Abstract
Heparan sulfate (HS) serves as a cell-surface co-receptor for growth factors, morphogens, and chemokines. These HS and protein binding events depend on the fine structure and distribution of domains along an HS chain. A given domain can vary in terms of uronic acid epimer, N- and O-sulfate, and N-acetate content. The most highly sulfated regions of HS chains, N-sulfated (NS) domains, play prominent roles in HS and protein binding. We have analyzed HS oligosaccharides from various mammalian sources and provide evidence that NS domains residing at the nonreducing end (NRE) are, on average, longer than those residing in the internal regions of the chain. Additionally, they are more highly sulfated than their internal counterparts. These features are independent of the sulfation pattern of the bulk HS chains. From disaccharide analysis, it is clear that NS domains do not always occupy HS NREs. However, when they do, they tend to terminate in a subset of N-sulfated disaccharides. Our observations are consistent with a significant role of NRE NS domains in HS-growth factor interactions.
Keywords: Extracellular Matrix, Glycosaminoglycan, Growth Factors, Heparan Sulfate, Mass Spectrometry (MS), Glycomics
Introduction
Heparan sulfate (HS)2 is covalently linked to HS proteoglycans that are expressed on the surface of nearly all adherent animal cells (1). HS chains interact with signaling molecules, including fibroblast growth factors (FGFs) (2, 3), hepatocyte growth factor (4), vascular endothelial growth factor (5), interferon-γ (6), and sonic hedgehog (7). The binding of HS to growth factors, cytokines, and chemokines makes it a key regulator of an extraordinary range of biological processes (8), and its ubiquity at the cell surface underscores its importance in cell signaling events.
The interaction of HS with its more than 200 protein partners (9) is possible because of the domain structure superimposed on the molecule during its biosynthesis. This multistep process, coordinated by many enzymes and isoforms thereof (8), is initiated on a tetrasaccharide structure, GlcUAβ1–3Galβ1–3Galβ1–4Xylβ1-, linked to a core protein via a Ser residue. Chain elongation occurs by alternating addition of GlcNAc and GlcUA monosaccharides directed by the exostosin family of enzymes (10). A number of subsequent enzymatic reactions modify the nascent heparan chain, and these are thought to occur simultaneously with chain polymerization.
The replacement of a subset of GlcNAc N-acetate groups with N-sulfate groups by N-deacetylase/N-sulfotransferase enzymes is a prerequisite reaction for subsequent modifications. These reactions create regions of contiguous N-sulfated disaccharides (NS domains) as well as regions of alternating N-sulfated and N-acetylated disaccharides (NA/NS domains). The majority of the HS chain, however, does not receive any N-sulfate modifications (NA domains). The NS domains are the principal substrates for additional reactions, the most common of which includes epimerization of GlcUA to IdoUA and 2O- and 6O-sulfation of IdoUA and GlcNS, respectively. More rarely, 3O-sulfation of GlcNS can occur. In total, 48 unique disaccharide structures can be described, but due to the specificities of the biosynthetic enzymes, not all occur naturally (10). HS biosynthesis is a nontemplate-directed process; mature structures are heterogeneous in nature and can be thought of as a distribution of glycoforms. Chain structure differs among cell, tissue, and organ types (11), but the organ-specific patterns are conserved from one individual to another (12). Mature HS chains are also edited by the post-biosynthetic actions of endosulfatases and heparanases, both of which affect the co-receptor function of HS in cell signaling events (13, 14).
According to the current model, NS domains, typically ranging from degree of polymerization (dp) 6 to dp16 (11), are flanked by NA/NS domains (15). In turn, these sulfated regions are spaced by NA domains containing repeats with predominantly acetylated GlcN residues (15, 16). The organization of HS domains in this fashion creates a variety of motifs available for protein binding. For example, FGFs have been shown to bind to the NS domains of HS chains (17), whereas binding of interleukin-8 and vascular endothelial growth factor occurs via multiple NS domains spaced by lowly sulfated domains (18, 19).
NS domains are structurally diverse (11) and typically include a small proportion of the length of a chain, relative to NA and NA/NS domains (16). They are primarily composed of GlcUA-GlcNS and IdoUA2S-GlcNS as well as other less abundant disaccharide units. About half of the total IdoUA and nearly half of the total 6O-sulfate content within a given chain are found in NS domains. Of the IdoUA present in these domains, most occur as IdoUA2S. NS domains of various lengths are found in HS chains, a feature that may be directed by the availability of the sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (20). Both the structural variability and spatial distribution of NS domains are essential for HS and protein binding events (21).
The nonreducing end (NRE) of HS chains is likely involved in many protein binding events due to its accessibility. However, the NRE of HS chains has not been extensively studied. We have employed two recently developed mass spectrometric methods in an investigation of the domain structure of HS from bovine and murine organs. These HS samples represent structures present within the different spatial contexts of an organism. The results show that HS chains terminate in both N-acetylated and N-sulfated disaccharide units. In addition, HS populations contain unique NS domains at the NRE. This pattern appears to be a feature of HS domain structure and may play a role in biochemical events regulated by HS and protein binding.
