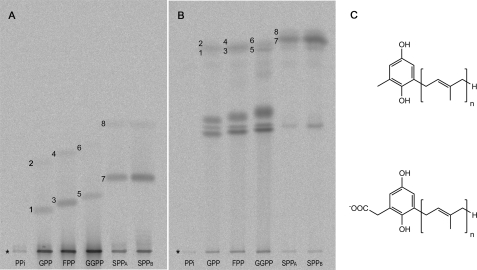
Fig. 3 depicts the results of chromatography experiments to further investigate the nature of these unexpected products. In these experiments, assays were carried out using C. reinhardtii HST and prenyl donors of different chain lengths. We previously reported that the addition of a detergent stimulated the SPP-dependent HST activity (6). To facilitate comparison, all reaction mixtures contained 0.1 mm n-dodecyl β-d-maltoside. This detergent concentration is sufficient for the SPP reaction to run without significantly inhibiting the rate of reaction with shorter chain prenyl donors (6). With no prenyl donor present but only diphosphate in its place, there was no detectable reaction. With geranyl diphosphate (GPP), FPP, or GGPP, bands consistent with the corresponding 2-methyl-6-prenyl-1,4-benzoquinol/quinone products were each detected on TLC plates developed in dichloromethane (Fig. 3A). However, from all three of these reactions, by far the major products again appeared to be more polar 14C-labeled species separating near the baseline. Further TLC using the toluene:isoamyl alcohol:acetic acid system indicated that these unknown products were likely to be prenylated because they separated distinct from one another according to the prenyl donor used in the reaction (Fig. 3B). Given the limited range of reasonable possibilities, we concluded that these unknown GPP-, FPP-, and GGPP-derived products were most likely the corresponding 6-prenyl-1,4-benzoquinol-2-methylcarboxylates that would result from HST-catalyzed prenylation of homogentisate occurring without concomitant decarboxylation (Fig. 3C). This assignment of the major polar products as acids is consistent with their observed lack of TLC movement with dichloromethane as mobile phase as well as the pH dependence of their movement in the toluene:isoamyl alcohol:acetic acid TLC system. The identity of the major GGPP-derived polar products as 6-geranylgeranyl-1,4-benzoquinol/quinone-2-methylcarboxylate was confirmed by mass spectrometry analyses (see supplemental Data 1). No detectable amounts of 14C-labeled 2-methylquinol (MQ)/quinone were found in either the chloroform or the aqueous extracts. Thus HST did not significantly catalyze the decarboxylation of homogentisate uncoupled from prenylation. Whether or not HSTs exhibit any phosphatase activity without catalyzing the prenylation of the aromatic substrate, as was reported for the 4-hydroxybenzoate prenyltransferase from E. coli (8), was not investigated here.
FIGURE 3.
TLC analyses of C. reinhardtii HST products from assays with various prenyl donors. Assays were conducted in the presence of 0.1 mm n-dodecyl β-d-maltoside with the exception of sample SPPB that contained 0.2 mm detergent in the reaction mixture. The chloroform soluble products were separated by TLC using dichloromethane (A) or toluene:isoamyl alcohol:acetic acid (80:40:3) (B) as mobile phase. Besides the expected 2-methyl-6-prenyl-1,4-benzoquinols (marked by 1, 3, 5, and 7) and the respective quinones (marked by 2, 4, 6, and 8), chloroform extracts of assays with prenyl donors up to a chain length of C20 contained mainly so far unknown prenylated polar products. * indicates the origin; PPi, diphosphate. C, the structures for the 2-methyl-6-prenyl-1,4-benzoquinols (upper) and the more polar 6-prenyl-1,4-benzoquinol-2-methylcarboxylates (lower) are given with n = 2, n = 3, n = 4, and n = 9 for the respective GPP-, FPP-, GGPP-, and SPP-derived products.
