Abstract
Before a sperm can fertilize an egg it must undergo a final activation step induced by the egg termed the acrosome reaction. During the acrosome reaction a lysosome-related organelle, the acrosome, fuses with the plasma membrane to release hydrolytic enzymes and expose an egg-binding protein. Because NAADP (nicotinic acid adenine dinucleotide phosphate) releases Ca2+ from acidic lysosome-related organelles in other cell types, we investigated a possible role for NAADP in mediating the acrosome reaction. We report that NAADP binds with high affinity to permeabilized sea urchin sperm. Moreover, we used Mn2+ quenching of luminal fura-2 and 45Ca2+ to directly demonstrate NAADP regulation of a cation channel on the acrosome. Additionally, we show that NAADP synthesis occurs through base exchange and is driven by an increase in Ca2+. We propose a new model for acrosome reaction signaling in which Ca2+ influx initiated by egg jelly stimulates NAADP synthesis and that this NAADP acts on its receptor/channel on the acrosome to release Ca2+ to drive acrosomal exocytosis.
Keywords: Calcium, Inositol Phosphates, Nucleotide, Sea Urchin, Signal Transduction, Spermatozoa, ADP-ribosyl Cyclase, NAADP, Acrosome
Introduction
For sperm to become competent for fertilization they must undergo the acrosome reaction in which the acidic secretory organelle called the acrosome is exocytosed (1–3). This releases hydrolytic enzymes, which facilitate penetration of the egg jelly, and exposes proteins required for binding to the egg's plasma membrane (1–4). Although the acrosomal and plasma membranes fuse at multiple sites, membrane fusions are triggered by a sustained increase in Ca2+ (5). For sea urchin sperm, the currently accepted view is that voltage-gated Ca2+ channels give rise to a transient Ca2+ increase (2, 3, 6) that activates phospholipase Cδ (7–10). The subsequent increase in inositol 1,4,5-trisphosphate (9) activates its receptors (11) to increase cytosolic Ca2+. Because sperm lack endoplasmic reticulum, the identity of the internal Ca2+ store is unknown in sea urchin; however, the internal Ca2+ store is the acrosome itself in mammalian sperm (12, 13). Depletion of internal Ca2+ stores stimulates store-operated Ca2+ channels that give rise to a sustained Ca2+ entry (5, 14).
Although there is no disputing the evidence and role for inositol 1,4,5-trisphosphate in the above process, there remains scope and indirect evidence for involvement of the Ca2+-releasing messenger nicotinic acid adenine dinucleotide phosphate (NAADP).4 NAADP-induced Ca2+ release was discovered in the sea urchin egg (15, 16). Suggestively, NAADP is present in sea urchin sperm (17) and is rapidly synthesized upon exposure to either egg jelly (18) or fucose sulfate polymer (19). We initially suggested that NAADP is produced in the sperm entirely for injection into the sea urchin egg to help other messengers activate it (18). Such a transcellular messenger role for NAADP is supported by fertilization in the starfish (20–22). Nevertheless, NAADP may play a role in the sperm per se based on the following reasoning. The acrosome is a lysosome-related organelle (23), and NAADP releases Ca2+ from lysosome-related organelles in most but possibly not all cell types (24, 25). The pharmacological agents used to demonstrate a role for Ca2+ influx such as voltage-gated channel inhibitors (e.g. verapamil) (2, 3, 6, 26) and the store-operated channel inhibitor SKF96365 (5) also block NAADP-mediated Ca2+ release (21, 27, 28). Lastly, NAADP is known to trigger and coordinate inositol 1,4,5-trisphosphate-mediated responses in other systems (29–32). In summary, sperm-produced NAADP is injected into the sea urchin egg and participates in its activation (18, 33), but a direct role for NAADP in the acrosome reaction has not been explored.
We investigated whether NAADP plays a role during the acrosome reaction. We report the presence of NAADP-sensitive channels in sperm that release Ca2+ from the acrosome. Additionally, we found that an increase in Ca2+ stimulated NAADP synthesis. We conclude that the initial Ca2+ increase stimulates NAADP production, which releases Ca2+ from the acrosome that synergistically contributes to acrosomal exocytosis. Our results add to the accumulating evidence that mobilization of Ca2+ from intracellular stores is stimulated not just by inositol 1,4,5-trisphosphate but also by NAADP.
EXPERIMENTAL PROCEDURES
Materials
Lytechinus pictus sea urchins (Marinus Inc., Long Beach, CA) were used for all experiments. Fura-2 Ca2+-sensing dye was purchased from Molecular Probes (Paisley, UK), and complete EDTA-free protease inhibiter tablets were from Roche Applied Science (East Sussex, UK). All other chemicals were purchased from Sigma-Aldrich except where otherwise indicated below. NAADP was synthesized with Aplysia cyclase (34, 35); 13 mm NADP (Melford, UK), 100 mm nicotinic acid, pH 4.5, and 1 μg/ml ADP-ribosyl cyclase are combined in a final volume of 100 ml. The purity of NAADP was tested using the high-performance liquid chromatography procedure outlined below. Purified Aplysia cyclase was kindly provided by Prof. H. C. Lee (Dept. of Physiology, University of Hong Kong).
Marker Enzymes
The abundance of organelles in the subcellular fractions was determined with the marker enzymes Na+/K+-ATPase (plasma membrane), glucosaminidase (lysosomes), glucose-6-phosphatase (endoplasmic reticulum), succinate dehydrogenase (mitochondria), β-galactosidase (lysosomes), and acid phosphatase (lysosomes), as described previously (15, 16).
[32P]NAADP Binding
Sea urchin sperm were diluted 40% volume/volume in buffer containing 50 μm digitonin to permeabilize the membrane. 25 μl of a known amount of NAADP or NADP were added to the test tube. Following the first addition, 125 μl of sperm were added to this preparation, and the mixture was incubated at 25 °C for 10 min. Finally, 100 μl of [32P]NAADP, approximating 50,000 counts per min per tube, diluted in intracellular medium was added, and the mixture was incubated at 25 °C for a further 60 min. This preparation was then washed through a Brandel cell harvester, and the sperm containing the bound radioactivity was trapped in GF/B filter paper (28, 36).