EXPERIMENTAL PROCEDURES
Materials
Heparin lyases I, II, and III from Flavobacterium heparinum were purchased from IBEX (Montreal, Quebec, Canada). Bovine organ HS preparations were a generous gift from Dr. Keiichi Yoshida and were isolated as described previously (16). Briefly, tissues were minced and treated with proteases, followed by release of glycosaminoglycans from core protein by alkali treatment. Subsequently, DNA was removed by enzymatic treatment and precipitation. Intact HS chains were recovered by ion-exchange chromatography. Murine organ HS preparations were the generous gift of Dr. Fuming Zhang and Dr. Robert Linhardt and were isolated as described previously (22). Briefly, tissues were minced, homogenized, and defatted. Subsequently, the samples were protease-digested and centrifuged, and glycosaminoglycans were released by alkali treatment. Glycosaminoglycans were recovered by cetylpyridinium chloride and methanol precipitation. Galactosaminoglycans were removed by chondroitinase treatment, and DNA was removed by treatment with endonucleases. HS was then desalted by size-exclusion chromatography. Bovine HS samples were found to be of very high purity, as assessed by absorbance at UV 232 nm during quantification experiments. Murine samples, however, were observed to have high background absorbance, possibly due to protein contaminants. Thus, they were further purified before subsequent sample workup. The murine HS samples were applied to a 300-μl DEAE-Sephacel (Sigma) column packed into the bottom of a 1-ml pipette tip. The column was equilibrated with 10 volumes of 0.1 m NaCl, 20 mm NaAc, pH 6.0. The samples were loaded and washed with 30 volumes of the same buffer. Samples were eluted with 2 m NaCl and then passed over a C18 reversed-phase MacroSpin column (The Nest Group, Southborough, MA). Finally, the samples were desalted using PD-10 columns (GE Healthcare). Murine and bovine HS samples were treated in the same fashion from this point on.
MATERIALS AND METHODS
Preparation of HS Di- and Oligosaccharides
For production of disaccharides, HS samples (10 μg each) were treated with 5 mIU each of heparin lyases I–III in 100 μl of 100 mm NaCl, 20 mm Tris-Cl, 1 mm CaOAc, pH 7.4, at 37 °C for 2 h. After this, another aliquot of heparin lyases was added, and the digestion was allowed to proceed overnight. NS domain oligosaccharides were produced by digestion with heparin lyase III using the same conditions as above. The lyase III digests were dried by centrifugal evaporation, reconstituted in 10 μl of 30% methanol, and purified/profiled by SEC-HPLC using a SuperdexTM peptide PC 3.2/30 column (GE Healthcare). The column was equilibrated and operated using a 50 mm ammonium acetate buffer in 10% acetonitrile. The region of the chromatogram corresponding to oligosaccharides (as detected by UV absorbance at 232 nm) was pooled and dried by centrifugal evaporation three times, each time with reconstitution of the sample in 800 μl of water.
Disaccharide Analysis Using Size-exclusion LC/MS
Disaccharides produced by exhaustive lyase digestion were analyzed directly after depolymerization using size-exclusion chromatography (SEC)-mass spectrometry as described previously (23). Briefly, HS samples (1 μg of material) were injected onto a SuperdexTM peptide PC 3.2/30 column (GE Healthcare) on line with an ABI SCIEX Q-Star Pulsar TOF mass spectrometer operating in the negative-ion mode. The isocratic mobile phase was 12.5 mm formic acid, pH 4.4, in 10% acetonitrile. Differentiation between Δ-unsaturated disaccharides that occur as isobars in the mass spectrometry mode was accomplished using tandem mass spectrometry methods following the rationale of previously developed methods (24, 25).
Oligosaccharide Analysis Using Amide-HILIC LC/MS
After desalting, lyase III-generated oligosaccharides were analyzed by amide-HILIC LC/MS as described previously (26, 27). Briefly, the samples (0.8 μg of lyase III-digested material) were injected onto a HILIC HPLC chip that was on line with an Agilent 6520 QTOF 6520 mass spectrometer operating in the negative ion mode. Samples were loaded at a solvent composition of 74% acetonitrile, 26% water. A gradient that finished at 0% organic phase was reached after 39 min. A post-column make-up flow of acetonitrile was used to stabilize negative ion electrospray (28). The instrument parameters were optimized to minimize loss of sulfate, which was assessed using the pentasulfated oligosaccharide Arixtra. This molecule contains 3.2 sulfates per disaccharide and is thus much more highly sulfated than any of the species analyzed in this study. Of particular importance for minimization of sulfate loss on the instrument used in this study is a gentle fragmentor voltage. A value of 100 V was chosen for this parameter. LC/MS of Arixtra was characterized by sulfate loss in the 2–3% (of total) range. In all cases, only the loss of one sulfate was observable from the test molecule. As in a previous study (28), we observed that for a given compound higher charge states are accompanied by higher losses of sulfate, and lower charge states are accompanied by higher propensities for ammonium adduction. We therefore include only middle charge states of a given compound in our glycan quantification. LC/MS runs were inspected for HS compounds using the “find compounds by formula” function of Agilent MassHunter software. All extracted ion chromatograms were manually inspected and integrated. An example showing extracted ion chromatograms produced from bovine intestine HS is shown in supplemental Fig. S1, with a corresponding mass spectrum shown in supplemental Fig. S2. HILIC provides partial resolution separation of HS oligosaccharides that differ only by sulfate content, and thus the previously described optimization of the instrument to minimize loss of sulfate is extremely important for accurate analysis. Summed mass spectra were produced for each extracted ion chromatogram, and identification of HS peaks was only made if the m/z of the candidate peak matched the theoretical value within 5 ppm mass error.