In control experiments, the ratio of 6-prenyl-1,4-benzoquinol-2-methylcarboxylate to 2-methyl-6-prenyl-1,4-benzoquinol products formed from FPP or GGPP remained unaltered upon further incubation with control E. coli membranes that lacked HST. Neither was any of the methylcarboxylate decarboxylated to 2-methyl-6-prenyl-1,4-benzoquinol when the products from one round of HST reaction were reincubated with fresh HST. Hence, it was confirmed that HST, and not some other component of the E. coli membranes, was solely responsible for the generation of both carboxylated and decarboxylated products and that there was no membrane-associated nonspecific decarboxylase activity capable of converting the carboxylate to the respective 2-methyl-6-prenyl-1,4-benzoquinol.
The proportion of non-decarboxylated to decarboxylated prenyl products of the HST reaction changed according to the prenyl donor. With the C10 to C20 prenyl donors, GPP, FPP, and GGPP, up to 95% of the total prenylated reaction products formed were carboxylic acids, whereas with the C45 prenyl donor, SPP, the major product was MSBQ (Fig. 3B).
Enzyme Kinetics of HST-catalyzed Product Formation
Kinetic studies were pursued using only the C. reinhardtii enzyme because the A. thaliana enzyme was more difficult to express in easily assayable amounts. Assay conditions were established to ensure that initial rates could be measured and thus that rates were adequately linear with additions of between 0 and 30 μg of membrane protein and with assay reaction times between 0 and 20 min. It was also established from the outset that haloxydine was neither a slow binding nor a slow dissociating inhibitor and that, on the time scale of the kinetic experiments, it could be treated as being in rapid equilibrium with the enzyme and enzyme substrate complexes.
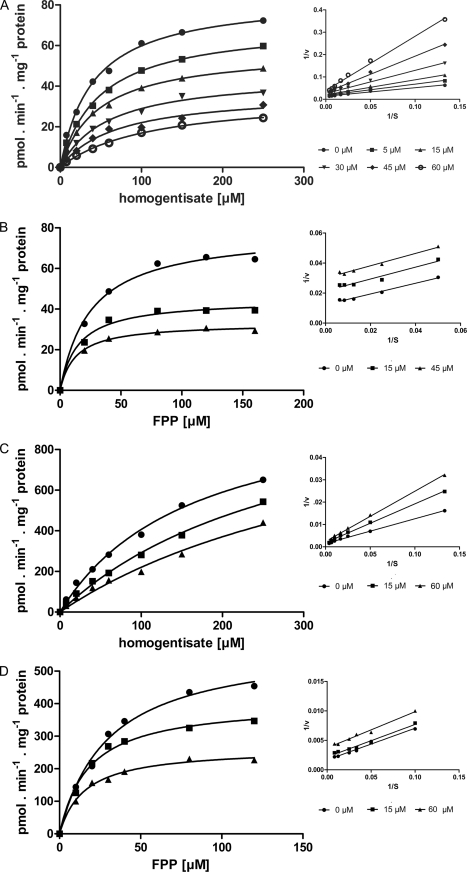
Fig. 4 describes the results of a number of experiments to investigate the kinetics of haloxydine inhibition of C. reinhardtii HST with respect to homogentisate and to FPP. Initial rate measurements were based on the amount of MFBQ formed. Consistent with our earlier observations (6), the C. reinhardtii HST exhibited an apparent Km of 40.5 ± 8 μm for homogentisate (at 200 μm FPP) and an apparent Km of 25 ± 10 μm for FPP (at 100 μm homogentisate). The Vmax for the C. reinhardtii HST membrane preparation used in these experiments was 84 ± 5 pmol min−1 mg−1. According to Western analysis versus a GST standard curve, HST expressed as a 63-kDa GST fusion protein constituted about 2% of the total membrane proteins. If it is assumed that all of the expressed HST is catalytically active, then the apparent kcat for MFBQ formation in vitro can be estimated as less than 0.005 s−1. Haloxydine exhibited mixed inhibition kinetics with respect to homogentisate (Ki