45Ca2+ Flux Assay
Regenerative sea urchin intracellular medium was prepared with Chelex-treated intracellular medium containing 75 mm potassium oxalate, 3% w/v polyethylene glycol, 10 mm dithiothreitol, 1 mm sodium azide, and 500 μm digitonin containing ∼117 nCi 45Ca2+. Uptake of radioactive Ca2+ in sperm was initiated by adding sperm to a final concentration of 10% (volume/volume) to the intracellular medium containing an ATP-regenerating system, which contained phosphocreatine 10 mm, creatine phosphokinase 10 units/ml, and MgATP 1 mm. Uptake was performed for 60 min, with or without various inhibitors. At the end of the uptake period, NAADP was added, and the whole preparation was filtered using a cell harvester (Brandel), and the radioactivity trapped on the filters was counted with the Typhoon phosphorimaging device as described previously (36). Briefly, filter papers containing radioactivity were carefully wrapped with cling film and then placed in a cassette along with a storage phosphor screen (Amersham Biosciences). The screen was exposed for 24 h for 45Ca2+ samples and 1–4 h for the NAADP binding assay. The screen was later scanned using a Typhoon 9400 scanner (Amersham Biosciences) at a resolution of 100 μm. The resulting image was later analyzed using ImageQuant (Amersham Biosciences).
Solutions
Normal artificial sea water contained NaCl (435 mm), MgCl2 (40 mm), MgSO4 (15 mm), 11 mm CaCl2, KCl (10 mm), NaHCO3 (2.5 mm), and Tris-Cl (20 mm), adjusted to pH 8.1. Zero-Ca2+ artificial sea water was the same as normal except that no CaCl2 was added. Zero-Ca2+-EGTA artificial sea water was the same as zero-Ca2+ artificial sea water but also contained 3.8 mm EGTA. Gluconate-based intracellular-like media contained: N-methylglucamine (250 mm), potassium gluconate (250 mm), Hepes free acid (20 mm), and MgCl2 (1 mm), adjusted to pH 7.4. Double strength (2×) acetate-based intracellular-like media contained: N-methylglucamine (500 mm), potassium acetate (500 mm), Hepes free acid (50 mm), MgCl2 (2 mm), adjusted to pH 7.2. Percoll (25%) separation solution contained acetate-based intracellular medium (250 mm), 25% Percoll, phosphocreatine (10 mm), creatine phosphokinase (10 units/ml), MgATP (1 mm), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (10 μm), and double-deionized H2O (56 m).
Egg and Sperm Collection
Eggs from Lytechinus pictus were shed by intracoelemic injection of 1–2 ml 0.5 m KCl and then collected by overturning the urchin onto a 50-ml tube filled with artificial sea water. Eggs were de-jellied using a 100-μm Nitex mesh filter and washed twice in zero-Ca2+-EGTA-artificial sea water and then twice in zero-Ca2+-artificial sea water by dilution at a ratio of 1:10 volume/volume eggs to wash solution. Eggs were gently pelleted using manual centrifugation after each wash, and the supernatant was removed. Sperm were shed from L. pictus by intracoelemic injection of 1–2 ml of 0.5 m KCl and collected by overturning the urchin onto a dry Petri dish. The sperm was then spun in a 1.5-ml microcentrifuge tube for 1.5 min at 1,500 × g to pellet and remove contaminating coelomocytes, which are shed along with the sperm. The supernatant was removed, and then the loose sperm pellet was washed once with zero-Ca2+-EGTA-artificial sea water (modified to pH 6.0 to prevent the acrosome reaction) at a ratio of 1:15 volume/volume sperm to wash solution. We centrifuged this mixture at 3,000 × g for 15 min at 11 °C to pellet the sperm and allow the supernatant to be removed.
Permeabilization of Sperm
Diluted sperm in permeabilization buffer was incubated with various concentrations of digitonin and then treated with Trypan Blue solution before investigation with a phase-contrast microscope as reported previously (4).
Egg Jelly
Egg jelly was prepared by filtering 3 ml of harvested eggs through 100-μm mesh into 15 ml of artificial sea water. We then gently pelleted the eggs with a hand-cranked centrifuge and decanted the supernatant. The supernatant was then centrifuged at 15,000 × g for 10 min to pellet any eggs or large sheets of plasma membrane. The supernatant was then used as “egg jelly.”
Preparation of 50% Sea Urchin Egg or Sperm Homogenate
A sperm or egg homogenate was made to a total volume of 1 ml by combining 500 μl of lightly packed, washed sea urchin eggs or sperm with MgATP (1 mm), phosphocreatine (10 mm), creatine phosphokinase (10 units/ml), Roche Complete EDTA-free protease inhibitor mixture (1×), sulfinpyrazone (200 μm) (to block anionic transporter pumping of fura-2), fura-2 (1 mm), and gluconate-based intracellular-like medium in a chilled 1-ml glass manual homogenizer (Fisher Scientific, Pittsburgh, PA). The components were homogenized together with six gentle strokes, on ice. The presence of fura-2 in the homogenization mix allows this dye to enter vesicles as the homogenization transiently ruptures them.
Percoll Density Gradient Centrifugation
Microsomal and yolk fractions, containing lysosomes and yolk platelets, which are lysosome-related organelles in sea urchin eggs, were isolated from sea urchin egg homogenate as previously described (15, 24). Briefly, 1 ml of 50% egg homogenate was layered above 7 ml of 25% Percoll solution and spun at 30,000 × g for 28 min at 11 °C. Acceleration and deceleration rates were kept to a minimum to prevent jolting of the bands. Microsomes were identified as a thin white colored band near the center of the tube, while the thicker yellow yolk fraction separated toward the bottom. Sperm vesicular fractions, which may represent acrosomal vesicles or resealed plasma membrane, were isolated by the same method, except that the spin time was decreased to 23 min, and vesicles were identified as the single white band in the lower half of the tube.