RESULTS
Analysis of Disaccharides from Bovine HS
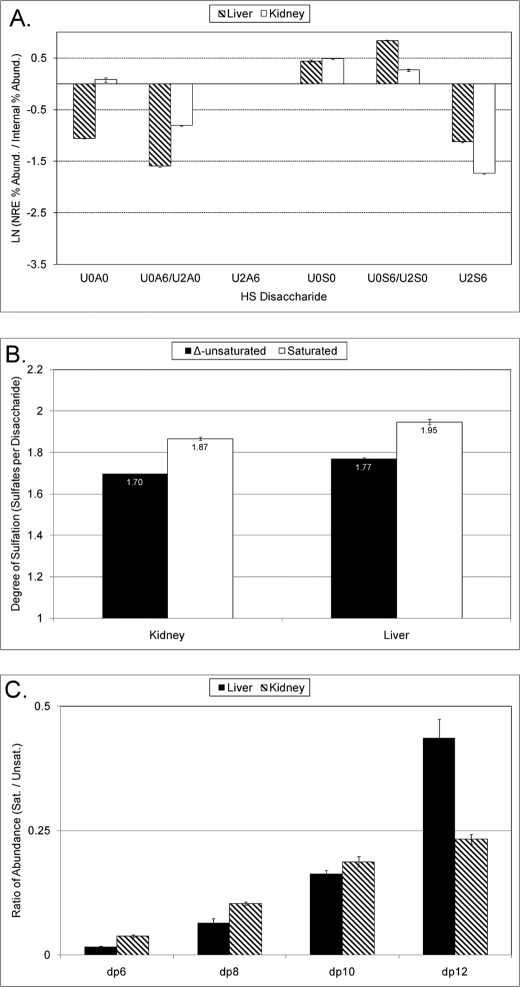
Bovine organ-derived HS populations (16) were digested to disaccharides by exhaustive treatment with heparin lyases I–III. The sample set included preparations from aorta, lung, intestine, and two fractions from kidney that were eluted from anion-exchange chromatography using either low (1.1 m) or high (1.25 m) salt concentrations. The disaccharide abundances were determined using SEC-MS (supplemental Fig. S2A), and the resultant structures are designated using the coding system of Lawrence et al. (29), summarized in Fig. 1A. Among the five preparations, aorta HS showed the highest abundance of the unsulfated disaccharide D0A0 and the lowest abundances of the N-sulfated disaccharides D2S6 and D0S6/D2S0. The abundances of disaccharides that constitute isomer pairs were determined by LC/tandem MS (supplemental Fig. S2, B and C) using the rationale of previously developed methods (24, 25). The SEC-MS disaccharide analysis was in good agreement with a previous analysis by Maccarana et al. (16) of HS samples from the same source.
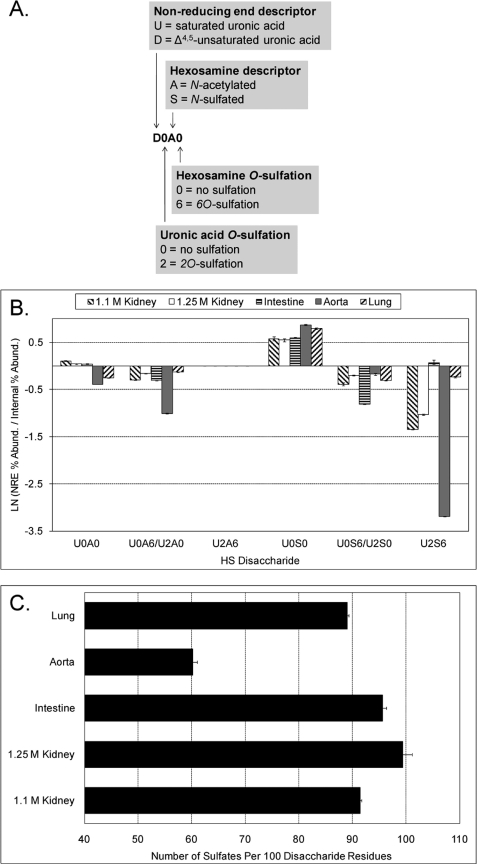
FIGURE 1.
Disaccharide analysis of bovine HS samples. Disaccharides were generated by exhaustive digestion with heparin lyases I–III and subsequently analyzed using SEC-MS. The profiles of Δ-unsaturated and saturated disaccharides are shown in supplemental Figs. 2 and 3, respectively. A, structural code used to designate the disaccharide structures detected in this study. The code is adapted from Lawrence et al. (29). The position within an HS chain (internal versus NRE) as well as sulfate and/or acetate modifications are specified with four characters. B, comparison of disaccharides at the NRE versus internal regions of the HS chain. These probabilities were calculated as a ratio of the abundance (Abund.) of a given saturated disaccharide divided by the abundance of its Δ-unsaturated counterpart and are expressed as a natural logarithm for more facile visualization. C, number of sulfates (N- and O-combined) per 100 disaccharide residues.
In addition to Δ-unsaturated structures, the SEC-MS platform quantifies saturated disaccharides that originate from the NRE of the HS chain (supplemental Fig. S3). By comparing the saturated and Δ-unsaturated disaccharide profiles, the probability that a given disaccharide will appear at the NRE compared with the internal region of the chain can be assessed (Fig. 1B) (23). Strikingly, all five of the organ HS preparations exhibit enrichment of the structure U0S0 at the NRE. Organ-specific variations in the abundances of other disaccharides at internal or NRE positions were also observed. For example, it can be seen that U2S6 has a very low probability of residing at the NRE of bovine aorta HS chains.
The disaccharide abundance data from supplemental Fig. S2A were used to calculate the number of sulfate groups per 100 disaccharide residues (Fig. 1C). It can be seen that aorta HS has a far lower average sulfation level than the other HS populations. Organ-specific differences in the amount of N-sulfation were also observed (supplemental Fig. S4).