SLOPE ∼8.4 μm (3.4–15.2) and Ki INTERCEPT ∼45 μm (39–52) and inhibition that appeared uncompetitive (Ki ∼38 μm (25–61) at 100 μm homogentisate) with respect to FPP (Fig. 4). The enzyme kinetics of HST appeared significantly different when analyzed with respect to initial rates of total product formation rather than in respect of MFBQ. Although the apparent Km value for FPP seemed to remain nearly unchanged (∼30 μm), it was difficult to obtain convincing saturation kinetics with respect to homogentisate. Both the Km value for homogentisate and the Vmax were clearly much higher for total product formation than for MFBQ formation. Based on data from homogentisate concentrations up to 0.25 mm, the Km for homogentisate was estimated as >170 μm, and Vmax was estimated as >1 nmol min−1 mg−1 (Fig. 4C). These kinetics indicated that although 6-farnesyl-1,4-benzoquinol-2-methylcarboxylate (FBQC) was the major component of the total product, the exact proportion of non-decarboxylated to decarboxylated product would vary according to substrate concentration. In respect of total product, inhibition by haloxydine was, again, uncompetitive with respect to FPP (apparent Ki ∼55 μm (33–70) at 100 μm homogentisate) and apparently competitive with a Ki ∼53 μm (22–90) with respect to homogentisate (Fig. 4C). We did not pursue the kinetics with respect to FBQC to the point of definitive determination. Nevertheless, it was clear that MFBQ formation and FBQC formation were each associated with quite distinct reaction kinetics and, furthermore, that haloxydine inhibition was uncompetitive with respect to FPP and either mixed or competitive with respect to homogentisate.
FIGURE 4.
Kinetics of inhibition of C. reinhardtii HST by haloxydine. The effects of haloxydine on HST-catalyzed MFBQ formation at 200 μm FPP (A) and at 100 μm homogentisate (B) as well as on total product formation at 200 μm FPP (C) and 100 μm homogentisate (D) are shown.
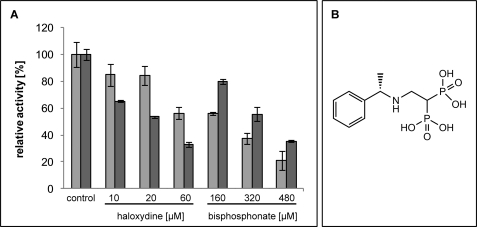
A bisphosphonate chemical, [2-((R)-1-phenyl-ethylamino)-1-phosphono-ethyl]-phosphonic acid, was also tested as a likely structural analogue and inhibitory mimic of FPP (Fig. 5). The data indicated that, in contrast to haloxydine, the bisphosphonate consistently inhibited the formation of total product (FBQC) more strongly than it did the formation of MFBQ.
FIGURE 5.
Inhibition of C. reinhardtii HST by haloxydine and a bisphosphonate. A, the inhibitor concentrations are indicated. Total product (FBQC, light gray bars) and MFBQ (dark gray bars) formation is affected to different extents depending on the inhibitor. Data represent mean values and standard deviations of quadruple estimations. The structure of the bisphosphonate is shown in B. Note that this compound was an S enantiomer; the R enantiomer also inhibited similarly.