Mn2+ Quenching of Luminal Fura-2
Intravesicular Ca2+ was measured in freshly prepared fura-2-containing vesicles (microsomes, lysosomes, and sperm vesicles) using a Mn2+ quenching technique. In brief, vesicles were diluted to 20% of their original concentration in 100 μl of a simple sampling media composed of Chelexed glucosamine intracellular medium. The ATP-regenerative system was excluded from this media to minimize Ca2+ reloading into vesicles. Extravesicular dye was quenched by the direct addition of 1 mm Mn2+, a competitive inhibitor of the Ca2+ binding site on fura-2, and the remaining intravesicular fluorescence was measured in a Novostar plate reader (BMG Labtechnologies, Offenberg, Germany) set to 340 nm excitation and 510 ± 30 nm emission. Fluorescence readings were taken at 8-s intervals separated by 1-s shaking times but paused briefly to allow for additions of various drugs.
NAADP Levels
As reported in detail previously (36), to determine NAADP levels we used the NAADP-binding protein from sea urchin (L. pictus) egg homogenate, which is highly selective for NAADP (37–40). First, we added 25 μl of test sample to each tube and then added 125 μl of 1% (volume/volume) sea urchin egg homogenate in intracellular medium and incubated the reaction for 10 min at 25 °C. To each tube we then added 0.2 nm of [32P]NAADP (∼50,000 cpm) diluted in 100 μl of intracellular medium and incubated the reaction for 10 min at 25 °C. Bound NAADP was then trapped onto Whatman GF/B filter papers using a Brandel Cell Harvester. We washed the filters 3 times with 1 ml of a buffer containing 20 mm Hepes and 500 mm potassium acetate, pH 7.4. We determined the amount of NAADP in each test sample with a standard curve containing known amounts of NAADP. All NAADP was synthesized in-house as described previously (28).
NAADP Synthesis by Base Exchange in Vitro
Michaelis-Menten kinetics was used to assess the ability of nicotinamide to inhibit the base exchange synthesis of NAADP. All samples contained 500 mm KCl, 20% sperm (volume/volume), 40 mm Hepes, pH 7, and were incubated at 22 °C for 1 h. To evaluate the effect of NADP, nicotinic acid was held at 100 mm and NADP was varied from 1 μm to 10 mm. To evaluate the effect of nicotinic acid, NADP was held at 10 mm, and nicotinic acid was varied from 50 μm to 100 mm. To evaluate the mechanism of nicotinamide inhibition, complete concentration curves for each substrate were generated in the presence of 50 mm nicotinamide. To evaluate the concentration-inhibition relationship, samples contained 100 mm nicotinic acid, 5 mm NADP, and nicotinamide were varied from 100 nm to 100 mm. Reactions were stopped by sonication, emersion of tubes into boiling water for 1 min, and then centrifugation at 13,000 × g for 5 min to pellet the denatured protein. The supernatant was injected onto an anion exchange column (3 × 30 mm) packed with AG-MP1 resin (Bio-Rad) and eluted with a concave upward gradient of trifluoroacetic acid (150 mm) at 4 ml min−1 as reported previously (28). Nucleotides were identified by elution time, and amounts were quantified by peak area. As the absolute amounts of NAADP synthesis varied between experiments likely due to sperm quality, we normalized the data for comparison, analysis, and presentation. Data were plotted and curve fitted with GraphPad Prism.
RESULTS AND DISCUSSION
NAADP Activates Mn2+-permeable Channels in Sea Urchin Eggs
Although fluorometry has been enormously successful in identifying and characterizing Ca2+-signaling pathways using sea urchin egg homogenates (15, 16, 28, 41, 42), we obtained only erratic and inconsistent results with sea urchin sperm homogenates. We were concerned that the sperm Ca2+ stores were too small relative to the volume of the cuvette, so that if only a small amount of Ca2+was released it would be buffered and not detected by the dye. Additionally, we worried that the Ca2+ pumps were damaged during the homogenization, which would prevent proper loading of organelles with Ca2+. Therefore, we turned to a technique that does not rely on stored Ca2+, but rather follows Mn2+, a surrogate for Ca2+, by monitoring the quenching of the metal-binding fluorescent dye fura-2 (43). In this technique, fura-2 is trapped either in the cytosol (44) or the lumen of an organelle (45), and Mn2+ is added to the bathing solution. Activation of cation channels allows Mn2+ to flow into the compartment and quench the fluorescence of fura-2.
To establish the validity of using a Mn2+-quenching technique to study NAADP activation of cation channels, we first turned to the established system of the sea urchin egg homogenate. Because L. pictus sea urchins do not have sufficient esterase activity to enable loading via incubation with the ester form of carboxylic acid dyes (28), we introduced the carboxylate form of the dye (fura-2 potassium salt) into the lumen of the vesicles via a homogenization step. Following homogenization of eggs in the presence of 1 mm fura-2 and Percoll-gradient separation of organelles, addition of the lysosomal fraction (containing trapped and free fura-2) to the well of a plate reader resulted in a large increase in fluorescence (Fig. 1A). Both to quench the extraorganellar dye and to provide a Mn2+ gradient, we added 1 mm MnCl2. The resulting quenching was biphasic (Fig. 1A). We attribute the first phase to quenching of extravesicular fura-2 and the second phase to quenching of luminal fura-2.
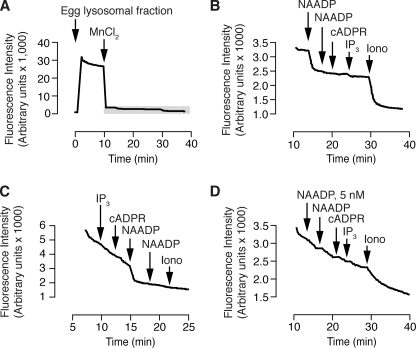
FIGURE 1.