Analysis of NS Domains from Bovine HS
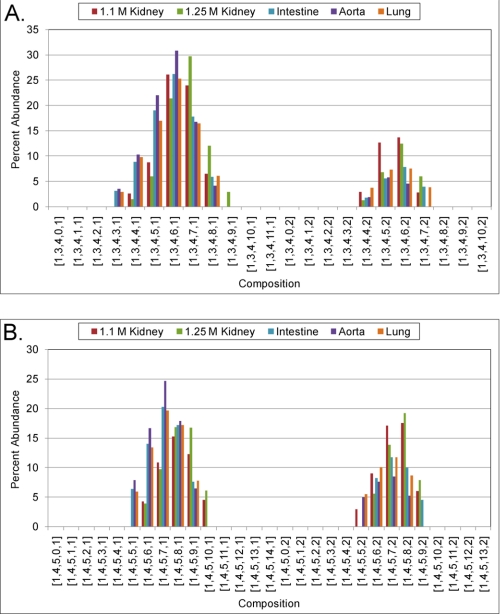
U0S0 has been detected previously in NS domains of bovine organ HS (16). Because the abundance of this disaccharide was increased at the NRE of the HS chains, we expanded our analysis to include the structure of extended NS domains. These domains were generated by exhaustive digestion with heparin lyase III (15). The samples were profiled and desalted using SEC-HPLC and subsequently analyzed using chip-based amide-LC/MS (27). The data were used to calculate the abundances of dp8 and dp10 NS domains (Fig. 2, A and B). The data are summarized using a glycan composition shorthand (ΔHexA, HexA, GlcN, SO3 (sulfate), Ac). The quantification of the structures shown in Fig. 2 was extremely reproducible, and the individual dp8 and dp10 profiles for each organ HS sample are shown (with error calculated as ± S.D.) in supplemental Figs. S5 and S6. The data in Fig. 2A show that the most abundant dp8 composition for aorta HS is [1,3,4,6,1], although the 1.25 m kidney HS sample has [1,3,4,7,1] as its most abundant composition. Interestingly, compositions from 1.25 m kidney that contain two Ac groups are significantly higher in abundance than those of aorta HS. The 1.1 m kidney HS sample shows a lower degree of sulfation than the 1.25 m kidney HS sample (as expected) with a similarly high abundance of compositions with two Ac groups. Similar trends are observed in Fig. 2B for dp10s. These data demonstrate that oligosaccharide compositions originating from NS domains are expressed in an organ-specific pattern. The variabilities observed are likely to reflect binding properties of the HS chains in different tissue environments and thus their biological activities.
FIGURE 2.
Oligosaccharide analysis of bovine HS NS domains. HS samples were digested exhaustively with heparin lyase III to produce oligosaccharides containing NS domains (15). Oligosaccharides were desalted using SEC-HPLC and analyzed using amide-LC/MS. Detected glycans are represented by the shorthand (ΔHexA, HexA, GlcN, SO3 (sulfate) Ac). A, oligosaccharide profiles of dp8 NS domains. B, oligosaccharide profiles of dp10 NS domains. Oligosaccharides containing more than two acetate groups for dp8 and dp10 were not detected.
Degree of Sulfation as a Function of Location of NS Domains from Bovine HS
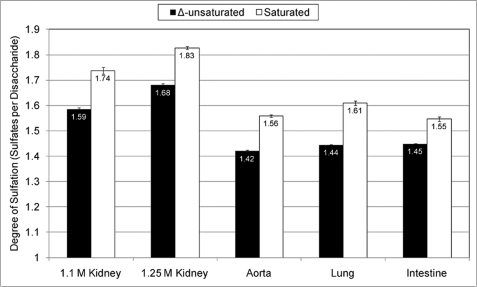
The amide-LC/MS platform used is sufficiently sensitive to detect saturated oligosaccharides derived from the NRE of HS chains (28). Compared with saturated disaccharides, saturated oligosaccharides serve as better approximations of the composition of the NRE of a given HS chain due to their increased length. We quantified the saturated counterparts of the one Ac containing dp8 oligosaccharides shown in Fig. 2A. In comparison with NS domain dp8 structures originating from the internal region of the chain, the NRE NS domain dp8 structures have, on average, a higher degree of sulfation (Fig. 3). From the data, it appears that the increase in degree of sulfation is relatively consistent for each of the preparations tested. We have previously observed such an increase for dp10 and dp12 NS domain oligosaccharides resultant from lyase III digestion of HS from porcine intestinal mucosa (27). On average, there is an 8.5% increase in the degree of sulfation for the dp8s at the NRE, with the intestine and lung samples having the smallest and largest increases, respectively.
FIGURE 3.
Degree of sulfation as a function of oligosaccharide location. The saturated counterparts of the dp8 oligosaccharides shown in Fig. 2A were quantified, and the average degree of sulfation for both sets of data were calculated. Saturated oligosaccharides originate from the NRE of the chain, whereas Δ-unsaturated oligosaccharides originate from the internal region of the chain.
Propensity for Longer NS Domains to Reside at the NRE of Bovine HS Chains
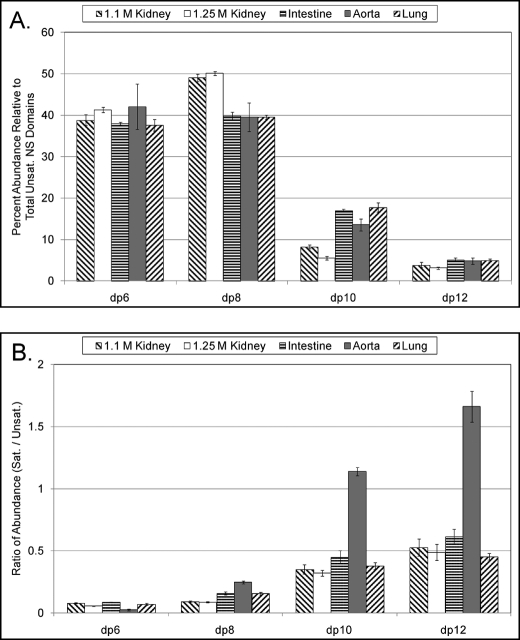
Digestion of the bovine organ HS samples with heparin lyase III produces a distribution of oligosaccharide lengths reflective of the polydispersity of the NS domains contained within the chain (15). We quantified the most abundant NS domain structure for dp6, dp8, dp10, and dp12 for each of the organ samples to assess the distribution of differently sized NS domains within the chains (Fig. 4A). For all samples, dp10 and dp12 NS domains are significantly lower in abundance than dp6 and dp8 NS domains. Additionally, we observed that the abundance of saturated versus Δ-unsaturated NS domain oligosaccharides differed depending on the oligosaccharide length. The ratios of saturated to unsaturated oligosaccharide abundances for dp6 to dp12 are shown in Fig. 4B. All of the preparations tested have in common a pattern whereby the ratio of the abundance of saturated NS domain: Δ-unsaturated NS domain oligosaccharides increases with increasing dp. This is consistent with the conclusion that the likelihood that an NS domain resides at the NRE increases with increasing oligosaccharide length. Although this feature is true for all bovine organs tested, there are organ-specific differences. In particular, for aorta HS, the ratio of abundance of saturated:Δ-unsaturated structures is greater than 1 for dp10 and dp12. This indicates that dp10 and dp12 NS domains have a very strong probability of residing at the NRE in this HS population.