DISCUSSION
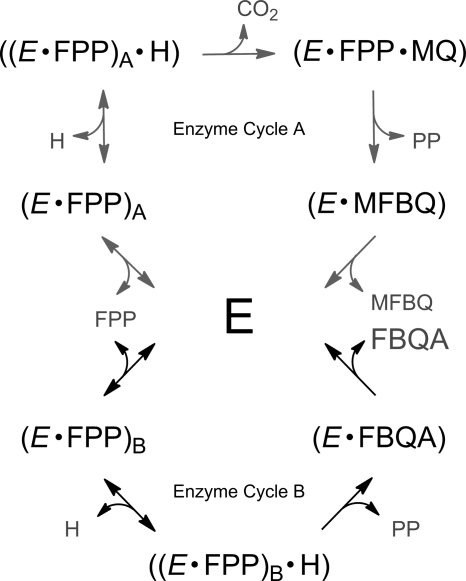
In vitro, HST-catalyzed prenylation of homogentisate resulted not only in the formation of the expected 2-methyl-6-prenyl-1,4-benzoquinol products but also in the corresponding 6-prenyl-1,4-benzoquinol-2-methylcarboxylates. Haloxydine, an anionic (pKa 2.15) and credible structural mimic of homogentisate, inhibited the formation of both MFBQ and FBQC consistent with them both being products of HST. Because HST preparations were not able to decarboxylate FBQC, we concluded that FBQC is not made as an exchangeable catalytic intermediate along the pathway of HST-catalyzed formation of MFBQ. Thus the 6-prenyl-1,4-benzoquinol-2-methylcarboxylate and 2-methyl-6-prenyl-1,4-benzoquinol products would appear to be formed via distinct and competing pathways. The scheme of Fig. 6 summarizes our proposal for the order and nature of intermediates along two concurrent pathways of HST-catalyzed prenylation. The essential features are (a) that prenyl diphosphate, in this case FPP, binds to the enzyme to form the (E·FPP) complex; (b) that homogentisate binds to the (E·FPP) complex; and (c) that especially the smaller prenyl donors such as FPP have enough “wobble” room within the enzyme to partition between at least two binding orientations and form alternative productive enzyme complexes, here designated (E·FPP)A and (E·FPP)B. These complexes react differently with homogentisate and go on to prenylate it either with (Enzyme Cycle A) or without (Enzyme Cycle B) it first being decarboxylated. It is supposed that the larger, cognate substrate of HST, SPP, which forms mainly the decarboxylated product (Fig. 3), has less steric freedom to move within the enzyme site and is constrained to predominantly bind in the orientation that takes it down reaction path A and thus to the metabolically productive MSBQ product.
FIGURE 6.
Suggested pathways for HST-catalyzed farnesylation of homogentisate. In Enzyme Cycle A, homogentisate is decarboxylated to 2-methylquinol prior to prenyl transfer, whereas in the major pathway, Enzyme Cycle B, prenylation occurs without decarboxylation. E, free HST enzyme; PP, pyrophosphate.
Enzyme Cycle A continues from (E·FPP)E with homogentisate binding to form ((E·FPP)A·H), which then decarboxylates to form a 2-methylquinol complex (E·FPP·MQ) prior to prenyl transfer to (E·MFBQ), which then dissociates to release product. Possible alternatives with prenylation preceding decarboxylation and thus having FBQC as an intermediate in MFBQ formation are difficult to reconcile with the observed inability of HST to convert FBQC into MFBQ. Equally, alternatives that have MFBQ and FBQC derived from a common ((E·FPP)·H) complex are difficult to reconcile with the observation that the apparent Km value for homogentisate in respect of the predominant and faster formed FBQC product (kcat >10-fold than for MFBQ) was significantly higher than that observed in respect of MFBQ formation (i.e. >170 μm as compared with 40 μm). Inhibition studies with haloxydine further complete the picture. Haloxydine was a competitive or near competitive inhibitor with respect to homogentisate and also an uncompetitive inhibitor with respect to FPP. This is consistent with an ordered mechanism in which homogentisate binds productively to the (E·FPP) complex. The fact that different Ki values for haloxydine, 8.5 and ∼53 μm, were observed in respect of MFBQ or FBQC formation is consistent with the proposed existence of at least two (E·FPP) complexes having distinct structures and commitments to forming the two alternate products. Similarly, the observed differences in the pattern of inhibition are also consistent with the scheme. For Enzyme Cycle B, (E·FPP)B is the only enzyme species that a homogentisate mimic such as haloxydine should bind to and, accordingly, haloxydine inhibition was observed to be competitive with respect to homogentisate in the formation of total product (FBQC). For reaction path A, as well as homogentisate-competitive binding to (E·FPP)A, there is the additional possibility of haloxydine binding to a later complex such as the presumptive methylquinol ternary complex ((E·FPP)A·MQ) that follows decarboxylation. The observed Ki INTERCEPT component and mixed nature of haloxydine inhibition of MFBQ formation would be consistent with it binding weakly to such a complex, and the observed mixed kinetics of haloxydine inhibition of MFBQ formation are thus consistent with the proposed scheme.