Effect of Ca2+-releasing messengers on Mn2+-mediated quenching of fura-2 trapped in the lumen of yolk platelets. Yolk platelets are lysosome-related organelles in sea urchin eggs. A, addition of 100 μm MnCl2 rapidly decreases fluorescence. The gray box highlights the area expanded in panel B. B, basal rate of fura-2 quenching is accelerated by NAADP (500 nm) and ionomycin (5 μm) but not a second addition of NAADP (500 nm) or inositol 1,4,5-trisphosphate (10 μm) or cADP-ribose (1 μm). C, the effect of a messenger is not affected by the order of addition (concentrations as in B). D, pre-treatment with a low concentration of NAADP (5 nm) abolishes the subsequent response to supramaximal NAADP (500 nm) demonstrating diagnostic self-desensitization. Other concentrations are as in panel B.
To determine whether NAADP activated cation channels in vesicles containing fura-2, we added NAADP. Addition of a supramaximal NAADP concentration (500 nm (16, 28, 41)) resulted in an increase in the rate of fura-2 quenching (Fig. 1B). A second addition of the same concentration of NAADP did not accelerate the rate of quenching, indicating that the action was saturable and mediated by a biological (receptor) process and not a physical-chemical process (ionophore). Addition of cADP-ribose (1 μm) and inositol 1,4,5-trisphosphate (10 μm) had no effect on the rate of quenching, but the cation ionophore ionomycin (5 μm) stimulated further quenching (Fig. 1B). Changing the order of addition of the Ca2+-releasing messengers also revealed that only NAADP accelerated the Mn2+ quenching of fura-2 (Fig. 1C). These results are entirely consistent with previous reports of NAADP action at a physically separate store (16, 46, 47) identified as a lysosome-related organelle (24). As a further test for specificity for the action of NAADP, we employed the unique and diagnostic desensitization exhibited by NAADP in which a low concentration of NAADP completely desensitizes the response to a second addition of even supramaximal NAADP in both homogenized and intact sea urchin eggs (37, 48, 49). Addition of 5 nm NAADP did not affect the rate of fura-2 quenching and prevented the increase in the rate of quenching by a subsequent addition of supramaximal NAADP (500 nm) (Fig. 1D). Combined, these data demonstrate that Mn2+ quenching of luminal fura-2 can be used to monitor NAADP activation of cation channels.
NAADP Activates Mn2+-permeable Channels in Sea Urchin Sperm
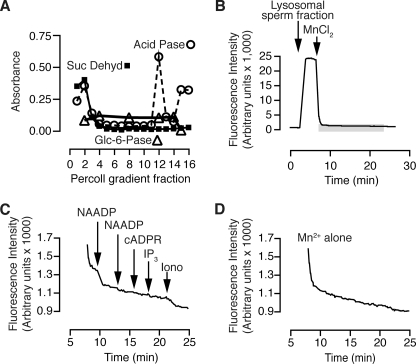
To determine whether NAADP can activate cation channels in sea urchin sperm, we applied the Mn2+ quenching method to homogenized sperm. Sperm were homogenized in the presence of 1 mm fura-2, and the resulting vesicles were run over a Percoll-density gradient both to remove extravesicular fura-2 and to enrich a vesicle fraction. To partially identify the Percoll fractions, we tested each fraction for enzymes accepted as markers of mitochondria (succinate dehydrogenase), endoplasmic reticulum (glucose-6-phosphatase), and lysosomes (acid phosphatase). We selected the fraction with the highest acid phosphatase activity (Fraction 12, Fig. 2A) and pipetted an aliquot into a plate reader well. As with the sea urchin egg lysosomal fraction (Fig. 1A), there was a large increase in fluorescence that then quenched in a biphasic manner (Fig. 2B). The addition of NAADP (500 nm) induced a rapid decrease in fluorescence, which was not induced with a second addition of NAADP (Fig. 2C). Neither cADP-ribose (1 μm) nor inositol 1,4,5-trisphosphate (10 μm) affected the rate of quenching, whereas the cation ionophore ionomycin (5 μm) did (Fig. 2C). Compared with the lysosomal fraction of eggs (Fig. 1B), the sperm fraction quenched more rapidly even in the absence of pharmacological agents (Fig. 2D), indicating leakier membranes. Nevertheless, NAADP and ionomycin clearly accelerated this rate of quenching (Fig. 2C). In summary, sea urchin sperm contain a cation channel that is selectively activated by NAADP.
FIGURE 2.
Effect of NAADP on Mn2+-mediated quenching of fura-2 trapped in the lumen of vesicles isolated from sperm. A, separation of fractions from homogenized sperm with density-gradient centrifugation and distribution of marker enzymes. Marker enzymes identify the endoplasmic reticulum (Glc-6-Pase, glucose-6-phosphatase), lysosomes (Acid Pase, acid phosphatase), and mitochondria (Suc Dehyr, succinate dehydrogenase). Fraction density increases from left to right. B, addition of 100 μm MnCl2 decreased fura-2 fluorescence. The area highlighted in the gray box is expanded in panel B. C, basal rate of fura-2 quenching is accelerated by supramaximal NAADP (500 nm) and ionomycin (5 μm) but not inositol 1,4,5-trisphosphate (10 μm) or cADP-ribose (1 μm). D, basal rate of fura-2 quenching in the absence of any second messenger additions.