FIGURE 4.
Propensity for longer NS domains to reside at the NRE. A, relative abundance of NS domains of increasing length within the HS chains. B, relative abundance of NRE NS domains of increasing length. To calculate these values, the most abundant saturated oligosaccharide for dp6, dp8, dp10, and dp12 was quantified, and a ratio between this value and the abundance of the corresponding Δ-unsaturated oligosaccharide was calculated. The structures used for the calculations were as follows: 1.1 m kidney, [1,2,3,4,1], [1,3,4,6,1], [1,4,5,8,1], and [1,5,6,10,1]; 1.25 m kidney, [1,2,3,5,1], [1,3,4,7,1], [1,4,5,8,1], and [1,5,6,10,1]; aorta, [1,2,3,4,1], [1,3,4,6,1], [1,4,5,7,1], and [1,5,6,8,1]; lung, [1,2,3,4,1], [1,3,4,6,1], [1,4,5,7,1], and [1,5,6,8,1]; intestine, [1,2,3,4,1], [1,3,4,6,1], [1,4,5,7,1], and [1,5,6,8,1].
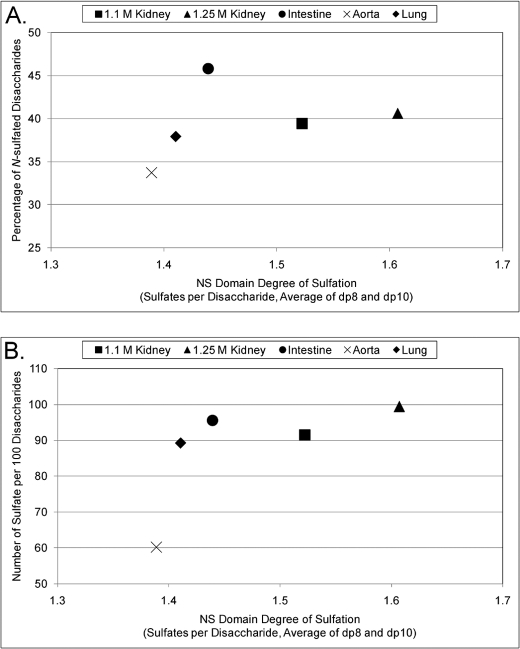
Comparisons between Disaccharide and Oligosaccharide Data for Bovine Organ HS
The combination of the disaccharide and oligosaccharide data for each of the bovine organ HS samples and the known specificity of the heparin lyases used in these experiments (30) enable an integrated view of HS domain structure. Specifically, the data on the degrees of NS domain sulfation (dp8 in supplemental Fig. S7A and dp10 in supplemental Fig. S7B), the percentages of N-sulfated disaccharides (supplemental Fig. S4), and the number of sulfates per 100 disaccharides (Fig. 1C) are combined as shown in Fig. 5, A and B. For each of these figures, we have averaged the degree of sulfation for the NS domains using the data from detected dp8s and dp10s. A similar pattern emerges from both figures, and this is likely due to a direct correlation between the number of N-sulfates and the total number of sulfates (a combination of N-sulfates and O-sulfates) for aorta, lung, and 1.1 m kidney HS. This correlation is not present, however, for 1.25 m kidney and intestine HS, and although 1.25 m kidney HS has fewer N-sulfated residues, it has the highest amount of O-sulfation.
FIGURE 5.
Comparisons of disaccharide and oligosaccharide data. Disaccharide and oligosaccharide data are visualized simultaneously in scatter plots. For both figures, the average degree of sulfation was calculated for dp8 and dp10 oligosaccharides (see supplemental Fig. 6, A and B for individual values). A, percentage of N-sulfated disaccharides (see supplemental Fig. 3) versus NS domain degree of sulfation. B, number of sulfates per 100 disaccharides (Fig. 1C) versus NS domain degree of sulfation.
The data also show that the most highly sulfated preparations (by disaccharide analysis: 1.25 m kidney, 1.1 m kidney, and intestine) differed considerably in the extent of sulfation of NS domains. It is therefore likely that the hybrid NA/NS domains of these preparations contain complementary differences in composition. Although the aorta, lung, and intestine samples have similar degrees of sulfation for their NS domains, they contain major differences in overall level of sulfation (with aorta having far fewer sulfates per 100 disaccharides than lung or intestine, see Fig. 1C). There are also major differences in N-sulfate content, where aorta and lung HS are more similar to one another than to intestine HS (Fig. 5A). Again, such differences point to organ-specific structures contained within NA/NS domains.
Analysis of Murine HS Reveals Consistent Features of NRE NS Domains
The enrichment of particular disaccharides at the NRE, as well as the existence of extended NS domains at this location, is a feature of HS that could play a role in protein binding events. As such, it was important to determine whether these features were present in HS from other mammalian sources and to rule out the possibility that the features we characterized in bovine HS were caused by the method of isolation. To this end, we tested murine liver and kidney HS, which were isolated using a completely different methodology than that used to isolate the bovine HS samples (see under “Materials and Methods”). In comparing the internal and NRE disaccharide profiles (Fig. 6A), we again observed an enrichment of the structure U0S0. In addition to this structure, the isomer pair U0S6/U2S0 was enriched. This observation is similar to our previous study of HS disaccharides from rat organs (23), and it may represent an evolutionary similarity between these two organisms.
FIGURE 6.