A bisphosphonate chemical, [2-((R)-1-phenyl-ethylamino)-1-phosphono-ethyl]-phosphonic acid, was a reasonable structural mimic of FPP that inhibited HST with an IC50 of ∼300 μm with 200 μm FPP present in the assay (Fig. 5). The data indicated that in contrast to haloxydine, which inhibited the formation of MFBQ considerably more potently than it did FBQC, the bisphosphonate exhibited the converse pattern and inhibited total (FBQC) product formation somewhat more strongly than it did MFBQ. In the context of the scheme of Fig. 6, which has FPP binding to free enzyme, it might be expected that a dead end inhibitory mimic of FPP should equally inhibit the formation of both products because (E·FPP)A and(E·FPP)B likely cannot co-exist, and so an inhibitor that occupied either site would equally prevent productive occupation of the other. Thus the observed slightly greater inhibition of FBQC formation than of MFBQ likely reflects a slightly higher Km value for FPP in respect of FBQC.
It is intriguing that, in vitro at least, HST catalyzes so efficiently the prenylation of homogentisate with a wide range of prenyl donors other than the presumptive natural donor, SPP, and, furthermore, forms both carboxylated and non-carboxylated products. Because of the need for detergent and uncertainty in the concentration of SPP in aqueous solution (nominally 200 μm), it is difficult to compare the rate at which SPP is converted to MSBQ with the equivalent rates for other prenyl donors. Assuming that the SPP concentration was at or above its Km, then the apparent rate (Fig. 3) would seem, at least, to be of a similar order to that observed with other prenyl donors. The question is raised therefore as to whether and how the HST reaction is controlled in planta to avoid the production of the kinds of side products observed in vitro or whether they are indeed produced but have yet to be detected. Possibly there is efficient substrate channeling to deliver SPP to HST. Alternatively, and consistent with the ordered mechanism suggested in the scheme of Fig. 6, it could simply be that the Km for SPP is much lower than for the other prenyl donors so that, effectively, there is little free enzyme and HST exists mainly bound up as the (HST·SPP) complex. In any case, plastoquinones with shorter prenyl side chains have been detected as minor components in higher plants such as plastoquinone-3 in spinach (15). The observed formation of MFBQ in assays with spinach chloroplast envelopes (Fig. 2) is thus consistent with the occurrence of plastoquinone-3 in this plant species.
Given the large and lipophilic nature of the SPP-derived reaction product it is reasonable to speculate that, as for many enzymes (16), product release or protein conformational changes that precede this rather than a bond-breaking or bond-forming step might be largely rate-limiting in the overall catalytic cycle. At least consistent with this notion is the observation that widely differing catalytic rates were observed according to the product formed.
In vitro, HST appeared to be such a poor catalyst for the production of MSBQ as to raise the question of whether the enzyme has any real role in PQ-9 biosynthesis in plants. However, the flux required in planta is likely very low, and the bleached seedling-lethal phenotype of the A. thaliana pds2 mutant (2), which maps to the same location (At3g11945) as the A. thaliana HST gene (3), clearly demonstrates that the product of this gene is indeed essential for PQ-9 biosynthesis. The biological bleaching effects of haloxydine on plants are also consistent with an essential role for HST; certainly other herbicides known to act at other sites in plastoquinone biosynthesis exhibit similar effects on plants (17).