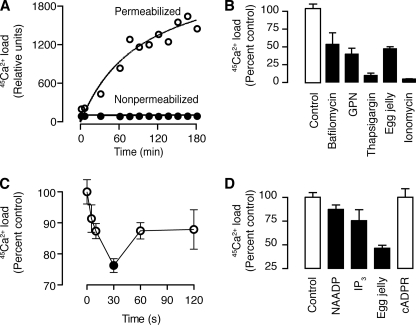
NAADP is known to activate, directly or indirectly, plasma membrane Ca2+ channels (18, 20, 50), and our homogenized sperm fraction might have contained hybrid vesicles formed by plasma membrane and lysosomes, because these two membranes fuse during membrane repair (51) and during the acrosome reaction itself (2). To determine whether the NAADP-sensitive channel is on a physiologically relevant organelle rather than an artifact of homogenization, we used permeabilized sperm and followed Ca2+ uptake and release with 45Ca. We permeabilized sea urchin sperm with digitonin (50 μm) as reported previously (52) and found increasing 45Ca2+uptake with time (Fig. 3A). Neither non-permeabilized sperm (Fig. 3A) nor sperm incubated without ATP (not shown) exhibited 45Ca2+uptake. Ca2+ uptake was partially inhibited by bafilomycin (1 μm), an inhibitor of the V-type H+-ATPase (Fig. 3B), which is required to maintain the proton gradient necessary for Ca2+ uptake into acidic organelles (53), including lysosome-related organelles (yolk platelets) in sea urchin eggs (24, 42). Ca2+ uptake was also partially inhibited by glycylphenylalanine 2-naphthylamide (50 μm) (Fig. 3B), an osmotic disruptor of lysosomes (54), and almost fully inhibited by thapsigargin (Fig. 3B), an inhibitor of the sarcoplasmic-endoplasmic reticulum calcium ATPase Ca2+ pump (55). Although sea urchin sperm do not express sarcoplasmic-endoplasmic reticulum calcium ATPase, they do express a secretory Ca2+ pump that is inhibited by thapsigargin at concentrations over 1 μm (56). This may suggest the presence of an H+/Ca2+ATPase (53). Ionomycin (5 μm) completely inhibited Ca2+ uptake (Fig. 3B), indicating that uptake was via sequestration into organelles and not just binding to the surface of the organelles over time. In summary, these data demonstrate active Ca2+ uptake by permeabilized sperm into a store with properties consistent with acidic organelles, likely the acrosome itself.
FIGURE 3.
45Ca2+ flux in permeabilized sea urchin sperm. A, time course of 45Ca2+ accumulation in sea urchin sperm. B, effect on 45Ca2+ uptake of thapsigargin (10 μm, inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase), bafilomycin (1 μm, inhibits V-H+-ATPase), GPN (50 μm, bursts lysosomes), and ionomycin (5 μm, Ca2+ ionophore). C, time course of 45Ca2+ release induced by 1 μm NAADP. D, effect of second messengers and egg jelly on 45Ca2+ release from permeabilized sea urchin sperm. For B–D, the error bars are mean ± S.E., n = 3, and filled bars or symbols are significantly different from the control (one-tailed t test, p ≤ 0.5).
NAADP Activates Ca2+-permeable Channels in Sea Urchin Sperm
To determine whether NAADP released Ca2+ from permeabilized sperm, we loaded permeabilized sperm with 45Ca2+for 2 h and then added 1 μm NAADP and filtered at various times. NAADP released Ca2+ within 10 s, the first time point (Fig. 3C). Similarly, inositol 1,4,5-trisphosphate also released Ca2+ (Fig. 3D), as reported previously for mammalian sperm (13). Inositol 1,4,5-trisphosphate sensitivity is also consistent with the presence of inositol 1,4,5-trisphosphate receptors on the acrosome of sea urchin sperm (13). In contrast, cADP-ribose (1 μm), did not release Ca2+ (Fig. 3D). To determine whether the stored Ca2+ was in the acrosome, we added egg jelly to permeabilized sperm loaded with 45Ca2+ for 2 h and found a large release of Ca2+ (Fig. 3D). Combined, these results demonstrate that in sea urchin sperm the stored Ca2+ is sensitive to inositol 1,4,5-trisphosphate receptors, NAADP and egg jelly, suggesting that the store is the acrosome.
NAADP Binds Specifically with High Affinity to Permeabilized Sperm
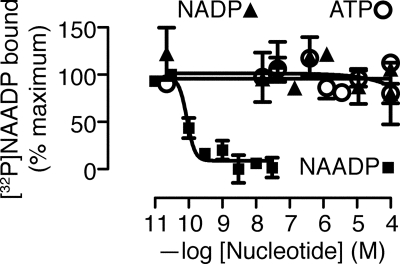
Given that NAADP is functionally active in sea urchin sperm, sperm must have an NAADP-binding protein. Using a competitive binding assay, we found that [32P]NAADP bound to permeabilized sperm with a Kd of 0.089 nm (confidence interval 0.054–0.147 nm) and a Hill coefficient of −3.6 (Fig. 4). Such high affinity is consistent with the binding site reported previously for NAADP in sea urchin eggs (37–39). In contrast to our current results, previous attempts to find an NAADP-binding protein were unsuccessful (17, 18). If the high endogenous level of NAADP were evenly distributed throughout the sperm, all the NAADP receptors would be bound with NAADP, and binding sites would not be detectable. Therefore, we suggest that in intact sperm the high amount of NAADP (17) is compartmentalized away from its receptor. Thus, when sperm are homogenized, as in the previous studies (17, 18), the NAADP receptors become occupied with endogenous NAADP, and not detectable with [32P]NAADP binding. In contrast, permeabilizing only the plasma membrane preserves the compartmentalized endogenous NAADP, maintaining the free receptors and making them detectable with [32P]NAADP binding. We base this explanation on one that has been proposed as an explanation for the 20 μm inositol 1,4,5-trisphosphate measured in pancreatoma cells, even though its receptor has nanomolar sensitivity for its ligand inositol 1,4,5-trisphosphate (57).
FIGURE 4.
Sea urchin sperm show specific NAADP binding. NAADP competes with [32P]NAADP binding, but the related nucleotides NADP or ATP do not compete. Absolute values of radioactivity vary between experiments and with time of exposure during detection of radioactivity with a phosphorimaging device and were therefore normalized. These particular data were obtained with 0.2 nm [32P]NAADP (1000 Ci/mmol) and had a Bmax of 9.1 × 107 absorbance units with a zero of 6.4 × 107 absorbance units.
NAADP Synthesis Is Stimulated by Ca2+
In sea urchin sperm, egg jelly induces an increase in NAADP levels (18, 19), and NAADP releases Ca2+ from the acrosome (this report). Therefore, we next investigated how NAADP integrates into what is known about the other Ca2+ signaling events that occur during the acrosome reaction. For inositol 1,4,5-trisphosphate, it has been suggested that phospholipase Cδ (7), which is Ca2+-sensitive (8), is stimulated by the initial Ca2+ influx (1, 2, 6, 13). The NAADP synthase in sea urchin sperm is also regulated by Ca2+ (52).