Analysis of murine liver and kidney HS. HS samples from two murine organs were analyzed to determine whether the NRE features discovered in bovine samples were present in the HS of other mammals and to eliminate the possibility that the isolation procedures used to purify the bovine HS created artifactual results. A, comparison of disaccharides at the NRE versus internal regions of the HS chain. These probabilities were calculated as a ratio of the abundance of a given saturated disaccharide divided by the abundance of its Δ-unsaturated counterpart and are expressed as a natural logarithm for more facile visualization. B, saturated counterparts of the dp8 oligosaccharides shown in supplemental Figs. S8 and S9 were quantified, and the average degree of sulfation for both sets of data were calculated. Saturated oligosaccharides originate from the NRE of the chain, whereas Δ-unsaturated oligosaccharides originate from the internal region of the chain. C, relative abundance of NRE NS domains of increasing length. To calculate these values, the most abundant saturated oligosaccharide for dp6, dp8, dp10, and dp12 was quantified, and a ratio between this value and the abundance of the corresponding Δ-unsaturated oligosaccharide was calculated. The structures used for the calculations were as follows: kidney, [1,2,3,4,1], [1,3,4,7,1], [1,4,5,9,1], and [1,5,6,11,1]; liver, [1,2,3,4,1], [1,3,4,7, 1], [1,4,5,9, 1], and [1,5,6,12,1].
We analyzed dp8 and dp10 NS domains from murine HS by chip-based amide-LC/MS (supplemental Figs. S8 and S9). The degree of sulfation for these structures (supplemental Fig. S10) was observed to depend on organ of origin, and murine liver HS had the highest degree of sulfation of any of the organ HS samples analyzed presently. A comparison between the degree of sulfation of internal versus NRE dp8 structures from murine HS is shown in Fig. 6B. Similar to the bovine HS samples, the NRE structures have a higher degree of sulfation, with 9.1 and 9.2% increases for kidney and liver, respectively. We also compared the abundance of saturated versus Δ-unsaturated NS domain oligosaccharides. The results (Fig. 6C) parallel the findings for the bovine samples, where the abundance of saturated NS domain structures increases with increasing dp. Organ-specific differences within this trend are present in murine kidney versus liver HS. Based on the analysis of bovine and murine HS, it appears that the presence of extended NS domains at the NRE is a general feature of HS chains from mammals.
DISCUSSION
Little is known about the manner in which the terminus of an HS chain is formed during biosynthesis. There has been some speculation that the enzyme exostosin-like 3 (EXTL3) is involved in this process (31). However, although small interfering RNAs directed against EXTL3 resulted in synthesis of longer HS chains, overexpression of EXTL3 did not significantly alter chain length (32). The mechanism of chain termination is a difficult issue to solve, especially in light of the many exostosin family members, and there is the possibility that sulfation, acetylation, and epimerization reactions may play a role. Few structural analyses of the NRE of HS have been completed. One study provided evidence that bovine kidney HS terminated in NS domains at least a hexamer in size and that these structures contained a GlcUA residue at the NRE (33). The epimerization state of the chain terminus was based on glucuronidase digestion of the only saturated disaccharide that was detected in a complete lyase depolymerization of HS.
Our analysis of bovine and murine HS demonstrates the presence of multiple saturated disaccharides corresponding to the NRE, some of which are N-sulfated and some of which are N-acetylated. This indicates that more than one type of domain can appear at the NRE. For bovine HS, the disaccharide composition at the NRE contained increased proportions of U0S0 when compared with the internal region of the chain. For murine HS, increased proportions of U0S0 and U0S6/U2S0 were observed.
U0S0 and U0S6/U2S0 are likely to be derived from NS domains, and these structures have in fact been observed by Maccarana et al. (16). Previously, we detected saturated dp10 and dp12 NS domain structures in HS from porcine intestinal mucosa (27). These NRE oligosaccharides were more highly sulfated than their corresponding Δ-unsaturated structures. We observe the same phenomenon for dp8 NRE NS oligosaccharides from all of the bovine and murine organ HS samples investigated (Figs. 3 and 6B). The increases in sulfate content range from 7 to 11% and represent a feature of NS domains at the NRE of HS. These data are consistent with the conclusion that there is a conserved mechanism of chain termination for cases where an NS domain appears at the NRE. Indeed, this has been proposed for chondroitin sulfate chains, where a 60-fold enrichment of 4,6SGalNAc has been observed (34).
In addition to their higher degree of sulfation, it appears that NRE NS domains can vary in length. Strikingly, the data suggest that as the length of the NS domain oligosaccharide increases, so does the probability that the domain will reside at the NRE (Figs. 4B and 6C). This trend was true for HS from all preparations tested, and the data are characterized by organ-specific differences. The most extreme of these is for bovine aorta HS, where dp10 and dp12 NRE NS domains are actually more abundant than the corresponding internal NS domains. This is evidence that NRE NS domains are a common feature for HS populations, even those with a low overall degree of sulfation. Like the increase in degree of sulfation, the means by which longer NS domains are formed at the NRE during HS biosynthesis is unknown. We show that NS domains more than dp8 in size are a relatively rare occurrence within HS chains (Fig. 4A). Despite this, the presence of long NS domains at the NRE is a consistent feature of HS. Such domains would be poised to bind to protein partners.
Combining the present data with information contained in previous reports (27, 33), we are able to propose a model for NRE NS domains. (a) HS chains that terminate in NS domains have a high probability of ending with particular N-sulfated disaccharides, and we observe enrichment of U0S0 in bovine, murine, and rat samples (present data and see Ref. 23). Despite this enrichment, it is clear from our disaccharide analysis that HS chains do not always terminate in NS domains. Therefore, the overall frequency with which NS domains occur at the NRE could be a feature dependent on cellular context. (b) NRE NS domains are, on average, more highly sulfated than those of the same length that originate from the internal regions of the molecule. (c) On average, NS domains that reside at the NRE of an HS chain are longer than those that originate from the internal portion of the chain.