Supplementary Material
Acknowledgments
We gratefully acknowledge expert help from Richard Wood (Syngenta Ltd.), Dalila Menguellet (Syngenta Ltd.), and Dr. Peter Howe (Syngenta Ltd.) in the analysis and characterization of chemicals used and products made in the course of this study.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Data 1.
- PQ-9
- plastoquinone-9
- HST
- homogentisate solanesyltransferase
- SPP
- solanesyl diphosphate
- MSBQ
- 2-methyl-6-solanesyl-1,4-benzoquinol
- GST
- glutathione S-transferase
- FPP
- farnesyl diphosphate
- GGPP
- geranylgeranyl diphosphate
- MFBQ
- 2-methyl-6-farnesyl-1,4-benzoquinol
- GPP
- geranyl diphosphate
- FBQC
- 6-farnesyl-1,4-benzoquinol-2-methylcarboxylate
- MQ
- 2-methylquinol
- H
- homogentisate
- HPLC
- high pressure liquid chromatography
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- Bis-Tris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane.
REFERENCES
- 1.Foyer C. H., Noctor G. D. (2009) Antioxid. Redox Signal. 11, 861–905 [DOI] [PubMed] [Google Scholar]
- 2.Norris S. R., Barrette T. R., DellaPenna D. (1995) Plant Cell 7, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian L., DellaPenna D., Dixon R. A. (2007) Planta 226, 1067–1073 [DOI] [PubMed] [Google Scholar]
- 4.Cheng Z., Sattler S., Maeda H., Sakuragi Y., Bryant D. A., DellaPenna D. (2003) Plant Cell 15, 2343–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soll J., Schultz G., Joyard J., Douce R., Block M. A. (1985) Arch. Biochem. Biophys. 238, 290–299 [DOI] [PubMed] [Google Scholar]
- 6.Sadre R., Gruber J., Frentzen M. (2006) FEBS Lett. 580, 5357–5362 [DOI] [PubMed] [Google Scholar]
- 7.Heide L. (2009) Curr. Opin. Chem. Biol. 13, 171–179 [DOI] [PubMed] [Google Scholar]
- 8.Bräuer L., Brandt W., Schulze D., Zakharova S., Wessjohann L. (2008) Chembiochem. 9, 982–992 [DOI] [PubMed] [Google Scholar]
- 9.Kawahara K., Koizumi N., Kawaji H., Oishi K., Aida K., Uchida K. (1991) Agric. Biol. Chem. 55, 2307–2311 [Google Scholar]
- 10.Melzer M., Heide L. (1994) Biochim. Biophys. Acta 1212, 93–102 [DOI] [PubMed] [Google Scholar]
- 11.Ohara K., Yamamoto K., Hamamoto M., Sasaki K., Yazaki K. (2006) Plant Cell Physiol. 47, 581–590 [DOI] [PubMed] [Google Scholar]
- 12.Tomlin C. D. S., Slater J. W., Hartley D. (August13, 1969) U. K. Patent 1,161,491
- 13.Fisher K. J., Woolard F. X., Leadbetter M. R., Gerdes J. M. (April7, 1995) U. S. Patent 5,728,650
- 14.Douce R., Joyard J. (1982) in Methods in Chloroplast Molecular Biology (Edelman M., Hallick R., Chua N. H. eds.), pp. 239–256, Elsevier Science Publishers B.V., Amsterdam [Google Scholar]
- 15.Misiti D., Moore H. W., Folkers K. (1965) J. Am. Chem. Soc. 87, 1402–1403 [DOI] [PubMed] [Google Scholar]
- 16.Cleland W. W. (1982) CRC Crit. Rev. Biochem. 13, 385–428 [DOI] [PubMed] [Google Scholar]
- 17.Hawkes T. R. (2007) in Modern Crop Protection Compounds (Kramer W., Schirmer U. eds.), pp. 211–221, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.