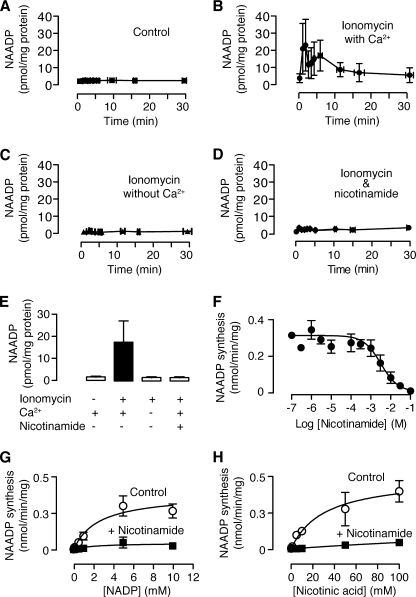
To determine whether Ca2+ stimulates NAADP synthesis, we increased cytosolic Ca2+ with the ionophore ionomycin. Dilution of sperm into artificial sea water, which is known to initiate motility (1–3), did not alter NAADP levels (Fig. 5A). In contrast, dilution of sperm into artificial sea water containing ionomycin (5 μm), which is known to trigger the acrosome reaction (1–3), resulted in an increase in NAADP (Fig. 5B). Sperm did not increase NAADP when diluted into artificial sea water containing ionomycin with no added Ca2+ and 1 mm EGTA (Fig. 5C), demonstrating the effect was due to Ca2+ and not ionomycin per se. Ca2+-stimulated synthesis of NAADP is consistent with our previous finding of an NAADP synthase enzyme that is regulated by Ca2+ (52).
FIGURE 5.
Ca2+ stimulates NAADP synthesis in sea urchin sperm via base exchange. A, NAADP levels in sperm diluted into normal artificial sea water (n = 7). B, NAADP levels in sperm diluted into normal artificial sea water containing the Ca2+ ionophore ionomycin (5 μm) with (11 mm Ca2+) (n = 11) or (C) without (no added Ca2+ and 1 mm EGTA) extracellular Ca2+ (n = 3). D, NAADP levels in sperm diluted into normal (11 mm Ca2+) artificial sea water containing the Ca2+ ionophore ionomycin (5 μm) and the base exchange inhibitor nicotinamide (50 mm) (n = 6). E, summary of the NAADP levels following the indicated treatments. Data are the means ± S.E. of the mean based on all values after time zero from panels A–D (n = 3–11). Filled bars are significantly different from the control (p < 0.01, Dunnett's multiple comparisons test after one-way analysis of variance, p = 0.006, performed on the logged data). F, nicotinamide concentration-inhibition relationship for NAADP synthesis by base exchange monitored by addition of exogenous substrates. G and H, Michaelis-Menten plots demonstrating mixed inhibition of NAADP synthesis monitored by exogenous substrate addition.
The NAADP synthase makes NAADP via a base exchange reaction in which the nicotinamide group on NADP is replaced with nicotinic acid (52), similar to that first reported for Aplysia ADP-ribosyl cyclase and CD38 (58). One product of this reaction, nicotinamide, is an inhibitor of both cyclization (59, 60) and base exchange (61). Because the characteristics of the NAADP synthase in sea urchin sperm (52) differ from those of the characterized ADP-ribosyl cyclases (62–64), we determined whether nicotinamide could inhibit NAADP synthase. In these experiments exogenous substrates (NADP and nicotinic acid) were added to the sperm. Nicotinamide inhibited NAADP synthase by a mixed mechanism (Fig. 5, G and H), in common with the characterized cyclases (60, 65, 66). We also determined the concentration-inhibition relationship for nicotinamide and found that the NAADP synthase has an IC50 of 3.1 mm (Fig. 5F), which is much higher than the tens of micromolar IC50 values of other cyclases (59, 61).
To determine whether the Ca2+-stimulated production of NAADP in intact sperm is due to base exchange, we preincubated sperm in artificial sea water containing 50 mm nicotinamide. Nicotinamide eliminated the Ca2+-stimulated production of NAADP (Fig. 5, D and E). Taken together, these data suggest that in sea urchin sperm NAADP synthesis can be increased by Ca2+ stimulation of the base exchange reaction.
NAADP May Play a Role in the Acrosome Reaction
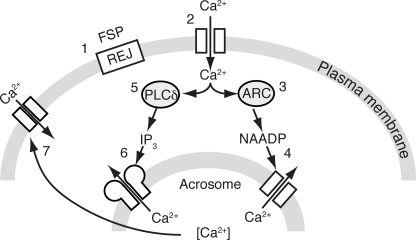
This is the first report detailing NAADP's role in sperm in any species. In regard to the acrosome reaction in sea urchin sperm, we suggest that NAADP plays a role that parallels that of inositol 1,4,5-trisphosphate as diagrammed in Fig. 6. Interaction of the fucose sulfate polymer with the receptor for egg jelly results in an initial transient Ca2+ increase (67). The Ca2+ increase stimulates not only phospholipase Cδ (8), but also NAADP synthase (52), possibly an ADP-ribosyl cyclase (62–64). As with inositol 1,4,5-trisphosphate in mammalian sperm (13), NAADP also releases Ca2+ from the acrosome. Ca2+ influx is then triggered either directly by the second messenger-mediated increase or indirectly via store-operated influx. Both Ca2+ entry and mobilization could contribute to Ca2+-mediated exocytosis of the acrosome. This model is consistent with the demonstration in pancreatic β-cells that NAADP may stimulate the release of Ca2+ from the secretory vesicles to stimulate insulin secretion (68). In conclusion, in the acrosome reaction NAADP plays a role that occurs alongside and synergizes with cyclic nucleotides, nitric oxide, and inositol 1,4,5-trisphosphate (1, 2).
FIGURE 6.