These findings have important implications for HS and protein binding events that may occur at the NRE of the chain. This is especially relevant to FGF signaling through FGFR. A model proposed by Schlessinger et al. (35) consists of a symmetric heparin-stabilized dimer of two 1:1:1 FGF2·FGFR1·heparin complexes. In this model, the NREs of the two separate HS chains are responsible for high affinity assembly of the FGF·FGFR·heparin complex. There are also reports containing additional evidence for the role of the NRE of heparin or HS in the formation of the FGF signaling complex (36, 37). Within the constraints of the Schlessinger model, it has been proposed that the binding specificity of HS within the complex is resultant from interaction with a positively charged groove formed at the interface of the two FGF·FGFR dimers. Following this rationale, the individual HS binding specificities of either FGF or FGFR are secondary compared with the binding specificity of the molecules in complex (35, 38). Such an idea is appealing because the specificity of interactions involved in complex formation depends not only on the fine structure of HS but also on the particular FGF and FGFR variants that form the signaling complex.
It is important to note that there is a second asymmetric model of ternary complex formation that has been proposed by Pellegrini et al. (39). The authors describe a 2:2:1 complex that is not limited to the NRE of HS chains. Evidence exists for both the Schlessinger and Pellegrini complexes (40, 41), and it has recently been proposed that both complexes may be physiologically relevant, depending on the HS structures present (42).
For either model of FGF·FGFR·HS ternary complexes, it would seem reasonable that the positioning of NS domains at the end of an HS chain is a means by which cells regulate FGF signaling. Additional regulation would be imposed by the length of the NS domains at the NRE. A variety of reports have documented the ability of longer (≥dp8) HS or heparin structures to facilitate formation of ternary FGF signaling complexes (43, 44). In mitogenic assays, heparin oligosaccharides ranging in size from dp4 to dp16 were able to promote mitogenesis, but the longer oligosaccharides promoted activity to a much higher degree than did the shorter ones (36). Longer heparin oligosaccharides have been shown to be essential for the binding of FGF:FGFR pairs that interact with low affinity, for example FGF7 and FGFR2 IIIb (45). Another layer of regulation could be imposed by the total length of HS chains and by likely changes in the NA/NS domains flanking NRE NS domains. Changes in chain length are known to correlate with binding of specific FGFs during embryogenesis (46), and recent data showed that FGF2 signaling is only supported when HS chains are of a sufficient length (47). Finally, the proportion of NS domains (with respect to NA or NA/NS domains) at the NRE could change as a function of developmental state or other factors, serving to further fine-tune protein binding events at the chain terminus.
Our data contain unique structural information on the NRE domains that are applicable to the formation of HS-growth factor-receptor complexes such as FGF·FGFR·HS. It can be envisioned that this approach will be useful to shed light on the HS domain structures that participate in these binding events. It is likely that tandem mass spectrometric experiments will be useful for these determinations, which should include a comparison of the HS structures able to bind different combinations of FGF and FGFR variants.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant P41RR10888. This work was also supported by the Agilent Technologies Foundation through a Research Project Gift.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–10.
- HS
- heparan sulfate
- dp
- degree of polymerization
- FGF
- fibroblast growth factor
- FGFR
- fibroblast growth factor receptor
- GlcNS
- N-sulfoglucosamine
- HexA
- hexuronic acid
- HILIC
- hydrophilic interaction chromatography
- HPLC
- high performance liquid chromatography
- LC/MS
- liquid chromatography-mass spectrometry
- NA domain
- contiguously N-acetylated domain
- NA/NS domain
- alternating N-acetylated/N-sulfated domain
- NRE
- nonreducing end
- NS domain
- N-sulfated domain
- SEC
- size-exclusion chromatography
- Ac
- acetate.
REFERENCES
- 1.Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. (1992) Annu. Rev. Cell Biol. 8, 365–393 [DOI] [PubMed] [Google Scholar]
- 2.Rapraeger A. C., Krufka A., Olwin B. B. (1991) Science 252, 1705–1708 [DOI] [PubMed] [Google Scholar]
- 3.Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. (1991) Cell 64, 841–848 [DOI] [PubMed] [Google Scholar]
- 4.Lyon M., Deakin J. A., Mizuno K., Nakamura T., Gallagher J. T. (1994) J. Biol. Chem. 269, 11216–11223 [PubMed] [Google Scholar]
- 5.Ono K., Hattori H., Takeshita S., Kurita A., Ishihara M. (1999) Glycobiology 9, 705–711 [DOI] [PubMed] [Google Scholar]
- 6.Lortat-Jacob H., Turnbull J. E., Grimaud J. A. (1995) Biochem. J. 310, 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dierker T., Dreier R., Petersen A., Bordych C., Grobe K. (2009) J. Biol. Chem. 284, 8013–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esko J. D., Lindahl U. (2001) J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ori A., Wilkinson M. C., Fernig D. G. (2008) Front. Biosci. 13, 4309–4338 [DOI] [PubMed] [Google Scholar]
- 10.Esko J. D., Selleck S. B. (2002) Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 11.Turnbull J., Powell A., Guimond S. (2001) Trends Cell Biol. 11, 75–82 [DOI] [PubMed] [Google Scholar]