Signaling pathway model showing the role of NAADP in the acrosome reaction in sea urchin sperm. The fucose sulfate polymer (FSP) in the egg jelly activates the receptor of egg jelly (REJ, 1), which stimulates a plasma membrane channel resulting in a transient Ca2+ increase (2). The initial Ca2+ increase stimulates NAADP synthesis possibly by ADP-ribosyl cyclase (ARC, 3). NAADP binds to its receptor (Two-Pore Channel) on the acrosome and releases Ca2+ (4). These steps parallel the traditional pathway in which the Ca2+ increase stimulates phospholipase Cδ (PLCδ, 5) to produce inositol 1,4,5-trisphosphate that activates its receptor (6). Ca2+ release from the acrosome might trigger exocytosis directly or indirectly via store-activated Ca2+ entry (7).
Acknowledgments
We thank Clive Garnham (Dept. of Pharmacology, University of Oxford) for maintaining the sea urchins and helping with the plate reader and fluorometer, Prof. H. C. Lee (Dept. of Physiology, University of Hong Kong) for the gift of the ADP-ribosyl cyclase, and Drs. Sandip Patel and Dev Churamani (University College London) for help with synthesizing [32P]NAADP.
This work was supported in part by the Wellcome Trust Grant 075203/Z/04/Z.
- NAADP
- nicotinic acid adenine dinucleotide phosphate.
REFERENCES
- 1.Beltran C., Galindo B. E., Rodriguez-Miranda E., Sanchez D. (2007) Signal Transduction 7, 103–117 [Google Scholar]
- 2.Darszon A., Acevedo J. J., Galindo B. E., Hernández-González E. O., Nishigaki T., Treviño C. L., Wood C., Beltrán C. (2006) Reproduction 131, 977–988 [DOI] [PubMed] [Google Scholar]
- 3.Darszon A., Labarca P., Nishigaki T., Espinosa F. (1999) Physiol. Rev. 79, 481–510 [DOI] [PubMed] [Google Scholar]
- 4.Vacquier V. D., Moy G. W. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 2456–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirohashi N., Vacquier V. D. (2003) Biochem. Biophys. Res. Commun. 304, 285–292 [DOI] [PubMed] [Google Scholar]
- 6.Darszon A., Lüpez-Martínez P., Acevedo J. J., Hernández-Cruz A., Treviño C. L. (2006) Cell Calcium 40, 241–252 [DOI] [PubMed] [Google Scholar]
- 7.Coward K., Owen H., Poustka A. J., Hibbitt O., Tunwell R., Kubota H., Swann K., Parrington J. (2004) Biochem. Biophys. Res. Commun. 313, 894–901 [DOI] [PubMed] [Google Scholar]
- 8.Allen V., Swigart P., Cheung R., Cockcroft S., Katan M. (1997) Biochem. J. 327, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domino S. E., Garbers D. L. (1988) J. Biol. Chem. 263, 690–695 [PubMed] [Google Scholar]
- 10.Fukami K., Nakao K., Inoue T., Kataoka Y., Kurokawa M., Fissore R. A., Nakamura K., Katsuki M., Mikoshiba K., Yoshida N., Takenawa T. (2001) Science 292, 920–923 [DOI] [PubMed] [Google Scholar]
- 11.Zapata O., Ralston J., Beltrán C., Parys J. B., Chen J. L., Longo F. J., Darszon A. (1997) Zygote 5, 355–364 [DOI] [PubMed] [Google Scholar]
- 12.Herrick S. B., Schweissinger D. L., Kim S. W., Bayan K. R., Mann S., Cardullo R. A. (2005) J. Cell. Physiol. 202, 663–671 [DOI] [PubMed] [Google Scholar]
- 13.De Blas G., Michaut M., Treviño C. L., Tomes C. N., Yunes R., Darszon A., Mayorga L. S. (2002) J. Biol. Chem. 277, 49326–49331 [DOI] [PubMed] [Google Scholar]
- 14.González-Martínez M. T., Galindo B. E., de De La Torre L., Zapata O., Rodríguez E., Florman H. M., Darszon A. (2001) Dev. Biol. 236, 220–229 [DOI] [PubMed] [Google Scholar]
- 15.Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. (1987) J. Biol. Chem. 262, 9561–9568 [PubMed] [Google Scholar]
- 16.Lee H. C., Aarhus R. (1995) J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 17.Billington R. A., Ho A., Genazzani A. A. (2002) J. Physiol. 544, 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churchill G. C., O'Neill J. S., Masgrau R., Patel S., Thomas J. M., Genazzani A. A., Galione A. (2003) Curr. Biol. 13, 125–128 [DOI] [PubMed] [Google Scholar]
- 19.Churamani D. (2006) Properties of the Nicotinic Acid Adenine Dinucleotide Phosphate Binding Protein in Sea Urchin Eggs. PhD thesis, University College London [Google Scholar]
- 20.Moccia F., Lim D., Kyozuka K., Santella L. (2004) Cell Calcium 36, 515–524 [DOI] [PubMed] [Google Scholar]
- 21.Moccia F., Billington R. A., Santella L. (2006) Biochem. Biophys. Res. Commun. 348, 329–336 [DOI] [PubMed] [Google Scholar]
- 22.Moccia F., Nusco G. A., Lim D., Kyozuka K., Santella L. (2006) Dev. Biol. 294, 24–38 [DOI] [PubMed] [Google Scholar]
- 23.Dell'Angelica E. C., Mullins C., Caplan S., Bonifacino J. S. (2000) FASEB J. 14, 1265–1278 [DOI] [PubMed] [Google Scholar]
- 24.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 25.Galione A., Petersen O. H. (2005) Mol. Interv. 5, 73–79 [DOI] [PubMed] [Google Scholar]
- 26.Schackmann R. W., Eddy E. M., Shapiro B. M. (1978) Dev. Biol. 65, 483–495 [DOI] [PubMed] [Google Scholar]
- 27.Genazzani A. A., Mezna M., Dickey D. M., Michelangeli F., Walseth T. F., Galione A. (1997) Br. J. Pharmacol. 121, 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan A. J., Churchill G. C., Masgrau R., Ruas M., Davis L. C., Billington R. A., Patel S., Yamasaki M., Thomas J. M., Genazzani A. A., Galione A. (2006) in Methods in Calcium Signalling (Putney J. W. ed) pp. 265–333, CRC Press, Boca Raton [Google Scholar]
- 29.Santella L., Kyozuka K., Genazzani A. A., De Riso L., Carafoli E. (2000) J. Biol. Chem. 275, 8301–8306 [DOI] [PubMed] [Google Scholar]
- 30.Churchill G. C., Galione A. (2000) J. Biol. Chem. 275, 38687–38692 [DOI] [PubMed] [Google Scholar]
- 31.Cancela J. M., Churchill G. C., Galione A. (1999) Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 32.Yamasaki M., Masgrau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J., Galione A. (2004) J. Biol. Chem. 279, 7234–7240 [DOI] [PubMed] [Google Scholar]
- 33.Morgan A. J., Galione A. (2007) J. Biol. Chem. 282, 37730–37737 [DOI] [PubMed] [Google Scholar]
- 34.Lee H. C., Aarhus R. (1991) Cell Regul. 2, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munshi C., Thiel D. J., Mathews, Aarhus R., Walseth T. F., Lee H. C. (1999) J. Biol. Chem. 274, 30770–30777 [DOI] [PubMed] [Google Scholar]
- 36.Lewis A. M., Masgrau R., Vasudevan S. R., Yamasaki M., O'Neill J. S., Garnham C., James K., Macdonald A., Ziegler M., Galione A., Churchill G. C. (2007) Anal. Biochem. 371, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarhus R., Dickey D. M., Graeff R. M., Gee K. R., Walseth T. F., Lee H. C. (1996) J. Biol. Chem. 271, 8513–8516 [DOI] [PubMed] [Google Scholar]
- 38.Billington R. A., Genazzani A. A. (2000) Biochem. Biophys. Res. Commun. 276, 112–116 [DOI] [PubMed] [Google Scholar]
- 39.Patel S., Churchill G. C., Galione A. (2000) Biochem. J. 352, 725–729 [PMC free article] [PubMed] [Google Scholar]
- 40.Churamani D., Carrey E. A., Dickinson G. D., Patel S. (2004) Biochem. J. 380, 449–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H. C. (2005) J. Biol. Chem. 280, 33693–33696 [DOI] [PubMed] [Google Scholar]
- 42.Morgan A. J., Galione A. (2007) Biochem. J. 402, 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 44.Hallam T. J., Rink T. J. (1985) FEBS Lett. 186, 175–179 [DOI] [PubMed] [Google Scholar]
- 45.Hajnüczky G., Thomas A. P. (1994) Nature 370, 474–477 [DOI] [PubMed] [Google Scholar]
- 46.Lee H. C. (2000) Sci. STKE 2000, PE1. [DOI] [PubMed] [Google Scholar]
- 47.Lee H. C., Aarhus R. (2000) J. Cell Sci. 113, 4413–4420 [DOI] [PubMed] [Google Scholar]
- 48.Genazzani A. A., Empson R. M., Galione A. (1996) J. Biol. Chem. 271, 11599–11602 [DOI] [PubMed] [Google Scholar]
- 49.Churchill G. C., Galione A. (2001) J. Biol. Chem. 276, 11223–11225 [DOI] [PubMed] [Google Scholar]
- 50.Brailoiu G. C., Brailoiu E., Parkesh R., Galione A., Churchill G. C., Patel S., Dun N. J. (2009) Biochem. J. 419, 91–97 [DOI] [PubMed] [Google Scholar]
- 51.McNeil P. L., Kirchhausen T. (2005) Nat. Rev. Mol. Cell Biol. 6, 499–505 [DOI] [PubMed] [Google Scholar]
- 52.Vasudevan S. R., Galione A., Churchill G. C. (2008) Biochem. J. 411, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Docampo R., Moreno S. N. (1999) Parasitol. Today 15, 443–448 [DOI] [PubMed] [Google Scholar]
- 54.Jadot M., Comant C., Wattiaux-De Conick S., Wattiaux R. (1984) Biochem. J. 219, 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunaratne H. J., Vacquier V. D. (2006) Biochem. Biophys. Res. Commun. 339, 443–449 [DOI] [PubMed] [Google Scholar]
- 57.Horstman D. A., Takemura H., Putney J. W., Jr. (1988) J. Biol. Chem. 263, 15297–15303 [PubMed] [Google Scholar]
- 58.Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. (1995) J. Biol. Chem. 270, 30327–30333 [DOI] [PubMed] [Google Scholar]
- 59.Sethi J. K., Empson R. M., Galione A. (1996) Biochem. J. 319, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauve A. A., Munshi C., Lee H. C., Schramm V. L. (1998) Biochemistry 37, 13239–13249 [DOI] [PubMed] [Google Scholar]
- 61.Bacher I., Zidar A., Kratzel M., Hohenegger M. (2004) Biochem. J. 381, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis L. C., Morgan A. J., Ruas M., Wong J. L., Graeff R. M., Poustka A. J., Lee H. C., Wessel G. M., Parrington J., Galione A. (2008) Curr. Biol. 18, 1612–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Churamani D., Boulware M. J., Geach T. J., Martin A. C., Moy G. W., Su Y. H., Vacquier V. D., Marchant J. S., Dale L., Patel S. (2007) PLoS ONE 2, e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Churamani D., Boulware M. J., Ramakrishnan L., Geach T. J., Martin A. C., Vacquier V. D., Marchant J. S., Dale L., Patel S. (2008) Cell Signal 20, 2347–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Love M. L., Szebenyi D. M., Kriksunov I. A., Thiel D. J., Munshi C., Graeff R., Lee H. C., Hao Q. (2004) Structure 12, 477–486 [DOI] [PubMed] [Google Scholar]
- 66.Graeff R., Lee H. C. (2002) Biochem. J. 361, 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vacquier V. D., Moy G. W. (1997) Dev. Biol. 192, 125–135 [DOI] [PubMed] [Google Scholar]
- 68.Mitchell K. J., Lai F. A., Rutter G. A. (2003) J. Biol. Chem. 278, 11057–11064 [DOI] [PubMed] [Google Scholar]