- 12.Ledin J., Staatz W., Li J. P., Götte M., Selleck S., Kjellén L., Spillmann D. (2004) J. Biol. Chem. 279, 42732–42741 [DOI] [PubMed] [Google Scholar]
- 13.Ai X., Kitazawa T., Do A. T., Kusche-Gullberg M., Labosky P. A., Emerson C. P., Jr. (2007) Development 134, 3327–3338 [DOI] [PubMed] [Google Scholar]
- 14.Xu X., Rao G., Quiros R. M., Kim A. W., Miao H. Q., Brunn G. J., Platt J. L., Gattuso P., Prinz R. A. (2007) J. Biol. Chem. 282, 2363–2373 [DOI] [PubMed] [Google Scholar]
- 15.Murphy K. J., Merry C. L., Lyon M., Thompson J. E., Roberts I. S., Gallagher J. T. (2004) J. Biol. Chem. 279, 27239–27245 [DOI] [PubMed] [Google Scholar]
- 16.Maccarana M., Sakura Y., Tawada A., Yoshida K., Lindahl U. (1996) J. Biol. Chem. 271, 17804–17810 [DOI] [PubMed] [Google Scholar]
- 17.Kreuger J., Salmivirta M., Sturiale L., Giménez-Gallego G., Lindahl U. (2001) J. Biol. Chem. 276, 30744–30752 [DOI] [PubMed] [Google Scholar]
- 18.Robinson C. J., Mulloy B., Gallagher J. T., Stringer S. E. (2006) J. Biol. Chem. 281, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 19.Spillmann D., Witt D., Lindahl U. (1998) J. Biol. Chem. 273, 15487–15493 [DOI] [PubMed] [Google Scholar]
- 20.Carlsson P., Presto J., Spillmann D., Lindahl U., Kjellén L. (2008) J. Biol. Chem. 283, 20008–20014 [DOI] [PubMed] [Google Scholar]
- 21.Kreuger J., Spillmann D., Li J. P., Lindahl U. (2006) J. Cell Biol. 174, 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warda M., Toida T., Zhang F., Sun P., Munoz E., Xie J., Linhardt R. J. (2006) Glycoconj. J. 23, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X., Zaia J. (2009) J. Biol. Chem. 284, 11806–11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad O. M., Ebel H., Uchimura K., Rosen S. D., Bertozzi C. R., Leary J. A. (2005) Glycobiology 15, 818–826 [DOI] [PubMed] [Google Scholar]
- 25.Hitchcock A. M., Costello C. E., Zaia J. (2006) Biochemistry 45, 2350–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staples G. O., Bowman M. J., Costello C. E., Hitchcock A. M., Lau J. M., Leymarie N., Miller C., Naimy H., Shi X., Zaia J. (2009) Proteomics 9, 686–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staples G. O., Naimy H., Yin H., Kileen K., Kraiczek K., Costello C. E., Zaia J. (2010) Anal. Chem, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staples G., Naimy H., Yin H., Kraiczek K., Killeen K., Costello C., Zaia J. (2009) Improved HILIC LC/MS Analysis of Heparinoids Using a Chip with Post-column Make-up Flow, in Proceedings of the 57th ASMS Conference on Mass Spectrometry and Allied Topics, Philadelphia, PA, June 1–5, 2009 [Google Scholar]
- 29.Lawrence R., Lu H., Rosenberg R. D., Esko J. D., Zhang L. (2008) Nat. Methods 5, 291–292 [DOI] [PubMed] [Google Scholar]
- 30.Ernst S., Langer R., Cooney C. L., Sasisekharan R. (1995) Crit. Rev. Biochem. Mol. Biol. 30, 387–444 [DOI] [PubMed] [Google Scholar]
- 31.Kim B. T., Kitagawa H., Tamura J., Saito T., Kusche-Gullberg M., Lindahl U., Sugahara K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7176–7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Busse M., Feta A., Presto J., Wilén M., Grønning M., Kjellén L., Kusche-Gullberg M. (2007) J. Biol. Chem. 282, 32802–32810 [DOI] [PubMed] [Google Scholar]
- 33.Wu Z. L., Lech M. (2005) J. Biol. Chem. 280, 33749–33755 [DOI] [PubMed] [Google Scholar]
- 34.Midura R. J., Calabro A., Yanagishita M., Hascall V. C. (1995) J. Biol. Chem. 270, 8009–8015 [DOI] [PubMed] [Google Scholar]
- 35.Schlessinger J., Plotnikov A. N., Ibrahimi O. A., Eliseenkova A. V., Yeh B. K., Yayon A., Linhardt R. J., Mohammadi M. (2000) Mol. Cell 6, 743–750 [DOI] [PubMed] [Google Scholar]
- 36.Wu Z. L., Zhang L., Yabe T., Kuberan B., Beeler D. L., Love A., Rosenberg R. D. (2003) J. Biol. Chem. 278, 17121–17129 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z., Coomans C., David G. (2001) J. Biol. Chem. 276, 41921–41929 [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi M., Olsen S. K., Goetz R. (2005) Curr. Opin. Struct. Biol. 15, 506–516 [DOI] [PubMed] [Google Scholar]
- 39.Pellegrini L., Burke D. F., von Delft F., Mulloy B., Blundell T. L. (2000) Nature 407, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 40.Harmer N. J., Ilag L. L., Mulloy B., Pellegrini L., Robinson C. V., Blundell T. L. (2004) J. Mol. Biol. 339, 821–834 [DOI] [PubMed] [Google Scholar]
- 41.Ibrahimi O. A., Yeh B. K., Eliseenkova A. V., Zhang F., Olsen S. K., Igarashi M., Aaronson S. A., Linhardt R. J., Mohammadi M. (2005) Mol. Cell. Biol. 25, 671–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodger S. J., Robinson C. J., Murphy K. J., Gasiunas N., Harmer N. J., Blundell T. L., Pye D. A., Gallagher J. T. (2008) J. Biol. Chem. 283, 13001–13008 [DOI] [PubMed] [Google Scholar]
- 43.Delehedde M., Lyon M., Gallagher J. T., Rudland P. S., Fernig D. G. (2002) Biochem. J. 366, 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loo B. M., Kreuger J., Jalkanen M., Lindahl U., Salmivirta M. (2001) J. Biol. Chem. 276, 16868–16876 [DOI] [PubMed] [Google Scholar]
- 45.Ostrovsky O., Berman B., Gallagher J., Mulloy B., Fernig D. G., Delehedde M., Ron D. (2002) J. Biol. Chem. 277, 2444–2453 [DOI] [PubMed] [Google Scholar]
- 46.Lindahl U., Kusche-Gullberg M., Kjellén L. (1998) J. Biol. Chem. 273, 24979–24982 [DOI] [PubMed] [Google Scholar]
- 47.Osterholm C., Barczyk M. M., Busse M., Grønning M., Reed R. K., Kusche-Gullberg M. (2009) J. Biol. Chem. 284, 34935–34943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.